Abstract
Cholecystokinin (CCK)-induced pancreatic growth in mice involves parallel increases in DNA and protein. The mammalian target of rapamycin (mTOR) signalling pathway regulates mRNA translation and its activation is implicated in growth of various tissues. The aim of this study was to elucidate whether mTOR activation is required for pancreatic growth in a mouse model of increased endogenous CCK release. In mice fed chow containing the synthetic protease inhibitor camostat, protein synthetic rates and phosphorylation of two downstream targets of mTOR, eukaryotic initiation factor 4E binding protein 1 (4E-BP1) and the ribosomal protein S6 (S6), increased in comparison with fasted controls. The camostat-induced increases in protein synthesis and 4E-BP1 and S6 phosphorylation were almost totally abolished by administration of the mTOR inhibitor rapamycin 1 h prior to camostat feeding. In contrast, the phosphorylation of ERK1/2 and JNK and the expression of the early response genes c-jun, c-fos, ATF3 and egr-1 induced by camostat feeding were not affected by rapamycin. In mice fed camostat for 7 days, the ratio of pancreatic to body weight increased by 143%, but when rapamycin was administered daily this was reduced to a 22% increase. Changes in pancreatic mass were paralleled by protein and DNA content following camostat feeding and rapamycin administration. Moreover, while BrdU incorporation, an indicator of DNA synthesis, was increased to 448% of control values after 2 days of camostat feeding, rapamycin administration completely inhibited this increase. We conclude that the mTOR signalling pathway is required for CCK-induced cell division and pancreatic growth.
Under physiological conditions in the adult, there is a low rate of cellular turnover and little change in the size of the pancreas. However, growth of the adult pancreas is possible in response to hyperphagia and/or increased dietary protein intake and in response to tissue injury, such as following pancreatitis (Pearson et al. 1977; Green et al. 1986; Sato et al. 2003). Gastrointestinal hormones can regulate cell-cycle progression and cellular hypertrophy in exocrine (acinar) cells of the pancreas. In particular, the hormone cholecystokinin (CCK) has been implicated in stimulating acinar cell growth (Logsdon, 1999). Feeding an oral trypsin inhibitor is associated with pancreatic growth (Chernick et al. 1948; Melmed & Bouchier, 1968; Goke et al. 1986; Tashiro et al. 2004) concomitant with increased concentrations of intestinal and circulating CCK (Melmed & Bouchier, 1968; Melmed et al. 1976; McGuinness et al. 1984; Goke et al. 1986), and exogenous CCK administration stimulates pancreatic growth in rodents (Dembinski & Johnson, 1980; Niederau et al. 1987) and cell division in acinar cell cultures (Logsdon, 1986). Furthermore, oral trypsin inhibitor-induced pancreatic growth is abated by coadministration of CCK antagonists (Wisner et al. 1988) and is absent in CCK (Tashiro et al. 2004) and CCK-A receptor-deficient mice (Sato et al. 2002, 2003).
The protein phosphatase calcineurin, and several protein kinases, including ERKs, JNKs and the mammalian target of rapamycin (mTOR), are activated in the pancreases of mice fed the synthetic trypsin inhibitor camostat (Sans & Williams, 2004; Tashiro et al. 2004, 2006). Administration of calcineurin inhibitors to these mice prevents pancreatic growth (Tashiro et al. 2004). Thus, the calcineurin signal transduction pathway appears to be necessary for CCK-induced pancreatic growth. However, the role of the aforementioned protein kinases in pancreatic growth has yet to be elucidated.
mTOR is an atypical protein kinase that integrates nutritional and mitogenic signals to control cell growth via phosphorylation of its downstream effectors (Avruch et al. 2005; Martin & Hall, 2005). Two of the most well-studied effectors of mTOR, the eukaryotic initiation factor (eIF)4E binding protein 1 (4E-BP1) and the ribosomal protein S6 kinase 1 (S6K1) are important regulators of protein synthesis (Shah et al. 2000). Protein synthesis is regulated primarily at the stage of mRNA translation initiation wherein an initiator-methionyl tRNA and mRNA are delivered to the ribosome (Merrick, 1992). A protein complex consisting of the initiation factors eIF4A, eIF4G and eIF4E facilitates delivery of mRNA to the ribosome. 4E-BP1 limits the formation of this complex through sequestration of eIF4E; however, increased phosphorylation of 4E-BP1 diminishes its binding capacity and leads to increased rates of translation initiation and protein synthesis (Graves et al. 1995). When S6K1 is highly phosphorylated its kinase activity towards the ribosomal protein S6 (S6) and eIF4B is increased (Dufner & Thomas, 1999; Holz et al. 2005). Although the mechanism remains poorly defined, phosphorylation of S6 and eIF4B results in enhanced translation of a subset of mRNAs that encode components of the translational apparatus (Meyuhas, 2000; Khan & Goss, 2005). Therefore, increased phosphorylation of S6K1 and its downstream targets is associated with an increased capacity for protein synthesis.
CCK stimulates global pancreatic protein synthetic rates in vitro and in vivo (Korc et al. 1981; Rausch et al. 1986; Williams, 2001; Sans et al. 2002). CCK stimulates the PI3K signalling pathway (Bi & Williams, 2004) and studies utilizing the PI3K inhibitor wortmannin have demonstrated that CCK-induced stimulation of protein synthesis requires activation of the mTOR pathway in a PI3K-dependent manner (Bragado et al. 1998; Sans & Williams, 2002). Moreover, CCK-induced stimulation of protein synthesis in vitro is associated with increased phosphorylation of 4E-BP1 and S6, and the highly specific mTOR antagonist rapamycin inhibits these phosphorylation changes and the increase in protein synthesis rates (Bragado et al. 1998). It is currently not known whether activation of the mTOR pathway is required for pancreatic growth in vivo. Therefore, the objective of this study was to elucidate whether inhibition of the mTOR signalling pathway prevents pancreatic growth in mice fed trypsin inhibitor to chronically elevate endogenous CCK levels.
