Abstract
To determine how the histaminergic system is implicated in vestibular compensation, we studied the changes in histidine decarboxylase (HDC; the enzyme synthesizing histamine) mRNA regulation in the tuberomammillary (TM) nuclei of cats killed 1 week, 3 weeks and 3 months after unilateral vestibular neurectomy (UVN). We also used one- and two-step bilateral vestibular neurectomized (BVN) cats to determine whether HDC mRNA regulation depended on the asymmetrical vestibular input received by the TM nuclei neurons. In addition, we analysed the HDC mRNA changes in the TM nuclei and the recovery of behavioural functions in UVN cats treated with thioperamide, a pure histaminergic drug. Finally, we quantified binding to histamine H3 receptors (H3Rs) in the medial vestibular nucleus (VN) by means of a histamine H3R agonist ([3H]N-α-methylhistamine) in order to further investigate the sites and mechanisms of action of histamine in this structure. This study shows that UVN increases HDC mRNA expression in the ipsilateral TM nucleus at 1 week. This increased expression persisted 3 weeks after UVN, and regained control values at 3 months. HDC mRNA expression was unchanged in the one-step BVN cats but showed mirror asymmetrical increases in the two-step BVN compared to the 1 week UVN cats. Three weeks' thioperamide treatment induced a bilateral HDC mRNA up-regulation in the UVN cats, which was higher than in the untreated UVN group. Binding to histamine H3Rs in the MVN showed a strong bilateral decrease after thioperamide treatment, while it was reduced ipsilaterally in the UVN cats. That such changes of the histaminergic system induced by vestibular lesion and treatment may play a functional role in vestibular compensation is strongly supported by the behavioural data. Indeed, spontaneous nystagmus, posture and locomotor balance were rapidly recovered in the UVN cats treated with thioperamide. These results demonstrate that changes in histamine levels are related to vestibular compensation.
The tuberomammillary (TM) nuclei of the posterior hypothalamus are the only structures within the central nervous system known to contain histaminergic neurons (Panula et al. 1984; Pollard & Schwartz, 1987). These histamine-containing neurons project axons to the whole vestibular nuclei complexes (VNCs) in rats and cats (Panula et al. 1989; Tighilet & Lacour, 1996). Histamine induced modulation of the activity of vestibular nucleus (VN) cells has been reported in vivo and in vitro. In vitro, histamine depolarizes the rat medial VN neurons, an effect that could be mediated by different histamine receptors (HRs), H1R (Inverarity et al. 1993) or H2R (Phelan et al. 1990; Serafin et al. 1993; Wang & Dutia, 1995). Microiontophoresis of histamine and histamine antagonists in the cat shows a more complex picture with both inhibitory and facilitatory actions on the medial and lateral VN neurons (Kirsten & Sharma, 1976; Satayavivad & Kirsten, 1977). A third type of histamine receptor, H3R, has been found at presynaptic sites on the histamine afferent fibres reaching the VNCs. Their stimulation inhibits histamine synthesis and release while their blockade by H3R antagonists leads to increased histamine turnover and release (Arrang et al. 1983, 1987). The central histaminergic system seems, therefore, to be involved in the regulation of vestibular functions (reviewed in Pollard & Schwartz, 1987; Lacour & Sterkers, 2001), and the vestibulo-hypothalamic loop very likely plays a significant role in these processes. Indeed, histamine release from the hypothalamus was induced by vestibular caloric stimulation (Horii et al. 1993) and 2 g hypergravity (Uno et al. 1997). Finally, the histaminergic system is also involved in vestibular autonomic responses; vestibular-induced hypothalamic neuronal activity was modified by antihistaminergic drugs after caloric stimulation in the guinea pig (Inokuchi et al. 1999).
The histaminergic system could also be implicated in vestibular compensation, i.e, the process of behavioural recovery occurring after unilateral damage of the peripheral vestibular system. In most of the species, the post-lesional vestibular syndrome is made of static and dynamic signs. The static signs include ocular motor (spontaneous nystagmus) and postural (head tilt, increase of the surface delimited by the four legs of the cat while standing erect) deficits that are compensated within a few days or weeks, while the dynamic signs (vestibulo-ocular reflex, locomotor performance on a rotating beam) are compensated much less completely or over a longer time (reviewed in Dieringer, 1995; Darlington & Smith, 2000). The static deficits appear to result from the spontaneous resting activity imbalance between the bilateral VNCs, and compensation approximately coincides with restoration of balanced electrical activity between the VNCs. These events were confirmed electrophysiologically in the alert guinea pig (Ris et al. 1995) and cat (Zennou-Azogui et al. 1993). They are supported also by the so-called Betcherew's phenomenon (Betcherew, 1883): after compensation of the static deficits following unilateral lesion of the peripheral vestibular system, a second lesion on the intact side leads to the reversal of the initial vestibular syndrome, i.e. to the mirror image of what was observed after the first lesion. By contrast, the compensation of dynamic signs seems more independent of rebalanced activity in the VNCs, and is attributed to a more global reorganization of the central nervous system (reviewed in Curthoys, 2000; Dieringer, 1995; Vidal et al. 1998).
This study aimed at determining first the effects of asymmetrical vestibular inputs resulting from unilateral vestibular loss on the histaminergic system in the TM nuclei. This was done by labelling histidine decarboxylase MRNA (HDC: the enzyme synthesizing histamine) by in situ hybridization in unilateral vestibular neurectomized (UVN) cats. The time course of HDC regulation changes was investigated during the recovery process in groups of cats tested 1 week, 3 weeks and 3 months after UVN. In addition, one- and two-step bilateral vestibular neurectomized (BVN) cats were used to confirm that HDC regulation changes depended on the asymmetrical vestibular input received by the TM nuclei neurons. The underlying hypothesis was that HDC mRNA would remain unchanged after one-step bilateral loss, and reversed after a two-step bilateral loss, compared to what happens after the first lesion, due to Betcherew's phenomenon. To investigate the effects of histaminergic drugs on the TM nuclei and their possible role in vestibular compensation, the effects of thioperamide, a pure H3R antagonist, on HDC mRNA expression were determined. To investigate the site and mechanisms of action of histamine in the medial VN, we quantified H3R binding of the H3R agonist ([3H]N-α-methylhistamine). Finally, we compared the recovery of vestibular function in untreated and thioperamide-treated UVN cats, using assays of posture and spontaneous nystagmus to test static compensation and locomotion on the rotating beam test to test dynamic compensation.
Methods
Animals
Experiments were performed on 50 adult pigmented domestic cats (3–4 kg) obtained from the Centre d'Élevage du Contigné, one of the French approved sources. All experiments were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and associated guidelines, the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the National Institutes of Health guide for the care and use of laboratory animals (NIH publications no. 8023, revised 1978). These normal cats were housed under a constant 12 h light–dark cycle with free access to food and water.
Twelve animals were submitted to one-step UVN on the left side and killed at three survival times: 1 week (n = 4), 3 weeks (n = 4), and 3 months (n = 4). Four animals were submitted to two-step bilateral vestibular neurectomy (BVN): they were first neurectomized on the left side, 3 months later on the right side, and then killed 7 days later. This two-step bilateral loss of vestibular function leading to the so-called Bechterew's phenomenon is of interest to understand the vestibular compensation process. Another group of animals (n = 4) were submitted to one-step BVN and killed seven days after. A group of sham-operated animals (n = 4) were used as control group; they were submitted to anaesthesia and surgical approach of the vestibular nerve without sectioning of the nerve. The survival times were selected from our previous behavioural and electrophysiological investigations, which had shown major postural deficits in acute cats (1 week) and nearly complete recovery in compensated animals (3 months). The histamine H3 receptor-binding study was performed in control (sham-operated) cats (n = 4), 1-week UVN cats (n = 4), and 1-week thioperamide-treated cats (n = 4). Concerning the behavioural investigations, 14 UVN cats were used for this study, seven receiving thioperamide treatment and seven serving as vehicle-control untreated animals.
Vestibular neurectomy
Vestibular nerve section was performed under aseptic conditions through a dissecting microscope. Under fluothane anaesthesia (2%), the cat underwent a mastoidectomy followed by destruction of the bony labyrinth, and surgical exposure of the internal auditory meatus. The vestibular nerve was then sectioned proximal to Scarpa's ganglion. The classical postural, locomotor, and oculomotor deficits displayed by the animals in the days after UVN were used as criteria indicating the effectiveness of the vestibular nerve lesion. Postoperatively, the animals were treated with antibiotics (7 days) and analgesics (1–2 days).
In situ hybridization and H3 receptor autoradiography methods for quantifying HDC mRNA and histamine H3 receptor binding
Tissue preparation
Cats of each group were deeply anaesthetized with ketamine dihydrochoride (20 mg kg−1, i.m., Merial, Lyon, France) and killed by decapitation. After removal from the skull, their brains were cut into several blocks containing the VN and the posterior hypothalamic nuclei. The blocks were rapidly frozen with CO2 gas and coronal sections (10 μm thick) were cut in a cryostat (Leica, Reuil-Malmaison, France), thawed onto ‘Superfrost ++’ glass slides (Fisher Scientific, Elancourt, France), and stored at −80°C until hybridization and radioautography.
