Abstract
The relationship between muscle sympathetic nerve activity (MSNA) and diastolic blood pressure has been used to describe two sites for arterial baroreflex control of MSNA. By determining both the likelihood of occurrence for sympathetic bursts and the area of each burst for a given diastolic blood pressure, both a ‘gating’ and an ‘area’ control site has been described in normothermic humans. Assessing the effect of heat stress on these mechanisms will improve the understanding of baroreflex control of arterial blood pressure under this thermal condition. Therefore, the purpose of this study was to test the hypothesis that heat stress enhances arterial baroreflex control of burst gating and area. In 10 normotensive subjects (age, 32 ± 2 years; mean ± s.e.m), MSNA (peroneal) was assessed using standard microneurographic techniques. Five minute periods of data were examined during normothermic and whole-body heating conditions. The burst incidence (i.e. number of sympathetic bursts per 100 cardiac cycles) and the area of each burst were determined for each cardiac cycle and were placed into 3 mmHg intervals of diastolic blood pressure. During normotheric conditions, there was a moderate, negative relationship between burst incidence and diastolic blood pressure (slope = −2.49 ± 0.38; r2 = 0.73 ± 0.06; mean ± s.e.m), while area per burst relative to diastolic blood pressure exhibited a less strong relationship (slope = −1.13 ± 0.46; r2 = 0.45 ± 0.09). During whole-body heating there was an increase in the slope of the relationship between burst incidence and diastolic blood pressure (slope = −4.69 ± 0.44; r2 = 0.84 ± 0.03) compared to normothermia (P < 0.05), while the relationship between area per burst and diastolic blood pressure was unchanged (slope = −0.92 ± 0.29; r2 = 0.41 ± 0.08) (P = 0.50). The primary finding of this investigation is that, at rest, whole-body heating enhanced arterial baroreflex control of MSNA through increased sensitivity of a ‘gating’ mechanism, as indicated by an increase in the slope of the relationship between burst incidence and diastolic blood pressure. This occurrence is likely to afford protection against potential decreases in arterial blood pressure in an effort to preserve orthostatic tolerance during heat stress.
Arterial baroreflex control of blood pressure is mediated, in part, through the modulation of sympathetic neural outflow. In 2001, Kienbaum et al. examined the relationship between muscle sympathetic nerve activity (MSNA) and diastolic blood pressure by determining both the likelihood of an occurrence for a sympathetic burst and the area of that burst for a given diastolic blood pressure (Kienbaum et al. 2001). They suggested that during resting steady-state conditions, baroreflex control of MSNA is dependent on a ‘gating’ mechanism, essentially influencing the opening (burst incidence) and closing (no burst) of a theoretical gate. In contrast, the area of a sympathetic burst exhibited minimal or no relationship to diastolic blood pressure, suggesting that during steady-state resting conditions baroreflex control of MSNA does not dictate the magnitude of a particular burst. Kienbaum et al. suggested that these differential ‘sites’ of regulation were subject to influence from other central (e.g. central command, temperature, etc.) and peripheral (e.g. mechanoreflex, metaboreflex, etc.) inputs. However, the potential influence of whole-body heat stress on baroreflex control of burst incidence and burst area has not been examined.
Whole-body heat stress has been referred to as a ‘hyperadrenergic’ state (Rowell, 1990), given accompanied increases in cardiac output, resistance of non-cutaneous vascular beds (Rowell et al. 1971; Rowell, 1974) and MSNA (Niimi et al. 1997; Crandall et al. 1999a; Cui et al. 2002; Kamiya et al. 2003; Yamazaki et al. 2003). This increase in MSNA is evidenced by an increase in burst frequency (i.e. bursts min−1), burst incidence (i.e. bursts per 100 cardiac cycles) and total activity (arbitrary units min−1). While this increase in MSNA has been observed in a number of investigations, the effect of whole-body heating on the sensitivity of the ‘gating’ and ‘area’ mechanisms described by Kienbaum et al. regarding baroreflex control of MSNA remains unknown.
Thus, the aim of this investigation was to examine the effect of whole-body heating on the ‘gating’ and ‘area’ mechanisms of arterial baroreflex control of MSNA using similar analyses to that utilized by Kienbaum et al. (2001). We hypothesize that whole-body heating enhances the sensitivity of these mechanisms, such that for a given change in blood pressure there will be a greater change in burst incidence and burst area.