Methods
Materials
Ono Pharmaceutical Company (Osaka, Japan) generously provided camostat (FOY-305). Rapamycin was obtained from LC Laboratories (Woburn, MA, USA), low viscosity carboxymethyl cellulose (CMC) was from Sigma-Aldrich (St Louis, MO, USA), protease inhibitors were from Roche (Indianapolis, IN, USA), 5-bromo-2′-deoxyuridine (BrdU) was from Calbiochem (San Diego, CA, USA), l-[2,3,4,5,6-3H]phenylalanine (3H-Phe) was from Amersham, and amino acid derivatization reagents were from Waters (Milford, MA, USA). RNAlater was obtained from Ambion (Austin, TX, USA), TRIzol reagent and all oligonucleotides were from Invitrogen (Carlsbad, CA, USA), RNeasy was from Qiagen (Valencia, CA, USA), and reverse transcription reagents, dNTPs and the Primer Express Primer software were from Applied Biosystems (Foster City, CA, USA). Bovine anti-goat horseradish-peroxidase-conjugated IgG was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), sheep anti-mouse and donkey anti-rabbit horseradish-peroxidase-conjugated IgG were from Amersham Pharmacia Biotech (Piscataway, NJ, USA), enhanced chemiluminescence reagents from Amersham and Pierce (Rockford, IL, USA), and pre-cast electrophoresis gels, SDS-PAGE molecular mass markers, nitrocellulose membranes and protein assay reagent were from Bio-Rad (Hercules, CA, USA). 4E-BP1 antibody was obtained from Calbiochem, S6 antibody was from Santa Cruz, BrdU antibody was from Accurate Chemical and Scientific Corporation (Westbury, NY, USA), and all other antibodies were from Cell Signaling (Beverly, MA, USA).
Animal care
Male ICR mice age 6–10 weeks from Harlan (Indianapolis, IN, USA) weighing approximately 30 g were maintained on a 12 h light:dark cycle with free access to water and chow (5001 Rodent Diet; PMI Nutrition International, St Louis, MO, USA). All animals were killed under carbon dioxide anaesthesia by decapitation. The University of Michigan Committee on Use and Care of Animals approved the animal facilities and the experimental protocol used in these studies.
Experimental design
In all experiments, animals were acclimated to chow in powdered form for 3 days prior to further experimental manipulation. Mice were food deprived overnight for 17 h prior to intraperitoneal injection of either a 2% CMC solution (1 ml (100 g body weight)−1) or rapamycin (0.2 mg (100 g body weight)−1) suspended in 2% CMC solution. One hour following this injection, half of the animals were provided powdered chow containing 0.1% camostat. For studies on acute biochemical changes, mice were killed 2 h later, with food deprivation continuing for control animals. For chronic growth studies, animals were injected daily with rapamycin and killed between 09.00 and 11.00 h after 2–7 days of camostat feeding, with control animals being fed standard chow. Preliminary studies demonstrated that a single injection of rapamycin significantly decreased the phosphorylation of both S6 and 4E-BP1 for 24 h (data not shown). In these experiments, some mice were given an intraperitoneal injection (1 ml (100 g body weight)−1) of 5 mg ml−1 BrdU solution 1 h prior to tissue collection. Blood was collected via cardiac puncture under carbon dioxide anaesthesia, and the pancreas was quickly excised and weighed. Portions of the pancreas were processed, either immediately or after freezing in liquid nitrogen, for further analysis.
Administration of metabolic tracer and measurement of protein synthesis
Fractional rates of protein synthesis in the pancreas were determined using the flooding dose method (Garlick et al. 1980) as modified for mice by Lundholm et al. (1991) and for pancreas by Sans et al. (2003). Briefly, mice were given an intraperitoneal injection of 3H-Phe (0.4 μCi (g body weight)−1) and unlabelled l-Phe (1.5 μmol (g body weight)−1). Ten minutes later, the pancreas was removed and frozen in liquid nitrogen. A portion of frozen pancreas was later homogenized in 0.6 M perchloric acid (1 ml (100 g body weight)−1) and processed as previously described. l-Phe was measured by HPLC, and protein synthesis was calculated from the rate of 3H-Phe incorporation into pancreatic protein using the specific radioactivity of pancreatic PCA-soluble l-Phe as the precursor pool.
Quantitative real-time PCR
Total RNA was isolated from pancreatic tissue stored in RNAlater and isolated using TRIzol and an RNeasy kit. Total RNA was reverse transcribed and quantitative real-time PCR was performed using an I-Cycler IQ real-time PCR detection system with 96-well plates from Bio-Rad as previously described (Sans et al. 2004a). In brief, samples were prepared by mixing 10.3 μl HPLC water, 2 μl 10× PCR buffer, 2.2 μl MgCl2 (50 mm), 1 μl flourescein, 0.4 μl dNTP (10 mm), 2 μl primer mixtures containing SYBR Green, and 2 μl cDNA. The primer sets employed in this study were identical to those previously described (Guo et al. 2004). Fluorescence resulting from the incorporation of SYBR Green dye into the double-stranded DNA produced during PCR was quantified to obtain the threshold cycle (CT) value for each sample. The final relative amounts of mRNA were determined from the ΔCT between experimental and control groups (Sans et al. 2004a).
Immunoblot analysis
Frozen tissue was homogenized in 2 ml of ice-cold lysis buffer (pH 7.4) containing (mm): 50 Tris-HCl, 5 ethylenediaminetetraacetic acid, 25 NaF, 10 sodium pyrophosphate, 50 β-glycerophosphate, 1 phenyl-methylsulphonyl fluoride, 0.2 Na3VO4, 1 dithiothreitol, 0.2% Triton X-100 (v/v), 5% 2-mercaptoethanol (v/v), 10 μg ml−1 aprotinin, and 10 μg ml−1 leupeptin. The homogenate was immediately centrifuged at 20 000 g for 15 min at 4°C. One portion of the resultant supernatant was used for immunoblot analysis using antibodies that specifically recognized Akt phosphorylated on Ser473, S6 phosphorylated on Ser240/244, ERKs phosphorylated on Thr202/Tyr204, p54 JNK phosphorylated on Thr183/Tyr185, and antibodies that recognized both phosphorylated and unphosphorylated forms of the respective proteins. Another portion of the supernatant was prepared for isoelectric focusing (IEF) gel electrophoresis and immunoblot analysis with CRHSP-24-specific antiserum as previously described (Schafer et al. 2003; Tashiro et al. 2004). An additional portion of the supernatant was boiled for 15 min and centrifuged at 10 000 g for 30 min at 4°C. This second supernatant was used for 4E-BP1 immunoblot analyses. Equal amounts of protein were subjected to SDS-PAGE or IEF gel electrophoresis, after which the protein was transferred to nitrocellulose membrane and Western blotting was performed as previously described (Gautsch et al. 1998). Proteins were visualized by enhanced chemiluminescence using an AlphaEase FC8900 imaging system (Alpha Innotech, San Leandro, CA, USA). Changes in the phosphorylation state of proteins other than 4E-BP1 were determined by normalizing the amount of protein in the phosphorylated form to the total amount of the respective protein prior to data transformation and are expressed as a percentage of fasted control values. In cases where the phosphorylation state of a protein in the fasted condition was too low for accurate measurement, results were expressed as a percentage of camostat-fed values. When subjected to SDS-PAGE, 4E-BP1 resolves into multiple electrophoretic forms whereby the most highly phosphorylated γ-form exhibits the slowest mobility, thus allowing for an assessment of both protein content and phosphorylation status. Changes in 4E-BP1 phosphorylation were assessed by calculating the proportion of 4E-BP1 in the γ-form.
