Abstract
The Epac family of cAMP-regulated guanine nucleotide exchange factors (cAMPGEFs, also known as Epac1 and Epac2) mediate stimulatory actions of the second messenger cAMP on insulin secretion from pancreatic β cells. Because Epac2 is reported to interact in vitro with the isolated nucleotide-binding fold-1 (NBF-1) of the β-cell sulphonylurea receptor-1 (SUR1), we hypothesized that cAMP might act via Epac1 and/or Epac2 to inhibit β-cell ATP-sensitive K+ channels (KATP channels; a hetero-octomer of SUR1 and Kir6.2). If so, Epac-mediated inhibition of KATP channels might explain prior reports that cAMP-elevating agents promote β-cell depolarization, Ca2+ influx and insulin secretion. Here we report that Epac-selective cAMP analogues (2′-O-Me-cAMP; 8-pCPT-2′-O-Me-cAMP; 8-pMeOPT-2′-O-Me-cAMP), but not a cGMP analogue (2′-O-Me-cGMP), inhibit the function of KATP channels in human β cells and rat INS-1 insulin-secreting cells. Inhibition of KATP channels is also observed when cAMP, itself, is administered intracellularly, whereas no such effect is observed upon administration N6-Bnz-cAMP, a cAMP analogue that activates protein kinase A (PKA) but not Epac. The inhibitory actions of Epac-selective cAMP analogues at KATP channels are mimicked by a cAMP agonist (8-Bromoadenosine-3′, 5′-cyclic monophosphorothioate, Sp-isomer, Sp-8-Br-cAMPS), but not a cAMP antagonist (8-Bromoadenosine-3′, 5′-cyclic monophosphorothioate, Rp-isomer, Rp-8-Br-cAMPS), and are abrogated following transfection of INS-1 cells with a dominant-negative Epac1 that fails to bind cAMP. Because both Epac1 and Epac2 coimmunoprecipitate with full-length SUR1 in HEK cell lysates, such findings delineate a novel mechanism of second messenger signal transduction in which cAMP acts via Epac to modulate ion channel function, an effect measurable as the inhibition of KATP channel activity in pancreatic β cells.
The regulation of ion channel function by cyclic adenosine 3′,5′-monophosphate (cAMP) is achieved via protein kinase A (PKA)-mediated phosphorylation of the channels, or by the direct binding of cAMP to the channels (Hille, 2001). These established actions of cAMP are likely to be complemented by a novel signalling mechanism that utilizes cAMP-regulated guanine nucleotide exchange factors (cAMPGEFs) designated as Epac (the exchange proteins activated by cyclic AMP) (de Rooij et al. 1998; Kawasaki et al. 1998). In mammalian cells, two variants of Epac are expressed (Epac1 and Epac2), each of which binds cAMP with micromolar affinity (Kd 1–3 μm) (de Rooij et al. 2000; Christensen et al. 2003). By binding cAMP, Epac couples cAMP production to the activation of Rap1 and Rap2, two small molecular weight GTPases of the Ras family (Bos, 2003; Rehmann et al. 2003a,b,c). Cellular processes stimulated by Epac include integrin-mediated cell adhesion (Rangarajan et al. 2003), gap junction formation (Somekawa et al. 2005), neurite outgrowth (Kiermayer et al. 2005), and phospholipase C-epsilon (PLC-ɛ) activation (Schmidt et al. 2001). An emerging body of evidence indicates that Epac may also link cAMP production to the regulation of ion channel function, Ca2+ signalling, and exocytosis in excitable cells (Renstrom et al. 1997; Kang et al. 2001, 2003, 2005; Eliasson et al. 2003; Kang & Holz, 2003; Holz & Chepurny, 2003, 2005; Miura & Matsui, 2003; Tsuboi et al. 2003; Holz, 2004a,b; Morel et al. 2005; Landa et al. 2005; Seino & Shibasaki, 2005; Hashiguchi et al. 2006). For these reasons, we have chosen to investigate the potential role of Epac as a determinant of ATP-sensitive K+ channel (KATP channel) activity in pancreatic β cells of the islets of Langerhans.
A role for Epac in the control of KATP channel function is indicated because earlier studies demonstrated an in vitro interaction of Epac2 with the isolated nucleotide-binding fold-1 (NBF-1) of the β-cell sulphonylurea receptor-1 (SUR1, an ATP-binding cassette protein). This interaction was studied within the context of a yeast two-hybrid screen using NBF-1 as bait (Ozaki et al. 2000), or in an immunoprecipitation assay utilizing bacterially expressed Epac2 and NBF-1 (Shibasaki et al. 2004a,b). Given that SUR1 oligomerizes with Kir6.2 to form inwardly rectifying KATP channels (Inagaki et al. 1995), such findings suggest that Epac2 might function as an accessory subunit of KATP channels. If this were to be the case, an interaction of Epac2 with SUR1 might explain earlier reports that cAMP-elevating agents including forskolin, isobutylmethylxanthine, glucagon, and the blood-glucose-lowering hormone glucagon-like peptide-1-(7–36-amide) (GLP-1) inhibit KATP channel function in β cells (Holz et al. 1993; Barnett et al. 1994; Gromada et al. 1998; He et al. 1998; Suga et al. 2000; Ding et al. 2001; Light et al. 2002).
Since the closure of KATP channels is established to be a stimulus for β-cell depolarization, Ca2+ influx and insulin secretion (Holz & Habener, 1992; Henquin, 2000; Ashcroft, 2005), any Epac-mediated action of cAMP to inhibit KATP channels would be of considerable interest. Despite this possibility, it has yet to be determined what effect, if any, Epac exerts at KATP channels. Similarly, it is not certain whether Epac2 interacts with full-length SUR1, nor has it been determined if Epac1 also interacts with SUR1. With these points in mind, we sought to determine if it is Epac that mediates the cAMP-dependent inhibition of KATP channel function in human β cells or rat INS-1 insulin-secreting cells. Our studies were facilitated by the availability of cAMP analogues 2′-O-Methyladenosine-3′, 5′-cyclic monophosphate, (2′-O-Me-cAMP); 8-(4-Chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate, (8-pCPT-2′-O-Me-cAMP); 8-(4-Methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate, (8-pMeOPT-2′-O-Me-cAMP) that activate Epac selectively (Enserink et al. 2002; Christensen et al. 2003; Rehmann et al. 2003a). We now demonstrate that Epac-selective cAMP analogues, but not a PKA-selective analogue (N6-Benzoyladenosine-3′,5′-cyclic monophosphate (N6-Bnz-cAMP), or a cGMP analogue 2′-O-Methylguanosine-3′, 5′-cyclic monophosphate (2′-O-Me-cGMP), inhibit KATP channel activity measured under conditions of whole-cell dialysis in which a low concentration of ATP is administered intracellularly. This inhibitory action of cAMP analogues is not observed in INS-1 cells transfected with a dominant negative Epac1 that fails to bind cAMP. Since both Epac1 and Epac2 are demonstrated to coimmunoprecipitate with full-length SUR1, it is concluded that in pancreatic β cells, the cAMP-dependent inhibition of KATP channels is Epacmediated.
Methods
Cell culture
Human islets of Langerhans were provided under the auspices of the National Institutes of Health, National Center for Research Resources, Islet Cell Resource Service. Single-cell suspensions of human islet cells were prepared by digestion of islets with trypsin-EDTA, and the single β cells were plated onto glass coverslips (25CIR 1; Fisher Scientific) coated with 1 mg ml−1 concanavalin A (type V; Sigma-Aldrich, St Louis, MO, USA) (Holz et al. 1995, 1999). Cell cultures were maintained in a humidified incubator (95% air, 5% CO2) at 37°C in CMRL-1066 modified culture medium (Mediatech, Inc., Herndon, VA, USA; catalogue no. 99-603-CV) containing 10% (v/v) fetal bovine serum (FBS). β Cells were identified by fluorescence microscopy after infection of the cultures with adenovirus directing expression of enhanced yellow fluorescent protein (EYFP) under the control of the rat insulin 2 gene promoter (Kang et al. 2003). INS-1 cells (passages 70–90) were maintained in RPMI 1640 culture medium containing 10 mm Hepes, 11.1 mm glucose, 10% FBS, 100 U ml−1 penicillin G, 100 μg ml−1 streptomycin, 2.0 mml-glutamine, 1.0 mm sodium pyruvate and 50 μm 2-mercaptoethanol (Asfari et al. 1992; Skoglund et al. 2000; Chepurny et al. 2002; Chepurny & Holz, 2002). INS-1 cells were passaged by trypsinization and subcultured once a week. All reagents for INS-1 cell culture were obtained from Invitrogen LifeTechnologies (Rockville, MD, USA).