Methods
Data from 10 subjects (6 men, 4 women) were used in this analysis. The subjects' mean age (± s.e.m), height and weight were 32 ± 3 years, 170 ± 3 cm and 76 ± 5 kg, respectively. All procedures conformed to the standards set by the Declaration of Helsinki. Each subject gave written informed consent that was approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center and Presbyterian Hospital of Dallas.
Instrumentation and measurements
Internal temperature was measured using either a thermistor placed in the sublingual sulcus (Tsl) or from a telemetry temperature pill (Tcore). The telemetry pill correlates well with other methods of internal temperature measurement (O'Brien et al. 1998). Upon entering the laboratory, subjects were dressed in a tube-lined perfusion suit enabling the control of skin temperature via changes in the temperature of the water perfusing the suit. Non-invasive measures of arterial blood pressure were measured continuously using finger cuff photoplethysmography (Finapres, Ohmeda, Louisville, CO, USA). Arterial blood pressure was also measured by auscultation of the brachial artery (SunTech, Medical Instruments, Raleigh, NC, USA). Auscultation-derived diastolic blood pressures were used to correct diastolic blood pressures measured by finger cuff photoplethysmography. This was accomplished by determining the average diastolic pressure over the ∼30 s period during which the auscultation-derived diastolic blood pressures were collected and correcting for the offset between the two measures (i.e. adjusting the finger diastolic pressures to the auscultation-derived pressures). Heart rate was collected from an electrocardiogram signal (SpaceLabs, Redmond, WA, USA) interfaced with a cardiotachometer (1000 Hz sampling rate, CWE, Ardmore, PA, USA).
Multifibre recordings of MSNA were obtained using a tungsten microelectrode positioned in the common peroneal nerve. A reference electrode was placed subcutaneously ∼2–3 cm from the recording electrode. The position of the recording electrode was adjusted until a site was attained in which bursts of MSNA were identified using previously established criteria (Vallbo et al. 1979). The nerve signal was amplified, passed through a bandpass filter with a bandwidth of 700–2000 Hz and integrated with a time constant of 0.1 s (Iowa Bioengineering, Iowa City, IA, USA).
Protocol
Subjects rested in the supine position during the entire study. The tube-lined suit was perfused with 34°C water for the normothermic condition, from which 5 min of data were collected and analysed. Following normothermia, whole-body heating was initiated by perfusing 46–48°C water through the tube-lined suit to increase Tsl, or Tcore∼0.6°C, after which data were collected and analysed over a 5 min period.
Data analysis
Data were sampled at 200 Hz via a commercial data acquisition system (Biopac System, Santa Barbara, CA, USA) and analysed using LabView software (National Instruments, Austin, TX, USA). Continuous, 5 min periods of data were analysed during both normothermia and whole-body heating. All MSNA data were evaluated using a computer program that identified bursts on the basis of fixed criteria, including an appropriate latency after the R-wave of the electrocardiogram and a signal-to-noise ratio. Each identified burst was then visually confirmed by an experienced investigator.
Baroreflex control of MSNA was determined using analysis similar to that described by Kienbaum et al. (2001). Briefly, diastolic blood pressures for each cardiac cycle during the 5 min of steady-state data collection were grouped into 3 mmHg intervals (bins). Burst incidence (i.e. number of bursts per 100 cardiac cycles) for a given 3 mmHg diastolic pressure bin was determined and plotted against the mean pressure of that bin. This value (i.e. burst incidence) represents the percentage of cardiac cycles in which a burst occurred for a given blood pressure bin. The slope of this relationship between burst incidence and mean diastolic blood pressure (as determined per bin) was identified using linear regression analysis (SigmaStat 8.0). Such an analysis assesses whether the likelihood of a burst (i.e. burst incidence) is related to diastolic blood pressure and, if so, the magnitude of that relationship.
The relationship between diastolic blood pressure and the area of each sympathetic burst, only when a burst occurred, was also determined. When a burst has occurred, this analysis identifies whether the area of that burst is related to diastolic blood pressure (i.e. baroreflex control of burst area). Similarly, the associated diastolic blood pressure for each burst was grouped into 3 mmHg bins and the slope of the relationship between these variables (i.e. mean area/burst versus mean diastolic blood pressure for each 3 mmHg bin) was determined using linear regression analysis. For this analysis, only cardiac cycles during which a burst occurred were used; therefore, no cardiac cycles with ‘zero’ burst area were included.