Quantification of pancreatic growth
Following determination of total pancreatic wet weight, a frozen portion was weighed and homogenized in a solution (2 ml (100 mg pancreas)−1) containing 0.1% Triton X-100 (v/v) and 5 mm MgCl2, and subsequently sonicated for 15 s. Protein was determined spectrophotometrically using Bio-Rad protein assay reagent. DNA was assayed using a DNA Quantification Kit (Sigma-Aldrich) and a Perkin Elmer LS 55 luminescence spectrometer (Perkin Elmer Instruments, Norwalk, CT, USA). Total pancreatic protein and DNA were then calculated.
To quantify cellular DNA synthesis, a portion of the pancreas from mice injected with BrdU was placed in 4% buffered formaldehyde, frozen, and processed for immunohistochemistry as previously described (Ohnishi et al. 1996). Sections were exposed to BrdU antibody at 1:50 dilution. Slides were then mounted using prolong gold antifade reagent containing DAPI (Molecular Probes, Carlsbad, CA, USA). Digital photographs were obtained of three separate ×40 fields per pancreas using an Olympus BX51 fluorescence microscope (Olympus Optical Co., Melville, NY, USA). For quantification of acinar cell nuclei, MetaMorph Offline version 6.1 software (Universal Imaging Corporation, Downingtown, PA, USA) was used to count DAPI-stained nuclei of a predetermined size limit within a field. BrdU-positive acinar cell nuclei within the same field were counted by hand. The percentage of BrdU-labelled acinar nuclei was determined by dividing the number of BrdU-labelled acinar nuclei by the number of DAPI-stained acinar nuclei within the three fields.
Statistical analysis
Data are expressed as means ± s.e.m. Data were analysed using the GraphPad Prism statistical software package (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was assessed using a one-way ANOVA and a Student-Newman-Keuls post test. P values <0.05 were considered significant.
Results
Rapamycin specifically inhibits stimulation of the mTOR pathway induced by camostat feeding
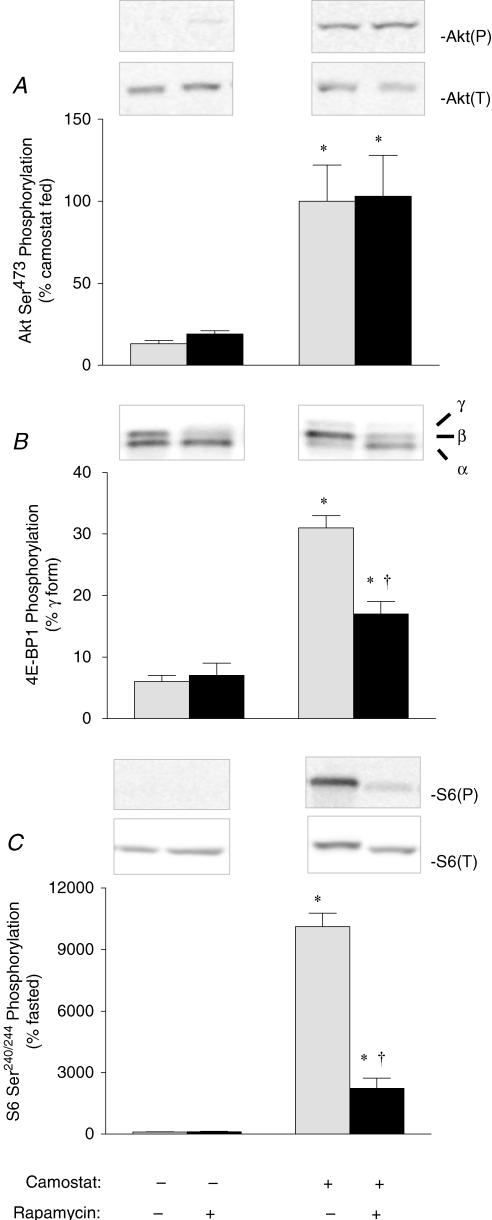
Activation of the mTOR signalling pathway is demarcated by changes in the phosphorylation state of key regulatory proteins along the pathway. Camostat feeding was associated with a more than fivefold increase above fasted control values in the phosphorylation of a kinase upstream of mTOR, Akt (Fig. 1A). Camostat feeding was also associated with a 5- and 100-fold increase above fasted control values in the phosphorylation of the downstream targets of mTOR, 4E-BP1 (Fig. 1B) and ribosomal protein S6 (Fig. 1C), respectively. Rapamycin administration had no effect on the phosphorylation of Akt, 4E-BP1 or S6 in the pancreas of fasted mice. Moreover, there was no effect of rapamycin administration on the increase of Akt phosphorylation in camostat-fed mice. However, camostat-induced increases in 4E-BP1 and S6 phosphorylation were inhibited by 56 and 79%, respectively, following rapamycin administration.
Figure 1. Effect of rapamycin on the phosphorylation of Akt (A), 4E-BP1 (B) and S6 (C), as biomarkers of mTOR pathway activation.
Insets, representative immunoblots. Akt(P), Akt phosphorylated on Ser473; Akt(T), total Akt content; S6(P), S6 phosphorylated on Ser240/244; S6(T), total S6 content; α, α form of 4E-BP1; β, β form of 4E-BP1; γ, γ form of 4E-BP1. Values represent means ± s.e.m.; n = 6–8. *Significantly different from fasted control value, †significantly different from camostat-fed value, P < 0.05.