Patch-clamp electrophysiology
Cells were bathed in a standard extracellular saline solution (SES) containing (mm): 138 NaCl, 5.6 KCl, 2.6 CaCl2, 1.2 MgCl2, 11.1 glucose, and 10 Hepes (295 mOsm pH adjusted to 7.4 with NaOH). Experiments were performed at 32°C using an inverted microscope (TE300; Nikon, Melville, NY, USA) equipped with a temperature-controlled stage (Medical Systems Corp., Greenvale, NY, USA), and fitted with a video imaging system (IonOptix Corp., Milton, MA, USA) for detection of EYFP epifluorescence. The KATP current was measured using the whole-cell, tight-seal configuration of the patch-clamp technique. Patch pipettes pulled from borosilicate glass (Kimax-51, tip resistance 2–3 MΩ) were fire-polished and back-filled with an intracellular solution containing (mm): 90 K2SO4, 10 NaCl, 1 MgCl2, 1.1 EGTA, 0.1 CaCl2, 0.3 ATP, 0.2 GTP, 5 Hepes (300 mOsm pH adjusted to 7.4 with NaOH). The free Ca2+ concentration of this solution was determined to be 160 nm. The patch pipette was connected to a Heka Electronik EPC-9 patch-clamp amplifier (Instrutech Corporation, Mineola, NY, USA) interfaced with a Macintosh G3 computer running Pulse version 8.31 software (Instrutech Corporation). Series resistance (RS) and membrane capacitance (CM) were updated on a continuous basis using the computer-controlled automated compensation features of the EPC-9 (Holz et al. 1993, 1995, 1999). The signal corresponding to the pipette current was low-pass filtered (0.5 kHz) and digitized (1 kHz). Raw data were analysed and statistical analyses were performed using IgorPro software version 5.03 (WaveMetrics Inc., Lake Oswego, OR, USA). Test solutions dissolved in SES were applied to individual cells from glass ‘puffer’ micropipettes (catalogue no. 1B150-6; World Precision Instruments, Sarasota, FL, USA), using a pressure ejection system (PicoSpritzer II; General Valve Corporation, NJ, USA) as described (Holz et al. 1993).
Generation and characterization of stably transfected cell lines
Human wild-type Epac1 (GenBank accession no. AAF103905) (de Rooij et al. 1998), and dominant-negative Epac1 (R279E) in pcDNA3.1 were obtained from Dr X. Cheng (Galveston, TX, USA) (Qiao et al. 2002). Epac cDNAs were subcloned into pCMV4-FLAG (Sigma-Aldrich) to insert the FLAG epitope at the N-terminus of the exchange factor. All Epac constructs were introduced into INS-1 cells using LipofectAMINE Plus (Invitrogen) (Chepurny et al. 2000; Chepurny & Holz 2002). Individual clones of stably transfected cells were selected for by treatment with geneticin (150 μg ml−1; Invitrogen). Expression of FLAG Epac1 or Epac2 was confirmed by immunoblot analysis (Kang et al. 2005), or by indirect immunofluorescence cytochemistry (ICC) using fixed and permeabilized INS-1 cells. The primary antiserum for ICC was mouse anti-FLAG M2 monoclonal antiserum (Sigma-Aldrich; catalogue no. F3165). A goat anti-mouse polyclonal antiserum conjugated to AlexaFluor 488 (Molecular Probes, Inc., Eugene, OR, USA; 1:1000 dilution) served as the secondary antiserum. Cells were imaged using a CARV2 spinning-disk confocal microscope (BD Biosciences, San Jose, CA, USA) or a Zeiss LSM510 confocal microscope equipped with a ×100 objective. Expression of endogenous Epac2 in INS-1 cells was confirmed by immunoblot analysis using an Epac2-specific monoclonal antiserum developed in the laboratory of J. L. Bos.
RT-PCR for detection of Epac mRNA
Detailed methods for RT-PCR of Epac have been described (Leech et al. 2000; Kang et al. 2001). For detection of rat Epac1 (accession no. U78167), RT-PCR was performed using primer sets: 1A (forward 5′-CGTCCCCGGTGCTGCTCTTAC-3′; reverse 5′-GTCCCCCTGGCTGAACAACACA3′), 1B (forward 5′-GGCCCGGAATGCACCTGTTTG-3′; reverse 5′-CTGGCCATCATTCGCATCTTCTCA3′), and 1C (forward 5′-TCTGGCCGGGAGCTAGTGGATGG-3′; reverse 5′-GGGTCGGAGGGCGGGAAGG-3′). Primer sets A, B and C for Epac1 generate PCR products that span nucleotides 22–708, 1479–2423 and 265–1015 of the coding sequence of U78167 (Kawasaki et al. 1998). RT-PCR for rat Epac2 (U78517) was performed using primer set 2 (forward 5′-GTGGGGACGTTTGAACTGATGAGC-3′; reverse 5′-AGCCTGTACGCCTTGTGATTTCTG3′). Primer set 2 generates a PCR product which spans nucleotides 568–1007 of U78517 within the coding sequence of Epac2 (Kawasaki et al. 1998).
Co-immunoprecipitation and detection of SUR1, Kir6.2 and Epac
HEK293T cells cultured in DMEM (Invitrogen; supplemented with 10% FBS) were transfected at 30–40% confluency using FuGENE6 (Roche Diagnostics, Alameda, CA, USA) and the following cDNAs: (1) mouse Kir6.2-HA (accession no. NM_010602; Inagaki et al. 1995) in pcDNA3.0 (from Dr S. Seino, Kobe University Graduate School of Medicine, Kobe, Japan) but C-terminally tagged with an HA epitope as described by Pountney et al. 2001); (2) hamster FLAG-SUR1 (accession no. L40623; Aguilar-Bryan et al. 1995) in pECE (from Dr S.-L. Shyng, Portland, OR, USA, but subcloned into pcDNA3.1 and N-terminally tagged with the FLAG epitope by Dr Pilyali Dhar Chowdhury, New York University School of Medicine, NY, USA); (3) human Epac1 (from Dr X. Cheng, (The University of Texas Medical Branch, Galveston, Texas, USA) but subcloned into pCMV2 (Sigma-Aldrich) and N-terminally tagged with a c-myc epitope); (4) mouse Epac2 (from Dr S. Seino, but subcloned into pCMV2 and N-terminally tagged with a c-myc epitope); and (5) pGFP-N1 (Clontech) which was used as a marker of transfection. Forty-eight hours post-transfection, cells washed in ice-cold PBS were lysed in ice-cold buffer containing (mm): 25 Tris-HCl (pH 7.4), 150 NaCl, 5 EDTA, and supplemented with Triton X-100 (1% v/v), phenylmethylsulphonylfluoride (1 mm) and a protease inhibitor cocktail (Sigma-Aldrich). Whole-cell lysates containing 800 μg of protein were incubated overnight at 4°C with a rabbit polyclonal anti-FLAG antiserum (Sigma-Aldrich; catalogue no. F7425). FLAG-SUR1 complexed to anti-FLAG antiserum was then immunoprecipitated with protein A/G Sepharose beads (Pierce, Rockford, IL, USA). Control samples of whole-cell lysates were incubated with non-specific rabbit serum IgG for immunoprecipitation using protein A/G Sepharose beads. Protein–antibody–bead complexes were washed three times in ice-cold buffer (same composition as above, but containing 0.1% Triton X-100), and resuspended in Laemmli SDS sample buffer (Bio-Rad, Hercules, CA, USA) without boiling. Proteins were separated by 10% SDS-PAGE, transferred to PVDF membranes (Bio-Rad), and immunoblotted with mouse monoclonal anti-FLAG M2 antiserum (Sigma-Aldrich; 1:500 dilution), or mouse monoclonal anti-c-myc antiserum (Sigma-Aldrich; catalogue no. M5546; 1:500 dilution). The secondary antiserum was HRP-conjugated donkey antimouse IgG (Amersham-Pharmacia, Piscataway, NJ, USA). Immunodetection was performed using enhanced chemiluminescence (Pierce).
