Abstract
Subjects quickly fatigue when they perform maximal voluntary contractions (MVCs). Much of the loss of force is from processes within muscle (peripheral fatigue) but some occurs because voluntary activation of the muscle declines (central fatigue). The role of central fatigue during submaximal contractions is not clear. This study investigated whether central fatigue developed during prolonged low-force voluntary contractions. Subjects (n = 9) held isometric elbow flexions of 15% MVC for 43 min. Voluntary activation was measured during brief MVCs every 3 min. During each MVC, transcranial magnetic stimulation (TMS) was followed by stimulation of either brachial plexus or the motor nerve of biceps brachii. After nerve stimulation, a resting twitch was also evoked before subjects resumed the 15% MVC. Perceived effort, elbow flexion torque and surface EMG from biceps, brachioradialis and triceps were recorded. TMS was also given during the sustained 15% MVC. During the sustained contraction, perceived effort rose from ∼2 to ∼8 (out of 10) while ongoing biceps EMG increased from 6.9 ± 2.1% to 20.0 ± 7.8% of initial maximum. Torque in the brief MVCs and the resting twitch fell to 58.6 ± 14.5 and 58.2 ± 13.2% of control values, respectively. EMG in the MVCs also fell to 62.2 ± 15.3% of initial maximum, and twitches evoked by nerve stimulation and TMS grew progressively. Voluntary activation calculated from these twitches fell from ∼98% to 71.9 ± 38.9 and 76.9 ± 18.3%, respectively. The silent period following TMS lengthened both in the brief MVCs (by ∼40 ms) and in the sustained target contraction (by ∼18 ms). After the end of the sustained contraction, the silent period recovered immediately, voluntary activation and voluntary EMG recovered over several minutes while MVC torque only returned to ∼85% baseline. The resting twitch showed no recovery. Thus, as well as fatigue in the muscle, the prolonged low-force contraction produced progressive central fatigue, and some of this impairment of the subjects' ability to drive the muscle maximally was due to suboptimal output from the motor cortex. Although caused by a low-force contraction, both the peripheral and central fatigue impaired the production of maximal voluntary force. While central fatigue can only be demonstrated during MVCs, it may have contributed to the disproportionate increase in perceived effort reported during the prolonged low-force contraction.
Human muscle fatigue can be defined as a loss of force-generating capacity in voluntary contractions. Defined in this way fatigue is not an abrupt event like exhaustion or task failure. It develops gradually from the start of the contraction. A quantitative measure of the fatigue can be obtained by interrupting the fatiguing exercise with brief maximal voluntary contractions (e.g. Bigland-Ritchie et al. 1986a; Lloyd et al. 1991; Zijdewind et al. 1998). This shows that the maximal force-generating capacity declines gradually during submaximal exercise, even though the target force can still be maintained.
Studies of contractions of the elbow flexors have shown that additional force can be evoked by stimulation of the motor nerve or the motor cortex during a maximal voluntary contraction (MVC, e.g. Gandevia et al. 1996). This indicates that despite maximal voluntary effort, not all motor units are driven at optimal firing rates (e.g. Merton, 1954; Belanger & McComas, 1981; Herbert & Gandevia, 1999). Furthermore, an increment in force evoked by stimulation of the motor cortex indicates that extra output from the motor cortex is available but is not being recruited by voluntary effort (Gandevia et al. 1996; Todd et al. 2003). During a sustained MVC, the increments in force evoked by both motor nerve and motor cortical stimuli increase (Gandevia et al. 1996). Thus, the ability to drive the muscle maximally decreases during the contraction. Therefore, while a large component of fatigue is due to peripheral intramuscular factors, central factors at spinal and supraspinal levels also play a role in the decline of maximal voluntary force (for review see Gandevia, 2001).
During a sustained submaximal isometric contraction, peripheral fatigue develops gradually (e.g. Bigland-Ritchie et al. 1986a; Zijdewind et al. 1998). At the same time, voluntary drive to the motoneurones is increased to recruit additional motor units or to increase the firing rate of active motor units in order to maintain the target force (Bigland-Ritchie et al. 1986a; Dorfman et al. 1990). Eventually the subject needs to make a maximal effort and can no longer maintain the task. At the moment of exhaustion, voluntary drive to the muscle is impaired (Löscher et al. 1996; Sacco et al. 1997). There may also be a progressive central contribution to fatigue before the moment of task failure (Zijdewind et al. 1998). One aim of this study was to determine if a fatiguing submaximal effort impairs voluntary drive measured during interposed brief MVCs and then to determine whether suboptimal output from the motor cortex contributes to the impairment.
EMG responses to transcranial magnetic stimulation of the motor cortex are altered during muscle fatigue produced by either maximal or submaximal voluntary contractions. During sustained or intermittent isometric MVCs, the short-latency excitatory response (motor evoked potential, MEP) increases in size and the subsequent period of EMG silence (silent period) lengthens (McKay et al. 1996; Taylor et al. 1996, 2000). As motoneurones become less excitable during a sustained MVC, the growth of the MEP implies that extra output is evoked from the motor cortex (Butler et al. 2003). In contrast, the increase in silent period duration indicates increased inhibition in the motor cortex (Taylor et al. 1996). MEP size also increases during sustained submaximal efforts, and lengthening of the silent period has been demonstrated in longer lasting submaximal (20% MVC) contractions which were held to the limit of endurance (Ljubisavljevic et al. 1996; Taylor et al. 1996; Sacco et al. 1997). While the lengthening of the silent period suggests that inhibitory changes within the cortex also occur with submaximal exercise, the increase in the MEP is not directly comparable to that occurring during maximal efforts but reflects the increasing motor cortical and motoneuronal excitability associated with voluntary motor unit recruitment.
Recovery from fatigue caused by weak contractions also differs from that caused by maximal contractions. After a sustained 2-min maximal effort, maximal voluntary force recovers over 10–15 min whereas after sustained submaximal contractions, which cause a similar force loss, recovery of maximal voluntary force and particularly twitch force are prolonged (Baker et al. 1993; Søgaard et al. 2003). After fatiguing maximal efforts, changes in the responses to stimulation of the motor cortex during contraction recover to control levels in ∼15 s and voluntary activation recovers in 1–2 min (Gandevia et al. 1996; Taylor et al. 1996; Todd et al. 2005). The recovery of these central changes after submaximal efforts is not well understood.
The purpose of this study was twofold: to study the relation between the development of peripheral and central fatigue during a prolonged submaximal contraction and to study the recovery of exercise-induced changes at both the peripheral and central levels. We hypothesized that part of the decrease in maximal voluntary force during a prolonged weak fatiguing contraction is due to central mechanisms including failure of supraspinal drive to the motoneurones, and that recovery of the central component of fatigue is faster than the peripheral component.
Methods
To examine fatigue caused by prolonged low-intensity exercise, subjects held a weak isometric contraction (15% MVC) of the elbow flexors for 43 min. At intervals during the prolonged contraction, subjects performed brief maximal efforts, and maximal voluntary force and voluntary activation, as well as EMG responses to transcranial magnetic stimulation, were measured. Recovery from fatigue was followed for 25 min after the prolonged contraction.
