Abstract
The human startle response produces muscle contractions throughout the body but the most brisk and synchronized contractions appear in the neck muscles. This response, which is greatest with the first exposure to a startling stimulus, could produce excessive and inappropriately directed muscle contractions that could explain the higher incidence of whiplash injuries in people who are unprepared for the collision. This study seeks neurophysiological evidence of startle responses in the neck muscles of 120 healthy subjects exposed to between 1 and 16 rear-end impacts or forward perturbations of different speeds. Startle responses were quantified by the synchronous electromyographic (EMG) activity between 10 and 20 Hz in bilaterally homologous sternocleidomastoid, scalene and cervical paraspinal neck muscles. Coherence analyses of EMGs from the left and right muscles were used to estimate synchrony for: (i) the first unexpected trial, (ii) subsequent habituated trials, and (iii) the superposition of habituated trials and a loud acoustic stimulus (40 ms, 124 dB sound). The peak in coherent EMG activity between contralateral muscle pairs in the 10–20 Hz bandwidth was related to startle. Synchrony in this bandwidth was observed between the left and right muscles during the first impact or whiplash-like perturbation. This synchrony decreased significantly in the habituated trials, but reappeared when the loud acoustic stimulus was introduced. Its presence in the first trial indicates that startle is part of the neuromuscular response to an unexpected rear-end impact. This startle component of the neuromuscular response could play a role in the aetiology of whiplash injuries.
Whiplash injuries are common musculoskeletal complaints following low-speed rear-end collisions (Spitzer et al. 1995) and being unprepared for the collision is one of the few factors associated with a greater risk of initial whiplash symptoms (Sturzenegger et al. 1994). The reason why injury risk is higher in unprepared individuals remains unclear, but could be related to an overreaction, i.e. a startle response, to the impact in unaware or unprepared individuals. In this study, our goal was to look for neurological evidence of startle in the response of unprepared subjects exposed to a rear-end impact.
Repeated exposures to rear-end impacts have been shown to attenuate the muscle response and alter the dynamic response of the head and neck (Blouin et al. 2003; Siegmund et al. 2003). These changes are due to habituation, and similar levels of neck muscle habituation have been observed during repeated whole body free-falls or repeated exposures to a loud sound (Brown et al. 1991; Bisdorff et al. 1994). Under all of these conditions, habituation appears to be the attenuation of an overreaction or startle response and suggests a shift from a startle-mediated muscle response when the stimulus is novel (Allum et al. 1992; Bisdorff et al. 1994) to a tuned muscle response with experience.
Grosse & Brown (2003) have shown that an acoustic startle evokes synchronized EMG activity in bilaterally homologous upper limb muscles. Using previously developed correlation techniques in the frequency domain (Rosenberg et al. 1989; Halliday et al. 1995, 1998; Halliday & Rosenberg, 2000), Grosse & Brown (2003) observed increased coherence between 10 and 20 Hz following an auditory startle, but not following a sham startle and not during a voluntary contraction. Increased coherence in this bandwidth is thought to represent increased reticulospinal activity (Grosse & Brown, 2003), since reticular structures are known to lie along the startle reflex pathway (Yeomans et al. 2002).
In this study, we examined whether increased coherence in the 10–20 Hz bandwidth was present between homologous neck muscles during perturbations simulating rear-end impacts. To do so, we conducted coherence analyses on three data sets to determine the following: (i) whether a single perturbation would evoke synchronous activity in neck motoneurons, (ii) whether habituation would extinguish this synchronous activity, and (iii) whether the superposition of an acoustic startle over a habituated postural response would reproduce the synchronous activity.
Methods
Three experiments involving 120 subjects were analysed (Table 1; Brault et al. 2000; Siegmund et al. 2003; Blouin et al. 2006). None of the subjects had a history of whiplash injury, medical conditions that impaired sensory or motor function, or prolonged neck or back pain during the preceding 2 years. Caffeine and nicotine were not permitted for 2 h prior to any of the experiments. All subjects gave their written informed consent and each experiment was reviewed and approved by either the Western Institutional Review Board, Olympia, WA, USA (experiment 1) or the University of British Columbia Clinical Ethics Review Board (experiments 2 and 3). All three experiments conformed to the Declaration of Helsinki.
Table 1.