In vitro Rap1 activation assay
Rap1B (200 nm) loaded with the fluorescent GDP analogue 2′-/3′-O-(N′-methylanthraniloyl)-guanosine-diphosphate (mantGDP) was incubated in the presence of 20 μm GDP and 100 nm Epac1. Then 2′-O-Me-cAMP or 2′-O-Me-cGMP was added as indicated for the individual experiments. Epac-mediated nucleotide exchange on Rap1B was measured in real time as the decay in fluorescence using a spectrofluorometer (Rehmann et al. 2003a). The decay in fluorescence initiated by cAMP is caused by the release of Rap1B-bound mantGDP, which shows a higher fluorescence intensity in the hydrophobic environment of Rap1B than in the buffer solution. All data analysis, fitting, and plotting were done with Grafit 3.0 (Erithacus Software Ltd, Surrey, UK).
Sources of reagents
8-pCPT-2′-O-Me-cAMP, 8-pMeOPT-2′-O-Me-cAMP, 2′-O-Me-cAMP, 2′-O-Me-cGMP, 8-Bromoadenosine-3′,5′-cyclic monophosphorothioate, Sp-isomer (Sp-8-Br-cAMPS), Rp-8-Br-cAMPS, and N6-Bnz-cAMP were obtained from Biolog Life Science Institute (Bremen, Germany). ATP, GTP, cAMP and glyburide were from Sigma. H-89, myr-PKI and the anti-alpha-tubulin mouse monoclonal antibody were from Calbiochem (San Diego, CA, USA). Anti-Epac2 mouse monoclonal antibody was generated in the laboratory of J. L. Bos by F. J. T. Zwartkruis and J. Zhao.
Results
Inhibition of the KATP current of human β cells by an Epac-selective cAMP analogue
Primary cultures of human islet cells were prepared, and individual β cells were identified on the basis of their expression of EYFP, the synthesis of which was placed under the control of the rat insulin 2 gene promoter. Prior studies demonstrate that this method of selection is an accurate means by which to identify fluorescent β cells in a mixed population of islet cells (Kang et al. 2003). The macroscopic KATP current was measured under conditions of voltage clamp using the whole-cell, tight-seal configuration of the patch-clamp technique. β cells were equilibrated in a standard extracellular saline solution containing 11.1 mm glucose, and the cells were dialysed with a pipette solution that included 0.3 mm ATP, 0.2 mm GTP and 160 nm Ca2+. This pipette solution was chosen because prior reports demonstrate that the inhibitory action of cAMP at KATP channels is facilitated by Ca2+ (He et al. 1998; Ding et al. 2001).
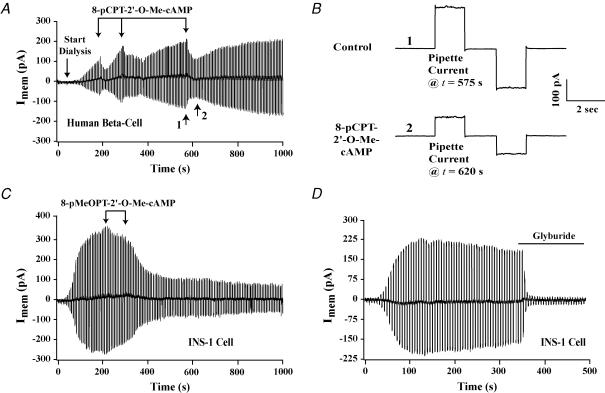
The membrane conductance was measured by determining the amplitude of the whole-cell current evoked by a ±20 mV command potential from a holding potential of −70 mV. Upon rupture of the patch, the membrane conductance and the associated whole-cell current increased gradually (Fig. 1A). This increase signifies the opening of KATP channels, and it is a consequence of the intracellular dialysis of cells with a pipette solution containing a low concentration of ATP (Trube et al. 1986; Williams et al. 1993). Extracellular application of the cell-permeant Epac-selective cAMP analogue 8-pCPT-2′-O-Me-cAMP (100 μm; three 30 s applications indicated by vertical arrows) inhibited the whole-cell KATP current of human β cells measured under these conditions (n = 5 cells). This action of 8-pCPT-2′-O-Me-cAMP is depicted on a time scale that is compressed (Fig. 1A) or expanded (Fig. 1B). The whole-cell current measured in this manner corresponded to KATP current because it was abolished by the sulphonylurea glyburide applied extracellularly (10 nm; n = 5 cells; data not shown).
Figure 1. Inhibition of the whole-cell KATP current by Epac-selective cAMP analogues.
A and B, 8-pCPT-2′-O-Me-cAMP (100 μm; individual 30 s applications indicated by vertical arrows) inhibited the KATP current of a human β cell, as depicted on a compressed (A) and an expanded (B) time scale. Time points 1 and 2 in A are illustrated in B as the pipette currents measured at t = 575 and t = 620 s. Outward currents are indicated by upward current deflections. The membrane conductances at t = 575 and t = 620 s, respectively, were 10.0 and 4.7 nS. C, the KATP current of an INS-1 cell was inhibited by 8-pMeOPT-2′-O-Me-cAMP (300 μm; individual 30 s applications indicated by arrows). D, glyburide (10 nm, continual application indicated by the horizontal line) abolished the KATP current of an INS-1 cell. All test solutions were applied extracellularly to individual cells using a ‘puffer’ pipette.
Epac-selective cAMP analogues inhibit the KATP current of rat INS-1 cells
The KATP current of INS-1 cells was also inhibited by Epac-selective cAMP analogues. This was the case for 8-pCPT-2′-O-Me-cAMP (100–300 μm; n = 5 cells; data not shown) and cell-permeant 8-pMeOPT-2′-O-Me-cAMP (300 μm; n = 6 cells) (Fig. 1C). Unlike human β cells, the inhibitory actions of both cAMP analogues in INS-1 cells were not reversible within 5–10 min. Why this is the case was not investigated, but it may reflect a difference in the level of cAMP phosphodiesterase (PDE) activity in the two cell types. 8-pCPT-2′-O-Me-cAMP and 8-pMeOPT-2′-O-Me-cAMP are both susceptible to hydrolysis by PDE, so the duration of their effect may be extended in INS-1 cells exhibiting a low level of PDE activity.
Current-clamp measurements of the membrane potential obtained under conditions of whole-cell dialysis demonstrated that 100 μm 8-pCPT-2′-O-Me-cAMP depolarized INS-1 cells and generated action potentials (n = 4 cells; data not shown). Although not investigated, this depolarizing action of 8-pCPT-2′-O-Me-cAMP may explain its ability to produce a sustained increase of [Ca2+]i and to stimulate exocytosis in INS-1 cells (see Fig. 6D of Kang et al. 2003). Consistent with the findings obtained using human β cells, the KATP current of INS-1 cells was inhibited by glyburide applied extracellularly (10 nm; n = 5 cells) (Fig. 1D).
Inhibition of the KATP current by intracellularly applied cAMP
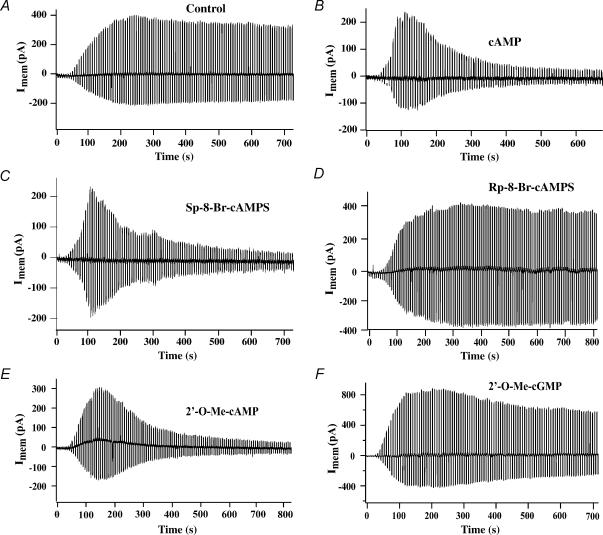
Although the 8-pCPT and 8-pMeOPT substitutions of cAMP analogues confer membrane permeability, they could in theory also confer a direct channel-blocking activity. Therefore, we sought to determine what effect cAMP, itself, exerted when it was administered intracellularly. To this end, experiments were repeated under conditions in which cAMP was included in the patch pipette solution. Under control conditions in which cAMP was not present, the whole-cell KATP current of INS-1 cells increased gradually upon rupture of the patch (Fig. 2A). The amplitude of this current reached a plateau, after which a slow decrease (‘run-down’) occurred (Fig. 2A). When cAMP (300 μm) was included in the pipette solution, the initial increase of whole-cell KATP current was followed by a prompt decrease not measured under control conditions (Fig. 2B). This action of cAMP to inhibit the KATP current was clearly distinguishable from the process of run-down, and it did not result from a re-sealing of the membrane within the patch since no increase of series resistance was measurable. Thus, cAMP, itself, inhibits the activity of KATP channels in INS-1 cells.