Rapamycin does not inhibit stimulation of calcineurin and the MAPK pathways induced by camostat feeding
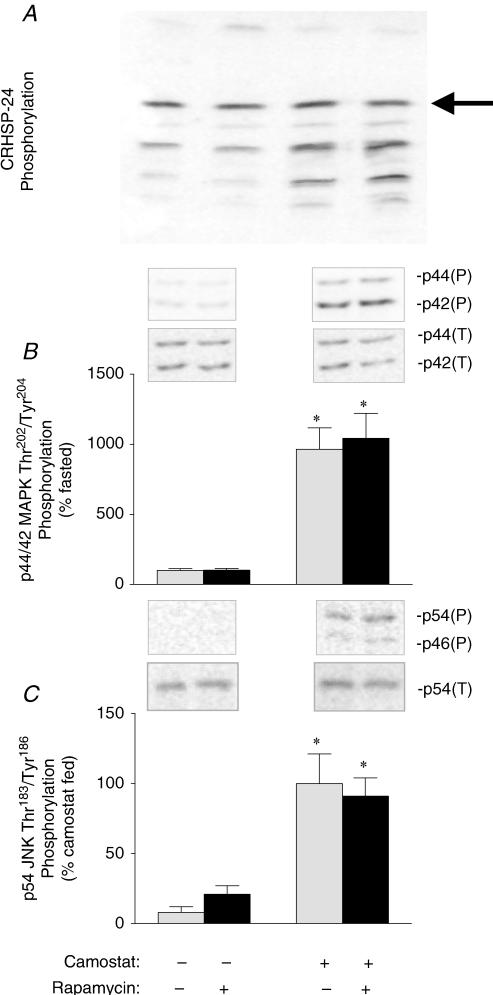
It has been demonstrated previously that camostat feeding not only stimulates the mTOR pathway, but also calcineurin and the MAPK pathways. To determine the specificity of rapamycin for the mTOR pathway following camostat-feeding, changes in calcineurin activity and MAPK phosphorylation were assessed. A major target of calcineurin in pancreatic tissue is the calcium-regulated heat-stable protein of 24 kDa (CRHSP-24), and dephosphorylation of CRHSP-24 thereby demarcates increased calcineurin activity (Groblewski et al. 1998). In the present study, dephosphorylation of CRHSP-24 was increased following camostat feeding (Fig. 2A), but was unaffected by rapamycin administration. Following camostat feeding there was a nearly 10-fold increase above fasted control values in the phosphorylation of p44/42 MAPK (ERK) (Fig. 2B) and p54 JNK (Fig. 2C), indicative of their activation. Similar to what was observed with changes in CRHSP-24 phosphorylation, neither the phosphorylation of JNK nor that of ERK was affected by rapamycin administration.
Figure 2. Effect of rapamycin on CRHSP-24 (A), p44/p42 MAPK (B) and p54 JNK (C) phosphorylation.
The most highly phosphorylated form of CRHSP-24 (arrow) was separated from increasingly dephosphorylated forms by isoelectric focusing and visualized by Western blotting. The amount of phosphorylated and total ERK was determined from the combined value of the p44 and p42 bands, while that of phosphorylated and total JNK was determined from the value of the p54 band only. Insets, representative immunoblots. p44/p42(P), p44/p42 MAPK phosphorylated on Thr202/Tyr204; p44/p42(T), total p44/p42 MAPK content; p54/p56(P), p54/p56 phosphorylated on Thr183/Tyr185; p54(T), total p54 JNK content. Values represent means ± s.e.m.; n = 6–8. *Significantly different from fasted control value, †significantly different from camostat-fed value, P < 0.05.
Rapamycin does not inhibit early response gene transcription induced by camostat feeding
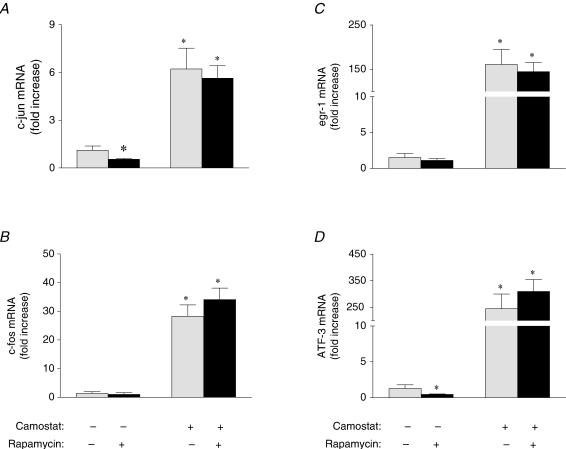
An additional hallmark of camostat-induced pancreatic growth is the induction of early response genes that peaks at one to two hours postfeeding (Guo et al. 2004). To evaluate whether rapamycin affected early response gene expression, quantitative real time PCR was employed to measure pancreatic mRNA levels of c-jun (Fig. 3A), c-fos (Fig. 3B), egr-1 (Fig. 3C) and ATF-3 (Fig. 3D). The mRNA levels of each of these genes was significantly increased above fasting control levels by 6- to 150-fold in camostat fed mice, as found previously (Guo et al. 2004), while there was no change in 18S RNA or GAPDH mRNA levels (data not shown). These increases in early response gene mRNA levels were unaffected by treatment with rapamycin. Rapamycin administration resulted in a small, but significant, inhibition of fasting c-jun and ATF-3 mRNA levels; however, the significance of this observation is not clear.
Figure 3. Effect of rapamycin on early response gene transcription.
Relative mRNA levels of c-jun (A), c-fos (B), egr-1 (C) and ATF-3 (D) in pancreas of fasted and camostat-fed mice administered carboxymethyl cellulose (CMC) or rapamycin as determined by quantitative PCR. Values represent means ± s.e.m.; n = 5–6. *Significantly different from fasted control value, †significantly different from camostat-fed value, P < 0.05.
Rapamycin inhibits pancreatic growth induced by camostat feeding
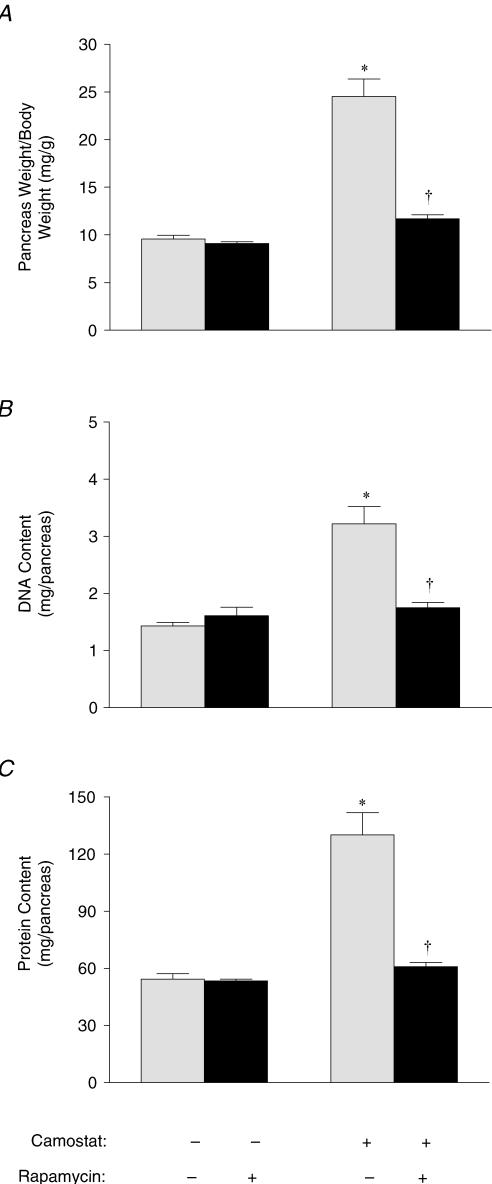
To address whether selective inhibition of the mTOR pathway can inhibit pancreatic growth, mice received daily injections of rapamycin while being fed camostat for 1 week. Similar to that reported previously (Tashiro et al. 2004), 1 week of camostat feeding did not affect body weight (data not shown), but resulted in pancreatic weight increasing from 9.5 ± 0.4 to 24.5 ± 1.8 mg (g body weight)−1 (Fig. 4A). Neither body weight nor pancreatic weight was affected following 1 week of rapamycin injections in mice fed control diet. However, 1 week of daily rapamycin injections almost completely blocked camostat-induced growth, with pancreatic weight only increasing to 11.7 ± 0.4 mg (g body weight)−1. Changes in the size of the pancreas were paralleled by changes in both DNA (Fig. 4B) and protein (Fig. 4C) content. DNA content increased from 1.4 ± 0.1 to 3.2 ± 0.03 (mg pancreas)−1 and protein content increased from 35.0 ± 1.3 to 73.2 ± 6.1 (mg pancreas)−1 and after 7 days of camostat feeding. In contrast, there were no significant differences in protein or DNA content between control animals, control diet-fed animals administered rapamycin, and camostat-fed animals administered rapamycin. Thus rapamycin completely blocked the increase in pancreatic protein and DNA content in response to camostat feeding.