Subjects
Experiments were performed on the right elbow flexor muscles of nine healthy subjects (five males, four females; aged 23–57 years). Subjects gave their informed written consent. All experiments were conducted at the Prince of Wales Medical Research Institute, Sydney, Australia. All procedures were approved by the local ethics committee and were performed according to the Declaration of Helsinki.
Experimental setup
Subjects sat with the right elbow flexed at 90 deg and the forearm supinated. The forearm was fixed at the wrist to a vertical isometric myograph (force transducer linear to 2 kN; X-tran, Melbourne, Australia) which measured elbow flexion torque. During the prolonged contraction each subject received torque feedback on an oscilloscope showing a 15% MVC target as well as two lines marking a deviation of 5% from the target torque. The subject was encouraged to keep the torque between the lines. Surface electromyograms (EMG) were recorded with electrodes (Ag–AgCl, 10 mm diameter) overlying the muscle bellies of biceps brachii, brachioradialis and the lateral head of the triceps brachii. EMG signals were amplified and filtered (16–1000 Hz). Torque and EMG signals were sampled (2000 Hz) for later analysis using a data acquisition system (CED 1401 interface, Spike 2 software, Cambridge Electronic Design, Cambridge, UK).
Rating of perceived effort
During the prolonged contraction subjects were asked after 1 min of contraction and thereafter every third minute to score the effort required to produce the target torque on a modified Borg scale from 0 (‘infinitely small’) to 10 (‘extremely large’) (Borg, 1990).
Motor nerve stimulation
Electrical stimuli were delivered to intramuscular nerve fibres of the biceps brachii and brachialis via a cathode located over the motor point of the muscle (midway between the anterior edge of deltoid and the proximal elbow crease with the elbow flexed to 90 deg) and an anode positioned over the bicipital tendon. For each subject the intensity of stimulation was set by gradually increasing the intensity until no further increase in twitch torque was evoked in the relaxed muscle by a single stimulus (100 µs duration, constant current; DS7A, Digitimer, Welwyn Garden City, UK). During the experiment, pairs of electrical stimuli with an interstimulus interval of 10 ms were delivered with an intensity set at 20% above that required for a single stimulus to produce a resting muscle twitch of maximal amplitude (120–400 mA).
Brachial plexus stimulation
Single electrical stimuli (100 µs duration) were delivered to the brachial plexus via a cathode in the supraclavicular fossa and an anode on the acromion (constant current, DS7). The stimulus intensity (55–240 mA) was set at least 30% above the level required to produce a resting maximal compound muscle action potential (Mmax) in the biceps, brachioradialis and triceps muscles. The average amplitude of the resting Mmax was 20.4 ± 8.8 mV for biceps, 9.9 ± 3.6 mV for brachioradialis and 8.2 ± 3.6 mV for triceps.
Motor cortical stimulation
Transcranial magnetic stimulation (Magstim 200, Magstim Co., Dyfed, UK) was used to stimulate the motor cortex. A circular coil (13.5 cm outside diameter) positioned over the vertex elicited motor evoked potentials (MEPs) in the biceps, brachioradialis and triceps muscles. The direction of current flow in the coil was selected to activate preferentially the left motor cortex. Stimulator output (55–85% of maximum) was set during brief MVCs to obtain a large MEP in the biceps (> 50–60% of Mmax) and a small MEP in the triceps (< 20%Mmax). The stimulus intensity remained constant throughout the protocol.
Protocol
The protocol consisted of baseline measurements in fresh muscle, a prolonged fatiguing contraction (43 min) and a recovery phase (23 min). Initially, six sets of control contractions were performed with at least 2 min rest between sets. Each set comprised a brief MVC (2–3 s), a paired motor nerve stimulus 3–4 s later with the muscle at rest, and a brief 50% MVC after another 4 s. During each brief contraction, two stimuli were delivered 1 s apart. In three sets of contractions, stimulation of the motor cortex was followed by stimulation of the brachial plexus. In the other contractions, motor cortical stimulation was followed by paired motor nerve stimulation. For each MVC, the mean torque over a 200 ms period prior to the cortical stimulus was calculated. The largest MVC was then used for calculation of the target torque of 15% MVC. Five brief 15% MVCs (∼5 s) were then performed. During each submaximal control contraction, a motor cortical stimulus was delivered followed by a brachial plexus stimulus.
The prolonged fatiguing contraction consisted of 43 min of isometric elbow flexion at 15% MVC. After 1 min of contraction and then at 3-min intervals, subjects performed brief MVCs during which a cortical stimulus was given followed by either a brachial plexus (one of three MVCs) or motor point stimulus (two of three MVCs; Fig. 1A). After brachial plexus stimulation, subjects immediately returned to the target torque of 15% MVC whereas after motor point stimulation subjects relaxed completely for a few seconds while a second motor point stimulus was given (after 3 s). Subjects then continued the contraction at the target level. During this contraction, cortical stimuli followed by brachial plexus stimuli were delivered at 30-s intervals. At 30 s prior to each MVC, no stimuli were given but subjects rated their perceived effort. Subjects were verbally encouraged to keep the torque steady on the target and to regain the target torque as fast as possible after cortical stimuli. During the brief MVCs, subjects were urged to produce a maximal effort and to pull up hard and fast after the stimulation. As the wrist strap caused discomfort during the prolonged contraction it was loosened for 2 min at 13 and 28 min between MVCs. During these periods the flexion torque was generated via a strap held by the hand. No stimulation was performed in these two periods.
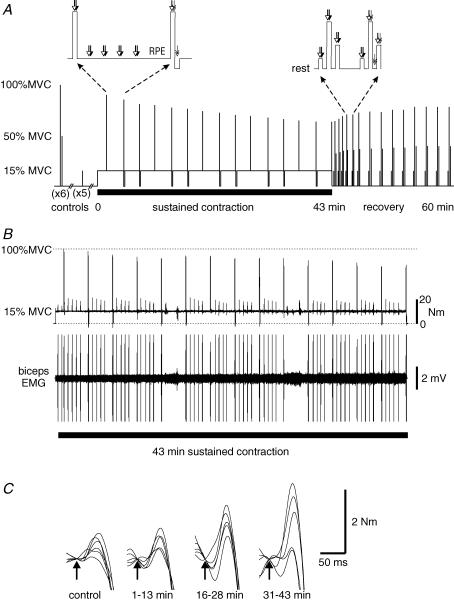
Figure 1. Experimental protocol, and torque and EMG traces.
A, experimental protocol. After performing 6 pairs of brief control contractions (100 and 50% maximal voluntary contractions (MVCs)) followed by a set of 5 brief 15% MVC efforts, subjects maintained a 15% MVC for 43 min. This sustained effort was interrupted by a brief MVC every 3 min. Sets of 15, 100 and 50% MVCs were performed during a recovery period. Motor cortex (white arrow), brachial plexus (black arrow) and motor nerve (double ended arrow) stimuli were delivered during the brief and sustained contractions as indicated in the insets. RPE, rating of perceived effort. B, raw traces of elbow flexion torque and EMG recorded from biceps brachii in one subject throughout the sustained 15% MVC. The torque record shows the maintained 15% maximal torque and the fall in voluntary torque in the brief MVCs. The EMG record shows the progressive increase in EMG required to maintain the torque. The EMG trace has been truncated during the MVCs and with stimulation. C, raw traces of elbow flexion torque responses to motor cortical stimulation (superimposed twitch) during brief MVCs in the same subject as in B. Superimposed twitches were seen during control MVCs and increased in amplitude during MVCs performed during the sustained low-force effort (5 overlaid traces in each set).