In situ hybridization histochemistry
As previously described by Tighilet et al. (2002), a DNA fragment encoding amino acids 503–639 of the human HDC gene sequence was selectively amplified by PCR from human genomic DNA and subcloned in pGEM-4Z (Promega, Charbonnieres, France). The subcloned DNA was checked against a limited restriction map to determine its identity and the orientation of the insert in the vector. Then, 33P-labelled antisense- and sense-strand RNA probes were prepared by in vitro transcription using a Riboprobe kit (Promega).
The brain sections were fixed in paraformaldehyde 4% in 0.1 m phosphate buffered saline (PBS) (pH 7.2–7.4) for 45 min at 4°C, rinsed three times in 0.1 m PBS, dehydrated in graded alcohols, air-dried for 30 min, and stored at −80°C until processing.
The sections were rinsed for 5 min in Tris (0.1 m, pH 7.5)–EDTA (0.5 m) buffer, treated with proteinase K (10 μg ml−1 buffer) at 37°C for 10 min, and rinsed in 0.1 m Tris buffer (2 × 5 min). They were washed in 2× standard saline citrate (SSC; 17.53 g l−1 NaCl, 8.82 g sodium citrate-2H2O, pH 7.0) for 2 × 5 min, immersed in 0.1 m triethanolamine, pH 8, for 5 min and then in 0.1 m triethanolamine + 0.25% acetic anhydride for 10 min, and rinsed in 2× SSC (2 × 5 min). After being washed in glycine buffer (7 mg ml−1 in 0.1 m Tris buffer, pH 7.5) for 60 min, they were rinsed in 2× SSC, dehydrated in graded alcohols, and air-dried for 30 min. 33P-labelled cRNA probes (sense and antisense) were denatured by heating at 70°C for 6 min, cooled in ice, and mixed in the hybridization buffer (50% deionized formamide, 10% dextran sulphate, 1× Denhart's solution, 2× SSC, 0.1% sodium pyrophosphate, 100 μg ml−1 yeast tRNA, 100 μg ml−1 denatured salmon sperm DNA). Each section was covered with 75 μl of diluted probe at the final concentration of 2 × 106 c.p.m., coverslipped with a sterile piece of Nescofilm, placed in a humidified box, and kept overnight at 58°C. The following day, the sections were rinsed in 2× SSC (2 × 5 min), incubated in RNAse buffer (200 μg ml−1 in 2× SSC) for 60 min at 37°C, and rinsed in 2× SSC (3 × 15 min). They were successively washed in 0.5× SSC for 30 min at 58°C, in 0.1× SSC for 30 min at 60°C, and in 0.1× SSC for 30 min at room temperature, and then they were dehydrated through 30, 50, 80 and 95% ethanol containing 300 mm ammonium acetate and 100% ethanol, and finally air dried.
Autoradiography films (Hyperfilm β-max, Amersham, Orsay, France) were apposed to the sections and stored at 4°C for 6 days. The films were developed for 6 min in Kodak D-19 (Eastman Kodak, Rochester, NY, USA) and fixed in GBX (Eastman Kodak) for 15 min. Slides were dipped in Amersham LM 141 emulsion at 43°C and stored in a light-proof box (containing dessicator) at 4°C for 6–10 days. They were developed, counterstained with haemalun (Merck, Fontenay-sous-Bois, France) or 0.1% cresyl violet, and mounted with Permount. Controls of hybridization histochemistry using the sense strand probe at the same final concentration gave no specific hybridization signal.
H3 receptor autoradiography
The binding of [3H]N-α-methylhistamine (80 Ci mmol−1, NEN Life Science Products, Boston, MA, USA) to histamine H3 receptors was performed on tissue sections as previously described (Tighilet et al. 2002). The brain sections (from fresh frozen tissue) were incubated with 4 nm[3H]N-α-methylhistamine, at 4°C in a 150 mm sodium phosphate buffer, pH 7.4, containing 2 mm magnesium chloride, and 100 μm dithiothreitol (Sigma, Saint Quentin, France). The non-specific binding component was measured by adding a large excess of thioperamide (2 mm, Tocris Cookson Ltd, Bristol, UK) 30 min before adding [3H]N-α-methylhistamine. After 45 min incubation, the sections were rinsed 3 times (each wash lasting 20 s) in a 4°C buffer, and then rinsed once in 4°C water for 3 s. The slices were dried with a stream of cold air and exposed to tritium-sensitive film ([3H]Hyperfilm, Amersham). The same brain level sections from the three groups of cats were performed together in the same binding experiment and exposed side by side on the same autoradiographic film. After 9 months of exposure at −80°C, the films were processed in Kodak Industrex developer at room temperature for 2 min, fixed, and then washed. Azur II stained sections were used for reference.
TM surface determined by HDC mRNA-containing cells
The TM nuclei of the posterior hypothalamus were identified using Berman's stereotaxic atlas (Berman & Jones, 1982). Neurons expressing HDC mRNA were analysed. To produce consistent results of positively labelled TM cell bodies on the surface of sections taken from control, lesioned and treated cats, all tissue was processed in parallel with 33P-labelled HDC probe. In addition, all TM tissue was exposed on the same autoradiographic film. Seventy-five serial sections were quantified in the TM nuclei of each animal, 15 sections being used for each of the five main levels examined (rostrocaudal planes of A12, A11, A10.2, A9.5, and A8.3). Autoradiographic signals were captured from the films through a high-resolution video camera (1024 × 1024 pixels) linked to a computer-image analyser (NIH, Image 1,62b7). Because of the scattered distribution of TM neurons containing HDC mRNA, we chose to binalize in the digitized images a constant surface unit containing the TM, after thresholding. The thresholding value was identical for all the sections. Data analysis consisted in evaluating the labelled HDC mRNA surface, expressed in pixel2. Reproducibility was assessed by comparing the data collected independently by two researchers. They were blinded to the animal groups they analysed with the image analysis system. The specific hybridization signal was measured in each section as a labelled surface and was automatically computed and evaluated thereafter as the mean (±s.e.m.) for each side, each cat, and each subgroup of cats (see Tighilet et al. 2002).
To determine the cause of the labelled surface changes, if any, histidine decarboxylase mRNA expression in tuberomammillary neurons was quantified at the cellular level on adjacent dipped sections. For each group of cats, we first counted the number of HDC radiolabelled neurons in the TM nuclei on both sides (left/right). Positive 33P-radiolabelled neurons were defined as those displaying at least four silver grains around the nucleus. For each cat, we examined an average of 50 rostro-caudal sections. Grain counts over individual cells were then analysed in the TM nuclei of these rostro-caudal sections. The number of grains per cell was quantified using an image analyser system (LUCIA G, Nikon, Champigny sur Marne, France). To eliminate quantification problems due to possible asymmetrical slides, data were collected only from symmetrical slides, on the basis of the total number of cells stained with cresyl violet on each side. With the aim of appreciating the background silver grain density, we counted the silver grains on a surface outside the neuron. Then we subtracted the background value to give the total value measured in a cell to obtain the specific labelling.
H3 receptor binding measurement
The VNs were identified with Berman's stereotaxic atlas (Berman, 1968). The autoradiograms of the binding to histamine H3Rs were analysed and quantified using NIH Image software. 3H Plastic standards (Amersham) were used to calibrate 3H concentrations. Receptor density was expressed in fmol/mg of protein and evaluated for the VN. A mean receptor density value was calculated for each nucleus from 60 serial sections. The specific binding value was determined as the difference between total and non-specific binding components for a given area and was evaluated as the mean ±s.e.m. The density of [3H]N-α-methylhistamine binding sites was evaluated in the medial VN (MVN). This nucleus: (1) is known to be the main target of direct histaminergic afferents from the TM nuclei (Takeda et al. 1987); (2) shows the highest density of histaminergic varicosities (Tighilet & Lacour, 1996, 1997) and the highest level of [3H]N-α-methylhistamine binding density (Tighilet et al. 2002); (3) is known to receive convergent semicircular and otolith afferents and to be involved in both the oculomotor and postural functions which are together concerned in our behavioural study (cf. Wilson & Melvill Jones, 1979).
Statistical analysis
Analysis of variance (ANOVA) was used to test the effects of the vestibular lesion (intact, UVN versus bilateral-lesioned cats), the survival period (1 week, 3 weeks versus 3 months), the side (left versus right, deafferented versus intact), and the treatment (treated versus untreated) on, and to determine the interactions between, the following: (1) HDC mRNA expression (labelled surface) in the tuberomammillary nuclei, (2) the number of histidine decarboxylase-radiolabelled neurons in the tuberomammillary nuclei, (3) the number of grains per cell in the tuberomammillary nuclei, and (4) the histamine H3R binding sites in the MVN. ANOVA was followed by post hoc analysis with Scheffé's test and multicomparison Fisher's test (StatView II, SAS Software Inc., Cary, NC, USA).
Behavioural investigations
Spontaneous nystagmus recovery
The frequency of horizontal spontaneous nystagmus (HSN) was measured in three untreated and three treated UVN cats. Prior to UVN, cats were chronically equipped with a head fixation device and Ag–AgCl electrodes implanted on both sides of the eyes in the horizontal plane (see Borel & Lacour, 1992 for details). Implantation was done with the cats under fluothane anaesthesia (2%). After 2 days of recovery, the cats underwent UVN, and horizontal eye movements were recorded as early as 1 day post-lesion. For nystagmus recording, the cat was placed on an apparatus with its head fixed and bent forward 23 deg, thus maintaining the horizontal semicircular canals in the horizontal plane, and its body was wrapped in a hammock so as to minimize head movements relative to the trunk. The frequency of the HSN was measured in the light as the number of quick phase beats towards the contralateral side relative to UVN in 10 s (five repeated measures per animal per sampling time).