Total MSNA was also determined for each diastolic blood pressure bin by calculating the average MSNA associated with each bin (i.e. total burst area of all cardiac cycles within a given diastolic blood pressure bin divided by the number of cardiac cycles that occurred within that bin). For this analysis, if a burst did not occur then a burst area value of zero was entered for that cardiac cycle, such that the average burst area of all cardiac cycles, regardless of whether a burst occurred, was determined.
For all linear regression analyses, the data were weighted for the number of cardiac cycles for each diastolic blood pressure bin. Weighting data in this manner is critical to remove bias caused by bins containing very few (i.e.< 5 cycles) cardiac cycles that otherwise would have equal contribution to the linear regression analyses relative to bins containing a large number of cardiac cycles (e.g.> 50 cycles).
Statistics
Data are expressed as means ± s.e.m Paired t tests were used to compare group data between normothermia and whole-body heating conditions. A P value of < 0.05 was considered significant.
Results
Thermal and cardiovascular responses to whole-body heating
Heart rate, systolic and diastolic blood pressures, as well as mean arterial pressure during normothermia and whole-body heating are presented in Table 1. Thermal responses are presented in Table 2. Typical thermal and cardiovascular responses associated with whole-body heating were observed, including a slight reduction in diastolic blood pressure.
Table 1.
Cardiovascular responses to whole-body heating
| Normothermia | Whole-body heating | |
|---|---|---|
| Heart rate (beats min−1) | 61.0 ± 2.9 | 81.1 ± 3.9* |
| MAP (mmHg) | 90.0 ± 2.6 | 86.2 ± 2.1† |
| DBP (mmHg) | 74.8 ± 2.6 | 66.8 ± 2.4* |
| SBP (mmHg) | 120.4 ± 3.0 | 125.1 ± 3.2* |
| Resp. rate (breaths min−1) | 17.7 ± 1.0 | 20.5 ± 1.6* |
MAP, mean arterial blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
P < 0.05
P = 0.06.
Table 2.
Thermal responses to whole-body heating
| Normothermia | Whole-body heating | |
|---|---|---|
| Skin blood flux (a.u.) | 24.4 ± 7.8 | 87.4 ± 11.5* |
| ΔSweat rate (mg cm−2 min−1) | — | 0.41 ± 0.1 |
| Mean skin temp. (°C) | 34.3 ± 0.3 | 37.5 ± 0.2* |
| Core temp. (°C) | 36.7 ± 0.1 | 37.3 ± 0.1* |
a.u., arbitrary units.
P < 0.05.
MSNA control mechanisms: gating and area
Basal MSNA data during normothermia and whole-body heating are presented in Table 3. Total activity, MSNA expressed as bursts per minute, and bursts per 100 cardiac cycles were significantly elevated by whole-body heating, whereas the average area of the bursts was not altered by heat stress. During normothermic conditions, the average slope of the relationship between sympathetic bursts per 100 cardiac cycles relative to diastolic blood pressure was −2.49 ± 0.38 (r2 = 0.73 ± 0.06). Whole-body heating increased this slope by approximately 2-fold (−4.69 ± 0.44, r2 = 0.84 ± 0.03; P < 0.05) (see Fig. 1). Representative data from one subject during normothermic and whole-body heating conditions are presented in Fig. 2.
Table 3.
Muscle sympathetic nerve activity responses to whole-body heating
| Normothermia | Whole-body heating | |
|---|---|---|
| Total activity (a.u.) | 239 ± 31 | 381 ± 41* |
| Bursts min−1 | 16.9 ± 2.8 | 29.9 ± 4.1* |
| Bursts (100 c.c.)−1 | 27.5 ± 4.5 | 36.2 ± 3.5* |
| Area burst−1 (a.u.) | 14.5 ± 0.9 | 13.1 ± 1.2 |
a.u., arbitrary units; c.c., cardiac cycles.
P < 0.05.
Figure 1.