Recovery was followed for 23 min. Fourteen sets of brief (2–3 s) contractions at 15, 50 and 100% MVC were performed with ∼8 s between contractions. The first four sets were performed at 35–40 s intervals. Later sets were performed at 1-, 2- and then 3-min intervals. Timing of the contractions is shown in Fig. 1A. During every second MVC, cortical stimulation was followed by motor point stimulation. These MVCs were followed by motor point stimulation with the muscle at rest. In the alternate MVCs, cortical stimulation was followed by brachial plexus stimulation. Cortical stimulation followed by brachial plexus stimulation was also carried out during each 15 and 50% MVC contraction.
Data analysis
Mean elbow flexion torque was calculated over 200 ms prior to each cortical stimulus. This gave measures of performance of the sustained 15% MVC and changes in maximal voluntary torque. Increments in torque evoked by each motor cortical stimulus (cortical superimposed twitch) were also measured. Increments in torque evoked by motor nerve stimulation were measured during the brief MVCs (motor nerve superimposed twitch) and in the relaxed muscle (resting twitch) which was potentiated by a preceding MVC. For motor nerve stimulation, voluntary activation was calculated by comparison of the amplitude of the superimposed twitch to that of the subsequent resting twitch using the equation:
(e.g. Gandevia et al. 1996; Herbert & Gandevia, 1999).
In the control and recovery periods, voluntary activation during MVCs was also calculated by comparison of the superimposed twitch evoked by cortical stimulation to an estimated resting twitch using the expression given above (Todd et al. 2003). The response to stimulation of the motor cortex during rest is estimated rather than measured directly because both motor cortical neurones and motoneurones are less excitable with the muscle at rest than during voluntary activity (e.g. Ugawa et al. 1995; Di Lazzaro et al. 1998). The amplitude of the resting twitch can be estimated by extrapolation of the linear regression between the amplitude of the cortical superimposed twitch and voluntary torque for contractions of 50 to 100% MVC.
The y-intercept is taken as the amplitude of the estimated resting twitch (Todd et al. 2003, 2004). Here, one regression was performed for each set of brief contractions (i.e. MVC followed by a 50% MVC) (Todd et al. 2004). Voluntary activation for the MVC which ended the sustained 15% contraction was calculated from the resting twitch estimated from the first set of contractions in the recovery period.
For each muscle, the area of MEPs and Mmax were measured between set cursors that encompassed all phases of the potentials. To account for activity-dependent changes in the muscle fibre action potentials, the area of the MEP in each muscle was normalized to the area of Mmax elicited nearby in time and during a contraction of similar strength. The duration of the silent period following cortical stimulation was measured as the time from the stimulus to the resumption of voluntary EMG. For each contraction strength, duration of the silent period is expressed as the difference from that measured during similar strength control contractions. Root mean square EMG (rms EMG) was measured during the 15% MVC sustained voluntary contraction and the test MVCs over 200 ms prior to each cortical stimulus. For each muscle, rms EMG was expressed as a percentage of the maximal EMG measured during control MVCs (EMGmax). For triceps, this represents a measure of a changed antagonist involvement rather than an actual percentage of this muscle's maximal activity. Over the prolonged 15% MVC contraction, triceps activity doubled from 14.5 ± 4.4% to 26.5 ± 9.0% of EMG during maximal elbow flexion. However, measurement of EMG from triceps during a maximal elbow extension (4 subjects), showed that this activity was equivalent to a change from < 1% to ∼2% of maximal triceps EMG.
Statistics
Prior to further analysis, the four time points between each MVC were averaged for parameters measured during the sustained 15% MVC. To test for time effects during the sustained voluntary contraction and the recovery period a one-way analysis of variance (ANOVA) for repeated measures approached by general linear modelling was performed for each parameter. Significance level was set at P < 0.05 level. If the ANOVA revealed significant changes a Bonferroni post hoc test comparing all values to a control was used to identify differences between baseline and time-specific means. To examine whether any of the measured parameters correlated with subjects' reported perceived effort during the sustained 15% MVC, a step-wise multiple linear regression was performed. Factors entered into the regression were change in silent period duration and rms EMG of biceps and brachioradialis measured during the 15% contraction, as well as maximal voluntary torque, cortical superimposed twitch, motor nerve superimposed twitch and voluntary activation measured during the occasional MVCs, and the amplitude of the resting twitch. Results are given in the text as mean ± s.d. Figures show mean ± s.e.m.
Results
Sustained contraction at 15% MVC
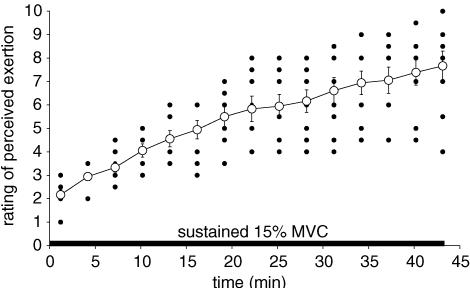
All subjects maintained the 15% MVC torque for 43 min. However, ratings of perceived effort increased progressively from 2.2 ± 0.6 (‘mild’) after the first 2 min of the prolonged contraction to 7.7 ± 1.9 (‘very large’) in the last minute (P < 0.001) with the increase reaching significance after 4 min (Fig. 2). The highest rating of perceived effort varied between subjects with one reaching a score of 10 (‘extremely large’), whereas another only reached 4 (‘considerable’). No correlation was found between the absolute size of the target torque for each subject and the increase in subjective effort.
Figure 2. Rating of perceived effort during the sustained 15% maximal voluntary contraction (MVC).
Subjects rated the effort to maintain the target torque once every 3 min during the sustained contraction using a modified Borg scale. ○, mean ± s.e.m for the group (n = 9). •, individual ratings.
The increase in perceived effort during the 15% MVC contraction was accompanied by a steady increase in voluntary EMG from 6.9 ± 2.1 and 9.2 ± 5.4% to 20.0 ± 7.8 and 23.9 ± 8.4% of EMGmax for the biceps and the brachioradialis muscles, respectively (P < 0.001 for both muscles; see Figs 1B and 3A). This is consistent with a decline in peripheral force-generating capacity and an increased drive to the active motoneurone pools.