Posture recovery
Posture deficits and recovery were evaluated by measuring the surface delimited by the four legs of the cat while standing erect at rest, without walking. Support surface can be regarded as a good estimate of postural control because it reflects the cat's behavioural adaptation compensating the static vestibulospinal deficits induced by the vestibular lesion (cf. Tighilet et al. 1995). As a rule, the surface was very small in the normal cat (about 50 cm2) and greatly increased in the days following unilateral vestibular lesion. Practically, it was measured as the surface delimited by the four legs on the ground using chalk as a marker. Five repeated measurements were done for each cat tested at each postoperative time, and an average was calculated for each experimental session.
Equilibrium function recovery
Locomotor balance function was quantified using the rotating beam experimental device (see Xerri & Lacour, 1980 for details). Two compartments (0.5 m × 0.6 m × 0.5 m) were connected by a horizontal beam (length: 2 m; diameter: 0.12 m) situated in a tunnel whose walls were covered by a pseudo-random visual pattern. The beam, placed 1.2 m off the ground, could be rotated along its longitudinal axis with a constant angular velocity ranging from 0 to 750 deg s−1 (linear tangential speed: 0–0.785 m s−1). A light signal in the arrival compartment conditioned the cat to cross the beam, which was equipped with a safety net to ensure the animals were protected in case they fell. The animal reward consisted of a small piece of fish (or meat) placed in a small bowl in the target compartment.
Animals were conditioned to cross over the beam when the light signal was lit in the opposite compartment. First crossings were made on the immobile beam and, thereafter, on the rotating beam. As a rule, rotation velocity of the beam was progressively increased after four consecutive trials without fall. Equilibrium function was quantified by measuring the highest speed of beam rotation that did not induce a fall. This maximal rotation speed determined the maximal locomotor balance performance (Max P).
Preoperative training on the rotating beam necessitated 6–10 training periods of 1 h per day, depending on the cats. Behavioural training on the rotating beam consisted in depriving the animals of food for 24 h before the first training session; thereafter, they were fed at the end of each of the following sessions. This procedure was enough to motivate the cats and to condition them relatively rapidly. Training was stopped when the cats' Max P was reached and stabilized at its highest level, which was found to be remarkably similar from one cat to another.
Eight animals were submitted to a unilateral vestibular neurectomy on the left side, after which postoperative locomotor balance was measured every 2 days beginning on the second postoperative day and until complete recovery. It was verified that animals did not change their food preference postoperatively. Data recorded after vestibular lesion (Max P) were related to individual references, each animal taken as its own control.
Experimental protocol
The cats were subdivided into two groups according to the treatment they received after UVN. The treated groups (n = 7) received intraperitoneal injections of thioperamide (a pure histamine H3 receptor antagonist) purchased from Tocris Cookson Ltd (UK) at daily doses of 3.5 mg kg−1. Conditions of the thioperamide treatment were similar to those in our previous study (Tighilet & Lacour, 1997). The vehicle control group (n = 7), which served as reference, received saline water intraperitoneally. Thioperamide or saline water was administered until complete behavioural recovery. For the cats used in the posturo-locomotor investigation (n = 8), drug or saline water was administered until the animals reached their Max P (100% of the preoperative values) as quantified by the rotating beam test. In the additional group of UVN cats used for measurement of the HSN recovery (N = 6), pharmacological treatment was given until full disappearance of the nystagmus in the light.
Data analysis
Data collected in each group of cats were first pooled, and then the mean was calculated for each postoperative time delay. Linear regression curves fitting the experimental data were computed, and the determination coefficients (R2) were calculated. Differences in the dynamics of the recovery profiles in the two groups of cats were first evaluated by comparing the curve slope. Statistical comparisons were also performed by ANOVA. Global evaluation was done with the Fisher PLSD multicomparison test, while comparisons for groups were evaluated with the Student-Newman-Keuls test. This analysis of variance was particularly useful for comparing the dynamics of the recovery time courses when they did not follow a linear function (see Tighilet et al. 1995). Time benefit due to thioperamide treatment was evaluated by subtracting the time required for a full compensation in the untreated group from the corresponding values in the treated group.
Once the treated cats had reached their preoperative Max P (3 weeks), they were killed and their brains were subjected to the same in situ hybridization for quantifying HDC mRNA in the TM nuclei.
Results
All the cats that underwent UVN exhibited ocular nystagmus (fast phase directed to the intact side), head tilt towards the side of the lesion, postural asymmetry, enhancement of their support surface, and falling to the lesioned side in the first post-lesion week. Most of them recovered sufficiently in 2 or 3 days to feed themselves. Those killed at 3 weeks' survival time had recovered only partially while those killed at 3 months had shown complete behavioural recovery. The two-step BVN cats showed decompensation and exhibited similar deficits toward the side of the second lesion. The deficits, first described at the behavioural level by Betcherew (1883), are therefore the mirror image of those seen after the first lesion. The one-step BVN cats showed no nystagmus during the acute stage, but they had unsteady head movements, picking movements, a very large support surface, and wide gaits. Finally, the thioperamide-treated cats tended to have increased global sensorimotor activity.
In the control cats, expression of the mRNA coding for HDC was moderate in the TM nuclei. Among the VN, the MVN and the SVN showed the highest level of [3H]N-α-methylhistamine binding density (only results from the MVN are reported here). No significant differences were seen between the left and the right sides, and no significant interindividual differences were found in the different groups, as shown by the analysis of variance. Significant changes in HDC mRNA expression were observed in the UVN cats. No HDC mRNA changes were found in the one-step BVN cats and mirror images of what was reported after UVN were found in the two-step BVN animals. Repeated-measure analysis of variance demonstrated that group (controls versus UVN/BVN), treatment (T versus NT) and survival period (1 week, 3 weeks versus 3 months) constituted the main fixed effects providing the sources of variation among the animals. Significant changes were also found for the number of grains per labelled neuron in the TM nuclei and for the histamine H3Rs binding in the MVN. By contrast, the number of histidine decarboxylase positive neurons depended neither on the group, nor on the treatment or survival period (Table 1).
Table 1.
Statistical analysis of the effects of thioperamide treatment and vestibular lesions (unilateral, bilateral one or two steps) on the histaminergic system at the molecular level
| Source of variation | d.f. | F | P |
|---|---|---|---|
| HDC mRNA | |||
| Controls/UVN cats | 3 | 14.22 | 0.0001* |
| Controls/one-step bilateral cats | 1 | 0.006 | 0.94 |
| Controls/two-step bilateral cats | 1 | 26.28 | 0.0001* |
| Treatment (T/NT) | 1 | 9.07 | 0.003* |
| Survival period (1w, 3 w versus 3 months) | 2 | 11.91 | 0.0001* |
| Silver grain number per HDC neurons | |||
| Controls/UVN cats | 3 | 47.22 | 0.0001* |
| Controls/one-step bilateral cats | 1 | 2.75 | 0.1 |
| Controls/two-step bilateral cats | 1 | 65.82 | 0.0001* |
| Treatment (T/NT) | 1 | 86.89 | 0.0001* |
| Survival period (1w, 3 w versus 3 months) | 2 | 40.09 | 0.0001* |
| Number of HDC-radiolabelled neurons | |||
| Controls/UVN cats | 3 | 1.45 | 0.22 |
| Controls/one-step bilateral cats | 1 | 0.023 | 0.87 |
| Controls/two-step bilateral cats | 1 | 0.52 | 0.46 |
| Treatment (T/NT) | 1 | 0.27 | 0.59 |
| Survival period (1w, 3 w versus 3 months) | 2 | 1.44 | 0.23 |
| H3 receptor-binding sites | |||
| Controls/UVN cats (lesioned side) | 1 | 14.35 | 0.0002* |
| Controls/treated cats (both sides) | 1 | 548.13 | 0.0001* |
| UVN cats/thioperamide-treated cat | 1 | 450.45 | 0.0001* |
| UVN cats (intact side lesioned−1 side) | 1 | 47.87 | 0.0001* |
Repeated-measures analysis of variance of the HDC mRNA labelled surface (in square pixels), the number of silver grains per histidine decarboxylase radiolabelled neurons, the number of histidine decarboxylase radiolabelled neurons in the tuberomammillary nuclei, and the number of histamine H3 receptor binding sites (fmol (mg protein)−1) in the medial vestibular nuclei (VMN). Group, vestibular lesion, treatment, and survival period are the main fixed effects providing the sources of variation among cats
d.f.: degree of freedom; F: Scheffé's test; P: probability level.
