Abstract
Renal circulatory adjustments to stress contribute to blood pressure and volume regulation. Both handgrip (HG) and disengagement of baroreflexes with lower body negative pressure (LBNP) can engage the sympathetic nervous system (SNS). However, the effect of simultaneous HG and LBNP on the renal circulation in humans is not known. Eighteen young healthy volunteers were studied. Beat-to-beat changes in renal blood flow velocity (RBV; Duplex Ultrasound), mean arterial pressure (MAP; Finapres) and heart rate (ECG) were monitored during (a) 15 s HG at 30% maximum voluntary contraction (MVC); (b) LBNP at −10 and −30 mmHg (each level for 5 min); and (c) 15 s HG (at 30% MVC) during LBNP at both levels. Renal vascular resistance index (RVR units) was calculated by dividing MAP by RBV. The increases in RVR during HG alone (12 ± 6%) were not different from the responses noted during combined HG and LBNP (17 ± 6% at −10 mmHg and 25 ± 8% at −30 mmHg). These results suggest occlusion occurs between a neural circuit engaged during 15 s of HG (central command and/or the muscle mechanoreflex) and a circuit activated by LBNP. In additional experiments (n = 6), similar non-algebraic summation of RVR was seen during 15 s involuntary biceps contractions (engages only muscle reflexes) and LBNP. With respect to RVR, neural occlusion occurs between baroreflexes and the muscle mechanoreflex. Muscle mechanoreflex mediated renal vasoconstriction during short bouts of HG is not influenced by baroreflex disengagement.
Orthostatic stress or lower body negative pressure (LBNP) deactivates baroreceptors, which causes sympathetic nervous system (SNS) activation and vasoconstriction. This in turn serves to maintain blood pressure (Zoller et al. 1972; Sundlöf & Wallin, 1978; Abboud et al. 1979; Victor & Leimbach, 1987; Baily & Sinoway, 1990; Thompson et al. 1990; Edouard et al. 1994; Khan et al. 2002).
Static exercise stimulates muscle afferent nerves and activates central command. When these systems are activated, the SNS is activated causing peripheral vasoconstriction and a rise in blood pressure (Mitchell & Schmidt, 1983). A number of prior studies have examined how simultaneous deactivation of baroreflexes and activation of muscle reflexes affect sympathetic activity and vascular resistance to a given circulatory bed. Walker et al. (1980) and Nishiyasu et al. (1993) found that forearm exercise coupled with LBNP raised forearm vascular resistance more than exercise alone. These results suggest a positive interaction exists between the muscle reflex (and/or central command) and the baroreflex. Interestingly, several other reports demonstrated that exercise with and without LBNP led to a similar rise in forearm vascular resistance and/or muscle sympathetic nerve activity (Sanders & Ferguson, 1988; Scherrer et al. 1988; Seals, 1988; Arrowood et al. 1993). These reports suggest that muscle reflex control of the skeletal muscle circulation is not modulated by the baroreflexes.
Handgrip (HG) exercise (Middlekauff et al. 1997; Momen et al. 2003) and LBNP (Gilbert et al. 1966; Tidgren et al. 1990; Miller et al. 1991; Berdeaux et al. 1992; Würzner et al. 2001) both also cause renal vasoconstriction.
In humans, very little is known about the effects of simultaneous exercise and orthostatic stress on the renal circulation. This interaction may be particularly important since the renal circulation contributes to blood pressure and plasma volume regulation. In this report, we tested the hypothesis that the baroreflex does not modulate muscle reflex control of the renal circulation. To test this hypothesis we studied 18 young healthy individuals and evaluated renal blood flow velocity (RBV) responses in a beat-by-beat fashion employing Duplex ultrasound technology during short bouts of static HG and LBNP. Once these studies were completed, we performed additional studies examining whether the baroreflex would modulate muscle mechanoreflex mediated control of the renal circulation. To accomplish this goal, RBV was evaluated during involuntary biceps contractions with and without LBNP. The results of these studies suggest that muscle mechanoreflex mediated renal vasoconstriction is not modulated by baroreflex disengagement.