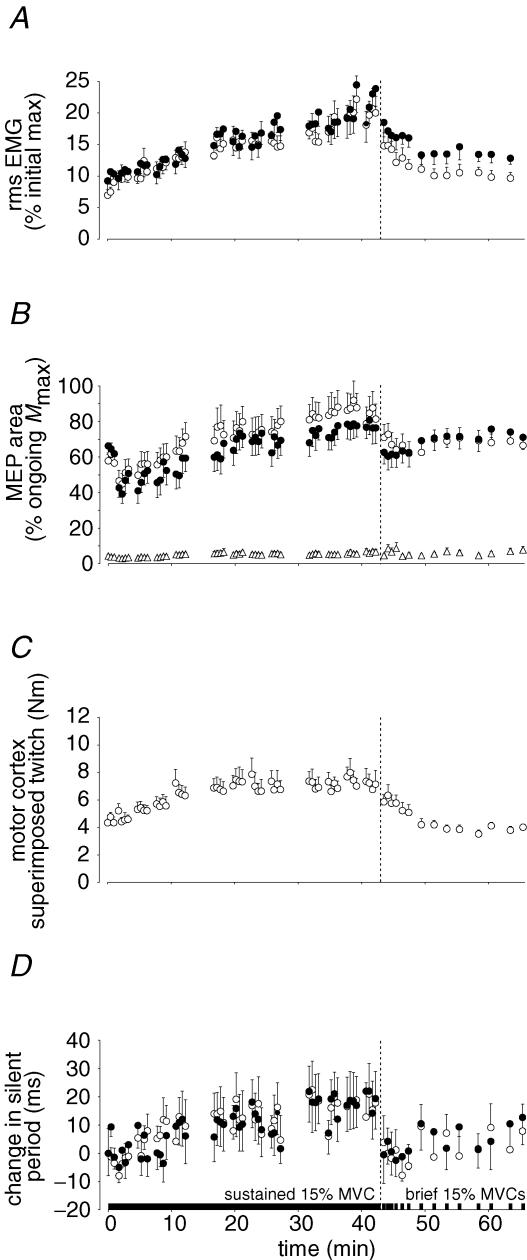
Figure 3. Voluntary EMG, and torque and EMG responses to motor cortical stimulation during the sustained 15% maximal voluntary contraction (MVC) and in brief recovery 15% MVCs.
All data are shown as mean ± s.e.m for the group of 9 subjects. Two breaks in each data set show periods when subjects pulled with the hand strap rather than the wrist strap and no stimuli were given. The end of the sustained 15% MVC is indicated by the vertical broken lines. A, voluntary EMG recorded from biceps brachii (○) and brachioradialis (•). Rms EMG recorded from both elbow flexor muscles increased throughout the sustained 15% MVC and recovered towards initial values during brief 15% MVCs performed in the recovery period. EMG is normalized to the maximum recorded during the brief control MVCs. B, motor evoked potentials (MEPs) elicited from biceps brachii (○), brachioradialis (•) and triceps brachii (▵) by stimulation of the motor cortex. The areas of the MEPs are expressed relative to the maximal M-wave (Mmax) recorded under the same conditions. In biceps and brachioradialis, an initial fall in the MEP area (probably due to potentiation of the muscle by the first brief MVC), was followed by a gradual increase throughout the 15% MVC. The MEP in triceps remained small throughout the experiment. C, torque response to motor cortical stimulation (superimposed twitch). The superimposed twitch increased during the sustained effort. D, change in the duration of the silent period following motor cortical stimulation in biceps brachii (○) and brachioradialis (•). The difference in the duration of the silent period from the mean measured during brief control contractions is shown. The silent period lengthened during the sustained contraction and recovered quickly.
The increased voluntary drive was also reflected in a concurrent increase in the MEP area (expressed relative to Mmax; Fig. 3B) in biceps (58 ± 13 to 81 ± 26%; P < 0.001) although the change in brachioradialis was not significant (66 ± 12 to 76 ± 26%). The superimposed twitch produced by the MEP also increased significantly (P < 0.001). It almost doubled in amplitude in the first 20 min with no further increase (Fig. 3C).
The occasional MVCs performed during the prolonged contraction had a minor effect on the size of Mmax and the MEP in both biceps and brachioradialis. Early in the sustained contraction, transient increases in Mmax occurred consistently after each brief MVC. The increase probably reflects potentiation of action potentials in muscle fibres recruited by the MVC but not engaged in the weak contraction. The MEPs showed the opposite tendency with a slight decrease after each MVC. This is consistent with the potentiation of muscle fibre force leading to a small drop in motor unit recruitment and motor cortex and/or spinal motoneurone excitability.
There was evidence for increased cortical inhibition during the submaximal contraction as the duration of the silent period increased (Fig. 3D). After 43 min, the silent period had increased by 18 ± 33 ms from 137 ± 31 ms in biceps (P < 0.05) and by 19 ± 32 ms from 147 ± 31 ms in brachioradialis (P < 0.05). Despite large individual variation the increase was seen for 7 of 9 subjects, including those with an initial silent period longer than 100 ms.
Brief maximal contractions performed during the sustained contraction at 15% MVC
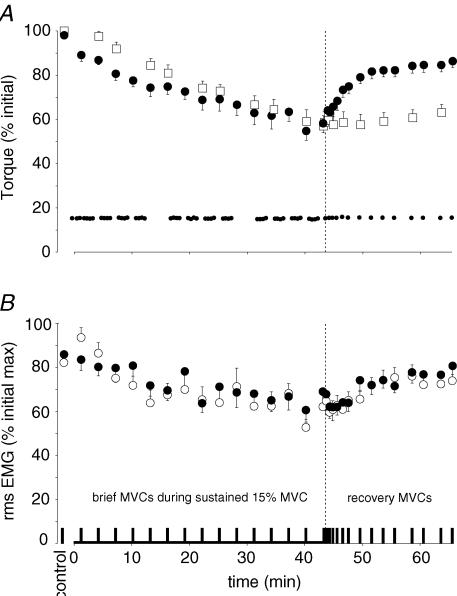
By the end of the sustained submaximal contraction, torque in the brief MVCs decreased to 58.2 ± 13.2% of the control MVCs (P < 0.001) and the resting twitch evoked by motor nerve stimulation declined to 58.6 ± 14.5% of the control value (P < 0.001; see Fig. 4A). There were also indications of a central component to the fatigue. The changes in MVC were accompanied by a decrease in voluntary EMG (Fig. 4B). In biceps, rms EMG in the MVCs decreased from 82.2 ± 5.0% of EMGmax in the control contractions to 62.2 ± 15.3% at the end of the sustained effort (P < 0.001). Similarly, brachioradialis rms EMG decreased from 85.8 ± 6.8% to 69.0 ± 23.5% of EMGmax (P = 0.047). This decrease was present although there was no change in Mmax.
Figure 4. Voluntary and evoked torque and EMG during brief maximal voluntary contractions (MVCs) performed during the sustained low-force effort and in the recovery period.
All data are shown as mean ± s.e.m (n = 9). The vertical broken lines indicate the end of the sustained contraction. A, voluntary torque during brief MVCs (large filled circles), 15% MVC target torque (small filled circles) and amplitude of the twitch evoked from the resting muscle by motor nerve stimulation (open square). Maximal voluntary torque and target torque are expressed relative to the maximal torque recorded during brief control MVCs. While subjects held the target torque for 43 min as planned, the torque produced during occasional brief MVCs declined. It returned to about 85% of control values during the recovery period. The twitch was evoked in the resting muscle by paired stimulation over biceps and was always potentiated by a preceding MVC. The amplitude of the twitch (expressed relative to the mean control amplitude) decreased during the sustained contraction and showed minimal recovery over 20 min. B, voluntary EMG recorded from biceps brachii (○) and brachioradialis (•). Rms EMG is expressed relative to the maximum recorded during brief control MVCs. EMG during MVCs declined in both elbow flexors over the course of the sustained low-force effort and recovered towards initial values after the end of the fatiguing contraction.