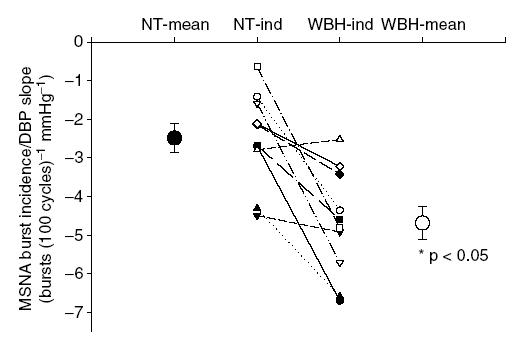
Individual and mean slopes of the relationship between muscle sympathetic nerve activity (MSNA) burst incidence (bursts (100 cardiac cycles)−1) and diastolic blood pressure (DBP) during normothermic (NT) and whole-body heating (WBH) conditions for each subject (lines and ‘-ind’) and for each thermal condition (large symbols; mean ± s.e.m).
Figure 2.
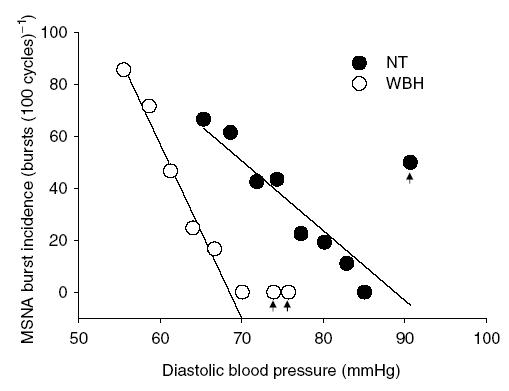
Slope of the relationship between MSNA burst incidence (bursts (100 cardiac cycles)−1) and diastolic blood pressure (DBP) during normothermic (NT) and whole-body heating (WBH) in one subject. Arrows indicate ‘potential’ outlier data that were included in regression analysis. However, the data points within these bins were 4 of 283 total points for the NT regression and 4 of 383 total points for the WBH regression for this subject.
The average slope of the relationship between changes in area per burst (considering only cardiac cycles when bursts occurred) relative to diastolic blood pressure during normothermia was −1.13 ± 0.46 (r2 = 0.45 ± 0.09). Whole-body heating did not change the slope of this relationship (−0.92 ± 0.29; r2 = 0.41 ± 0.08; P = 0.50) (see Fig. 3).
Figure 3.
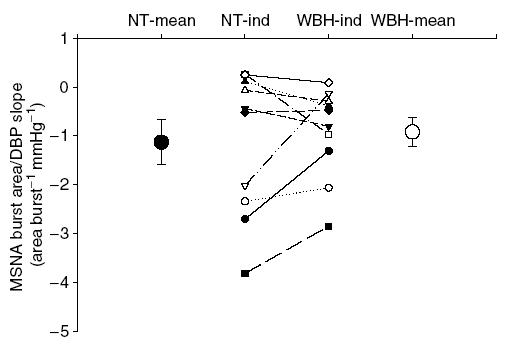
Individual and mean slopes of the relationship between MSNA burst area (area burst−1) and diastolic blood pressure (DBP) during normothermic (NT) and whole-body heating (WBH) conditions for each subject (lines and ‘-ind’) and for each group (large symbols; mean ± s.e.m).
When all cardiac cycles were considered, the average slope of the relationship between total activity per cardiac cycle and diastolic blood pressure was −0.37 ± 0.05 (r2 = 0.73 ± 0.06). Whole-body heating increased the slope of this relationship (−0.67 ± 0.07; r2 = 0.84 ± 0.03) compared to normothermia (P < 0.05) (see Fig. 4). Considering whole-body heating did not change the relationship between diastolic blood pressure and burst area, the effect of whole-body heating on the relationship between total activity and blood pressure can be entirely described by changes in burst incidence.
Figure 4.
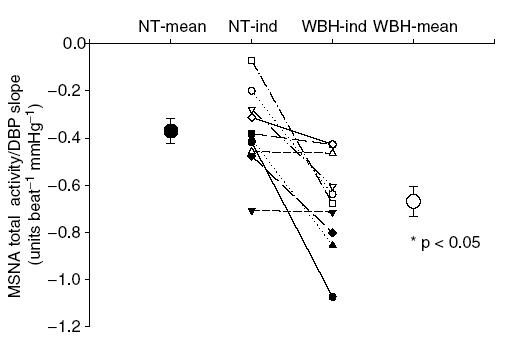
Individual and mean slopes of the relationship between MSNA total activity (arbitrary units (cardiac cycle)−1) and diastolic blood pressure (DBP) during normothermic (NT) and whole-body heating (WBH) conditions for each subject (lines and ‘-ind’) and for each thermal condition (large symbols; mean ± s.e.m).