Methods
Study population
Twenty-one healthy volunteers were studied (12 male, 9 female; age 26 ± 1 years, mean body mass index 24 ± 1 kg m−2). Subjects signed an informed written consent approved by the Penn State Hershey Institutional Review Board. A physical examination was performed on all subjects before they were studied. No volunteers were on medications, were smokers or were hypertensive. The study conformed to the Declaration of Helsinki.
Renal blood flow velocity
Duplex ultrasound (HDI 5000, ATL Ultrasound, Bothell, WA, USA) was used to determine renal blood flow dynamics. The renal artery was scanned using the anterior abdominal approach with a curved-array transducer (2–5 MHz) and a 2.5 MHz pulsed Doppler frequency was used. The probe insonation angle to the renal artery was < 60 deg and the focal zone was set at the depth of the renal artery. In order to obtain optimum velocity tracings, the transducer was held in a constant position. Therefore, the data were obtained in the same phase of the respiratory cycle of the respective subject. Care was taken to ensure that the subject did not perform Valsalva's manoeuvres during the HG protocols. Mean RBV was obtained by analysing the cardiac cycle Doppler tracings with HDI 5000 ATL software.
Based on the following considerations, RBV will be used as an index of renal blood flow.
where r is vessel radius. Thus if vessel diameter changes dramatically with an intervention, velocity will not be reflective of flow. Unfortunately, it is not possible to make accurate measurements of renal artery diameter using duplex methods. Accordingly, it is important to note that prior studies (Marraccini et al. 1996) have shown that renal artery diameter does not change as vasoconstrictor agents are infused into the renal artery and RBV is a good surrogate for renal blood flow. In turn, we used mean arterial pressure (MAP; mmHg)/RBV (cm s−1) as an index of renal vascular resistance (RVR). In this report, RVR is expressed in arbitrary units. All subjects were post-absorptive and studied in the supine position.
In addition to continuous recordings of RBV, heart rate (HR; electrocardiogram) and MAP (Finapres; Ohmeda, Madison, WI, USA) were also obtained continuously during each protocol. The MAP values obtained by Finapres were adjusted to baseline MAP values obtained by a Dinamp device (Criticon, Tampa, FL, USA).
Static handgrip
Static HG was performed using an adjustable HG dynamometer (Stoelting, Wood Dove, IL, USA). Maximum contractions were performed three times by each subject. The largest of these was considered the maximal voluntary contraction (MVC).
Lower body negative pressure
The method for LBNP has been previously described (Baily & Sinoway, 1990). Briefly, each subject was placed on a padded table with their lower body (up to the level of the iliac crests and umbilicus) positioned inside a sealed chamber. The upper surface of the LBNP chamber was below the Doppler probe. Suction was applied to the lower body with a vacuum cleaner. The amount of suction was quantified with a pressure gauge.
Involuntary biceps contraction
Involuntary biceps contractions were induced by electrical stimulation of the biceps muscle. Electrical pads (5 cm × 5 cm) were placed ∼3 cm apart over the skin of the biceps muscle. The biceps muscle was then electrically stimulated (200 V; phase duration, 0.3 ms; phase interval, 0.1 ms). Electrical biceps contraction evoked contractions with a tension of ∼20% of MVC that were sustained for ∼15 s without eliciting pain. Fifteen seconds of involuntary contractions preferentially engages mechanosensitive and not metabosensitive muscle afferent nerves and does not engage central command (Kaufman & Forster, 1996).
Plasma catecholamines and plasma renin activity measurements
Venous blood samples were obtained at rest and during LBNP of −30 mmHg. Plasma noradrenaline (NA) and adrenaline (Adr) were measured by high performance liquid chromatography. Plasma renin activity was measured by radioimmunoassay technique.