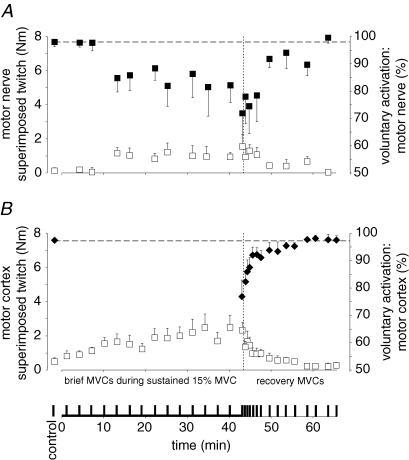
The declines in voluntary torque and EMG in the brief MVCs were accompanied by increases in the superimposed twitches evoked by motor nerve stimuli and motor cortical stimuli (Figs. 5A and B, and Fig. 1C). The superimposed twitch from motor nerve stimulation increased from 0.4 ± 0.5% MVC in the control MVCs to 2.3 ± 2.9% in the final MVC (P = 0.017) during the sustained submaximal contraction. The superimposed twitch evoked by motor cortical stimulation increased from 1.2 ± 1.5 to 3.6 ± 2.4% MVC (P < 0.001). When voluntary activation was calculated from the responses to motor nerve stimulation, it dropped from 98.0 ± 5.1% in the control MVCs to 71.9 ± 38.9% in the MVC at the end of the prolonged contraction (Fig. 5A). For the group of subjects, the decline in voluntary activation was not related to the absolute level of target torque. Voluntary activation evaluated with cortical stimulation also declined during the sustained submaximal contraction (Fig. 5B). It decreased from 97.5 ± 2.5% in the control MVCs to 76.9 ± 18.3% in the final MVC in the sustained contraction (P < 0.001). This suggests that maintenance of a 15% MVC contraction progressively impaired subjects' ability to produce optimal output from the motor cortex during brief maximal voluntary efforts.
Figure 5. Voluntary activation and the superimposed twitches evoked by motor nerve and motor cortex stimulation during brief maximal voluntary contractions (MVCs).
All data are shown as mean ± s.e.m (n = 9). The vertical broken lines indicate the end of the sustained contraction. A, motor nerve stimulation. The increments in torque (superimposed twitch, □) evoked by paired motor nerve stimulation during brief control MVCs, MVCs during the sustained contraction and in the recovery period are shown (left axis). Voluntary activation (▪, right axis) was calculated by comparing the superimposed twitch to the twitch in the resting muscle. B, motor cortex stimulation. The increments in torque (superimposed twitch, □) evoked by motor cortex stimulation during MVCs are shown (left axis). For the control and recovery period, voluntary activation (♦, right axis) was calculated by comparing the superimposed twitch to an estimated resting twitch (see Methods).
During the control MVCs the duration of the silent period following cortical stimulation was 115 ± 43 ms in biceps and 114 ± 41 ms in brachioradialis. It lengthened gradually (by 41 ± 57 ms in biceps, 39 ± 47 ms in brachioradialis; P < 0.001) from the first to the last brief MVC performed occasionally during the submaximal contraction (Fig. 6B). The lengthening of the silent period was similar to that observed during the prolonged 15% MVC contraction (compare Figs 3D and 6B).
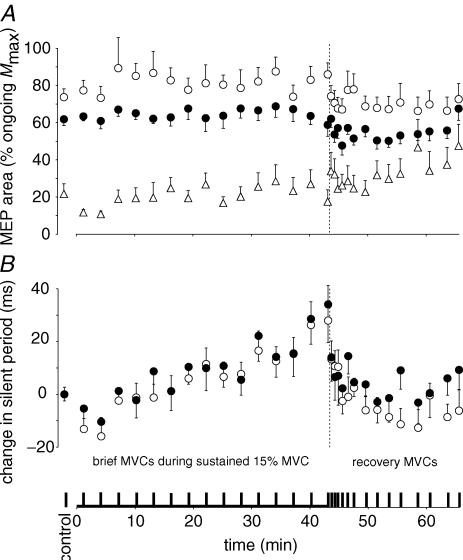
Figure 6. EMG responses to motor cortical stimulation during brief maximal voluntary contractions (MVCs).
All data are shown as mean ± s.e.m (n = 9). The vertical broken lines indicate the end of the sustained contraction. A, motor evoked potentials (MEPs) in biceps brachii (○), brachioradialis (•) and triceps brachii (▵). The areas of the MEPs are expressed relative to the maximal M-waves (Mmax) recorded in the same conditions and at close to the same time. B, change in the duration of the silent period in biceps brachii (○) and brachioradialis (•) following motor cortical stimulation. The difference in duration of the silent period from the mean during brief control MVCs is shown. Silent periods in both elbow flexors lengthen through the sustained low-force contraction and recover quickly.
MEPs (expressed relative to Mmax) in biceps and brachioradialis did not change in the brief MVCs during the 43-min contraction (Fig. 6A). For triceps, the MEP became larger. Although its size remained less than 30% of Mmax on average, the increment in elbow flexion torque evoked by the cortical stimulation may have been underestimated due to an antagonist extension twitch and consequently, voluntary activation may be overestimated.
Recovery after the sustained contraction at 15% MVC
During the recovery period, maximal voluntary torque recovered rapidly during the first 10 min but thereafter improved little. Thus, after 25 min, the MVC was still significantly decreased (86.4 ± 8.7% of control value, P = 0.043; Fig. 4A). In contrast, the resting twitch showed minimal recovery. Its amplitude did not increase significantly from that in the last minute of the sustained contraction. Therefore, as judged from the resting twitch, peripheral fatigue continued over the 25 min of rest (Fig. 4A). This suggests that the partial recovery of the MVC reflects recovery from central fatigue. The time course of the recovery of the voluntary activation supports this interpretation. Voluntary activation measured by motor nerve or motor cortex stimulation showed a steep increase during the initial few minutes after the end of the sustained contraction (Fig. 5A and B). The reduction in rms EMG in the elbow flexors during the brief MVCs returned towards control levels in ∼10 min (Fig. 4B).
At the end of the sustained 15% MVC, rms EMG was high. In the recovery period, it dropped during the brief 15% MVCs (made to the target torque) and was close to control values after ∼8 min. This is consistent with the continued recruitment of additional motor units to compensate for continuing peripheral fatigue. The MEP in biceps followed a similar time course of recovery. In contrast to the slower recovery of other parameters, the silent period recovered almost immediately on cessation of the sustained contraction. In biceps and brachioradialis, the duration of the silent period during both the 15% MVC and the maximal efforts returned to control values with the first brief 15% MVC and first MVC in the recovery period (Figs. 3B and D, and Fig. 6).