Mean and standard deviation (S.D.) of subject age and selected anthropometry
| Experiment 1 | Experiment 2 | Experiment 3 | ||||
|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | |
| N | 21 | 21 | 34 | 30 | 8 | 6 |
| Age (year) | 27 (5) | 26 (4) | 24 (5) | 27 (7) | 24 (5) | 27 (5) |
| Mass (kg) | 62 (9) | 75 (10) | 61 (10) | 78 (15) | 57 (7) | 77 (12) |
| Height (cm) | 164 (5) | 175 (5) | 166 (6) | 176 (8) | 162 (7) | 181 (4) |
Surface EMG electrodes were applied bilaterally to the subject's sternocleidomastoid (SCM), scalenus (SCAL, experiment 3 only) and cervical paraspinal (PARA, experiment 1 and 3) muscles. Reusable (experiment 1: In Vivo Metrics, Healdsburg, CA, USA) or disposable Ag–AgCl electrodes (experiment 2: H69P, Kendall-LTP, CA, USA; experiment 3: Bortec Biomedical, Calgary, AB, Canada) were taped to the skin and connected to an EMG amplifier (experiment 1: Konigsberg Instruments Inc., Pasadena, CA, USA; experiment 2: Bortec Biomedical AMT-8, Calgary, AB, Canada; experiment 3: Noraxon Myosystem 1400, AZ, USA). EMG signals acquired during experiment 1 were bandpass filtered at 40–500 Hz before being digitally sampled at 1 kHz. For experiments 2 and 3, the EMG signals were bandpass filtered at 10–1000 Hz prior to being sampled at 2 kHz. To standardize the EMG data, the data acquired during experiments 2 and 3 were digitally bandpass filtered (40–500 Hz) with a second order dual-pass Butterworth filter and decimated to 1 kHz prior to subsequent analyses.
For experiments 1 and 3, head acceleration was measured with a nine accelerometer array (Kistler 8302B20S1; ± 20 g, Amherst, NY, USA) arranged in a 3-2-2-2 configuration (Padgaokar et al. 1975; Siegmund et al. 1997) and sampled at 2 kHz. Head displacements were measured with an OmniSpeed HS motion capture system (Speed Vision Technologies, Solana Beach, CA, USA) and high speed camera (experiment 1: JCLabs 250, Mountain View, CA, USA) or an Optotrak motion analysis system (experiment 3: Northern digital 3020; Waterloo, ON, Canada) and sampled at 250 and 100 Hz per marker for experiments 1 and 3, respectively. The location and orientation of the head instrumentation was measured relative to anatomical landmarks using a 3-D digitizer (FaroArm B08–02, Lake Mary, FL, USA). Data from the head transducers were reduced into global reference frames with origin at the atlanto-occipital joint (AOJ). The AOJ was assumed to be 24 mm posterior and 37 mm inferior to the head's centre of mass, which was estimated to lie in the mid-sagittal plane, rostral to the interaural axis by 17% of the distance between the interaural axis and the vertex (NASA, 1978). All linear accelerometers were corrected for the time-varying orientation of the earth's gravity field prior to computing the six degree-of-freedom kinematics. The x-axis was horizontal and positive forward, the z-axis was vertical and positive down, and the y-axis was horizontal and positive to the right (extension was positive about the y-axis). Similarly detailed kinematics were not recorded in experiment 2.
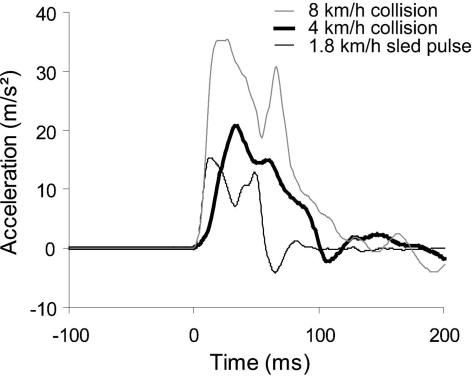
In all three experiments, subjects were instructed to sit normally, face forward with their head level, place their hands on their lap, and relax prior to impact. In experiment 1, subjects underwent a single, aligned, bumper-to-bumper collision between a rolling bullet vehicle and a stationary target vehicle (1991 Honda Accord). The target vehicle was accelerated to 4 km h−1 over 136 ms (2.1 g peak at 36 ms). In experiments 2 and 3, the same front passenger seat used in experiment 1 was mounted on a feedback-controlled sled and accelerated to 1.8 km h−1 over 60 ms (1.5 g peak at 16 ms). The speed change was lower for experiments 2 and 3 because subjects were exposed to multiple sequential impacts. Figure 1 shows, however, that the initial parts of the 1.8 and 4 km h−1 pulses are similar to that of a more severe vehicle-to-vehicle collision (speed change of 8 km h−1). Each subject underwent 11 perturbations (experiment 2) or 16 perturbations (experiment 3). For trials 12–16 of experiment 3, subjects were also exposed to a loud auditory tone (1 kHz; 124 dB, 40 ms duration) beginning 18 ms after the onset of the perturbation.
Figure 1. Sample acceleration pulses used in different experiments.
Note that the initial parts of the 1.8 km h−1 (thin black line) and 4 km h−1 (thick black line) pulses are similar to that of a more severe 8 km h−1 vehicle-to-vehicle collision (thin grey line).