Study protocols
Protocol 1. Static HG (n = 18)
Baseline ECG, RBV and MAP data were recorded over 5 min. Each subject performed 15 s static HG exercise at 30% MVC. This voluntary contraction protocol was utilized to raise sympathetic outflow by preferentially engaging central command and/or the muscle mechanoreflex without engaging the metaboreflex.
Protocol 2. Graded LBNP with static HG (n = 18)
After a 15 min rest period, 5 min of baseline ECG, RBV, and MAP data were recorded. LBNP was applied in a graded fashion. It was started at −10 mmHg and was increased by 20 mmHg every 5 min to −50 mmHg. LBNP was discontinued if: (1) hypotensive symptoms developed (nausea, diaphoresis, etc.); or (2) a sustained fall of > 10 mmHg in MAP was noted.
All 18 subjects tolerated −10 mmHg LBNP. Sixteen subjects tolerated −30 mmHg, and 12 tolerated −50 mmHg of LBNP. Subjects performed 15 s HG (30% MVC) at the end (5 min) of −10 and −30 mmHg LBNP.
Protocol 3. Involuntary biceps contraction with and without LBNP (n = 6)
Protocol 3 was performed on a separate day from protocols 1 and 2. After determining MVC for voluntary biceps contractions, involuntary biceps contractions were performed at ∼20% MVC for ∼15 s.
After a ∼15 min rest period, baseline data were recorded (5 min) and −10 mmHg LBNP was applied. Involuntary biceps contraction was then performed at the end of 5 min of LBNP.
Data analysis and statistics
Variables obtained during baseline and LBNP are presented as the mean values during each 5 min period of baseline and LBNP.
Repeated measures one-way ANOVA was applied to variables during the graded LBNP protocol. Student's paired t test was used to compare responses from baseline in the different protocols. Responses during handgrip/involuntary biceps contraction and during combined LBNP with HG/involuntary contraction were analysed using paired t tests. Data are presented as means ± s.e.m. P < 0.05 was considered significant.
Results
Graded LBNP
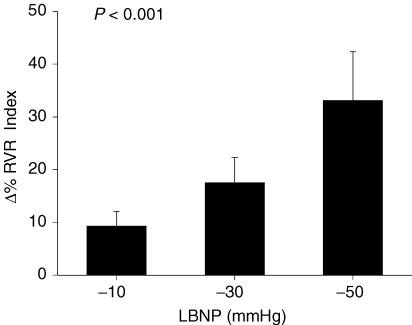
Graded LBNP led to progressive increases in RVR (P < 0.001; Fig. 1), decreases in RBV (P < 0.01), and increases in HR (P < 0.001; Table 1). MAP did not change during LBNP. Plasma NA rose with LBNP (baseline: 1.54 ± 0.19 nmol l−1 versus LBNP: 2.37 ± 0.19 nmol l−1; P < 0.0001). Plasma Adr and PRA did not rise with LBNP (Adr baseline: 0.23 ± 0.04 nmol l−1 versus LBNP: 0.30 ± 0.11 nmol l−1; not significant (NS); PRA baseline: 0.91 ± 0.14 ng ml−1 h−1 versus LBNP: 0.89 ± 0.18 ng ml−1 h−1; NS).
Figure 1. Renal vascular resistance responses during graded LBNP.
Data are presented as means ± s.e.m. and are shown as percentage change from baseline in renal vascular resistance index (RVR; Y-axis) as a function of graded LBNP (X-axis). P-value reflects statistical analysis using one-way ANOVA performed on Δ% data.
Table 1.