Vestibular lesion-induced changes of HDC mRNA expression in the TM nuclei
Labelled surface
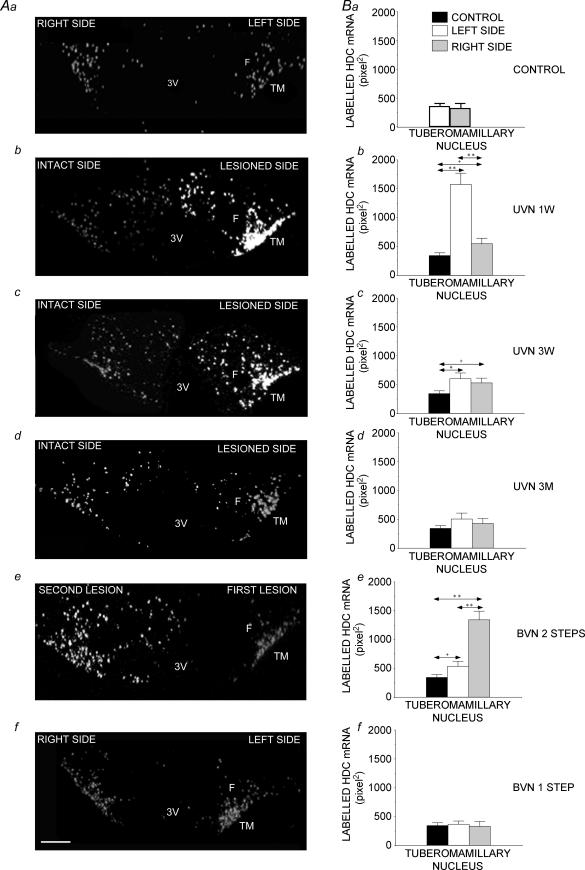
Figure 1 shows photomicrographs of autoradiographically labelled sections taken from the posterior hypothalamus area of six animals: one control, three UVN cats killed 1 week (Fig. 1Ab), 3 weeks (Fig. 1Ac), and three months (Fig. 1Ad) after a left UVN, and two BVN cats operated in two steps (Fig. 1Ae) or one step (Fig. 1Af). Compared to the controls, which exhibited moderate and symmetrical expression of HDC mRNA, UVN induced a strong increase in surface label of TM nuclei on the lesioned side, at the 1- and 3-week post-lesion times. Three months later, HDC mRNA expression regained control levels on both sides. Mirror images were observed in the two-step BVN animals with increased HDC mRNA labelled surface on the side of the second lesion. By contrast, no changes were seen in the one-step BVN cats.
Figure 1. Expression of histidine decarboxylase (HDC) cRNA probe in coronal sections of the posterior hypothalamus of cats subjected to unilateral vestibular neurectomy (UVN) or bilateral vestibular neurectomy (BVN) performed in one or two steps.
A, illustration of the typical labelling in a representative control cat (a) and three experimental animals submitted to unilateral vestibular neurectomy and observed 1 week (b), 3 weeks (c), and 3 months (d) after the lesion, and two representative cats subjected to a two- (e) or a one-step (f) bilateral vestibular neurectomy. Note that the lesion induced a strong increase in histidine decarboxylase mRNA expression in the tuberomammillary nuclei with predominance on the lesioned side at the 1- and 3-week survival times (b and c). Three months after UVN, HDC mRNA expression regained control levels on both sides (d). The two-step bilateral vestibular neurectomized cats (e), which are compensated left UVN cats killed 1 week after a second neurectomy on the right side, showed mirror images of what was observed after left UVN (b). Cats killed 1 week after a one-step bilateral vestibular lesion (f) showed HDC mRNA expression similar to that of control cats (a). F: fornix; TM: tuberomammillary nucleus; 3V: third ventricle. Bar, 1 mm. B, quantitative evaluation. Data are the mean values (±s.e.m.) of the labelled surface expressed in pixel2 for the control cats (N = 4), unilateral neurectomized cats (N = 12), and bilateral neurectomized cats (N = 8). The values recorded on the right (grey bars) and left (white bars) sides are given separately for all the subgroups of cats. The data from both sides were pooled for the controls (black bars) for a direct comparison with the subgroups of cats killed 1 week (b), 3 weeks (c) and 3 months (d) after a left unilateral vestibular neurectomy, and with the two-step (e) and the one-step (f) bilateral vestibular neurectomized cats. *P < 0. 01; **P < 0.0001; +P < 0.04. HDC: histidine decarboxylase; UVN: unilateral vestibular neurectomy; BVN: bilateral vestibular neurectomy; 1 W: 1 week; 3 W: 3 weeks; 3M: 3 months.
The quantitative analysis of these data is shown on the bar graphs of Fig. 1B. The labelled surface was 340.9 ± 51.1 pixels2 on average in the TM nuclei of the control cats (356.3 ± 55.1 and 325.5 ± 87.4 on the left and right sides, respectively: NS). For the subgroup of cats examined 1 week after UVN, the statistical analysis indicated a significant increase on both sides, with a higher labelling on the lesioned side (1572.9 ± 199.5; 362%; P < 0.0001) than the intact side (547.4 ± 95.7; 60%; P < 0.04). HDC mRNA expression was 287% larger on the lesioned side than on the intact side (P < 0.0001). The labelled surface remained significantly increased on both sides in the cats examined 3 weeks after UVN, but HDC mRNA expression was strongly reduced on the lesioned side compared to that 1 week post-lesion. In addition, no differences between the intact (528 ± 83.1) and the lesioned (600.3 ± 102.5) sides were observed. Three months after UVN, HDC mRNA expression regained control values on both the intact (427 ± 88.8) and lesioned (504 ± 80.7) sides. The quantitative analysis confirmed the reversed pattern of HDC mRNA expression in the two-step BVN compared to the 1 week UVN cats. A significant bilateral increase in the labelled surface was seen relative to the controls, with a more pronounced effect on the side of the second lesion (1339.7 ± 151.4; 300%; P < 0.0001) than on the side of the first lesion (534.8 ± 88.8; 56%, P < 0.04). After one-step BVN, the mean values on the left (364.1 ± 58.1) and right (328.6 ± 84.8) sides were not significantly modified with respect to controls.
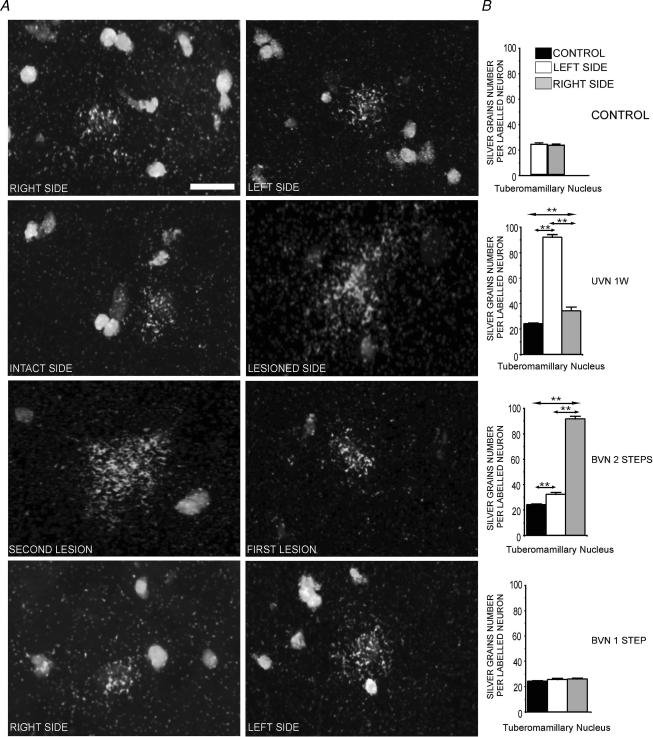
Silver grain number per labelled neuron
Figure 2A illustrates the changes in the number of silver grains per HDC-radiolabelled neuron in the TM nuclei after vestibular lesion. A higher number was in particular found on the side of the lesion 1 week after UVN and on the side of the second lesion in the two-step BVN cats, demonstrating again mirror images in these two subgroups. By contrast, the number of silver grains per labelled neuron remained unchanged in the one-step BVN animals. The quantitative analysis of the data is shown in Fig. 2B. The bar graphs correspond to those concerning the labelled surface (Fig. 1B) for the UVN cats examined at 1 week, and the one- and two-step BVN animals.
Figure 2. Changes in the number of silver grains per HDC radiolabelled neuron in the TM nuclei after vestibular lesion.
A, high magnification photomicrographs from emulsion autoradiograms showing hybridization of cRNA specific for histidine decarboxylase mRNAs to neurons in the tuberomammillary nucleus (TM), illustrating the number of silver grains per labelled neuron in the tuberomammillary nuclei (TMN) on the right and left sides of a representative control cat. One week after unilateral vestibular neurectomy (UVN), the number of silver grains on the intact side was lower than that on the lesioned side. In the two-step bilateral vestibular neurectomized cats (BVN), mirror images were obtained with an increased labelling on the side of the second lesion as compared with the TMN on the side of the first lesion. The number of silver grains per labelled neuron remained unchanged in the one-step BVN cats. Scale bar, 10 μm. B, quantitative analysis of the effects of unilateral vestibular neurectomy (UVN) and bilateral vestibular neurectomy (BVN) performed in one or two steps on the number of silver grains per radiolabelled neurons in the tuberomammillary nuclei. Data are expressed as mean values (±s.e.m.). The values recorded on the right (grey bars) and left (white bars) sides are given separately for all the vestibular lesioned cats. The data from both sides were pooled for the controls (black bars) for a direct comparison with the subgroups of cats killed 1 week after a left unilateral vestibular neurectomy, and with the two-step and the one-step bilateral vestibular neurectomized cats. The bar graphs correspond to those of Fig. 1B for the UVN cats examined at 1 week, and the one- and two-step BVN cats. **P < 0.0001. UVN: unilateral vestibular neurectomy; BVN: bilateral vestibular neurectomy; 1 W: 1 week.