Discussion
The primary finding of this investigation is that whole-body heating enhances arterial baroreflex control of MSNA through increased sensitivity of the ‘gating’ mechanism, as indicated by an increase in the slope of the relationship between sympathetic bursts per 100 cardiac cycles and diastolic blood pressure. Furthermore, whole-body heating did not alter arterial baroreflex control of burst area. These data suggest that for a given change in diastolic blood pressure there is a greater change in burst incidence during heat stress relative to normothermia. For example, for a given decrease diastolic blood pressure in a heat-stressed subject, there is a greater likelihood that a burst will occur relative to normothermic conditions. In contrast, for a given decrease in diastolic blood pressure, when a burst occurred, heating did not alter the size of that burst (expressed as burst area).
In the current study, the relationship between changes in MSNA and spontaneous changes in diastolic blood pressure was assessed and used as an index of arterial baroreflex function. A number of studies have utilized this analysis to reflect arterial baroreflex control of MSNA (Donadio et al. 2002; Ichinose et al. 2004b, c). During whole-body heating, arterial baroreflex control of burst incidence and total activity was enhanced compared to normothermia (see Figs 1 and 4). These findings are in contrast to the work of Cui et al. (2002) who demonstrated no change in arterial baroreflex control of total MSNA during whole-body heating when blood pressure was perturbed via pharmacological agents (i.e. modified Oxford technique). One possible explanation for the difference between these findings is the different techniques used to assess baroreflex function (i.e. pharmacologically induced versus spontaneous fluctuations in arterial blood pressure). Another possible explanation for this discrepancy is that central venous pressure can be altered during bolus infusions of sodium nitroprusside and phenylephrine (Martin & Charkoudian, 2005) and therefore cardiopulmonary baroreceptor stimulation may have contributed to the findings of Cui et al. In the present study, while steady-state central venous pressure is expected to have been reduced by whole-body heating, it is unlikely to have changed during the baroreceptor ‘stimulation’ within a given condition outside of changes that occur during spontaneous breathing.
Consistent with the findings of Kienbaum et al. (2001), the current investigation demonstrated a strong, negative relationship between burst incidence and diastolic blood pressure. During whole-body heating, the slope of the relationship between burst incidence and diastolic blood pressure was markedly increased (Fig. 1). Therefore, for a given decrease in diastolic blood pressure, there is a greater probability that a burst will occur during whole-body heating compared to normothermic conditions. Conversely, a given increase in diastolic blood pressure would result in a greater probability that a burst will not occur during whole-body heating due to the hypothesized changes in the gating mechanism.
In the current investigation, burst area and diastolic blood pressure also exhibited an inverse relationship during both normothermic and whole-body heating conditions, which is in contrast to that previously reported by Kienbaum et al. (2001) in normothermic subjects. However, there is a key difference in the analytical technique that probably explains the disparity between these findings. In the current investigation, MSNA data (i.e. burst incidence and burst area) were pooled into 3 mmHg diastolic blood pressure interval bins. This is in contrast to the method used by Kienbaum et al. (2001) in which MSNA data were pooled into 0.5 mmHg diastolic blood pressure bins, or were not binned at all. The present technique resulted in an increased r2, and therefore, a slightly stronger relationship for either dependent variable (i.e. burst incidence, or burst area). It is important to note that when the present data were analysed in a similar manner to that of Kienbaum et al. (2001), similar low r, and therefore, r2, values were observed (data not shown). As it pertains to the question addressed in the current investigation, the interpretation of the findings were unaffected by the analytical technique. That is, regardless of the method of data analysis (i.e. weighted in 3 mmHg intervals versus not pooling the data into diastolic blood pressure intervals), whole-body heating did not alter baroreflex control of burst area (i.e. slopes or r2) while baroreflex control of burst incidence was elevated.