For each trial, EMG data for the left and right homologous cervical muscles were analysed from 24 ms before to 487 ms after the onset of the forward acceleration (512 data points per trial). All EMG signals were full wave rectified and the DC offset was removed. For experiment 1, EMG data from all subjects during their single impact were concatenated separately for the left and right muscles (total of 21504 points per muscle). For experiment 2, two different concatenated data sets were analysed: (i) EMG data from the first trial of all subjects (EMGfirst; 32768 points per muscle) and (ii) EMG data from trials 7–11 of 61 subjects (EMGhab; 156160 points per muscle). Previous observations have shown that a stable habituated postural response is reached by trial 7 (Blouin et al. 2003; Siegmund et al. 2003). For experiment 3, three different concatenated sets of data were analysed: (i) EMG data from the first trial (EMGfirst; 7168 points per muscle), (ii) EMG data from trials 7–11 (EMGhab; total of 35840 points per muscle), and (iii) EMG data from trials 12–16 (EMGstartle; total of 35840 points per muscle).
Using finite fast Fourier transforms (FFTs), the auto-spectra, flMusclelMuscle(λ) and frMusclerMuscle(λ), and cross-spectrum, flMusclerMuscle(λ), were computed for each set of left and right homologous muscle data (λ denotes frequency). The spectra were estimated by averaging non-overlapping windows of 512 points, and thus the frequency resolution of the spectra was 1.95 Hz. The components of the spectra at 0 and 1.95 Hz were not considered because of the concatenation methods. Coherence, |RlMusclerMuscle(λ)|2, between the left and right homologous muscles was then computed using eqn (1) (Rosenberg et al. 1989; Halliday et al. 1995). Coherence is a unitless measure bounded from 0 to 1 which indicates the linear relationship between two processes at various frequencies.
| (1) |
Frequency-specific coherence estimates were considered significant when they exceeded the 95% confidence interval computed according to Halliday et al. (1995). A difference of coherence (DoC) test was used to test for significant changes in coherence between EMGfirst and EMGhab in experiment 2 and between EMGfirst, EMGhab and EMGstartle in experiment 3. The DoC test applies a Fisher transform (tanh−1) to the square root of the coherence estimate (termed coherency) and normalizes these corrected values based on the number of non-overlapping windows used to compute the auto- and cross-spectra (Amjad et al. 1997). The DoC test was computed at each frequency and compared to a χ2 distribution with k – 1 degrees of freedom (k is the number of experimental conditions). Here, we were interested in coherence differences in a local maximum occurring between 10 and 20 Hz. A statistical significance level of P≤ 0.05 was chosen. For experiment 3, an omnibus DoC test was first performed, followed by pair-wise post hoc comparisons with a Bonferroni correction.
For each trial of experiments 1 and 3, head accelerations (the linear ax and αy) were analysed from 200 ms before to 824 ms after the onset of the forward acceleration (2048 data points per trial). The power spectra of these kinematic signals were computed using finite FFTs with a frequency resolution of 0.98 Hz. From each power spectrum, two dependent variables were computed: (i) the average power between 5 and 10 Hz (5.88–9.8 Hz), and (ii) the frequency below which resides 85% of the power between 1 and 20 Hz. For experiment 3, changes in the dependent variables between the three conditions (first trial, habituated trials, and perturbation + auditory tone trials) were compared using one-way repeated-measures ANOVAs and post hoc Tukey tests. Statistical significance was set at P = 0.05.
Results
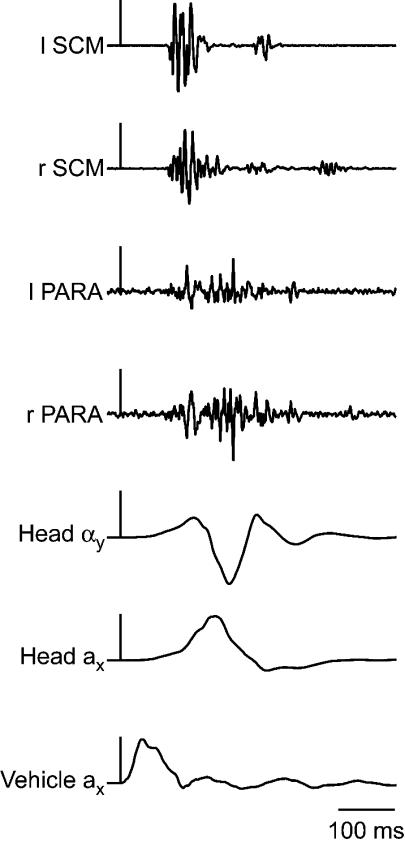
Subjects exhibited stereotypic kinematic and muscle responses to the single vehicle collision (Fig. 2). In experiment 1, peak head forward acceleration ranged from 1.6 to 5.0 g, peak head angular acceleration from 158 to 534 rad s−2 and peak rearward horizontal translation of the head relative to the C7–T1 from 21 to 72 mm. Neck muscle onset occurred 91 ± 9 and 96 ± 11 ms after the impact for the SCM and PARA muscles, respectively. For experiment 2, habituation of the neck postural responses yielded a 41–64% decrement in EMG amplitude between the first trial and the mean of the last five trials. Baseline levels of EMG immediately prior to the perturbation did not change. Habituation-related kinematic changes ranged from a 21% increase in head extension angle to a 29% decrease in forward acceleration at the forehead. These muscle and kinematic changes occurred as early as the second exposure in some variables. A similar habituation was observed in experiment 3; however, the subsequent superposition of the startling tone and perturbation reversed the habituation-related attenuation of the muscle responses. The increased neck muscle activity observed with the addition of the startling tone did not change with repeated exposures to the combined perturbation–tone stimuli.