Number of labelled neurons
The quantitative analysis of the number of HDC radiolabelled neurons in the TM nuclei showed no changes after vestibular lesion (UVN, BVN in one or two steps) compared to the controls (data not shown). The mean number in the controls (115.17 ± 4.75) remained unchanged whatever the type of vestibular lesion and the survival time.
Taken together, the data demonstrate that the larger labelled surface observed after vestibular lesion is due to an increase in silver grain number per labelled neuron and not to an increase in the number of labelled cells.
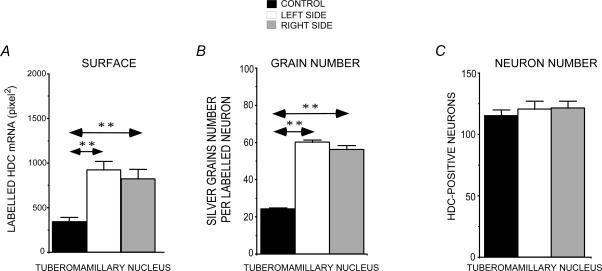
Effects of thioperamide treatment
Effects on HDC mRNA in the TM nuclei of UVN cats
Figure 3 illustrates the quantitative analysis of the HDC mRNA labelled surface in the TM nuclei of UVN cats subjected to 3 week thioperamide treatment. The TM nuclei showed a bilateral and symmetrical increase in the HDC mRNA labelled surface relative to the TM nuclei of the controls. Indeed, the staining on both the lesioned (921.5 ± 99.4 pixel2) and the intact (820.1 ± 108.8 pixel2) sides of this subgroup of cats was significantly larger than that of the control group (270%, P < 0.0001, and 240%, P < 0.0001, for the lesioned and intact sides, respectively). In addition, the HDC mRNA labelled surface in the TM nuclei was significantly larger in the UVN cats treated with thioperamide for 3 weeks than in the untreated UVN cats tested at the same post-lesion time. The increase due to the treatment was 153% on the lesioned side (P < 0.03) and 155% on the intact side (P < 0.04) compared to the untreated cats (Fig. 1Bc right panel).
Figure 3. Effects of thioperamide treatment on HDC mRNA expression in the tuberomammillary nuclei.
Quantification of the HDC mRNA labelled surface (A), silver grain number per HDC labelled neuron (B), and the number of HDC radiolabelled neurons (C) on the right (grey bars) and left (white bars) TM nucleus of thioperamide-treated cats compared to the control cats (black bars). Note that the HDC mRNA labelled surface and the number of silver grains per HDC-labelled neurons are bilaterally and significantly increased in the TM nuclei of thioperamide-treated cats. By contrast, the number of radiolabelled neurons in the TM nuclei is not affected by the thioperamide treatment. **P < 0.0001. HDC: histidine decarboxylase.
The bar graphs again correspond to those concerning the labelled surface. The quantitative analysis showed a bilateral and symmetrical increase in the grain number after a 3 week thioperamide treatment. By contrast, the number of radiolabelled neurons in the TM nuclei was not affected by the thioperamide treatment in this subgroup of cats (Fig. 3).
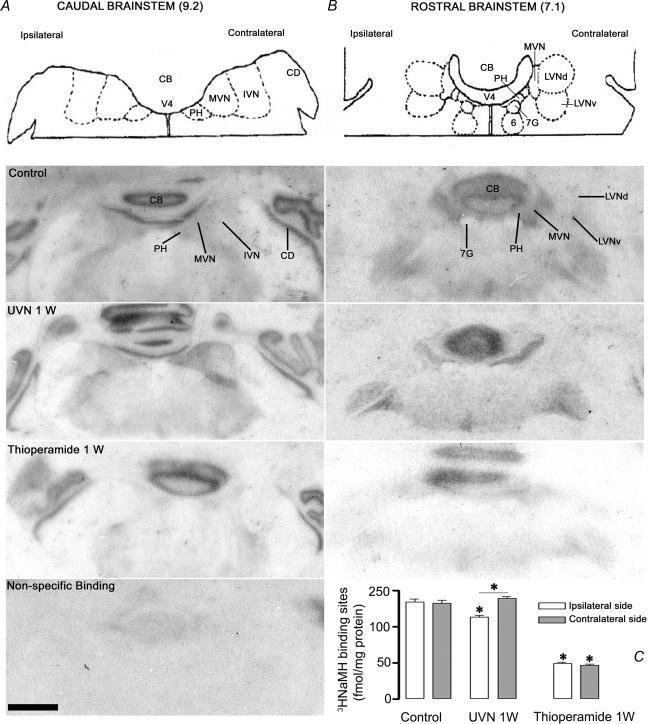
Effects on H3 receptor binding sites in the medial vestibular nuclei
Figure 4A illustrates the spatial distribution of H3 binding density in representative serial frontal sections collected from the caudal part of the brainstem in one control cat (top), one animal killed 1 week after UVN (centre), and one cat treated for one week with thioperamide (bottom). The pictures show a drastic bilateral decrease after thioperamide treatment and an asymmetrical pattern with an ipsilateral decrease after UVN. Figure 4B shows similar data in representative coronal sections collected from the rostral part of the brainstem. The statistical analysis of [3H]N-α-methylhistamine binding site density points to a significant decrease in the MVN on the deafferented side for the UVN cats (16%; P < 0.0002) and to a significant bilateral reduction after 1 week thioperamide treatment (64%; P < 0.0001) (Fig. 4C). It can be mentioned that asymmetrical labellings after UVN were found not only in the MVN, but also in the prepositus hypoglossi, the inferior olive complex and the solitary nucleus, which exhibited decreased labellings on the ipsilateral side.
Figure 4. Illustration of [3H]N-α-methylhistamine binding sites in representative sections of the cat brainstem.
Coronal sections from three representative animals: a control cat, a 1-week unilateral neurectomized cat (UVN 1 W), and a 1 week thioperamide-treated cat. Histamine H3 receptor binding is decreased ipsilaterally in both caudal (A) and rostral (B) parts of the MVN 1 week after unilateral vestibular neurectomy, as compared to the control. Bilateral and symmetrical reductions are observed in the caudal and rostral MVN of the 1 week thioperamide-treated cat. A non-specific binding is represented at the bottom of the figure. IVN: inferior vestibular nucleus; MVN: medial vestibular nucleus; LVNd: lateral vestibular nucleus, dorsal part; LVNv: lateral vestibular nucleus, ventral part; PH: prepositus hypoglossi; CD: dorsal cochlear nucleus; CB: cerebellar cortex; 7G: genu of the facial nerve; 6: abducens nucleus. Scale bar: 1 mm. C, bar graph representing the quantitative evaluation of the effects of unilateral vestibular neurectomy or thioperamide treatment on the binding density of the agonist [3H]N-α-methylhistamine to H3 receptors in the medial vestibular nuclei. The data are means and standard errors of the mean fmol of [3H]N-α-methylhistamine specifically bound per mg of protein from autoradiograms taken from 4 animals in each group. Data from the MVN are provided separately for each side (left/ipsilateral side (white bars) versus right/contralateral side (grey bars)). *Significantly different from the control data (cf. Table 1).
Behavioural correlates
Horizontal spontaneous nystagmus (HSN)
The variance analysis (ANOVA) demonstrated significant effects depending on the groups (P < 0.0001), the postoperative time (P < 0.0001), and the interaction between these two factors (P < 0.0001) (Table 2). The statistical analysis pointed therefore to improvement of HSN, posture and equilibrium function during the recovery process, and to acceleration of the behavioural recovery under thioperamide treatment.
Table 2.
Statistical analysis of the effects of thioperamide treatment on vestibular compensation at the behavioural level
| Source of variation | d.f. | F | P |
|---|---|---|---|
| Horizontal nystagmus | |||
| Group (UVN treated/UVN untreated) | 1 | 168.09 | 0.0001* |
| Post operative time | 7 | 616.98 | 0.0001* |
| Group × post operative time | 7 | 35.38 | 0.0001* |
| Posture | |||
| Group (UVN treated/UVN untreated) | 1 | 979.69 | 0.0001* |
| Post operative time | 22 | 530.17 | 0.0001* |
| Group × post operative time | 22 | 84.87 | 0.0001* |
| Equilibrium function | |||
| Group (UVN treated/UVN untreated) | 1 | 238.80 | 0.0001* |
| Post operative time | 23 | 305.06 | 0.0001* |
| Group × post operative time | 23 | 57.67 | 0.0001* |
Repeated-measures analysis of variance of the horizontal spontaneous nystagmus, posture recovery and equilibrium function recovery. Group (treated unilateral vestibular neurectomized cats versus untreated unilateral vestibular neurectomized cats), and survival period are the main fixed effects providing the sources of variation among cats, as also illustrated by the significant interaction between these two variables
d.f.: degree of freedom; F: Scheffé's test; P: probability level.