Figure 2. Inhibition of the KATP current of INS-1 cells by intracellularly applied cyclic nucleotide analogues.
A, dialysis with a pipette solution containing no cyclic nucleotide resulted in the appearance of the whole-cell KATP current under control conditions. B, inclusion of cAMP (300 μm) in the patch pipette did not prevent the appearance of the KATP current once dialysis had commenced at t = 0, but it resulted in a prompt decrease of the KATP current measured at later time points. C–F, the action of cAMP was reproduced by 100 μm each of Sp-8-Br-cAMPS (C) and 2′-O-Me-cAMP (E), but not Rp-8-Br-cAMPS (D) or 2′-O-Me-cGMP (F).
Inhibition of the KATP current by Sp-8-Br-cAMPS but not Rp-8-Br-cAMPS
We next compared the efficacy of Sp- and Rp- isomers of 8-Br-subsituted cAMPS in order to determine if these cAMP analogues act in a stereospecific manner when administered intracellularly. Consistent with prior studies in which the Sp- but not Rp- isomers of cAMPS were demonstrated to activate Epac1 (Christensen et al. 2003), we found that the KATP current of INS-1 cells was inhibited by 100 μm Sp-8-Br-cAMPS but not Rp-8-Br-cAMPS (Fig. 2C and D). Thus, these cAMP analogues interact with a binding site that discriminates between Sp- and Rp-isomers, and which might correspond to the cAMP-binding pocket of Epac (Rehmann et al. 2003b,c).
KATP channels are inhibited by 2′-O-Me-cAMP but not 2′-O-Me-cGMP
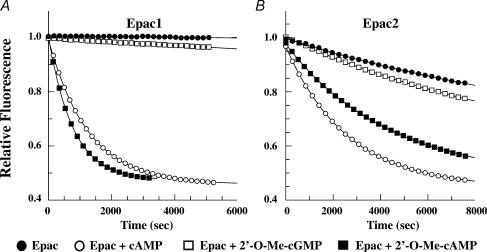
Given that Epac is activated by cAMP but not cGMP (Rehmann et al. 2003a), we sought to determine whether an appropriate cyclic-3′,5′-adenosine monophosphate specificity existed for the inhibitory actions of cyclic nucleotides described here. For this purpose, we examined the effects of cyclic nucleotides that are not 8-substituted, but which contain the 2′-O-Me substitution necessary for selective activation of Epac. We found that the KATP current of INS-1 cells was inhibited by intracellular administration of 2′-O-Me-cAMP (Fig. 2E), but not 2′-O-Me-cGMP (Fig. 2F). Because the relative efficacies of 2′-O-Me-cAMP and 2′-O-Me-cGMP as activators of Epac has not been reported, we validated that 2′-O-Me-cAMP activates both Epac1 and Epac2, whereas 2′-O-Me-cGMP is ineffective, as demonstrated in an in vitro assay of Epac-mediated guanyl nucleotide exchange on Rap1 (Fig. 3A and B). Therefore, it may be concluded that the KATP channels of INS-1 cells are inhibited in an adenine- but not guanine-nucleotide-specific manner.
Figure 3. cAMP and 2′-O-Me-cAMP but not 2′-O-Me-cGMP act via Epac to stimulate guanyl nucleotide exchange on Rap1.
Rap1B loaded with a fluorescent GDP analogue was incubated in the presence of non-fluorescent GDP and either Epac1 (A) or Epac2 (B). cAMP, 2′-O-Me-cAMP or 2′-O-Me-cGMP was then added at a final concentration of 500 μm. Exchange of fluorescent GDP for non-fluorescent GDP was measured spectrophotometrically in real time. When bound to Rap1B, fluorescent GDP exhibits greater fluorescence than when it is simply dissolved in buffer solution. Thus, the decay of fluorescence measured in this assay reflects Epac-mediated stimulation of guanyl nucleotide exchange on Rap1B. Note that when tested at a saturating concentration (500 μm), the Epac1-mediated action of 2′-O-Me-cAMP proceeds at a faster rate than that mediated by Epac2.
Comparison of the average rates of decay of whole-cell KATP currents
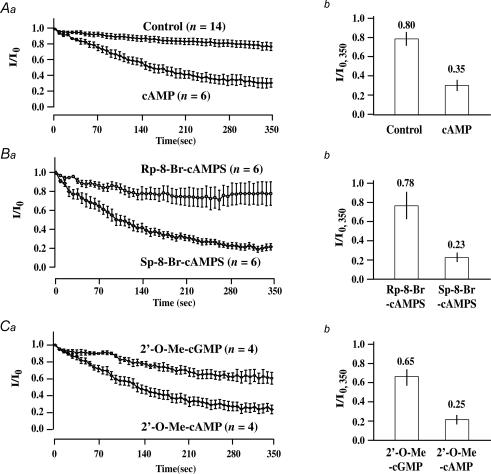
The effects of cyclic nucleotide analogues administered via the patch pipette were analysed by performing population studies of INS-1 cells so that the average rates of decay of the KATP currents could be determined (Fig. 4Aa, Ba and Ca). Simultaneously, the average amplitudes of the currents were measured 350 s after the currents had reached their initial maxima (Fig. 4Ab, Bb and Cb). These analyses demonstrated inhibitory actions of cAMP (Fig. 4Aa and b), Sp-8-Br-cAMPS (Fig. 4Ba and b), and 2′-O-Me-cAMP (Fig. 4Ca and b). However, Rp-8-Br-cAMPS (Fig. 4Ba and b) and 2′-O-Me-cGMP (Fig. 4Ca and b) exerted no such inhibitory effect. To quantify these effects in greater detail, the time constant of KATP current decay was determined. Under control conditions in which the pipette solution contained no cAMP, the time constant of KATP current decay was 318 s. This value decreased to 129, 142 and 130 s when the pipette solution contained cAMP, Sp-8-Br-cAMPS or 2′-O-Me-cAMP, respectively. In contrast, the time constant of KATP current decay did not differ from the control value when evaluating the actions of Rp-8-Br-cAMPS and 2′-O-Me-cGMP (see online Supplemental material Fig. 1). Such findings validate that cAMP analogues exhibit a stereospecificity (Sp-8-Br-cAMPS versus Rp-8-Br-cAMPS) and adenine nucleotide specificity (2′-O-Me-cAMP versus 2′-O-Me-cGMP) consistent with an action of cAMP mediated by Epac.
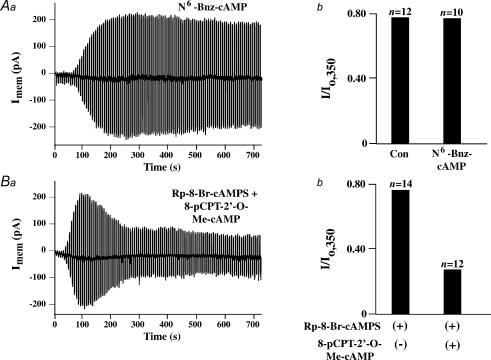
Figure 4. Comparison of the rates of KATP current decay measured under conditions in which INS-1 cells were dialysed with a pipette solution containing cyclic nucleotides.
Aa, Ba and Ca, the time course of whole-cell KATP current decay is illustrated under control conditions, or conditions in which the pipette solution contained cyclic nucleotides. The current measured at t = 0 is the maximal current (Io) achieved following the initiation of dialysis. The current (I) measured at subsequent time points is normalized relative to Io. Values of n correspond to the number of cells studied under each experimental condition. Ab, Bb and Cb, the amplitude of the normalized KATP current measured at t = 350 s (I/Io,350) is illustrated under control conditions, or conditions in which the pipette solution contained cyclic nucleotides. All cyclic nucleotides were administered at a concentration of 100 μm, except for cAMP (300 μm). Error bars indicate means ±s.e.m.