Figure 4. Effect of rapamycin on pancreatic growth in mice fed camostat for 7 days.
Pancreatic weight (A), DNA content (B) and protein content (C) of mice given daily injections of CMC or rapamycin and fed standard chow or camostat-enriched chow for 7 days. Values represent means ± s.e.m.; n = 6–7. *Significantly different from fasted control value, †significantly different from camostat-fed value, P < 0.05.
Rapamycin inhibits pancreatic DNA synthesis induced by camostat feeding
The close correlation between increased pancreatic protein and DNA content observed following 1 week of camostat feeding in the present study is indicative of cellular proliferation. To determine whether inhibition of the mTOR pathway prevents camostat-induced DNA synthesis, mice were injected with the uridine analogue BrdU, which is incorporated into newly synthesized DNA (Fig. 5A). A time course analysis (Fig. 5B) indicated that DNA synthesis in the pancreas was increased slightly after 1 day of camostat feeding, peaked after 2 days, and then declined but remained elevated after 5 days of camostat feeding. Therefore, the effect of rapamycin was studied in mice after 2 days of camostat feeding and daily rapamycin injections. Camostat feeding for 2 days resulted in a 45-fold increase in BrdU incorporation (Fig. 5A and C) above control levels. Rapamycin had no effect on incorporation in mice fed control diet, but inhibited camostat-induced BrdU incorporation by more than 98%.
Figure 5. Effect of rapamycin on pancreatic DNA synthesis.
A, immunostaining of BrdU in pancreatic acinar cell nuclei. B, time course of BrdU incorporation in the pancreas of camostat-fed mice. C, BrdU incorporation in pancreas of mice given daily injections of CMC or rapamycin and fed standard chow or camostat-enriched chow for 2 days. Values represent means ± s.e.m.; n = 9. *Significantly different from fasted control value, †significantly different from camostat-fed value, P < 0.05.
Rapamycin inhibits stimulation of global protein synthetic rates induced by camostat feeding
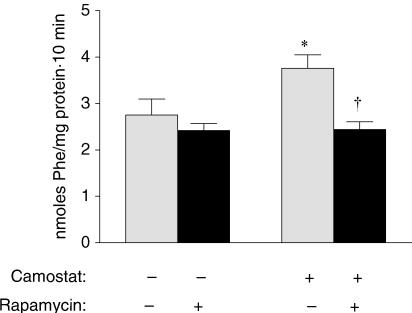
Previous studies in our laboratory have shown that pancreatic weight and protein increase in parallel after camostat feeding. These increases necessitate an increase in pancreatic protein synthetic rates and/or an inhibition of digestive enzyme secretion. The latter possibility seems unlikely, however, as CCK is a well-established pancreatic secretagogue and rapamycin does not block amylase secretion in vitro (Bragado et al. 1998). We therefore measured pancreatic protein synthesis (Fig. 6) 2 h after the initiation of camostat feeding and found that global rates were increased from 2.75 ± 0.35 nmol Phe (mg protein)−1 (10 min)−1 in fasted mice to 3.76 ± 0.35 nmol Phe (mg protein)−1 (10 min)−1 in camostat fed animals. Rapamycin administration had no effect on synthesis rates in fasted mice; however, it completely prevented the camostat-induced stimulation of pancreatic protein synthesis.
Figure 6. Effect of rapamycin on pancreatic protein synthesis.
Rates of global pancreatic protein synthesis in fasted and 2 h camostat-fed mice administered CMC or rapamycin as assessed by the incorporation of 3H-Phe into pancreatic protein during a 10 min period. Values represent means ± s.e.m.; n = 5–6. *Significantly different from fasted control value, †significantly different from camostat-fed value, P < 0.05.
Discussion
The present study documents that activation of the mTOR pathway is required for camostat-induced pancreatic growth. Administration of the mTOR inhibitor rapamycin did not affect camostat-induced calcineurin and MAPK activation or early response gene transcription. Moreover, previous studies have demonstrated that rapamycin does not affect CCK-induced amylase secretion in isolated pancreatic acini (Bragado et al. 1998). However, rapamycin administration significantly attenuated the increased phosphorylation of 4E-BP1 and ribosomal protein S6, as well as the increased rates of pancreatic protein synthesis, observed acutely following camostat feeding. Daily rapamycin administration also abolished the increases in pancreatic DNA synthesis and growth observed following prolonged camostat feeding.
Activation of the serine/threonine-specific protein phosphatase calcineurin was previously identified as a necessary step in the induction of pancreatic growth following camostat feeding (Tashiro et al. 2004). It was not known, however, whether activation of calcineurin by itself was sufficient to cause pancreatic growth. The results from this study demonstrating that rapamycin administration inhibited pancreatic growth, but not calcineurin-mediated dephosphorylation of CRHSP-24, make it likely that camostat-feeding-induced activation of calcineurin alone is not sufficient to induce pancreatic growth. A possible caveat to this conclusion, however, is that mTOR may be regulated by calcineurin following camostat feeding. In a recent study involving isolated pancreatic acinar cells, administration of the calcineurin inhibitor FK506 resulted in the dephosphorylation of 4E-BP1, but not S6 (Sans & Williams, 2004). In a more recent in vivo study, FK506 decreased, but did not completely inhibit camostat feeding-induced phosphorylation of both 4E-BP1 and S6 (Tashiro et al. 2006). Calcineurin activity may thus affect 4E-BP1 and S6 phosphorylation through a hitherto unknown mTOR-dependent pathway. However, the present results clearly demonstrate that inhibition of the mTOR pathway inhibits growth independent of calcineurin following camostat feeding. Therefore, activation of both calcineurin and the mTOR pathway appear to be necessary for camostat-induced pancreatic growth.