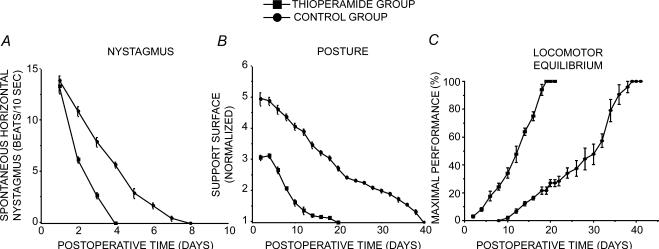
The HSN fully recovered in both the treated and the untreated UVN cats, but the recovery was significantly accelerated in the thioperamide-treated group. Figure 5 illustrates the time course of this decline in HSN frequency recorded in the light. One day after UVN, the HSN frequency was similar in the untreated control group and the thioperamide group (14 ± 0.4 beats and 13.5 ± 0.6 beats, respectively; NS). The HSN was significantly decreased 2 days after UVN and thioperamide treatment compared to the control untreated cats (6.25 ± 0.25 and 11 ± 0.4, respectively; P < 0.0001). The HSN totally disappeared 4 days after UVN in the treated group while it persisted until 8 days in the untreated group.
Figure 5. Effects of thioperamide treatment on horizontal spontaneous nystagmus (HSN), posture function, locomotor balance recovery.
For all the behavioural data, the thioperamide group is shown as filled squares and the control group as filled circles. A measurement of horizontal spontaneous nystagmus (HSN) after unilateral vestibular neurectomy as an index of vestibular compensation. Time course of disappearance of HSN frequency after UVN. Each data point represents the mean number of HSN quick-phase movements in 10 s across 3 animals (five repeated measures per animal per sampling). Error bars represent s.e.m. Note the strong increase in HSN 2 days after unilateral vestibular neurectomy and the faster recovery under thioperamide treatment. B, mean postoperative development of the support surface in the two groups of cats. The support surface evaluated in cm2 and normalized with respect to the preoperative values referred to unity (1 being close to 50 cm2), is reported on the ordinate as a function of the postoperative time in days on the abscissae. Each point represents the mean value (n = 5 measurements per cat) calculated in the treated (N = 4) and untreated (N = 4) groups of cats. Standard errors of the mean are reported as vertical lines. Note the strong increase in support surface in the days following unilateral vestibular neuretomy and the faster recovery under thioperamide treatment. C, mean recovery curves illustrating maximal performance of the cat on the rotating beam, expressed as a percentage of the preoperative maximal performance (on the ordinates) as a function of the postoperative time in days (on the abscissa). Standard errors of the mean are reported as vertical lines. The linear regression curves fitting the experimental data are plotted on each graph. Note the acceleration of the recovery time under thioperamide treatment as compared to the controls, illustrated both by the slope of the regression curves and by the shorter time required to achieve full compensation (3 weeks instead of almost 6 weeks).
Posture function recovery
Figure 5 shows the time course of posture function recovery as revealed by the changes in the surface delimited by the four legs of the cat standing erect at rest in the group treated postoperatively with thioperamide and in the untreated group. UVN induced a strong increase in the support surface in both the treated and untreated groups of cats. The mean normalized values recorded 2 days postoperatively reached 3.09 ± 0.07 in the thioperamide-treated cats and 5 ± 0.21 in the controls (P < 0.0001). The recovery profile followed an almost linear function in the control group, by contrast, the recovery time course in the thioperamide group fitted better with an exponential regression curve, indicating that postural function was restored more quickly and non-linearly in this group of cats. Accordingly, comparisons were performed using the variance analysis (Table 2). Improvement of posture function recovery under thioperamide treatment was reflected by a faster return of support surface toward normal values. This required 20 days in the thioperamide group while a full recovery necessitated 40 days in the control group of cats.
Locomotor balance recovery
Recovery of locomotor balance (Max P) was complete in the treated and the untreated groups of cats, but it was also significantly accelerated in the thioperamide-treated group, as shown by the mean recovery profiles (Fig. 5). The untreated UVN cats were unable to walk on the beam up to 8 days after vestibular lesion. By contrast, the thioperamide-treated UVN group were able to cross as soon as 2–4 days postoperatively. Later, Max P increased in the two groups of cats, as indicated by the regression curves best fitting the experimental data and by the determination coefficients (cf. Table 3). However, restoration of locomotor balance was significantly accelerated in the thioperamide-treated cats, as shown by the higher slope (5.8%) recorded in this group compared to the controls (3.2%: P < 0.01).
Table 3.
Statistical evaluation of locomotor balance recovery (maximal performance) in the thioperamide-treated group and the control group of cats
| Statistical analysis (ANOVA) | ||||
|---|---|---|---|---|
| Linear regression | Coefficient of | |||
| Groups | curve | determination | Slope | ANOVA |
| Control group | y = 3.2x− 37.5 | R2= 0.95 | 0.01 | 0.0001 |
| Thioperamide-treated group | y = 5.8x− 16.4 | R2= 0.98 | ||
Shown are the linear regression equations fitting the experimental data concerning the maximal performance of the cats on the rotating beam, with coefficients of determination. The statistical evaluation was performed using both the slope comparison test and a variance analysis (ANOVA).
Finally, the treated cats fully recovered in 21 days while 41 days were required for the controls, a time benefit of 20 days due to the treatment.
Discussion
This study shows that unilateral vestibular neurectomy (UVN) increases HDC mRNA expression in the TM nuclei acutely (1 week), with asymmetrical changes characterized by a greater up-regulation in the ipsilateral TM nucleus. This significantly increased expression persisted 3 weeks after the lesion, while fully compensated cats (3 months post-lesion) exhibited labelling that returned to control values. HDC mRNA expression was not modified in the one-step BVN cats but still showed asymmetrical increases after two-step surgery. In these latter animals, the asymmetrical HDC mRNA expression was the mirror image of that found in the 1 week UVN cats, and thus consistent with the so-called Betcherew's phenomenon. UVN cats treated for 3 weeks with thioperamide showed bilateral and symmetrical HDC mRNA up-regulation, which was higher than in the untreated UVN group killed at the same post-lesion time. In addition, binding to histamine H3Rs in the MVN showed a strong bilateral decrease in the unlesioned thioperamide-treated cats, while binding density was also reduced but restricted to the ipsilateral MVN in the UVN cats. That such changes of the histaminergic system induced by vestibular nerve lesion and treatment may play a functional role in vestibular compensation is strongly supported by the behavioural data. Indeed, spontaneous nystagmus, posture, and locomotor balance were rapidly recovered in the UVN cats treated with thioperamide. Taken together, these results confirm changes in HDC mRNA expression and lend further support to the notion that changes in histamine levels may be related to vestibular compensation.
Unilateral vestibular lesion-induced modification of HDC mRNA expression in the TM nuclei
The UVN strongly increased HDC mRNA expression in the TM nucleus on both sides, with a predominant expression on the lesioned side at 1 week. The vestibular imbalance generated in the VN after UVN (reviewed in Smith & Curthoys, 1989) most likely activates a vestibulo-hypothalamic loop responsible for HDC mRNA up-regulation. This hypothesis is supported by the increased histamine release from the hypothalamus after vestibular caloric (Horii et al. 1993) or hypergravity (Uno et al. 1997) stimulations, the lack of histamine changes after vestibular stimulations in bilateral labyrinthectomized animals (Uno et al. 1997), and the responses of hypothalamic neurons to electrical stimulation of the vestibular nerve or the lateral VN (Grigoryan et al. 1999). The reverse pattern of HDC mRNA expression seen acutely in the two-step BVN compared to the UVN cats and the absence of modification in the one-step BVN animals are supplementary arguments strengthening the idea of vestibulo-hypothalamic loop activation due to VNC electrical asymmetry. Quantification of the autoradiograms demonstrated that the increase in HDC mRNA was due to an increased expression per cell and not to a greater number of cells expressing HDC. Anatomical data underline the existence of vestibulo-hypothalamic connections. Polysynaptic and bilateral projections from the VN to the hypothalamus have been reported (Ericson et al. 1991). But direct and predominantly contralateral projections from the MVN to the posterior hypothalamic area have been found in the monkey (Matsuyama et al. 1996). The asymmetrical firing rate of the VN cells in acute UVN cats, with reduced activity on the lesioned side and increased activity on the intact side for both the MVN (Precht et al. 1966) and the LVN (Zennou-Azogui et al. 1993) can therefore account for the HDC mRNA up-regulation, particularly pronounced in the TM nucleus on the lesioned side (ipsilaterally in the UVN cats, contralaterally in the two-step BVN animals).
The time course of HDC mRNA expression in the TM nuclei of the UVN cats correlates with electrophysiological and behavioural data. Electrophysiological investigations in the UVN cat still revealed, 3 weeks post-lesion, asymmetrical spontaneous firing rates between the bilateral VNCs, but the imbalance was attenuated. This attenuated imbalance may account for the lower asymmetry in HDC mRNA expression observed between the two TM nuclei at this compensatory stage. Finally, the HDC mRNA labelling regained control values in fully compensated UVN cats (3 months) in which the electrical activity of VNCs was rebalanced (Precht et al. 1966; Zennou et al. 1993). A two-step BVN again up-regulates HDC mRNA expression and creates asymmetrical changes with increased labelling in the TM nuclei predominantly on the side of the second lesion. This mechanism correlates with Betcherew's phenomenon, i.e. the mirror image of the vestibular deficits seen after the first lesion. This phenomenon is clearly attributed to resumption of normal discharge by VN neurons on the side of the first lesion. Reduction in the severity of the ocular motor and postural syndrome over time is correlated with the restoration of neuronal activity within the ipsilateral VNC. The time to restoration varies with species (Zennou-Azogui et al. 1993; Ris et al. 1995), but a close correlation was always found between the electrophysiological and behavioural data (Smith & Curthoys, 1989; Curthoys, 2000). Behaviourally, the UVN cats still exhibit head tilt 3 weeks post-lesion (Lacour et al. 1991) and their static as well as dynamic equilibrium functions are only partially recovered (Tighilet et al. 1995; Lacour et al. 1997). The static vestibular syndrome is totally compensated 5–6 weeks post-lesion in the cat (Lacour et al. 1991, 1997; Tighilet et al. 1995), a result that is confirmed by our behavioural data collected in the UVN untreated group of cats (see below).