N6-Bnz-cAMP fails to inhibit the KATP current
Prior studies of insulin-secreting cells demonstrate a role for PKA as an inhibitor of KATP channel activity (Light et al. 2002). In contrast, studies of non-insulin-secreting cells that express Kir6.2 and SUR1 seem to indicate that PKA stimulates rather than inhibits KATP channel function (Beguin et al. 1999; Lin et al. 2000). Thus, we examined whether the KATP current of INS-1 cells is affected by N6-Bnz-cAMP, a cAMP analogue that activates PKA but not Epac (Christensen et al. 2003). We found that N6-Bnz-cAMP (100 μm) failed to alter the KATP current when it was included in the patch pipette solution (Fig. 5Aa and b; n = 10 cells). For this reason, a role for PKA as an intermediary linking cAMP to the regulation of KATP channel activity was not substantiated in the study of INS-1 cells reported here.
Figure 5. Assessment of the actions of N6-Bnz-cAMP, Rp-8-Br-cAMPS and 8-pCPT-2′-O-Me-cAMP in INS-1 cells.
A, inclusion of N6-Bnz-cAMP (100 μm) in the patch pipette solution was without effect on the rate of decay (Aa) or absolute magnitude (Ab) of the KATP current measured under conditions of whole-cell dialysis. See the legend of Fig. 4 for an explanation of how the value of I/Io,350 was calculated. B, inclusion of Rp-8-Br-cAMPS (100 μm) in the patch pipette solution failed to influence the action of 8-pCPT-2′-O-Me-cAMP (100 μm) to increase the rate of decay of the KATP current (Ba), and to decrease its absolute magnitude (Bb).
Rp-8-Br-cAMPS fails to block the inhibitory action of 8-pCPT-2′-O-Me-cAMP
Rp-8-Br-cAMPS is a cAMP analogue that prevents activation of PKA by cAMP (Dostmann et al. 1990). In marked contrast, recent studies demonstrate that Rp-8-Br-cAMPS fails to block activation of Epac by cAMP (Christensen et al. 2003; Branham et al. 2006). In the present study of INS-1 cells, we found that the action of 8-pCPT-2′-O-Me-cAMP (100 μm) to inhibit KATP channels was unaffected by inclusion of Rp-8-Br-cAMPS (100 μm) in the patch pipette solution (Fig. 5B, n = 5 cells). Thus, available information indicates that it is Epac that is targeted by cAMP in this assay of whole-cell KATP current.
The action of 8-pCPT-2′-O-Me-cAMP is blocked by dominant-negative Epac1
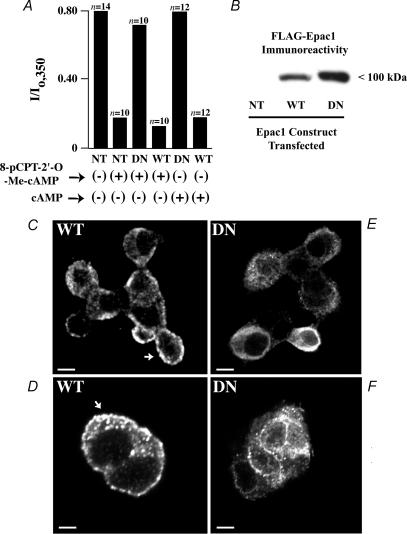
If cAMP acts via Epac to inhibit KATP channels, such an effect should persist after exposure of cells to compounds that suppress the catalytic activity of PKA. To evaluate this possibility, INS-1 cells were treated with H-89 (1 μm) or myr-PKI (10 μm), two inhibitors of PKA catalytic activity (Hidaka & Kobayashi, 1992). Unfortunately, we found that PKA inhibitors, alone, inhibited the KATP current measured under conditions of whole-cell dialysis (data not shown). A similar finding has been reported in studies of KATP channel activity in excised patches of plasma membrane derived from RINm5F insulin-secreting cells (Ribalet et al. 1989; RINm5F cells are the parental cell line of INS-1 cells). To circumvent this problem, we adopted a molecular biological approach in which INS-1 cells were stably transfected with wild-type (WT) or dominant-negative (DN) FLAG epitope-tagged Epac1. DN FLAG-Epac1 incorporates an amino acid substitution (R279E) that prevents the binding of cAMP, and it is reported to block Epac-mediated mobilization of intracellular Ca2+ in INS-1 cells (Kang et al. 2005). Using this approach, it was demonstrated that transfection with DN but not WT FLAG-Epac1 nearly abolished the inhibitory actions of 8-pCPT-2′-O-Me-cAMP (100 μm) and cAMP (300 μm) at KATP channels (Fig. 6A). Immunoblot analysis confirmed the expression of WT and DN FLAG-Epac1 in these stably transfected INS-1 cells, as detected using a specific anti-FLAG antiserum (Fig. 6B). Indirect immunofluorescence cytochemistry also demonstrated FLAG immunoreactivity corresponding to WT and DN FLAG-Epac1 expressed in these cells, as detected by confocal microscopy. This punctate immunoreactivity was detected at or near the plasma membrane and in the cytoplasm, but not within the nucleus (Fig. 6C–F).
Figure 6. Dominant-negative Epac1 diminishes the action of 8-pCPT-2′-O-Me-cAMP.
A, the amplitude of the normalized whole-cell KATP current of INS-1 cells measured at t = 350 s (I/Io,350) is illustrated for INS-1 cells not transfected (NT) or stably transfected with either wild-type (WT) FLAG-Epac1 or dominant-negative (DN) FLAG-Epac1. Cells dialysed with 8-pCPT-2′-O-Me-cAMP (100 μm) or cAMP (300 μm) are indicated as (+), whereas cells not dialysed with 8-pCPT-2′-O-Me-cAMP or cAMP are indicated as (−). B, expression of WT and DN FLAG-tagged Epac1 in lysates of stably transfected INS-1 cells was confirmed by immunoblot analysis, whereas no such immunoreactivity was detected in INS-1 cells not transfected (NT). C–F, immunofluorescence cytochemistry for detection of WT and DN FLAG-Epac1 in stably transfected INS-1 cells. Punctate immunoreactivity (arrows) corresponding to WT or DN FLAG-Epac1 was apparent at the plasma membrane and in the cytoplasm of a cluster of cells (C, E and F) or a pair of cells (D). No such immunoreactivity was detected in cells not transfected with FLAG-Epac1. Calibration bars: 3.4 μm for C, E and F; 2.5 μm for D.
Expression of endogenous Epac in INS-1 cells and human β cells
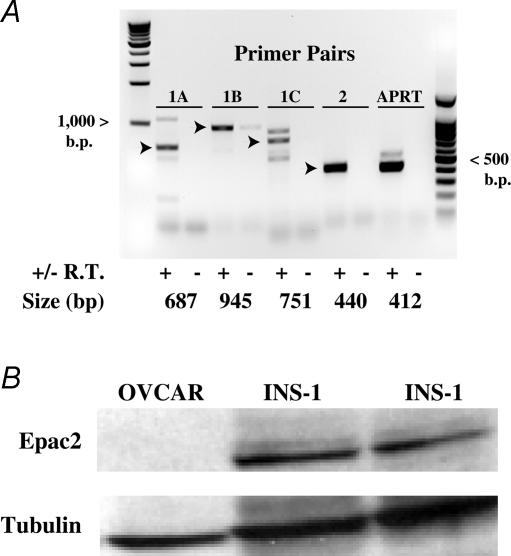
Semi-quantitative RT-PCR analysis confirmed the expression of endogenous Epac1 and Epac2 mRNA in INS-1 cells (Fig. 7A). Nearly identical findings were obtained using human islets (see Fig. 2 of Supplemental material). Although prior studies have focused on the role of Epac2 as a determinant of β cell stimulus–secretion coupling (Kang et al. 2001; Kashima et al. 2001; Eliasson et al. 2003), the presence of Epac1 mRNA in INS-1 cells is not surprising. In fact, mRNA corresponding to Epac1 can also be detected in rat islets (Leech et al. 2000). However, it should be noted that the predominant PCR product detected in INS-1 cells corresponded to Epac2 (Fig. 7A). Furthermore, Western blot analysis using a specific anti-Epac2 monoclonal antiserum demonstrated significant Epac2 immunoreactivity in INS-1 cell lysates (Fig. 7B). No such immunoreactivity was detected in OVCAR cells, an ovarian carcinoma cell line serving as a negative control (Fig. 7B). Although not shown, Epac1 immunoreactivity recognized by an Epac1 monoclonal antiserum was present only in trace amounts in INS-1 cells (data not shown). Thus, available evidence indicates that it is Epac2 that is expressed at the highest levels in INS-1 cells.
Figure 7. Expression of endogenous Epac in INS-1 cells.