Whole-body heating slightly increases respiratory rate (Cui et al. 2004). In the current investigation data were obtained while subjects were spontaneously breathing. Therefore, potential changes in respiration on baroreflex function cannot be eliminated. However, due to the relatively small changes in respiratory rate that occurred during this modest heat stress (see Table 2), it is unlikely that this would entirely account for the observed changes in baroreflex function.
A limitation of the interpretation of these data pertains to the relatively small range of pressure used to assess baroreflex function (i.e. ∼15 mmHg). It is possible that the mechanism(s) by which the arterial baroreflex modulates MSNA (i.e. baroreflex control of burst incidence and burst area), as well as the effect of whole-body heating on this mechanism(s), are different at different locations of the baroreflex function curve relative to that which was assessed during spontaneous breathing (e.g. closer to threshold or saturation of the curve). The findings of the current investigation therefore must be interpreted as arterial baroreflex control of MSNA at (and around) the operating point of the baroreflex function curve.
Whole-body heating markedly enhanced baroreflex control of MSNA via increased sensitivity of the ‘gating’ mechanism. However, the exact means for this increase remains unknown. One explanation for this response is a direct effect of elevated core temperature on central baroreflex pathways. Previous findings have demonstrated that increased temperature enhances neuronal firing rate in thermosensitive neurons (Boulant, 1998). While these findings were limited to the hypothalamic region of the brain, it remains possible that neurons involved in the pathways of baroreflex control are also thermosensitive and are responsible for the changes in control of MSNA with heating. Another possible explanation for the change in baroreflex responsiveness during heating is related to the slight decrease in diastolic blood pressure commonly observed when compared to normothermia (see Table 1). If in normothermia subjects are functioning near the saturation point of the baroreflex curve, heat stress may move the operating point to a steeper portion (i.e. greater gain) of the same baroreflex curve as diastolic blood pressure decreases. This could be interpreted as heat stress increasing the gain of the baroreflex when in fact the maximal gain has not been altered; rather, the operating point has shifted to a point closer to the maximum gain. Such a response, however, would not completely explain the observation of enhanced baroreflex control of burst incidence with heating. For example, data from a representative subject in Fig. 2 clearly illustrate very different burst incidences between thermal conditions within the diastolic blood pressure range of 65–70 mmHg. Such a response cannot be explained by a shift of the operating point on the same baroreflex curve. Rather, these data strongly suggest that heating shifted the entire baroreflex curve to lower diastolic blood pressures and increased the gain of that new curve.
Previous findings have demonstrated that unloading of the cardiopulmonary baroreceptors enhances arterial baroreflex control of the heart rate and arterial blood pressure (Ogoh et al. 2002, 2003, 2006; Ichinose et al. 2004a). Given that reductions in central venous pressure occur during whole-body heating (Rowell, 1986; Minson et al. 1998; Crandall et al. 1999b), it is possible that enhanced sensitivity of the ‘gating’ mechanism is the result of unloading the cardiopulmonary baroreceptors. While this may partially explain the effect of whole-body heating on baroreflex function, returning central venous pressure, and thus presumably cardiopulmonary baroreceptor loading, to pre-heat-stress values did not reduce MSNA (Crandall et al. 1999a). Further studies are needed to specifically address the potential role of these individual mechanisms in modulating MSNA response during whole-body heating.
In summary, whole-body heating increased arterial baroreflex control of MSNA via increased sensitivity of the ‘gating’ mechanism, while arterial baroreflex control of burst area was unaltered. Thus, during whole-body heating there is a greater probability that a sympathetic burst will occur for a given fall in diastolic blood pressure relative to normothermia, although the size of that burst (i.e. area) will not be affected. An increased sensitivity of burst incidence is likely to afford protection against potential decreases in arterial blood pressure in an effort to preserve orthostatic tolerance during heat stress.
Acknowledgments
The authors thank all of the participants involved with the study. This work was supported by NIH grant's HL61388 and HL67422.