Effects of histamine H3 receptor antagonist treatment
At the molecular level
UVN cats treated with thioperamide, an H3R antagonist/inverse agonist, exhibited a strong bilateral up-regulation of HDC mRNA in the TM nuclei. The histamine H3Rs are considered located in the presynaptic neurons of a variety of neuronal systems, including the histaminergic neurons, and act as autoreceptors for regulating the release of histamine from the presynaptic neurons. Histamine H3Rs are involved in the regulation of the synthesis and release of histamine. Hence, it is likely that the blockade of the H3Rs by thioperamide may increase the synthesis and release of histamine from the presynaptic histamine neurons.
Thioperamide increases histamine synthesis in the TM nuclei neurons and histamine release in their cerebral targets, as shown in rat brain slices (Arrang et al. 1983, 1987). The VNCs are one of the histamine brainstem target structures for which we suggested histamine release is increased in the cat after treatment with thioperamide or with betahistine, another H3R antagonist (Tighilet & Lacour, 1997). As suggested by Lozada et al. (2004), H3R may be an essential part of presynaptic mechanisms for re-establishing resting activities after unilateral vestibular lesion. Release of histamine likely participates in restoring balanced activity of the VN cells on both sides. Indeed, in vitro intracellular recordings from neurons in the MVN have revealed several classes of neurons, all of which are depolarized by histamine via an action at postsynaptic H1R (Inverarity et al. 1993) or H2R (Phellan et al. 1990; Serafin et al. 1993; Wang & Dutia, 1995).
Our autoradiographic data show a strong decrease in the binding density of the agonist [3H]N-α-methylhistamine to histamine H3Rs in the MVN of thioperamide-treated and UVN cats. This effect was seen bilaterally after treatment with thioperamide, confirming what we previously described with betahistine (Tighilet et al. 2002), and only in the ipsilateral MVN after UVN. The data strongly suggest that treatment with H3R antagonists increases histamine turnover and release, very likely by blocking presynaptic histamine H3 autoreceptors. The high endogenous histamine release due to thioperamide administration may decrease the number of H3 receptor sites, consistent with a selective down-regulation of these receptors. Another possibility is that thioperamide causes a decrease in [3H]N-α-methylhistamine binding solely because residual compounds remain in the tissue. This possibility, however, can be excluded since residual thioperamide in the brain tissue is presumably not present because of (i) the long time (24 h) elapsed between the last drug administration and killing of the animals, and (ii) the likely dissociation of any residual thioperamide from the histamine H3 receptor during the tissue section procedure (incubation and washing).
Interestingly, the VN complexes are known to contain H1Rs, H2Rs and H3Rs and in addition to the H3 autoreceptors on their afferent fibres, histamine H3Rs have been found on the perykaria of MVN neurons themselves (Pillot et al. 2002). This could explain why local perfusion of the VN on one side with histamine H3R agonist was found to induce a postural and oculomotor syndrome mimicking that seen after unilateral labyrinthectomy (Yabe et al. 1993). The data also support the behavioural facilitation of vestibular compensation observed after systemic administration of H3R antagonists or inverse agonists (Yabe et al. 1993; Tighilet et al. 1995), a result confirmed by the present study for the thioperamide-treated UVN cats.
Since the original demonstration by Arrang et al. (1983) that histamine H3 receptors inhibit histamine synthesis and release, histamine has been found to inhibit via this receptor the release of many other transmitters, including glutamate, GABA, noradrenaline, dopamine, acetylcholine, serotonin, and various peptides (reviewed in Brown et al. 2001). Interestingly, these different classes of neurotransmitters are present in the VNCs and are involved in vestibular functions and vestibular compensation (reviewed in De Waele et al. 1995).
At the behavioural level
Our data demonstrate that behavioural recovery after UVN is strongly accelerated in cats treated with thioperamide. The data confirm our previous work with betahistine in the same animal model (Tighilet et al. 1995) and the results of Pan et al. (1998) in unilateral labyrinthectomized (UL) rats receiving various H3R antagonist compounds, including betahistine and thioperamide. By contrast, inhibition of the histaminergic system was found also to facilitate the functional recovery after UL in the goldfish (Piratello & Mattioli, 2004). Taken together, the data suggest that histamine could play an opposite role in quadrupeds and in fish through a mechanism other than its impact on the vestibular system.
Thioperamide accelerated the recovery of the post-lesional horizontal nystagmus since it disappeared as early as 4 days in the light compared to 8 days in the untreated UVN group. In addition, time to recovery of posture was also reduced in the same proportion (20 days versus 40 days) under treatment. The histaminergic system seems therefore to have a specific effect on the compensation of the static deficits. Interestingly, a close relationship was found between the compensation of the static (HSN, posture) and dynamic (locomotor balance) deficits in both the treated and untreated cats: Walking again on the beam in the rotarod test coincides with nystagmus disappearance (4 days and 8 days, respectively), and full locomotor balance recovery is correlated with regaining a nearly normal support surface (20 days and 40 days, respectively). The data strongly suggest that acceleration of dynamic compensation is highly dependent on the static compensation, which itself is shortened by H3R antagonist compounds. It is known, however, that thioperamide heavily modifies vigilance by increasing alertness in cats at doses similar to those used in our study (Lin et al. 1990). By contrast, mice that lack histamine by disrupting the histidine decarboxylase gene show a deficit in waking and cognitive functions (attention, interest in new environments: see Parmentier et al. 2002). Conversely, pharmacological blockade of central H3Rs exerts pro-cognitive activity in tasks such as olfactory social memory, five-trial inhibitory avoidance test (Fox et al. 2003), and attention (Komater et al. 2003). By promoting vigilance, thioperamide could also favour sensorimotor and cognitive activity and explain partly the acceleration of vestibular compensation (Xerri & Lacour, 1980).
Acknowledgments
The study was supported by grants from Ministère de l'Enseignement Supérieur et de la Recherche and CNRS (UMR Université de Provence/CNRS No. 6149) and from the European Union (FEDER). We thank Dr Carole Chotard for technical assistance. We also thank Valérie Gilbert and Patrick Gélign for having taken care of the animal.