A, RT-PCR was performed using three sets of Epac1 primer pairs (designated as 1A, 1B and 1C) and a single Epac2 primer pair (designated as 2). PCR product size is indicated in base pairs. Arrowheads indicate PCR products of the expected sizes. Abbreviations: +/− R.T., template generated with or without reverse transcriptase added to the cDNA synthesis reaction; APRT, adenine phosphoribosyltransferase control. A PCR product corresponding to APRT can be derived by PCR of cDNA derived from mRNA but not genomic DNA when using the rat APRT forward (5′-TCCGAATCTGAGTTGCAGC-3′) and reverse primers (5′-CTGCACACATGGTTC-CTCC-3′). B, Epac2 was detected in INS-1 cells (two different platings) by Western blot analysis using an anti-Epac2 monoclonal antiserum. No such immunoreactivity was detected in an ovarian carcinoma cell line (OVCAR). Anti-tubulin antiserum was used to verify loading of the wells with equal amounts of proteins derived from whole-cell lysates.
Epac1 and Epac2 interact with full-length SUR1
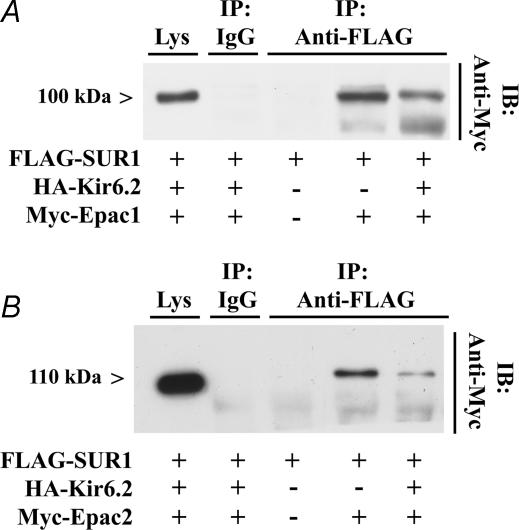
To assess whether Epac interacts with SUR1 in living cells, immunoprecipitation (IP) assays were performed using the supernatant fractions of lysates obtained from HEK cells transfected with FLAG-SUR1, and either myc-epitope-tagged Epac1 or Epac2. These studies demonstrated that both myc-Epac1 and myc-Epac2 interacted with FLAG-SUR1 (Fig. 8A and B). Interestingly, a complex of FLAG-SUR1 and either myc-Epac1 or myc-Epac2 was detected in lysates obtained from HEK cells transfected, or not transfected, with haemagglutinin (HA)-tagged Kir6.2 (Fig. 8A and B). Given that SUR1 is known to be confined to the endoplasmic reticulum in the absence of Kir6.2 (Zerangue et al. 1999), such findings indicate that both forms of Epac may associate with SUR1 at a step prior to the translocation of KATP channels to the plasma membrane.
Figure 8. Epac1 and Epac2 interact with SUR1.
A, anti-FLAG antiserum affixed to protein A/G Sepharose beads was used to immunoprecipitate FLAG-SUR1 from lysates of HEK cells transfected with FLAG-SUR1, HA-Kir6.2 and myc-Epac1. The immunoprecipitate (IP) was subjected to immunoblot (IB) analysis using anti-myc antiserum. A 100 kDa immunoreactivity corresponding to myc-Epac1 was detected in lysates of cells transfected with FLAG-SUR1 and myc-Epac1, but not in lysates obtained from cells transfected with FLAG-SUR1 only. Control experiments demonstrated that myc-Epac1 was expressed in whole-cell lysates (Lys), whereas no immunoreactivity was detected in lysates subjected to immunoprecipitation with non-specific rabbit IgG. B, identical findings to those presented in A were obtained when HEK cells were transfected with myc-Epac2 appearing as 115 kDa immunoreactivity. Lower bands correspond to non-specific myc immunoreactivity.
Discussion
A role for Epac in β cell KATP channel regulation
Although cAMP-elevating agents such as forskolin, isobutylmethylxanthine, glucagon and GLP-1 inhibit KATP channels in β cells, it has remained uncertain until now whether such an effect is achieved via cAMP-dependent activation of PKA and/or Epac. Here we present evidence that Epac does in fact contribute to this process. We find that the activity of KATP channels is inhibited by Epac-selective cAMP analogues, whereas no such effect is observed after transfection with a dominant-negative Epac1. The analogues we tested inhibit KATP channels in a stereospecific and cyclic adenosine monophosphate-specific manner, as expected for a specific interaction of cAMP with the cyclic nucleotide-binding domain of Epac. Furthermore, both Epac1 and Epac2 are shown to interact with full-length SUR1. On the basis of these findings, we propose that Epac mediates a major inhibitory effect of cAMP at KATP channels.
It remains to be established which isoform of Epac subserves inhibitory actions of cAMP at KATP channels. Although the dominant-negative Epac1 we tested is likely to interfere with actions of cAMP mediated by Epac1, it may also disrupt signalling mediated by Epac2. This possibility exists due to the structurally conserved nature of the DEP, cAMP-binding, REM and GEF domains of Epac1 and Epac2 (Holz, 2004a). Thus, a contribution of both Epac1 and Epac2 to KATP channel regulation is not excluded. In fact, we find that INS-1 cells express Epac1 and Epac2 mRNA. Since both Epac1 and Epac2 coimmunoprecipitate with SUR1, it is seems likely that these two cAMPGEFs subserve similar functions at KATP channels.
Epac activators are effective inhibitors of KATP channels
It is perhaps surprising that we find that cAMP analogues, alone, exert a strong inhibitory effect at KATP channels. Given that the conditions of whole-cell dialysis used in the present study disrupt β-cell glucose metabolism, and given that cAMP-elevating agents inhibit KATP channel activity in a glucose-dependent manner (Holz et al. 1993), why is the efficacy of cAMP preserved? We believe a simple explanation for this finding is that the experimental design reported here allows us to study effects of cAMP under conditions that recapitulate the glucose-dependent increase of cytosolic ATP/ADP concentration ratio. This metabolic signal is known to support KATP channel inhibition by cAMP (Holz & Habener, 1992). Because our pipette solution contains 300 μm ATP but no added ADP, whole-cell dialysis with this solution results in an ATP/ADP concentration ratio that is elevated and which may approximate or even exceed the ratio achieved when β cells are bathed in a high concentration of glucose. For this reason, the strong inhibitory effect of cAMP analogues we report is not at odds with the established action of cAMP as a potentiator of glucose-dependent insulin secretion (Holz, 2004b). It will be of interest to determine in future studies exactly how alterations of intracellular [ATP] or [ADP] influence the potency and/or efficacy of Epac-selective cAMP analogues as inhibitors of KATP channels.
On the potential importance of PKA to KATP channel regulation
A prior report of Light and coworkers offers a competing hypothesis to explain the inhibition of β-cell KATP channels by cAMP. It was proposed that PKA-mediated phosphorylation of SUR1 decreases the channel's sensitivity to Mg2+-ADP, thereby closing the channel (Light et al. 2002). In that study, the catalytic subunit of PKA (cPKA) inhibited KATP channel activity under conditions in which inside-out patches of plasma membrane were exposed to an intracellular solution containing 0.2 mm ADP. In marked contrast, channel activity was stimulated by cPKA in the presence of 0.5 mm ADP. Since glucose metabolism lowers cytosolic levels of ADP, it was suggested that the inhibitory action of cAMP at KATP channels might be most prominent under conditions in which β cells are exposed to elevated concentrations of glucose.
We believe that the study of Light and coworkers is not necessarily at odds with the new findings presented here. Although we find that the KATP current of INS-1 cells is not affected by N6-Bnz-cAMP, an activator of PKA, it is possible that our use of whole-cell dialysis results in ‘wash-out’ of PKA. If so, no action of N6-Bnz-cAMP is expected. Similarly, the wash-out of cAMP might preclude our ability to detect actions of endogenous cAMP that are PKA-mediated and which should be blocked by cAMP antagonist Rp-8-Br-cAMPS. In this regard, it is interesting to note that no such wash-out phenomenon exists when assessing the inhibitory action of Epac-selective cAMP analogues. Thus, it would appear that Epac is tightly associated with KATP channels, a conclusion that is supported by our observation that both Epac1 and Epac2 co-immunoprecipitate with SUR1 in HEK cell lysates.