Discussion
Both peripheral and central fatigue increased progressively during a sustained voluntary contraction of 15% maximal torque with the elbow flexor muscles. The sustained low-force contraction reduced the maximal voluntary torque. As expected, part of this was peripheral as the resting twitch decreased by ∼40%. However, central fatigue also developed throughout the 43-min submaximal contraction. An increase in the superimposed twitch evoked during brief MVCs by motor nerve stimulation indicated that voluntary drive to the muscle decreased. Furthermore, an increase in the superimposed twitch evoked by motor cortical stimulation indicated that a component of this decrease occurred because of suboptimal output from the motor cortex. While central and peripheral fatigue developed together during the sustained contraction, recovery from central fatigue was much faster than recovery in the periphery. The discussion focuses on three main areas: first, the development of peripheral fatigue during the prolonged contraction; second, the development of central fatigue and the changes in cortically evoked EMG responses; and third, the dissociation of central and peripheral changes during recovery.
Fatigue produced by the sustained 15% voluntary effort was revealed by a decrease in the torque produced by occasional brief MVCs. While the initial fall of ∼8% between the control efforts and the first MVC (after 30 s of sustained contraction) may be a consequence of starting the MVC from contraction at 15% MVC, the incremental decreases in the remainder of the low-force contraction indicate the progressive development of fatigue. The amplitude of the twitch evoked by paired stimulation of the resting muscle also decreased progressively. The decrease in twitch amplitude with little change in the Mmax indicates impairment of processes within the muscle fibres. As the decrease in the resting twitch was of a similar extent to torque during the MVC, it might suggest that most of the loss of voluntary torque was due to fatigue of the muscle. However, muscle fatigue from low work loads (either intermittent contractions or sustained weak contractions) is largely attributed to decreased calcium release (Westerblad et al. 1998; Allen et al. 2002; Lamb, 2002) and impairs force production more with activation of muscle fibres at low frequencies (e.g. Edwards et al. 1977; Bigland-Ritchie et al. 1986b; Blangsted et al. 2005). Thus, if peripheral fatigue were the sole cause of impairment of force production then twitch torque should be reduced more than the MVC in which muscle fibres are activated repetitively.
Voluntary EMG increased during the sustained low-force contraction. Thus, to maintain the target, either the firing frequencies of motor units were increased or additional motor units were recruited by increased descending drive (Bigland-Ritchie et al. 1986b; Dorfman et al. 1990; Krogh-Lund, 1993; Kuchinad et al. 2004; Adam & De Luca, 2005). This implies that the motor units engaged in the l5% contraction fatigued and produced less force. This supports the idea that the fatigue resulted from the sustained low-force effort and not from the MVCs (performed every 3 min) which would be expected to affect the high-threshold fast-fatigable units. The gradual increase in the MEP elicited during the 15% effort is consistent with increasing voluntary drive (see also Sacco et al. 1997). Increased voluntary activity results in increased motoneuronal and cortical excitability and, in the elbow flexor muscles, leads to increased MEPs for contractions less than ∼50% MVC (Ugawa et al. 1995; Di Lazzaro et al. 1998; Taylor et al. 2002; Todd et al. 2003).
The present results show that central fatigue develops progressively throughout a prolonged low-force isometric contraction and are consistent with previous studies which have demonstrated central fatigue during and at the limit of endurance of sustained submaximal efforts (Löscher et al. 1996; Sacco et al. 1997; Zijdewind et al. 1998). During the low-force contraction, subjects became less able to activate the muscle maximally in occasional MVCs. Both motor nerve and motor cortical stimulation evoked increasing increments in torque despite maximal efforts. An increase in the superimposed twitch elicited by motor nerve stimulation indicates that some motor units are no longer firing fast enough to produce fused force from their muscle fibres but gives no clue as to the mechanism of this impairment. Possible mechanisms include inhibition or disfacilitation of the motoneurone pool through altered afferent or descending input (see Gandevia, 2001 for review). However, the increase in the superimposed twitch elicited by motor cortical stimulation indicates that voluntary output from the motor cortex has become suboptimal. Some motor cortical output is untapped by the maximal effort and this can evoke extra force from the muscle. Thus, some of the impairment in motor unit discharge can be ascribed to supraspinal mechanisms (Gandevia et al. 1996; Taylor et al. 2000; Todd et al. 2003). Supraspinal fatigue has also been described during sustained and intermittent isometric maximal contractions, as well as during concentric and eccentric MVCs (Gandevia et al. 1996; Taylor et al. 2000; Löscher & Nordlund, 2002; Todd et al. 2003, 2005).
Voluntary activation was calculated from the responses to motor nerve stimulation by comparing the superimposed twitch to the twitch of the whole muscle at rest. It fell by ∼25% over the course of the sustained contraction although there was variation between subjects. For motor cortical stimulation, the changes in cortical and spinal excitability related to voluntary contraction make a twitch evoked from the resting muscle inappropriate for comparison. However, an estimated resting twitch calculated by linear regression of superimposed twitches against voluntary torque for contractions of greater than 50% MVC can be used (Todd et al. 2003, 2004). This method was used before and after the sustained low-force contraction and showed a fall in activation of ∼20%. Quantitative comparison of voluntary activation measured with stimulation at the two sites in the motor pathway is problematic. Activation measured with motor cortex stimulation has a direct relationship with voluntary force, but that measured with motor nerve stimulation is curvilinear (Todd et al. 2003). Furthermore, because of low-frequency fatigue, the resting twitch to motor nerve stimulation probably overestimates the effect of peripheral fatigue on maximal voluntary torque (e.g. Blangsted et al. 2005). Thus, although there is a supraspinal component to the central fatigue its magnitude is not clear. As the relationship between activation measured with motor cortical stimulation and voluntary force remains linear with fatigue, it is possible to estimate how much of the total torque loss is due to supraspinal mechanisms. If activation had remained at 97.5%, maximal voluntary torque at the end of the sustained contraction would have fallen to 74% of maximum. The further fall to 58% MVC means that about 40% of the total loss of torque could be due to supraspinal fatigue. Indeed, this figure may underestimate supraspinal fatigue. MEPs in the triceps brachii grew during the fatiguing protocol, so it is likely that the evoked extensor torque acted to decrease the measured flexor superimposed twitch more with fatigue.
The silent period elicited by cortical stimulation during the brief MVCs increased in duration during the sustained low-force contraction although the MEP did not change. The lengthening of the silent period is similar to changes seen during fatigue produced by sustained or intermittent maximal contractions (Taylor et al. 1996, 2000). However, the immediate recovery of the silent period on cessation of the low-force contraction indicates that its lengthening is due to the sustained effort and not the brief MVCs. The silent period initially reflects events at a spinal level but this recovers by 100 ms after stimulation (Fuhr et al. 1991; Inghilleri et al. 1993). The latter part of the silent period results from inhibition of voluntary cortical output (Di Lazzaro et al. 2002). For five of the subjects in the current study, silent periods were initially longer than 100 ms so that their duration reflects intracortical inhibition. As the silent periods lengthened for all subjects, the increase in duration probably represents extra inhibition within the motor cortex. The silent period elicited during the 15% MVC effort also lengthened progressively although by a small amount. Previous studies have shown variable changes in the silent period during sustained submaximal contractions (Ljubisavljevic et al. 1996; Taylor et al. 1996; Sacco et al. 1997). Instructions to the subject can alter the silent period and may account for some of the variation (Mathis et al. 1998).