Hemodynamic responses during graded lower body negative pressure (LBNP; n = 18)
| LBNP −10 mmHg | LBNP −30 mmHg | LBNP −50 mmHg | Significance | |
|---|---|---|---|---|
| RBV | −7.1 ± 2.2 | −11.9 ± 3.0 | −18.8 ± 5.1 | P < 0.006 |
| MAP | 0.6 ± 0.9 | 1.6 ± 1.4 | 3.6 ± 1.9 | NS |
| HR | 2.0 ± 1.1 | 15.5 ± 2.8 | 39.3 ± 7.4 | P < 0.001 |
Δ%s data are presented as mean ± s.e.m. P-values denote results from one-way analysis of variance performed on Δ% data. RBV, renal blood flow velocity; MAP, mean arterial pressure; HR, heart rate.
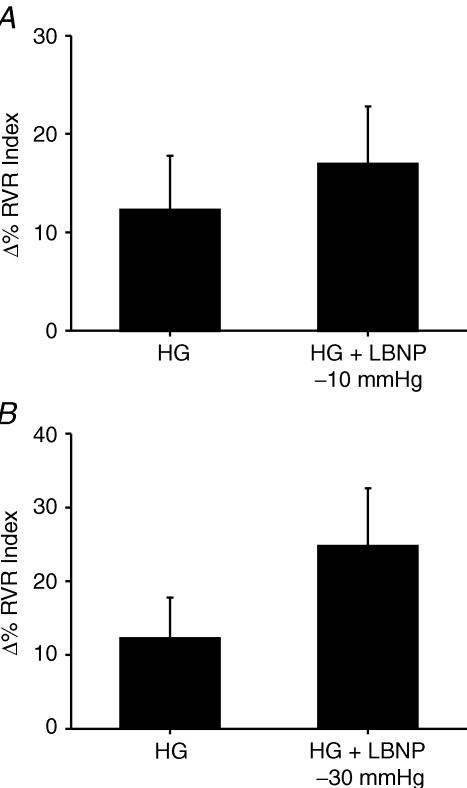
Responses during HG with or without LBNP protocol (Fig. 2; Table 2)
Figure 2. Renal vascular resistance responses during handgrip with and without LBNP.
Data are presented as means ± s.e.m. and are shown as percentage change from baseline in renal vascular resistance index (RVR; Y-axis) during 15 s static handgrip at 30% of maximum voluntary contraction (MVC) and during handgrip at 30% MVC with lower body negative pressure (A, LBNP −10 mmHg; B, LBNP −30 mmHg; X-axis). Note, no significant differences in terms of RVR responses between handgrip versus handgrip + LBNP.
Table 2.
Hemodynamic responses to static handgrip alone and to static handgrip during lower body negative pressure (LBNP)
| Handgrip | Handgrip + LBNP −10 mmHg | Handgrip + LBNP −30 mmHg | |
|---|---|---|---|
| RBV | −2.3 ± 4.7 | −8.1 ± 4.6 | −10.9 ± 5.2 |
| MAP | 6.7 ± 1.3 | 4.2 ± 1.3 | 6.2 ± 1.8 |
| HR | 5.1 ± 1.8 | 11.0 ± 2.0* | 23.0 ± 4.0* |
Δ% data are presented as mean ± s.e.m.
Significantly different than values during handgrip (at 30% MVC) alone. RBV, renal blood flow velocity; MAP, mean arterial pressure; HR, heart rate.
Both static HG and LBNP −10 mmHG and −30 mmHg LBNP raised RVR (12 ± 6%; P < 0.02 during HG; 9 ± 3%; P < 0.005 during LBNP −10 mmHg; 18 ± 5%; P < 0.005 during LBNP −30 mmHg). MAP and HR rose during HG, whereas RBV did not rise with HG (Table 2). RVR rose when HG was performed during LBNP at −10 mmHg (17 ± 6%; P < 0.005) and at −30 mmHg (25 ± 8%; P < 0.005). RVR responses during HG were not statistically different from RVR responses during combined HG and LBNP (Fig. 2A and B). Changes in MAP, HR and RBV values during HG and HG + LBNP are shown in Table 2. Of note, increases in HR during HG were less than increases in HR seen with HG + LBNP.