Interpretation of findings obtained using PKA inhibitors
In the present study we found that H-89, an inhibitor of PKA, nearly abolished the KATP current of INS-1 cells. It remains to be determined if this effect of H-89 results from its selective inhibitory action at PKA, or a non-specific action unrelated to PKA. Perhaps a more meaningful finding obtained using PKA inhibitors was our observation that Rp-8-Br-cAMPS failed to block the inhibition of KATP channels by 8-pCPT-2′-O-Me-cAMP. This is a key finding because Rp-8-Br-cAMPS is poor antagonist of the cAMP-dependent activation of Epac (Christensen et al. 2003; Branham et al. 2006). Thus, the maintained efficacy of 8-pCPT-2′-O-Me-cAMP under conditions of Rp-8-Br-cAMPS treatment is not surprising, and in fact it is exactly what one would expect if it is Epac that mediates the cAMP-dependent inhibition of KATP channel function.
Signal transduction properties of Epac relevant to KATP channel regulation
Given that KATP channels are inhibited by ATP, and stimulated by Mg2+-ADP, it is not unreasonable to propose that cAMP acts via Epac to influence the adenine-nucleotide sensitivity of KATP channels. Such an effect of cAMP might be conferred by a direct interaction of Epac with NBF-1 of SUR1 (Shibasaki et al. 2004a,b). For example, Epac bound to SUR1 might influence allosteric interactions of NBF-1 with its partner NBF-2. Such interactions dictate Mg2+-ADP-dependent stimulation of KATP channel activity (Gribble et al. 1997). Simultaneously, SUR1 might act as a scaffold protein, recruiting Epac to the plasma membrane where it interacts with the Rap GTPases. In this regard, cAMP is reported to activate a signalling complex comprised of Epac1, Rap2B and PLC-ɛ in HEK cells (Schmidt et al. 2001). Since PLC-ɛ catalyses the hydrolysis of membrane-bound polyphosphatidylinositol 4,5-bisphosphate (PIP2), and because PIP2 stimulates the activity of KATP channels by reducing the channel's sensitivity to ATP (Baukrowitz et al. 1998; Shyng & Nichols, 1998), an ability of Epac to promote PIP2 hydrolysis in β cells might explain the inhibitory action of cAMP reported here. Indeed, expression of PLC-ɛ (Kelley et al. 2001) has been confirmed in INS-1 cells (G. Kelley, personal communication). Thus, it will be of interest to assess whether there is a loss of Epac-mediated signal transduction in β cells derived from PLC-ɛ knockout mice (Wang et al. 2005).
Potential physiological significance
It is of interest to relate the findings presented here to current concepts regarding GLP-1 receptor-mediated signal transduction in pancreatic β cells. GLP-1 is a cAMP-elevating hormone and it stimulates insulin secretion, an action downregulated following exposure of islets to Epac2 antisense deoxyoligonucleotides (Kashima et al. 2001). Thus, speculation has centred on the possibility that cAMP-dependent and Epac-mediated inhibition of KATP channels might explain, at least in part, the ability of GLP-1 to act as an insulin secretagogue (Holz, 2004a,b). One report that might seem to contradict this hypothesis is the finding that the inhibitory action of GLP-1 at KATP channels is not blocked by cAMP antagonist Rp-cAMPS (Suga et al. 2000). However, it should be noted that although Rp-cAMPS will block activation of PKA by cAMP, it is not a particularly effective inhibitor of Epac. For example, Rp-cAMPS fails to block Epac-mediated activation of Rap1 by cAMP in living cells (Christensen et al. 2003). Similarly, Rp-cAMPS fails to block the Epac-mediated stimulation of exocytosis by cAMP in sperm (Branham et al. 2006). Thus, an Rp-cAMPS-insensitive action of GLP-1 at KATP channels is expected if it is Epac that is the primary transducer of GLP-1 action.
With these concluding points in mind, it will be of special interest to determine if the previously reported PKA-independent insulin secretagogue action of GLP-1 in isolated islets of Langerhans (Kashima et al. 2001; Nakazaki et al. 2002) results, at least in part, from Epac-mediated inhibition of KATP channels. This possibility is advanced because GLP-1 is reported to be a less effective insulin secretagogue in knockout mice where expression of SUR1 has been disrupted (Nakazaki et al. 2002; Shiota et al. 2002; Eliasson et al. 2003; Doliba et al. 2004). Similarly, studies of Kir6.2 knockout mice demonstrate that the insulin secretagogue action of GLP-1, while being present, is markedly reduced relative to that which is measurable in wild-type mice (see Fig. 5D of Miki et al. 2005).
Supplementary Material
Acknowledgments
G.G.H. acknowledges the support of the NIH (R01-DK45817) and the American Diabetes Association (Research Grant Award). M.J.R. acknowledges the support of the NIH (R21-DK067283). W.A.C. was supported by the NIH (R01-HL064838). F.S. acknowledges the support of BIA, Bremen, Germany. H.R. was supported by the Chemical Sciences of the Netherlands Organization for Scientific Research (NWO-CW) and is a recipient of the Otto-Hahn-Medaille of the Max-Planck-Gesellschaft. We thank Joost Das for technical assistance, and F. J. T. Zwartkruis and J. Zhao for generation of the Epac2 monoclonal antibody.
References
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, 4th, Boyd AE, 3rd, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115:2047–2058. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett DW, Pressel DM, Chern HT, Scharp DW, Misler S. cAMP-enhancing agents ‘permit’ stimulus–secretion coupling in canine pancreatic islet beta-cells. J Membr Biol. 1994;138:113–120. doi: 10.1007/BF00232639. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Beguin P, Nagashima K, Nishimura M, Gonoi T, Seino S. PKA-mediated phosphorylation of the human K (ATP) channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J. 1999;18:4722–4732. doi: 10.1093/emboj/18.17.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- Branham MT, Mayorga LS, Tomes CN. Calcium-induced acrosomal exocytosis requires cAMP acting through a PKA-independent, EPAC-mediated pathway. J Biol Chem. 2006;281:8656–8666. doi: 10.1074/jbc.M508854200. [DOI] [PubMed] [Google Scholar]
- Chepurny OG, Holz GG. Over-expression of the glucagon-like peptide-1 receptor on INS-1 cells confers autocrine stimulation of insulin gene promoter activity: a strategy for production of pancreatic beta cell lines for use in transplantation. Cell Tissue Res. 2002;307:191–201. doi: 10.1007/s00441-001-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepurny OG, Hussain MA, Holz GG. Exendin-4 as a stimulator of rat insulin I gene promoter activity via bZIP/CRE interactions sensitive to serine/threonine protein kinase inhibitor Ro 31–8220. Endocrinology. 2002;143:2303–2313. doi: 10.1210/endo.143.6.8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, Doskeland SO. cAMP analog mapping of Epac1 and cAMP-kinase. Discriminating analogs demonstrate that Epac and cAMP-kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- Ding WG, Kitasato H, Matsuura H. Involvement of calmodulin in glucagon-like peptide-1-(7–36)-amide induced inhibition of the ATP-sensitive K+ channel in mouse pancreatic beta-cells. Exp Physiol. 2001;86:331–339. doi: 10.1113/eph8602173. [DOI] [PubMed] [Google Scholar]
- Doliba NM, Qin W, Vatamaniuk MZ, Li C, Zelent D, Najafi H, Buettger CW, Collins HW, Carr RD, Magnuson MA, Matschinsky FM. Restitution of defective glucose-stimulated insulin release of sulfonylurea type 1 receptor knockout mice by acetylcholine. Am J Physiol Endocrinol Metab. 2004;286:E834–E843. doi: 10.1152/ajpendo.00292.2003. [DOI] [PubMed] [Google Scholar]
- Dostmann WRG, Taylor S, Genieser H-G, Jastorff B, Døskeland SO, Øgreid D. Probing the cyclic nucleotide binding sites of cAMP-dependent protein kinase I and II with analogs of adenosine 3′,5′-cyclic phosphorothioates. J Biol Chem. 1990;265:10484–10491. [PubMed] [Google Scholar]
- Eliasson L, Ma X, Renstrom E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic beta cells. J Gen Physiol. 2003;121:181–197. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, Triest MV, Schwede F, Genieser HG, Døskeland SO, Blank JL, Bos JL. A novel Epac-selective cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Ashcroft FM. The essential role of the Walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P. Glucagon-like peptide-1 stimulates exocytosis in human pancreatic beta cells by both proximal and distal regulatory steps in stimulus–secretion coupling. Diabetes. 1998;47:57–65. doi: 10.2337/diab.47.1.57. [DOI] [PubMed] [Google Scholar]
- Hashiguchi H, Nakazaki M, Koriyama N, Fukudome M, Aso K, Tei C. Cyclic AMP/cAMP-GEF pathway amplifies insulin exocytosis induced by Ca2+ and ATP in rat islet beta cells. Diabetes Metab Res Rev. 2006;22:64–71. doi: 10.1002/dmrr.580. [DOI] [PubMed] [Google Scholar]