While peripheral and central fatigue developed together during the long low-force contraction, they recovered differently. The silent period recovered during the first MVC ∼30 s after the sustained contraction (see also Taylor et al. 1996, 2000). The silent period in a 15% contraction had also recovered. Voluntary activation measured with both methods started to recover immediately but took several minutes to recover completely. This is slower than the 1–2 min seen after shorter, more intense exercise (Bigland-Ritchie et al. 1986b; Taylor et al. 1996, 2000). The slower recovery of voluntary activation than the silent period provides further evidence that cortical changes represented by the increased silent period are not directly linked to central fatigue (Gandevia et al. 1996; Taylor et al. 2000). Peripheral fatigue was long-lasting, with minimal change of the twitch evoked by paired stimulation of the resting muscle over the 20-min recovery period, whereas maximal voluntary torque recovered to about 85% of its initial value over 10 min with little further improvement. The lack of recovery of the twitch is consistent with the prolonged low-frequency fatigue that has been demonstrated even after relatively short (10 min) low-force contractions and has been attributed to impaired excitation–contraction coupling (Blangsted et al. 2005). There are two alternative explanations for the recovery of maximal voluntary torque. First, an intramuscular process which impairs the force produced by repetitive activation has recovered whereas the response to paired stimulation has not. However, the continued depression of both a twitch and the response to 20 Hz stimulation with low-frequency fatigue argues against this (Blangsted et al. 2005). Alternatively, the recovery of the MVC can be attributed to the recovery of voluntary activation while the remaining decrement in torque represents continued peripheral fatigue.
It is intriguing that the ability to drive a muscle maximally is impaired by a sustained low-force effort. The motor units that are not driven optimally in an MVC are likely to be the high-threshold ones, not the low-threshold ones that are active in the fatiguing 15% MVC. Even at the end of the contraction, the required contraction represented only 25% of the final MVC. It is also unlikely that cortical activity will be sustained at the same level during a weak contraction as during a strong one. One possible explanation for the development of central fatigue is the action of group III and IV muscle afferents at a supraspinal level. When muscles are held ischaemic at the end of a fatiguing maximal contraction to maintain the firing of group III and IV muscle afferents, voluntary activation measured with motor nerve or motor cortical stimulation remains impaired although EMG responses to motor cortical stimulation return to normal (Bigland-Ritchie et al. 1986b; Gandevia et al. 1996). Furthermore, experimental muscle pain can reduce cortical excitability (Le Pera et al. 2001). As all subjects in the present study reported pain in the elbow flexor muscles during the sustained low-force contraction, it is likely that small-diameter muscle afferents, which are sensitive to the metabolic products of fatigue, were activated, and may have influenced voluntary activation.
The effect of central fatigue measured during brief MVCs on the performance of the sustained low-force contraction is uncertain. One measure that suggests that it is important is the subjects' perceived effort. On average, this increased from ‘mild’ (score of 2 out of 10) to ‘very large’ (8 out of 10) while the contraction increased from 15% to 25% of the current maximum torque. Rms EMG increased threefold but remained ∼20% of the initial maximum or ∼30% of that during the final MVC. Thus, perceived effort at the end of the low-force contraction is disproportionately high. Many variables changed progressively during the low-force contraction and are correlated with perceived effort and with each other. However, step-wise multiple linear regression indicates that the perceived effort is best predicted by a combination of the rms EMG measured in biceps during the 15% contraction and expressed relative to the initial maximum, the decrease in amplitude of the resting twitch, and failure of voluntary activation measured with motor nerve stimulation (r = 0.8). Each of these variables contributes significantly to the correlation (P < 0.001). Thus, along with peripheral factors, central fatigue appears to contribute to the perceived effort of producing a submaximal isometric torque. A second measure which suggests an influence of central fatigue on the generation of the low-force contraction is the rms EMG. After increasing throughout the low-force contraction, the rms EMG decreases with the cessation of the sustained contraction. As twitch torque recovers minimally, the recovery of EMG is unlikely to be because of improved contractile force of the muscle fibres. One possibility is that motor unit firing rates are limited during the sustained contraction so that the forces produced by the low-threshold units are submaximal, and it is the recovery of firing rates that generates more force from these units and results in fewer units overall being active. Decreased firing rates in low-threshold units have been observed previously in sustained submaximal efforts (Garland et al. 1997; Jensen et al. 2000) and specific changes in the continuously active motor units might be explained by an effect of repetitive activation such as late adaptation of the motoneurones (e.g. Kernell & Monster, 1982).
In summary, a sustained low-force contraction produces progressive peripheral and central fatigue, and some of the central fatigue is due to suboptimal drive from the motor cortex. Although caused by a low-force contraction, both the peripheral and central fatigue impair the production of maximal voluntary force. The fatigue is accompanied by some of the changes in the motor cortex which occur with fatigue caused by maximal efforts. Although central fatigue can only be demonstrated formally during maximal efforts our results suggest that the neural processes involved in its generation also occur during submaximal exercise and are likely to contribute to the marked increase in effort.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia.