Activation of the mTOR pathway is associated with increased translation of mRNAs containing a 5′-terminal oligopyrimidine sequence and mRNAs with highly structured 3′ untranslated regions in particular (Meyuhas, 2000; Khan & Goss, 2005). The aforementioned mRNAs encode many proteins required for cell growth, such as ribosomal proteins and translation factors (Meyuhas, 2000; Kimball & Jefferson, 2004). mTOR also modulates cell growth via phosphorylation of the nucleolar transcription factor UBF which stimulates ribosomal DNA transcription (Hannan et al. 2003). Recently, it has been demonstrated that mTOR activity also affects various cell cycle regulators. For example, rapamycin administration inhibits serum-stimulated increases in cyclin D1 protein and the resultant induction of CDK-4 kinase activity (Nader et al. 2005). Induction of CDK-4 kinase activity is required for entry of a cell into the G1 phase of the cell cycle, and, in the present study, rapamycin administration inhibited camostat feeding-induced increases in BrdU incorporation, indicating that camostat-induced cell cycle entry was indeed blocked.
The changes in MAPK activation observed in the current study are likely to have an impact upon changes in the pancreatic proteome following camostat feeding. The MAPKs are important regulators of transcription, including that of the early response genes (Minet et al. 2001). Therefore, the camostat feeding-induced changes in early response gene expression observed both previously (Guo et al. 2004) and in the present study are likely to be mediated, at least in part, by ERK and JNK. As the early response genes encode factors that regulate the proliferation of many cell types, ERK and JNK activation is likely to be important in the regulation of pancreatic growth. Neither camostat feeding-induced ERK and JNK phosphorylation, nor activation of the early response genes, were affected by rapamycin administration in the present study and these changes thus appear to be independent of the mTOR pathway. In a recent paper (Tashiro et al. 2006), it was demonstrated that administration of the calcineurin inhibitor FK506 blocks camostat feeding-induced JNK, but not ERK, phosphorylation. Thus, while JNK activation may be mediated by calcineurin in vivo, ERK activation appears to be independent of both mTOR and calcineurin and it will be important for future studies to address whether activation of the MAPKs is necessary or sufficient for pancreatic growth following camostat feeding or whether activation of calcineurin, mTOR and the MAPKs are all necessary for camostat-induced pancreatic growth.
Interestingly, the rates of pancreatic protein synthesis observed 2 h following the initiation of consumption of camostat-containing chow in the current study are no greater than those observed 2 h following the initiation of consumption of standard chow in a prior study (Sans et al. 2004a). Increased rates of protein synthesis are required for pancreatic growth, but as feeding standard chow is not associated with pancreatic growth in mice, there must be an underlying difference between the changes in protein synthesis initiated by feeding chow containing camostat and those initiated by feeding standard chow. One possible difference between these two diets is the duration for which they stimulate pancreatic protein synthetic rates. Pancreatic protein synthesis rates have been shown to be greater than fasting levels in mice allowed access to food for 1 or 2 h, but not 3 h, following 18 h of food deprivation (Sans et al. 2004a). In contrast, the stimulation of protein synthesis following consumption of a camostat-containing meal appears to be far more prolonged. We recently determined that after 2 days of camostat feeding, pancreatic protein synthetic rates are 32% greater per milligram of protein than for mice freely fed standard chow (unpublished data). These results are in keeping with prior observations that the duration, but not the magnitude, of CCK release is greater in rats fed soybean trypsin inhibitor than in those fed casein (Liddle et al. 1986). Thus, it may be that the stimulation of protein synthesis observed following normal feeding is sufficient to maintain cell size, while the prolonged stimulation observed following camostat feeding is necessary for cell division. An additional, and not mutually exclusive, explanation for the differences in pancreatic growth following standard chow feeding and feeding chow supplemented with camostat is variations in the synthesis of specific proteins. For example, given the camostat feeding-specific activation of MAPKs and the known transcriptional effects of the MAPKs, it is highly plausible that there are significant differences in the mRNAs available for translation between camostat-fed and standard chow-fed animals. Finally, it has been estimated that 90% of protein synthesis in the pancreas can be attributed to digestive enzymes (Scheele, 1993). Therefore, it is unlikely that the synthesis of proteins required for camostat-induced cell growth and proliferation would result in measurable changes in global protein synthetic rates, as measured by the flooding dose method, until after additional synthetic machinery had been synthesized and acinar cells were entering mitosis. As protein synthesis measurements in the present study were made 2 h after feeding, it is likely that the bulk of protein synthesis was directed towards digestive enzyme synthesis and not cell growth. Therefore, it is highly possible that the pancreatic proteome of camostat fed mice differs significantly from that of a standard chow fed mice 2 h after the initiation of feeding despite there being no difference in their protein synthetic rates.
While the effects of camostat feeding on pancreatic growth in this study have been primarily attributed to CCK, the gastrointestinal hormone secretin may also be playing an important role. Camostat feeding results in significant increases in circulating secretin (Watanabe et al. 1992). Moreover, it has been demonstrated that both CCK and secretin are required for maximal camostat-induced digestive enzyme secretion (Watanabe et al. 1992). While secretin alone has minimal effects on pancreatic growth, secretin enhances growth of the pancreas in response to administration of the CCK analogue caerulein (Solomon et al. 1978). Thus, the effects of camostat on pancreatic growth may be mediated by the combination of CCK and secretin. Activation of the mTOR pathway however, is unlikely to be modulated by secretin. The effects of secretin on intracellular signalling are mediated by adenylate cyclase and, unlike CCK, adenylate cyclase agonists and cAMP analogues do not effect the phosphorylation of downstream targets of mTOR in isolated acini (Bragado et al. 1997; Sans et al. 2004b).
In conclusion, the results presented herein demonstrate that the mTOR signalling pathway is necessary for pancreatic cell division and growth following camostat feeding. Moreover, the results demonstrate that the mTOR pathway plays a critical role in the regulation of both pancreatic protein and DNA synthesis in this model. Thus, mTOR joins calcineurin as an essential modulator of pancreatic growth in protease inhibitor-treated mice. These findings demonstrate that multiple signalling pathways are required to initiate pancreatic growth and an important avenue for future research will be to identify the other signalling pathways that are necessary for this process.
Acknowledgments
The authors thank Mr Brad Nelson and Dr Stephen Lentz for their assistance with immunohistochemical procedures and morphometric analysis, and Mr Steven Whitesall for his assistance with protein synthesis measurements. This research was supported by a National Institutes of Health grant to J. A. Williams (DK-59578) and by the Michigan Gastrointestinal Peptide Center (DK-34933).