- He LP, Mears D, Atwater I, Kitasato H. Glucagon induces suppression of ATP-sensitive K+ channel activity through a Ca2+/calmodulin-dependent pathway in mouse pancreatic beta cells. J Membr Biol. 1998;166:237–244. doi: 10.1007/s002329900465. [DOI] [PubMed] [Google Scholar]
- Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- Hidaka H, Kobayashi R. Pharmacology of protein kinase inhibitors. Annu Rev Pharmacol Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 3. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- Holz GG. Epac – A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta cell. Diabetes. 2004a;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG. New insights concerning the glucose-dependent insulin secretagogue action of glucagon-like peptide-1 in pancreatic beta cells. Horm Metab Res. 2004b;36:787–794. doi: 10.1055/s-2004-826165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG. Glucagon-like peptide-1 synthetic analogs: New therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem. 2003;10:2471–2483. doi: 10.2174/0929867033456648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG. Diabetes outfoxed by GLP-1? Sci STKE. 2005;268:pe2. doi: 10.1126/stke.2682005pe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Habener JF. Signal transduction crosstalk in the endocrine system: pancreatic beta cells and the glucose competence concept. Trends Biochem Sci. 1992;17:388–393. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Kuhtreiber WM, Habener JF. Pancreatic beta cells are rendered glucose competent by the insulinotropic hormone glucagon-like peptide-1-(7–37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Leech CA, Habener JF. Activation of a cAMP-regulated Ca2+-signaling pathway in pancreatic beta cells by the insulinotropic hormone glucagon-like peptide-1. J Biol Chem. 1995;270:17749–17757. [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic beta cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7–37) J Biol Chem. 1999;274:14147–14156. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, 4th, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor-II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta cells. J Physiol. 2001;536:375–385. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic beta cells. J Physiol. 2005;566:173–188. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Holz GG. Amplification of exocytosis by Ca2+-induced Ca2+ release in INS-1 pancreatic beta cells. J Physiol. 2003;546:175–189. doi: 10.1113/jphysiol.2002.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta cells. J Biol Chem. 2003;278:8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP-GEFII-Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276:46046–46053. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C-epsilon: a novel Ras effector. EMBO J. 2001;20:743–754. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiermayer S, Biondi RM, Imig J, Plotz G, Haupenthal J, Zeuzem S, Piiper A. Epac activation converts cAMP from a proliferative into a differentiation signal in PC12 cells. Mol Biol Cell. 2005;16:5639–5648. doi: 10.1091/mbc.E05-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa LR, Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem. 2005;280:31294–31302. doi: 10.1074/jbc.M505657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech CA, Holz GG, Chepurny O, Habener JF. Expression of cAMP-regulated guanine nucleotide exchange factors in pancreatic beta cells. Biochem Biophys Res Commun. 2000;278:44–47. doi: 10.1006/bbrc.2000.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light PE, Manning Fox JE, Riedel MJ, Wheeler MB. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol. 2002;16:2135–2144. doi: 10.1210/me.2002-0084. [DOI] [PubMed] [Google Scholar]
- Lin YF, Jan YN, Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J. 2000;19:942–955. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Minami K, Shinozaki H, Matsumura K, Saraya A, Ikeda H, Yamada Y, Holst JJ, Seino S. Distinct effects of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 on insulin secretion and gut motility. Diabetes. 2005;54:1056–1063. doi: 10.2337/diabetes.54.4.1056. [DOI] [PubMed] [Google Scholar]
- Miura Y, Matsui H. Glucagon-like peptide-1 induces a cAMP-dependent increase of [Na+]i associated with insulin secretion in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2003;285:E1001–E1009. doi: 10.1152/ajpendo.00005.2003. [DOI] [PubMed] [Google Scholar]
- Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, Lompre AM, Vandecasteele G, Lezoualc'h F. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res. 2005;97:1296–1304. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- Nakazaki M, Crane A, Hu M, Seghers V, Ullrich S, Aguilar-Bryan L, Bryan J. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes. 2002;51:3440–3449. doi: 10.2337/diabetes.51.12.3440. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- Pountney DJ, Sun ZQ, Porter LM, Nitabach MN, Nakamura TY, Holmes D, Rosner E, Kaneko M, Manaris T, Holmes TC, Coetzee WA. Is the molecular composition of KATP channels more complex than originally thought? J Mol Cell Cardiol. 2001;33:1541–1546. doi: 10.1006/jmcc.2001.1407. [DOI] [PubMed] [Google Scholar]
- Qiao J, Mei FC, Popov VL, Vergara LA, Cheng X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J Biol Chem. 2002;277:26581–26586. doi: 10.1074/jbc.M203571200. [DOI] [PubMed] [Google Scholar]
- Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehmann H, Prakash B, Wolf E, Rueppel A, De Rooij J, Bos JL, Wittinghofer A. Structure and regulation of the cAMP-binding domains of Epac2. Nat Struct Biol. 2003c;10:26–32. doi: 10.1038/nsb878. [DOI] [PubMed] [Google Scholar]
- Rehmann H, Rueppel A, Bos JL, Wittinghofer A. Communication between the regulatory and the catalytic region of the cAMP-responsive guanine nucleotide exchange factor Epac. J Biol Chem. 2003b;278:23508–23514. doi: 10.1074/jbc.M301680200. [DOI] [PubMed] [Google Scholar]
- Rehmann H, Schwede F, Doskeland SO, Wittinghofer A, Bos JL. Ligand-mediated activation of the cAMP-responsive guanine nucleotide exchange factor Epac. J Biol Chem. 2003a;278:38548–38556. doi: 10.1074/jbc.M306292200. [DOI] [PubMed] [Google Scholar]
- Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B, Ciani S, Eddlestone GT. ATP mediates activation and inhibition of KATP channel activity via cAMP-dependent protein kinase in insulin-secreting cell lines. J Gen Physiol. 1989;94:693–717. doi: 10.1085/jgp.94.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. 2004a;279:7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Sunaga Y, Seino S. Integration of ATP, cAMP, and Ca2+ signals in insulin granule exocytosis. Diabetes. 2004b;53:S59–S62. doi: 10.2337/diabetes.53.suppl_3.s59. [DOI] [PubMed] [Google Scholar]
- Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Hoy M, Lindner J, Kooptiwut S, Juntti-Berggren L, Gromada J, Berggren PO, Magnuson MA. Sulfonylurea receptor type 1 knock-out mice have intact feeding stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002;277:37176–37183. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Skoglund G, Hussain MA, Holz GG. Glucagon-like peptide-1 stimulates insulin gene promoter activity by protein kinase A-independent activation of the rat insulin I gene cAMP response element. Diabetes. 2000;49:1156–1164. doi: 10.2337/diabetes.49.7.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somekawa S, Fukuhara S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N. Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circ Res. 2005;97:655–662. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
- Suga S, Kanno T, Ogawa Y, Takeo T, Kamimura N, Wakui M. cAMP-independent decrease of ATP-sensitive K+ channel activity by GLP-1 in rat pancreatic beta cells. Pflugers Arch. 2000;440:566–572. doi: 10.1007/s004240000279. [DOI] [PubMed] [Google Scholar]
- Trube G, Rorsman P, Ohno-Shosaku T. Opposite effects of tolbutamide and diazoxide on the ATP-dependent K+ channel in mouse pancreatic beta cells. Pflugers Arch. 1986;407:493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, Rutter GA. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta cells. Biochem J. 2003;369:287–299. doi: 10.1042/BJ20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Oestreich EA, Maekawa N, Bullard TA, Vikstrom KL, Dirksen RT, Kelley GG, Blaxall BC, Smrcka AV. Phospholipase C epsilon modulates beta-adrenergic receptor-dependent cardiac contraction and inhibits cardiac hypertrophy. Circ Res. 2005;97:1305–1313. doi: 10.1161/01.RES.0000196578.15385.bb. [DOI] [PubMed] [Google Scholar]
- Williams BA, Smith PA, Leow K, Shimizu S, Gray DW, Ashcroft FM. Two types of potassium channel regulated by ATP in pancreatic B cells isolated from a type-2 diabetic human. Pflugers Arch. 1993;423:265–273. doi: 10.1007/BF00374405. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.