References
- Adam A, De Luca CJ. Firing rates of motor units in human vastus lateralis muscle during fatiguing isometric contractions. J Appl Physiol. 2005;99:268–280. doi: 10.1152/japplphysiol.01344.2004. [DOI] [PubMed] [Google Scholar]
- Allen DG, Kabbara AA, Westerblad H. Muscle fatigue: the role of intracellular calcium stores. Can J Appl Physiol. 2002;27:83–96. doi: 10.1139/h02-006. [DOI] [PubMed] [Google Scholar]
- Baker AJ, Kostov KG, Miller RG, Weiner MW. Slow force recovery after long-duration exercise: metabolic and activation factors in muscle fatigue. J Appl Physiol. 1993;74:2294–2300. doi: 10.1152/jappl.1993.74.5.2294. [DOI] [PubMed] [Google Scholar]
- Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol. 1981;51:1131–1135. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Cafarelli E, Vollestad NK. Fatigue of submaximal static contractions. Acta Physiol Scand Suppl. 1986a;556:137–148. [PubMed] [Google Scholar]
- Bigland-Ritchie B, Dawson NJ, Johansson RS, Lippold OCJ. Reflex origin for the slowing of motoneurone firing rates in fatigue of human voluntary contractions. J Physiol. 1986b;379:451–459. doi: 10.1113/jphysiol.1986.sp016263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangsted AK, Sjøgaard G, Madeleine P, Olsen HB, Søgaard K. Voluntary low-force contraction elicits prolonged low-frequency fatigue and changes in surface electromyography and mechanomyography. J Electromyogr Kinesiol. 2005;15:138–148. doi: 10.1016/j.jelekin.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work, Environ Health. 1990;16(Suppl. 1):55–58. doi: 10.5271/sjweh.1815. [DOI] [PubMed] [Google Scholar]
- Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci. 2003;23:10224–10230. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, Visocchi M, Colosimo C, Tonali PA, Rothwell JC. Direct demonstration of long latency cortico-cortical inhibition in normal subjects and in a patient with vascular parkinsonism. Clin Neurophysiol. 2002;113:1673–1679. doi: 10.1016/s1388-2457(02)00264-x. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman LJ, Howard JE, McGill KC. Triphasic behavioural response of motor units to submaximal fatiguing exercise. Muscle Nerve. 1990;13:621–628. doi: 10.1002/mus.880130711. [DOI] [PubMed] [Google Scholar]
- Edwards RHT, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhr P, Agostino R, Hallett M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:257–262. doi: 10.1016/0168-5597(91)90011-l. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland SJ, Griffin L, Ivanova T. Motor unit discharge rate is not associated with muscle relaxation time in sustained submaximal contractions in humans. Neurosci Lett. 1997;239:25–28. doi: 10.1016/s0304-3940(97)00885-9. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Gandevia SC. Twitch interpolation in human muscles: mechanisms and implications for measurement of voluntary activation. J Neurophysiol. 1999;82:2271–2283. doi: 10.1152/jn.1999.82.5.2271. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Manfredi M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. J Physiol. 1993;466:521–534. [PMC free article] [PubMed] [Google Scholar]
- Jensen BR, Pilegaard M, Sjøgaard G. Motor unit recruitment and rate coding in response to fatiguing shoulder abductions and subsequent recovery. Eur J Appl Physiol. 2000;83:190–199. doi: 10.1007/s004210000278. [DOI] [PubMed] [Google Scholar]
- Kernell D, Monster AW. Motoneurone properties and motor fatigue: an intracellular study of gastrocnemius motoneurones of the cat. Exp Brain Res. 1982;46:197–204. doi: 10.1007/BF00237177. [DOI] [PubMed] [Google Scholar]
- Krogh-Lund C. Myo-electric fatigue and force failure from submaximal static elbow flexion sustained to exhaustion. Eur J Appl Physiol Occup Physiol. 1993;67:389–401. doi: 10.1007/BF00376454. [DOI] [PubMed] [Google Scholar]
- Kuchinad RA, Ivanova TD, Garland SJ. Modulation of motor unit discharge rate and H-reflex amplitude during submaximal fatigue of the human soleus muscle. Exp Brain Res. 2004;158:345–355. doi: 10.1007/s00221-004-1907-0. [DOI] [PubMed] [Google Scholar]
- Lamb GD. Excitation-contraction coupling and fatigue mechanisms in skeletal muscle: studies with mechanically skinned fibres. J Muscle Res Cell Motil. 2002;23:81–91. doi: 10.1023/a:1019932730457. [DOI] [PubMed] [Google Scholar]
- Le Pera D, Graven-Nielsen T, Valeriani M, Oliviero A, Lazzaro VD, Tonali P, Arendt-Nielsen L. Inhibition of motor system excitability at cortical and spinal level by tonic muscle pain. Clin Neurophysiol. 2001;112:1633–1641. doi: 10.1016/s1388-2457(01)00631-9. [DOI] [PubMed] [Google Scholar]
- Ljubisavljevic M, Milanovic S, Radovanovic SS, Vukcevic I, Kostic V, Anastasijevic R. Central changes in muscle fatigue during sustained submaximal isometric voluntary contraction as revealed by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1996;101:281–288. doi: 10.1016/0924-980x(96)95627-1. [DOI] [PubMed] [Google Scholar]
- Lloyd AR, Gandevia SC, Hales JP. Muscle performance, voluntary activation, twitch properties and perceived effort in normal subjects and patients with the chronic fatigue syndrome. Brain. 1991;114:85–98. [PubMed] [Google Scholar]
- Löscher WN, Cresswell AG, Thorstensson A. Central fatigue during a long-lasting submaximal contraction of the triceps surae. Exp Brain Res. 1996;108:305–314. doi: 10.1007/BF00228103. [DOI] [PubMed] [Google Scholar]
- Löscher WN, Nordlund MM. Central fatigue and motor cortical excitability during repeated shortening and lengthening actions. Muscle Nerve. 2002;25:864–872. doi: 10.1002/mus.10124. [DOI] [PubMed] [Google Scholar]
- McKay WB, Stokic DS, Sherwood AM, Vrbova G, Dimitrijevic MR. Effect of fatiguing maximal voluntary contraction on excitatory and inhibitory responses elicited by transcranial magnetic motor cortex stimulation. Muscle Nerve. 1996;19:1017–1024. doi: 10.1002/mus.880190803. [DOI] [PubMed] [Google Scholar]
- Mathis J, de Quervain D, Hess CW. Dependence of the transcranially induced silent period on the ‘instruction set’ and the individual reaction time. Electroencephalogr Clin Neurophysiol. 1998;109:426–435. doi: 10.1016/s0924-980x(98)00042-3. [DOI] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco P, Thickbroom GW, Thompson ML, Mastaglia FL. Changes in corticomotor excitation and inhibition during prolonged submaximal muscle contractions. Muscle Nerve. 1997;20:1158–1166. doi: 10.1002/(sici)1097-4598(199709)20:9<1158::aid-mus11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Søgaard K, Blangsted AK, Jørgensen LV, Madeleine P, Sjøgaard G. Evidence of long term muscle fatigue following prolonged intermittent contractions based on mechano- and electromyograms. J Electromyogr Kinesiol. 2003;13:441–450. doi: 10.1016/s1050-6411(03)00075-0. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol. 2000;89:305–313. doi: 10.1152/jappl.2000.89.1.305. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM, Gandevia SC. Changes in motor cortical excitability during human muscle fatigue. J Physiol. 1996;490:519–528. doi: 10.1113/jphysiol.1996.sp021163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen NT, Butler JE, Gandevia SC. Interaction of transcranial magnetic stimulation and electrical transmastoid stimulation in human subjects. J Physiol. 2002;541:949–958. doi: 10.1113/jphysiol.2002.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Petersen NT, Taylor JL, Gandevia SC. Measurements of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol. 2003;551:661–671. doi: 10.1113/jphysiol.2003.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Butler JE, Gandevia SC. Hyperthermia: a failure of the motor cortex and the muscle. J Physiol. 2005;563:621–631. doi: 10.1113/jphysiol.2004.077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Reproducible measurement of voluntary activation of human elbow flexors with motor cortical stimulation. J Appl Physiol. 2004;97:236–242. doi: 10.1152/japplphysiol.01336.2003. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Terao Y, Hanajima R, Sakai K, Kanazawa I. Facilitatory effect of tonic voluntary contraction on responses to motor cortex stimulation. Electroencephalogr Clin Neurophysiol. 1995;97:451–454. doi: 10.1016/0924-980x(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG, Bruton JD, Andrade FH, Lannergren J. Mechanisms underlying the reduction of isometric force in skeletal muscle fatigue. Acta Physiol Scand. 1998;162:253–260. doi: 10.1046/j.1365-201X.1998.0301f.x. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Zwarts MJ, Kernell D. Influence of a voluntary fatigue test on the contralateral homologous muscle in humans? Neurosci Lett. 1998;253:41–44. doi: 10.1016/s0304-3940(98)00609-0. [DOI] [PubMed] [Google Scholar]