References
- Boulant JA. Cellular mechanisms of temperature sensitivity in hypothalamic neurons. Prog Brain Res. 1998;115:3–8. doi: 10.1016/s0079-6123(08)62026-9. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Etzel RA, Farr DB. Cardiopulmonary baroreceptor control of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol. 1999a;277:H2348–H2352. doi: 10.1152/ajpheart.1999.277.6.h2348. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Levine BD, Etzel RA. Effect of increasing central venous pressure during passive heating on skin blood flow. J Appl Physiol. 1999b;86:605–610. doi: 10.1152/jappl.1999.86.2.605. [DOI] [PubMed] [Google Scholar]
- Cui J, Wilson TE, Crandall CG. Baroreflex modulation of sympathetic nerve activity to muscle in heat-stressed humans. Am J Physiol Regul Integr Comp Physiol. 2002;282:R252–R258. doi: 10.1152/ajpregu.00337.2001. [DOI] [PubMed] [Google Scholar]
- Cui J, Zhang R, Wilson TE, Crandall CG. Spectral analysis of muscle sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2004;286:H1101–H1106. doi: 10.1152/ajpheart.00790.2003. [DOI] [PubMed] [Google Scholar]
- Donadio V, Karlsson T, Elam M, Wallin BG. Interindividual differences in sympathetic and effector responses to arousal in humans. J Physiol. 2002;544:293–302. doi: 10.1113/jphysiol.2002.020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Kitano A, Hayashi K, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during mild orthostatic stress in humans. J Physiol. 2004a;557:321–330. doi: 10.1113/jphysiol.2003.057133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of control of muscle sympathetic nerve activity during orthostatic stress in humans. Am J Physiol Heart Circ Physiol. 2004b;287:H2147–H2153. doi: 10.1152/ajpheart.00215.2004. [DOI] [PubMed] [Google Scholar]
- Ichinose M, Saito M, Wada H, Kitano A, Kondo N, Nishiyasu T. Modulation of arterial baroreflex control of muscle sympathetic nerve activity by muscle metaboreflex in humans. Am J Physiol Heart Circ Physiol. 2004c;286:H701–H707. doi: 10.1152/ajpheart.00618.2003. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Michikami D, Hayano J, Sunagawa K. Heat stress modifies human baroreflex function independently of heat-induced hypovolemia. Jpn J Physiol. 2003;53:215–222. doi: 10.2170/jjphysiol.53.215. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EA, Charkoudian N. Changes in central venous pressure with vasoactive drug injections in humans. Clin Auton Res. 2005;15:121–125. doi: 10.1007/s10286-005-0262-y. [DOI] [PubMed] [Google Scholar]
- Minson CT, Wladkowski SL, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. J Appl Physiol. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, et al. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/s0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- O'Brien C, Hoyt RW, Buller MJ, Castellani JW, Young AJ. Telemetry pill measurement of core temperature in humans during active heating and cooling. Med Sci Sports Exerc. 1998;30:468–472. doi: 10.1097/00005768-199803000-00020. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Brothers RM, Barnes Q, Eubank WL, Hawkins MN, Purkayastha S, et al. The cardiopulmonary baroreflex is reset during dynamic exercise. J Appl Physiol. 2006;100:51–59. doi: 10.1152/japplphysiol.00804.2005. [DOI] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Monteiro F, Wasmund WL, Raven PB. Haemodynamic changes during neck pressure and suction in seated and supine positions. J Physiol. 2002;540:707–716. doi: 10.1113/jphysiol.2001.013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Volianitis S, Nissen P, Wray DW, Secher NH, Raven PB. Carotid baroreflex responsiveness to head-up tilt-induced central hypovolaemia: effect of aerobic fitness. J Physiol. 2003;551:601–608. doi: 10.1113/jphysiol.2003.046029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation: Regulation During Physical Stress. New York: Oxford University Press; 1986. Thermal stress; pp. 174–212. [Google Scholar]
- Rowell LB. Hyperthermia: a hyperadrenergic state. Hypertension. 1990;15:505–507. doi: 10.1161/01.hyp.15.5.505. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Detry JR, Profant GR, Wyss C. Splanchnic vasoconstriction in hyperthermic man – role of falling blood pressure. J Appl Physiol. 1971;31:864–869. doi: 10.1152/jappl.1971.31.6.864. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Hagbarth KE, Torebjork HE, Wallin BG. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- Yamazaki F, Yamauchi K, Tsutsui Y, Endo Y, Sagawa S, Shiraki K. Whole body heating reduces the baroreflex response of sympathetic nerve activity during Valsalva straining. Auton Neurosci. 2003;103:93–99. doi: 10.1016/s1566-0702(02)00140-6. [DOI] [PubMed] [Google Scholar]