References
- Avruch J, Lin Y, Long X, Murthy S, Ortiz-Vega S. Recent advances in the regulation of the TOR pathway by insulin and nutrients. Curr Opin Clin Nutr Metab Care. 2005;8:67–72. doi: 10.1097/00075197-200501000-00010. [DOI] [PubMed] [Google Scholar]
- Bi Y, Williams JA. Receptor biology and signal transduction in pancreatic acinar cells. Curr Opin Gastroenterol. 2004;20:427–434. doi: 10.1097/00001574-200409000-00002. [DOI] [PubMed] [Google Scholar]
- Bragado MJ, Groblewski GE, Williams JA. p70s6k is activated by CCK in rat pancreatic acini. Am J Physiol. 1997;273:C101–C109. doi: 10.1152/ajpcell.1997.273.1.C101. [DOI] [PubMed] [Google Scholar]
- Bragado MJ, Groblewski GE, Williams JA. Regulation of protein synthesis by cholecystokinin in rat pancreatic acini involves PHAS-I and the p70, S6 kinase pathway. Gastroenterology. 1998;115:733–742. doi: 10.1016/s0016-5085(98)70153-2. [DOI] [PubMed] [Google Scholar]
- Chernick SS, Lepkovsky S, Chaikoff IL. A dietary factor regulating the enzyme content of the pancreas: Changes induced in size and proteolytic activity of the chick pancreas by the ingestion of raw soy-bean meal. Am J Physiol. 1948;155:33–41. doi: 10.1152/ajplegacy.1948.155.1.33. [DOI] [PubMed] [Google Scholar]
- Dembinski AB, Johnson LR. Stimulation of pancreatic growth by secretin, caerulein, and pentagastrin. Endocrinology. 1980;106:323–328. doi: 10.1210/endo-106-1-323. [DOI] [PubMed] [Google Scholar]
- Dufner A, Thomas G. Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res. 1999;253:100–109. doi: 10.1006/excr.1999.4683. [DOI] [PubMed] [Google Scholar]
- Garlick PJ, McNurlan MA, Preedy VR. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980;192:719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautsch TA, Anthony JC, Kimball SR, Paul GL, Layman DK, Jefferson LS. Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise. Am J Physiol. 1998;274:C406–C414. doi: 10.1152/ajpcell.1998.274.2.C406. [DOI] [PubMed] [Google Scholar]
- Goke B, Printz H, Koop I, Rausch U, Richter G, Arnold R, Adler G. Endogenous CCK release and pancreatic growth in rats after feeding a proteinase inhibitor (camostat) Pancreas. 1986;1:509–515. doi: 10.1097/00006676-198611000-00008. [DOI] [PubMed] [Google Scholar]
- Graves LM, Bornfeldt KE, Argast GM, Krebs EG, Kong X, Lin TA, Lawrence JC., Jr cAMP- and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1995;92:7222–7226. doi: 10.1073/pnas.92.16.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green GM, Levan VH, Liddle RA. Plasma cholecystokinin and pancreatic growth during adaptation to dietary protein. Am J Physiol. 1986;251:G70–G74. doi: 10.1152/ajpgi.1986.251.1.G70. [DOI] [PubMed] [Google Scholar]
- Groblewski GE, Yoshida M, Bragado MJ, Ernst SA, Leykam J, Williams JA. Purification and characterization of a novel physiological substrate for calcineurin in mammalian cells. J Biol Chem. 1998;273:22738–22744. doi: 10.1074/jbc.273.35.22738. [DOI] [PubMed] [Google Scholar]
- Guo LL, Sans MD, Gurda G, Lee SH, Williams JA. Trypsin inhibitor-induced pancreatic growth involves induction of early response genes (abstract) Pancreas. 2004;29:333. doi: 10.1152/ajpgi.00433.2006. [DOI] [PubMed] [Google Scholar]
- Hannan KM, Brandenburger Y, Jenkins A, Sharkey K, Cavanaugh A, Rothblum L, Moss T, Poortinga G, McArthur GA, Pearson RB, Hannan RD. mTOR-dependent regulation of ribosomal gene transcription requires S6K1 and is mediated by phosphorylation of the carboxy-terminal activation domain of the nucleolar transcription factor UBF. Mol Cell Biol. 2003;23:8862–8877. doi: 10.1128/MCB.23.23.8862-8877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Khan MA, Goss DJ. Translation initiation factor (eIF) 4B affects the rates of binding of the mRNA m7G cap analogue to wheat germ eIFiso4F and eIFiso4F. Biochem. 2005;44:4510–4516. doi: 10.1021/bi047298g. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Regulation of global and specific mRNA translation by oral administration of branched-chain amino acids. Biochem Biophys Res Commun. 2004;313:423–427. doi: 10.1016/j.bbrc.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Korc M, Bailey AC, Williams JA. Regulation of protein synthesis in normal and diabetic rat pancreas by cholecystokinin. Am J Physiol. 1981;241:G116–G121. doi: 10.1152/ajpgi.1981.241.2.G116. [DOI] [PubMed] [Google Scholar]
- Liddle RA, Green GM, Conrad CK, Williams JA. Proteins but not amino acids, carbohydrates, or fats stimulate cholecystokinin secretion in the rat. Am J Physiol. 1986;14:G243–G248. doi: 10.1152/ajpgi.1986.251.2.G243. [DOI] [PubMed] [Google Scholar]
- Logsdon CD. Stimulation of pancreatic acinar cell growth by CCK, epidermal growth factor, and insulin in vitro. Am J Physiol. 1986;251:G487–G494. doi: 10.1152/ajpgi.1986.251.4.G487. [DOI] [PubMed] [Google Scholar]
- Logsdon CD. Role of cholecystokinin in physiologic and pathophysiologic growth of the pancreas. In: Greeley GH, editor. Gastrointestinal Endocrinology. Totowa, NJ: Humana Press; 1999. pp. 393–422. [Google Scholar]
- Lundholm K, Ternell M, Zachrisson H, Moldawer L, Lindstrom L. Measurement of hepatic protein synthesis in unrestrained mice-evaluation of the ‘flooding technique’. Acta Physiol Scand. 1991;141:207–219. doi: 10.1111/j.1748-1716.1991.tb09069.x. [DOI] [PubMed] [Google Scholar]
- Martin DE, Hall MN. The expanding TOR signaling network. Curr Opin Cell Biol. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- McGuinness EE, Morgan RG, Wormsley KG. Effects of soybean flour on the pancreas of rats. Environ Health Perspect. 1984;56:205–212. doi: 10.1289/ehp.8456205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melmed RN, Bouchier IA. Effects of trypsin inhibitors on the acinar cells of rat pancreas. Gut. 1968;9:729. [PubMed] [Google Scholar]
- Melmed RN, El-Aaser AA, Holt SJ. Hypertrophy and hyperplasia of the neonatal rat exocrine pancreas induced by orally administered soybean trypsin inhibitor. Biochim Biophys Acta. 1976;421:280–288. doi: 10.1016/0304-4165(76)90294-4. [DOI] [PubMed] [Google Scholar]