Biceps contraction with and without LBNP
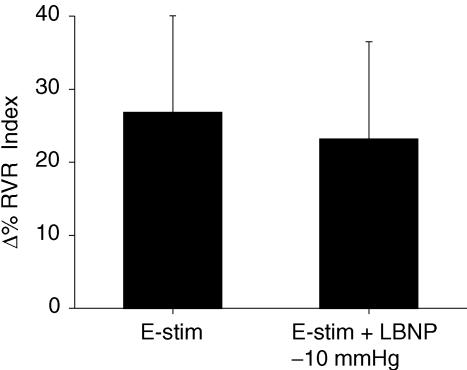
RVR responses during involuntary contraction alone are not significantly different from RVR responses during involuntary contractions + LBNP (Fig. 3). Changes in MAP, HR and RBV during involuntary contraction alone were also not different from changes in the corresponding values during involuntary contraction + LBNP. To exclude the possibility of a ceiling effect for RVR responses during involuntary biceps contraction, additional studies were performed in four subjects. In these studies, a similar protocol was followed to protocol 3 except that biceps muscle contraction was evoked with less electrical stimulation (∼10% of maximum voluntary contraction). One subject did not show any vasoconstrictor responses during e-stim nor during e-stim + LBNP. However, three other subjects showed small increases in renal vasoconstriction. The average magnitude of the vasoconstriction observed during e-stim alone was similar to the response during e-stim + LBNP (9 ± 4%versus 10 ± 9%, respectively; NS). These data suggest that a ceiling effect was not involved in the observed RVR responses during the involuntary muscle contraction protocol.
Figure 3. Renal vascular resistance responses during involuntry contraction with and without LBNP.
Data are presented as mean ± s.e.m. and are shown as percentage change from baseline in renal vascular resistance index (RVR; Y-axis) during 15 s electrical stimulation of biceps at ∼20% of maximum voluntary contraction (E-stim) and during E-stim with lower body negative pressure (LBNP −10 mmHg; X-axis). Note, no significant differences in terms of RVR responses between E-stim versus E-stim + LBNP.
Discussion
In this report LBNP did not augment the RVR responses seen with short bouts of HG. These findings suggest that static HG induced renal vasoconstrictor responses are not influenced by baroreflex engagement.
Importantly, the observed non-algebraic summation of renal vasoconstrictor responses observed in this report was not due to a ceiling effect since: (1) the RVR responses seen were less than RVR values previously reported for greater levels of HG (Momen et al. 2003); and (2) the RVR values seen at −10 and −30 mmHg LBNP were less than values seen during −50 mmHG (Fig. 1). Thus, the most likely explanation for our results is that the ‘occlusion’ occurred between some neural circuit engaged during HG exercise and the baroreflex. The occlusion phenomenon takes place when two different redundant neural mechanisms are simultaneously activated to modulate the cardiovascular system, and one system simply gets turned off (Goodwin et al. 1972; McRitchie et al. 1976; Rybicki et al. 1989). In the present report, isometric muscle contraction stimulated central command and/or the exercise pressor refex to increase SNS. Similarly, LBNP caused SNS activation by disengaging baroreflexes. Therefore, during combined static exercise and LBNP, two different mechanisms were activated for neurovascular control to the kidney. Under these circumstances, SNS activation and the resultant rise in RVR during combined HG and LBNP would be less than the algebraic sum of HG and LBNP alone.
Renal vasoconstriction during 15 s of HG is primarily due to muscle mechanoreflex and/or the central command mechanism (Krogh & Lindhard, 1913; Kaufman & Forster, 1996; Herr et al. 1999). Thus based on the HG and HG + LBNP data from this report, it is not possible to identify whether neural occlusion occurred between central command and the baroreflex or between the muscle mechanoreflex and the baroreflex. To address this issue, we examined renal blood flow responses during 15 s of involuntary contraction of biceps muscle with and without LBNP at −10 mmHg. Involuntary contraction eliminates any influences of central command on RVR responses (Goodwin et al. 1972). Since the RVR responses seen during involuntary contraction were not different from RVR during involuntary contractions coupled with LBNP, we suggest that occlusion occurred between the muscle mechanoreflex and the baroreflex.