References
- Arrang JM, Garbag M, Schwartz JC. Autoregulation of histamine synthesis in brain mediated by presynaptic H3 receptors. Neuroscience. 1987;23:149–157. doi: 10.1016/0306-4522(87)90279-x. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- Berman AL. A Cytoarchitechtonic Atlas with Stereotaxic Coordinates. Madison: Wisconsin University Press; 1968. The brain stem of the cat. [Google Scholar]
- Berman AL, Jones EG. A Cytoarchitectonic Atlas with Stereotaxic Coordinates. Madison: Wisconsin University Press; 1982. The thalamus and basal telencephalon of the cat. [Google Scholar]
- Betcherew W. Ergebnisse der durchschneidung des N. acusticus, nebst Erörterung der bedeutung der semicirculären canäle für das korpergleichgewicht. Pflüg Arch Ges Physiol. 1883;30:312–347. [Google Scholar]
- Borel L, Lacour M. Functional coupling of the stabilizing eye and head reflexes during horizontal and vertical linear motion in the cat. Exp Brain Res. 1992;91:191–206. doi: 10.1007/BF00231654. [DOI] [PubMed] [Google Scholar]
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. Vestibular compensation and substitution. Curr Opin Neurol. 2000;13:27–30. doi: 10.1097/00019052-200002000-00006. [DOI] [PubMed] [Google Scholar]
- Darlington CL, Smith PF. Molecular mechanisms of recovery from vestibular damage in mammals: recent advances. Prog Neurobiol. 2000;623:13–25. doi: 10.1016/s0301-0082(00)00002-2. [DOI] [PubMed] [Google Scholar]
- De Waele C, Muhlethaler M, Vidal PP. Neurochemistry of the central vestibular pathways. Brain Res Brain Res Rev. 1995;20:24–46. doi: 10.1016/0165-0173(94)00004-9. [DOI] [PubMed] [Google Scholar]
- Dieringer N. Vestibular compensation: neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol. 1995;46:97–129. [PubMed] [Google Scholar]
- Ericson H, Blomqvist A, Köhler C. Origin of neuronal inputs to the region of the tuberomammillary nucleus of the rat brain. J Com Neurol. 1991;311:45–64. doi: 10.1002/cne.903110105. [DOI] [PubMed] [Google Scholar]
- Fox GB, Pan JB, Radek RJ, Lewis AM, Bitner RS, Esbenshade TA, et al. Two novel and selective nonimidazole H3 receptor antagonists A-304121 and A-317920. II. In vivo behavioral and neurophysiological characterization. J Pharmacol Exp Ther. 2003;305:897–908. doi: 10.1124/jpet.102.047241. [DOI] [PubMed] [Google Scholar]
- Grigoryan SS, Baklavadzhyan OG, Minasyan SM, Adamyan TsI, Gevorkyan ES, Sarkisyan SG. Responses of hypothalamic neurons to stimulation of the vestibular nerve and lateral vestibular nucleus in the rabbit. Neurosci Behav Physiol. 1999;29:61–66. doi: 10.1007/BF02461359. [DOI] [PubMed] [Google Scholar]
- Horii A, Takeda N, Matsunaga T, Yamatodani A, Mochizuki T, Okakura-Mochizuki K, et al. Effect of unilateral vestibular stimulation on histamine release from the hypothalamus of rats in vivo. J Neurophysiol. 1993;70:1822–1826. doi: 10.1152/jn.1993.70.5.1822. [DOI] [PubMed] [Google Scholar]
- Inokuchi A, Liu F, Yokomitsu S, Ureshino M, Komiyama S. Effects of the antihistaminergic drugs diphenhydramine and zolantidine on vestibular-induced hypothalamic neuronal activity in the guinea pig. Eur Arch Otorhinolaryngol. 1999;256:S22–S266. doi: 10.1007/pl00014148. [DOI] [PubMed] [Google Scholar]
- Inverarity DJ, Johnston AR, McQueen DS, Dutia MB. Effects of histamine on rat medial vestibular nucleus neurones in vitro. J Physiol. 1993;459:466P. [Google Scholar]
- Kirsten EB, Sharma JN. Microiontophoresis of acetylcholine, histamine and their antagonists on neurones in the medial and lateral vestibular nuclei of the cat. Neuropharmacology. 1976;15:743–753. doi: 10.1016/0028-3908(76)90003-4. [DOI] [PubMed] [Google Scholar]
- Komater VA, Browman KE, Curzon P, Hancock AA, Decker MW, Fox GB. H3 receptor blockade by thioperamide enhances cognition in rats without inducing locomotor sensitization. Psychopharmacology. 2003;167:363–372. doi: 10.1007/s00213-003-1431-0. [DOI] [PubMed] [Google Scholar]
- Lacour M, Ez-Zaher L, Raymond J. Plasticity mechanisms in vestibular compensation in the cat are improved by an extract of Ginkgo biloba (Egb 761) Pharmacol Biochem Behav. 1991;40:367–379. doi: 10.1016/0091-3057(91)90568-m. [DOI] [PubMed] [Google Scholar]
- Lacour M, Sterkers O. Histamine and betahistine in the treatment of vertigo: elucidation of mechanisms of action. CNS Drugs. 2001;15:853–870. doi: 10.2165/00023210-200115110-00004. [DOI] [PubMed] [Google Scholar]
- Lacour M, Sun J, Harlay F. Kinematic analysis of locomotion in unilateral vestibular neurectomized cats. J Vest Res. 1997;7:101–118. [PubMed] [Google Scholar]
- Lin JS, Sakai K, Vanni-Mercier G, Arrang JM, Garbarg M, Schwartz JC, et al. Involvement of histaminergic neurons in arousal mechanisms demonstrated with H3-receptor ligands in the cat. Brain Res. 1990;523:325–330. doi: 10.1016/0006-8993(90)91508-e. [DOI] [PubMed] [Google Scholar]
- Lozada AF, Aarnisalo AA, Karlstedt K, Stark H, Panula P. Plasticity of histamine H3 receptor expression and binding in the vestibular nuclei after labyrinthectomy in rat. BMC Neurosci. 2004;5:32. doi: 10.1186/1471-2202-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T, Kayahara T, Nomura J, Nakano K. Direct projections from the medial vestibular nucleus to the posterior hypothalamic area in the monkey (Macaca fuscata) Neurosci Lett. 1996;219:199–202. doi: 10.1016/s0304-3940(96)13206-7. [DOI] [PubMed] [Google Scholar]
- Pan JB, O'Neill AB, Hancock AA, Sullivan JP, Brioni JD. Histaminergic ligands attenuate barrel rotation in rats following unilateral labyrinthectomy. Meth Find Exp Clin Pharmacol. 1998;20:771–777. [PubMed] [Google Scholar]
- Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibers in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci U S A. 1984;81:2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier R, Ohtsu H, Djebbara-Hannas Z, Valatx JL, Watanabe T, Lin JS. Anatomical, physiological and pharmacological characteristics of histidine decarboxylase Knock-out mice: evidence for the role of brain histamine in behavioral and sleep-wake control. J Neurosci. 2002;22:7695–7711. doi: 10.1523/JNEUROSCI.22-17-07695.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan KD, Nakamura J, Gallagher JP. Histamine depolarizes rat medial vestibular nucleus neurons recorded intracellularly in vitro. Neurosci Lett. 1990;109:287–292. doi: 10.1016/0304-3940(90)90009-x. [DOI] [PubMed] [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz J, et al. A detailed mapping of the histamine H3 receptor and its gene transcripts in rat brain. Neuroscience. 2002;114:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Piratello AC, Mattioli R. Effects of Chlorpheniramine and L-histidine on vestibular compensation in goldfish, Carassius auratus. Neurosci Lett. 2004;367:160–163. doi: 10.1016/j.neulet.2004.05.105. [DOI] [PubMed] [Google Scholar]
- Pollard H, Schwartz JC. Histamine neuronal pathways and their function. Trends Neurosci. 1987;10:86–89. [Google Scholar]
- Precht W, Shimazu H, Markham CH. A mechanism of central compensation of vestibular function following hemilabyrinthectomy. J Neurophysiol. 1966;29:996–1010. doi: 10.1152/jn.1966.29.6.996. [DOI] [PubMed] [Google Scholar]
- Ris L, De Waele C, Serafin M, Vidal PP, Godaux E. Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea pig. J Neurophysiol. 1995;74:2087–2099. doi: 10.1152/jn.1995.74.5.2087. [DOI] [PubMed] [Google Scholar]
- Satayavivad J, Kirsten EB. Iontophoretic studies of histamine and histamine antagonists in the feline vestibular nuclei. Eur J Pharmacol. 1977;41:17–26. doi: 10.1016/0014-2999(77)90366-1. [DOI] [PubMed] [Google Scholar]
- Serafin M, Khateb A, Vibert N, Vidal PP, Muhlethaler M. Medial vestibular nucleus in the guinea-pig: histaminergic receptors. I. An in vitro study. Exp Brain Res. 1993;93:242–248. doi: 10.1007/BF00228391. [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy. Brain Res Brain Res Rev. 1989;14:155–180. doi: 10.1016/0165-0173(89)90013-1. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Lacour M. Distribution of histaminergic axonal fibres in the vestibular nuclei of the cat. Neuroreport. 1996;7:873–878. doi: 10.1097/00001756-199603220-00008. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Lacour M. Histamine immunoreactivity changes in vestibular-lesioned and histaminergic-treated cats. Eur J Pharmacol. 1997;330:65–77. doi: 10.1016/s0014-2999(97)10124-8. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Leonard J, Lacour M. Betahistine dihydrochloride treatment facilitates vestibular compensation in the cat. J Vest Res. 1995;5:53–66. [PubMed] [Google Scholar]
- Tighilet B, Trottier S, Mourre C, Chotard C, Lacour M. Betahistine dihydrochloride interaction with the histaminergic system in the cat: neurochemical and molecular mechanisms. Eur J Pharmacol. 2002;446:63–73. doi: 10.1016/s0014-2999(02)01795-8. [DOI] [PubMed] [Google Scholar]
- Uno A, Takeda N, Horii A, Morita M, Yamamoto Y, Yamatodani A, et al. Histamine release from the hypothalamus induced by gravity change in rats and space motion sickness. Physiol Behav. 1997;61:883–887. doi: 10.1016/s0031-9384(96)00613-0. [DOI] [PubMed] [Google Scholar]
- Vidal PP, de Waele C, Vibert N, Muhlethaler M. Vestibular compensation revisited. Otolaryngol Head Neck Surg. 1998;119:34–42. doi: 10.1016/S0194-5998(98)70171-8. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Dutia MB. Effects of histamine and betahistine on rat medial vestibular nucleus neurones: possible mechanism of action of anti-histaminergic drugs in vertigo and motion sickness. Exp Brain Res. 1995;105:18–24. doi: 10.1007/BF00242178. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Melvill Jones G. Mammallian Vestibular Physiology. New York, London: Plenum Press; 1979. [Google Scholar]
- Xerri C, Lacour M. Compensation des déficits posturaux et cinétiques après neurectomie vestibulaire unilatérale chez le chat. Rôle de l'activité sensorimotrice. Acta-Oto-Laryngol. 1980;90:414–420. [PubMed] [Google Scholar]
- Yabe T, De Waele C, Serafin M, Vibert N, Arrang JM, Muhlethaler M, et al. Medial vestibular nucleus in the guinea-pig: histaminergic receptors. II. An in vivo study. Exp Brain Res. 1993;93:249–258. doi: 10.1007/BF00228392. [DOI] [PubMed] [Google Scholar]
- Zennou-Azogui Y, Borel L, Lacour M, Ez-Zaher L, Ouaknine M. Recovery of head postural control following unilateral vestibular neurectomy in the cat. Neck muscle activity and neuronal correlates in Deiters' nuclei. Acta Otolaryngolsupplement. 1993;509:1–19. [PubMed] [Google Scholar]