- Merrick WC. Mechanism and regulation of eukaryotic protein synthesis. Microbiol Rev. 1992;56:291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem. 2000;267:6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- Minet E, Michel G, Mottet D, Piret JP, Barbieux A, Raes M, Michiels C. c-JUN gene induction and AP-1 activity is regulated by a JNK-dependent pathway in hypoxic HepG2 cells. Exp Cell Res. 2001;265:114–124. doi: 10.1006/excr.2001.5180. [DOI] [PubMed] [Google Scholar]
- Nader GA, McLoughlin TJ, Esser KA. mTOR function in skeletal muscle hypertrophy: increased ribosomal RNA via cell cycle regulators. Am J Physiol Cell Physiol. 2005;289:C1457–C1465. doi: 10.1152/ajpcell.00165.2005. [DOI] [PubMed] [Google Scholar]
- Niederau C, Liddle RA, Williams JA, Grendell JH. Pancreatic growth: interaction of exogenous cholecystokinin, a protease inhibitor, and a cholecystokinin receptor antagonist in mice. Gut. 1987;28:63–69. doi: 10.1136/gut.28.suppl.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H, Ernst SA, Wys N, McNiven M, Williams JA. Rab3D localizes to zymogen granules in rat pancreatic acini and other exocrine glands. Am J Physiol. 1996;271:G531–G538. doi: 10.1152/ajpgi.1996.271.3.G531. [DOI] [PubMed] [Google Scholar]
- Pearson KW, Scott D, Torrance B. Effects of partial surgical pancreatectomy in rats. I. Pancreatic regeneration. Gastroenterology. 1977;72:469–473. [PubMed] [Google Scholar]
- Rausch U, Rudiger K, Vasiloudes P, Kern H, Scheele G. Lipase synthesis in the rat pancreas is regulated by secretin. Pancreas. 1986;1:522–528. doi: 10.1097/00006676-198611000-00010. [DOI] [PubMed] [Google Scholar]
- Sans MD, DiMagno MJ, D'Alecy LG, Williams JA. Caerulein-induced acute pancreatitis inhibits protein synthesis through effects on eIF2B and eIF4F. Am J Physiol Gastrointest Liver Physiol. 2003;285:G517–G528. doi: 10.1152/ajpgi.00540.2002. [DOI] [PubMed] [Google Scholar]
- Sans MD, Kimball SR, Williams JA. Effect of CCK and intracellular calcium to regulate eIF2B and protein synthesis in rat pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2002;282:G267–G276. doi: 10.1152/ajpgi.00274.2001. [DOI] [PubMed] [Google Scholar]
- Sans MD, Lee SH, D'Alecy LG, Williams JA. Feeding activates protein synthesis in mouse pancreas at the translational level without increase in mRNA. Am J Physiol Gastrointest Liver Physiol. 2004a;287:G667–G675. doi: 10.1152/ajpgi.00505.2003. [DOI] [PubMed] [Google Scholar]
- Sans MD, Williams JA. Translational control of protein synthesis in pancreatic acinar cells. Int J Gastrointest Cancer. 2002;31:107–115. doi: 10.1385/IJGC:31:1-3:107. [DOI] [PubMed] [Google Scholar]
- Sans MD, Williams JA. Calcineurin is required for translational control of protein synthesis in rat pancreatic acini. Am J Physiol. 2004;287:C310–C319. doi: 10.1152/ajpcell.00534.2003. [DOI] [PubMed] [Google Scholar]
- Sans MD, Xie Q, Williams JA. Regulation of translation elongation and phosphorylation of eEF2 in rat pancreatic acini. Biochem Biophys Res Commun. 2004b;319:144–151. doi: 10.1016/j.bbrc.2004.04.164. [DOI] [PubMed] [Google Scholar]
- Sato T, Niikawa J, Usui I, Imamura T, Yoshida H, Tanaka S, Mitamura K. Pancreatic regeneration after ethionine-induced acute pancreatitis in rats lacking pancreatic CCK-A receptor gene expression. J Gastroenterol. 2003;38:672–680. doi: 10.1007/s00535-003-1120-0. [DOI] [PubMed] [Google Scholar]
- Sato N, Suzuki S, Kanai S, Ohta M, Jimi A, Noda T, Takiguchi S, Funakoshi A, Miyasaka K. Different effects of oral administration of synthetic trypsin inhibitor on the pancreas between cholecystokinin-A receptor gene knockout mice and wild type mice. Jpn J Pharmacol. 2002;89:290–295. doi: 10.1254/jjp.89.290. [DOI] [PubMed] [Google Scholar]
- Schafer C, Steffen H, Krzykowski KJ, Goke B, Groblewski GE. CRHSP-24 phosphorylation is regulated by multiple signaling pathways in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G726–G734. doi: 10.1152/ajpgi.00111.2003. [DOI] [PubMed] [Google Scholar]
- Scheele G. Regulation of pancreatic gene expression in response to hormones and nutritional substrates. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele G, editors. The Pancreas: Biology, Pathobiology and Disease. New York: Raven Press; 1993. pp. 103–120. [Google Scholar]
- Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab. 2000;279:E715–E729. doi: 10.1152/ajpendo.2000.279.4.E715. [DOI] [PubMed] [Google Scholar]
- Solomon TE, Petersen H, Elashoff J, Grossman MI. Interaction of caerulein and secretin on pancreatic size and composition in rat. Am J Physiol. 1978;235:E714–E719. doi: 10.1152/ajpendo.1978.235.6.E714. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Dabrowski A, Guo LL, Sans MD, Williams JA. Calcineurin-dependent and independent signal transduction pathways activated with pancreatic growth. Pancreas. 2006 doi: 10.1097/01.mpa.0000218316.12577.c0. in press. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Samuelson LC, Liddle RA, Williams JA. Calcineurin mediates pancreatic growth in protease inhibitor-treated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G784–G790. doi: 10.1152/ajpgi.00446.2003. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Takeuchi T, Chey WY. Mediation of trypsin inhibitor-induced pancreatic hypersecretion by secretin and cholecystokinin in rats. Gastroenterology. 1992;102:621–628. doi: 10.1016/0016-5085(92)90111-b. [DOI] [PubMed] [Google Scholar]
- Williams JA. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol. 2001;63:77–97. doi: 10.1146/annurev.physiol.63.1.77. [DOI] [PubMed] [Google Scholar]
- Wisner JR, Jr, McLaughlin RE, Rich KA, Ozawa S, Renner IG. Effects of L-364,718, a new cholecystokinin receptor antagonist, on camostate-induced growth of the rat pancreas. Gastroenterology. 1988;94:109–113. doi: 10.1016/0016-5085(88)90617-8. [DOI] [PubMed] [Google Scholar]