Our findings do not support the data reported by Matsukawa et al. (1991). In this prior report, conscious cats performed static exercise while resting arterial pressure was raised by injecting noradrenaline. The investigators observed less of an increase in renal sympathetic nerve activity suggesting an inhibitory influence due to baroreflex engagement during exercise. The reason for the variances between this prior report and our current findings is not exactly known. However, fundamental differences in study design and/or species differences might play a role.
Interestingly, unlike RVR, increases in HR were greater during HG + LBNP than during HG alone. On the other hand, HR during involuntary biceps contraction was similar with and without LBNP. The exact explanation for these findings is unclear. However, we believe the most likely explanation is that increase in HR during HG is mediated predominantly by central command (Goodwin et al. 1972) and that occlusion does not occur between central command and the baroreflex.
LBNP disengages the afferent nerve activity of the baroreflex system and causes activation of the SNS. Renal vasoconstriction (as well as increased sodium and water reabsorption) has been previously seen during LBNP (Gilbert et al. 1966; Tidgren et al. 1990; Miller et al. 1991; Berdeaux et al. 1992; Würzner et al. 2001). However, unlike our findings, some of the previous reports did not observe increases in RVR at low (−10 or −15 mmHg) levels of LBNP (Miller et al. 1991; Berdeaux et al. 1992; Würzner et al. 2001). The exact reason for these differences is not clear although differences in study design as well as differences in the methods used to measure renal blood flow between prior reports and ours are likely to be important issues.
Finally, we observed increases in plasma NA but not PRA during the LBNP procedure. This suggests that the rise in RVR response during LBNP was due to sympathetic activation (Würzner et al. 2001) and not to a hormone related process.
In conclusion, our findings suggest that muscle mechanoreflex mediated renal vasoconstrictor responses are not influenced by baroreflex disengagement in healthy humans during short bouts of exercise.
Renal circulation importantly contributes to blood pressure as well as fluid volume regulation. During both static exercise and orthostatic stress, activation of SNS plays an important role in evoking renal vasoconstriction and this helps in maintaining blood pressure. During the activities of daily living (ADL), different physical activities involve short bouts of isometric muscle contraction (e.g. holding, gripping, lifting, etc.). These ADL often occur in the upright posture. Since exercise has a profound impact on the renal circulation, we believe that understanding renal circulatory control mechanisms during exercise combined with orthostatic stress is of major clinical relevance. Our findings indicate that renal vasoconstrictor responses seen during short bouts of exercise are not modulated by orthostatic stress induced baroreflex disengagement. However, it should be noted that in our study design the simulated orthostatic stress was non-hypotensive (up to −30 mmHg LBNP level). Therefore, our current data may not be reflective of the situation seen during exercise in the presence of moderate to severe hypovolumia (e.g. severe haemorrhage) or in disease processes associated with altered sympathetic function (e.g. hypertension, heart failure). Abnormalities in neural mechanisms in these conditions cause abnormal neurovascular control to the kidney. Accordingly, in these clinical settings, compensatory exaggerated renal vasoconstriction may occur to maintain blood pressure and extracellular body fluid volumes. Therefore, an important question raised by these studies is whether occlusion is seen to the same degree in conditions associated with heightened renal constriction such as hypertension and heart failure.
Acknowledgments
The authors are grateful to Jennifer Stoner for excellent manuscript preparation, to Brian Handly, Kristen Gray and Michael Herr for technical support, and the staff of the General Clinical Research Center. This work was supported by National Institutes of Health (NIH) grants R01 HL070222 (Sinoway), P01 HL077670 (Sinoway), R01 HL068699 (Leuenberger), NIH/NCRR grants M01 RR010732 and C06 RR016499, and Pennsylvania Tobacco Settlement Funds – Penn State College of Medicine.
References
- Abboud FM, Eckberg DL, Johannsen UJ, Mark AL. Carotid and cardiopulmonary baroreceptor control of splanchnic and forearm vascular resistance during venous pooling in man. J Physiol. 1979;286:173–184. doi: 10.1113/jphysiol.1979.sp012612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowood JA, Mohanty PK, McNamara C, Thames MD. Cardiopulmonary reflexes do not modulate exercise pressor reflexes during isometric exercise in humans. J Appl Physiol. 1993;74:2559–2565. doi: 10.1152/jappl.1993.74.5.2559. [DOI] [PubMed] [Google Scholar]
- Baily RG, Sinoway LI. Insight into human baroreceptor function using multiple indices of neural activity. Heart Fail. 1990;6:33–41. [PubMed] [Google Scholar]
- Berdeaux A, Duranteau J, Pussard E, Edouard A, Giudicelli JF. Baroreflex control of regional vascular resistances during simulated orthostatism. Kidney Intsupplement. 1992;37:S29–S33. [PubMed] [Google Scholar]
- Edouard AR, Degremont AC, Duranteau J, Pussard E, Berdeaux A, Samii K. Heterogeneous regional vascular responses to simulated transient hypovolemia in man. Intensive Care Med. 1994;20:414–420. doi: 10.1007/BF01710651. [DOI] [PubMed] [Google Scholar]
- Gilbert CA, Bricker LA, Springfield WT, Jr, Stevens PM, Warren BH. Sodium and water excretion and renal hemodynamics during lower body negative pressure. J Appl Physiol. 1966;21:1699–1704. doi: 10.1152/jappl.1966.21.6.1699. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr MD, Imadojemu V, Kunselman AR, Sinoway LI. Characteristics of the muscle mechanoreflex during quadriceps contractions in humans. J Appl Physiol. 1999;86:767–772. doi: 10.1152/jappl.1999.86.2.767. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Forster HV. Reflexes controlling circulatory, ventilatory and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 381–447. Chap. 10. [Google Scholar]
- Khan MH, Sinoway LI, MacLean DA. Effects of graded LBNP on MSNA and interstitial norepinephrine. Am J Physiol Heart Circ Physiol. 2002;283:H2038–H2044. doi: 10.1152/ajpheart.00412.2001. [DOI] [PubMed] [Google Scholar]
- Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraccini P, Fedele S, Marzilli M, Orsini E, Dukic G, Serasini L, L'Abbate A. Adenosine-induced renal vasoconstriction in man. Cardiovasc Res. 1996;32:949–953. [PubMed] [Google Scholar]
- Matsukawa K, Mitchell JH, Wall PT, Wilson LB. The effect of static exercise on renal sympathetic nerve activity in conscious cats. J Physiol. 1991;434:453–467. doi: 10.1113/jphysiol.1991.sp018480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRitchie RJ, Vatner SF, Boettcher D, Heyndrickx GR, Patrick TA, Braunwald E. Role of arterial baroreceptors in mediating cardiovascular response to exercise. Am J Physiol. 1976;230:85–89. doi: 10.1152/ajplegacy.1976.230.1.85. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Nitzsche EU, Nguyen AH, Hoh CK, Gibbs GG. Modulation of renal cortical blood flow during static exercise in humans. Circ Res. 1997;80:62–68. doi: 10.1161/01.res.80.1.62. [DOI] [PubMed] [Google Scholar]
- Miller JA, Floras JS, Skorecki KL, Blendis LM, Logan AG. Renal and humoral responses to sustained cardiopulmonary baroreceptor deactivation in humans. Am J Physiol Regul Integr Comp Physiol. 1991;260:R642–R648. doi: 10.1152/ajpregu.1991.260.3.R642. [DOI] [PubMed] [Google Scholar]
- Mitchell J, Schmidt R. Cardiovascular reflex control by afferent fibers from skeletal muscle receptors. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology section 2, The Cardiovascular System. III. Bethesda: American Physiological Society; 1983. pp. 623–658. part 1, [Google Scholar]
- Momen A, Leuenberger UA, Ray CA, Cha S, Sinoway LI. Renal vascular responses to static handgrip: role of the muscle mechanoreflex. Am J Physiol Heart Circ Physiol. 2003;285:H1247–H1253. doi: 10.1152/ajpheart.00214.2003. [DOI] [PubMed] [Google Scholar]
- Nishiyasu T, Shi X, Mack GW, Nadel ER. Forearm vascular responses to baroreceptor unloading at the onset of dynamic exercise. J Appl Physiol. 1993;75:979–985. doi: 10.1152/jappl.1993.75.2.979. [DOI] [PubMed] [Google Scholar]
- Rybicki KJ, Stremel RW, Iwamoto GA, Mitchell JH, Kaufman MP. Occlusion of pressor responses to posterior diencephalic stimulation and muscular contraction. Brain Res Bull. 1989;22:305–312. doi: 10.1016/0361-9230(89)90058-0. [DOI] [PubMed] [Google Scholar]
- Sanders JS, Ferguson DW. Cardiopulmonary baroreflexes fail to modulate sympathetic responses during isometric exercise in humans: direct evidence from microneurographic studies. J Am Coll Cardiol. 1988;12:1241–1251. doi: 10.1016/0735-1097(88)92607-1. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Vissing SF, Victor RG. Effects of lower-body negative pressure on sympathetic nerve responses to static exercise in humans. Circulation. 1988;78:49–59. doi: 10.1161/01.cir.78.1.49. [DOI] [PubMed] [Google Scholar]
- Seals DR. Cardiopulmonary baroreflexes do not modulate exercise-induced sympathoexcitation. J Appl Physiol. 1988;64:2197–2203. doi: 10.1152/jappl.1988.64.5.2197. [DOI] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. Effect of lower body negative pressure on human muscle nerve sympathetic activity. J Physiol. 1978;278:525–532. doi: 10.1113/jphysiol.1978.sp012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CA, Tatro DL, Ludwig DA, Convertino VA. Baroreflex responses to acute changes in blood volume in humans. Am J Physiol Regul Integr Comp Physiol. 1990;259:R792–R798. doi: 10.1152/ajpregu.1990.259.4.R792. [DOI] [PubMed] [Google Scholar]
- Tidgren B, Hjemdahl P, Theodorsson E, Nussberger J. Renal responses to lower body negative pressure in humans. Am J Physiol Renal Physiol. 1990;259:F573–F579. doi: 10.1152/ajprenal.1990.259.4.F573. [DOI] [PubMed] [Google Scholar]
- Victor RG, Leimbach WN., Jr Effects of lower body negative pressure on sympathetic discharge to leg muscles in humans. J Appl Physiol. 1987;63:2558–2562. doi: 10.1152/jappl.1987.63.6.2558. [DOI] [PubMed] [Google Scholar]
- Walker JL, Abboud FM, Mark AL, Thames MD. Interaction of cardiopulmonary and somatic reflexes in humans. J Clin Invest. 1980;65:1491–1497. doi: 10.1172/JCI109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würzner G, Chiolero A, Maillard M, Nussberger J, Hayoz D, Brunner HR, Burnier M. Renal and neurohormonal responses to increasing levels of lower body negative pressure in men. Kidney Int. 2001;60:1469–1476. doi: 10.1046/j.1523-1755.2001.00950.x. [DOI] [PubMed] [Google Scholar]
- Zoller RP, Mark AL, Abboud FM, Schmid PG, Heistad DD. The role of low pressure baroreceptors in reflex vasoconstrictor responses in man. J Clin Invest. 1972;51:2967–2972. doi: 10.1172/JCI107121. [DOI] [PMC free article] [PubMed] [Google Scholar]