Figure 2. Sample electromyographic and kinematic data from a single subject (experiment 1).
The vertical scale bars are aligned with the onset of vehicle impact and represent 20 m s−2 and 200 rad s−2. SCM, sternocleidomastoid; PARA, cervical paraspinal muscles; l, left; r, right; a, linear acceleration; α, angular acceleration; x, the x-direction; y, the y-direction;
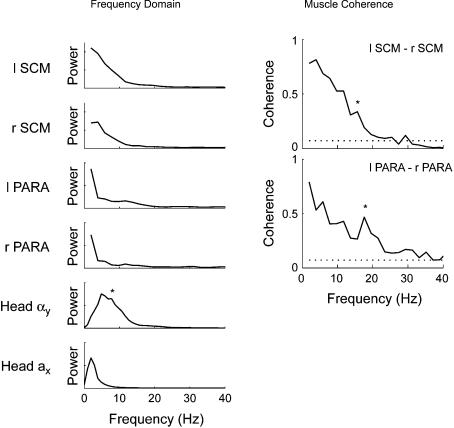
Exposure to a single 4 km h−1 perturbation (experiment 1) elicited significant bilaterally synchronous activity in the SCM and PARA muscles between 4 and 20 Hz (Fig. 3). A small increase in the region of significant coherence (indicated by the asterisk) centred around 15.6 Hz and 17.6 Hz for the SCM (R2= 0.34) and PARA (R2= 0.47), respectively, was associated with a relative increase in the power of the muscles' autospectra around the same frequency (Fig. 3, left panel).
Figure 3. Power and coherence of muscle and kinematic data recorded during a single 4 km h−1 rear-end collision (experiment 1).
Left panel, average power spectra for the left and right SCM muscles, left and right PARA muscles, head forward acceleration (Head ax) and head angular acceleration (Head αy). * denotes the secondary peak in the power of the head angular acceleration signal between 5 and 10 Hz. Right panel, average coherence between the left and right SCM and PARA muscles. The dotted lines represents the 95% confidence limit for the coherence estimates. Note the significant peaks in coherence at 15.6 Hz (SCM) and 17.6 Hz (PARA) depicted by * and the power associated with these increases in the averaged power spectra (left panel).
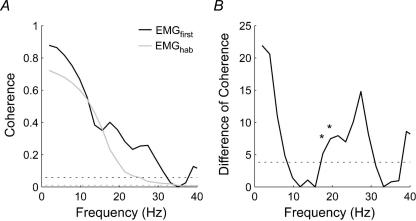
In experiment 2, the first perturbation also induced bilaterally synchronous activity in the SCM muscles between 4 and 30 Hz with a distinct secondary peak in coherence centred around 17.6 Hz (Fig. 4). This secondary peak in coherence was not present in the habituated data (trials 7–11). The DoC test confirmed a significantly lower coherence at 17.6 Hz (coherence decreased from 0.40 to 0.23, P = 0.02) and 19.5 Hz (coherence decreased from 0.35 to 0.16, P = 0.006).
Figure 4. Coherence between the left and right SCM muscles recorded during exposure to 1.8 km h−1 forward perturbations (experiment 2).
A, average coherence between the left and right SCM muscles for the first perturbation (EMGfirst; black line) and the habituated trials (EMGhab; grey line). The two dotted lines represents the 95% confidence limit for the coherence estimates (black line: EMGfirst; gray line: EMGhab). B, distribution of the difference of coherence test between the first and habituated trials for all frequencies. The dotted line indicates the P = 0.05 threshold for a χ2 distribution with 1 degree of freedom. Note the significant difference (depicted by *) in coherence at ∼17 Hz and ∼19 Hz associated with a secondary peak in coherence present in the first trial but absent in the habituated trials.
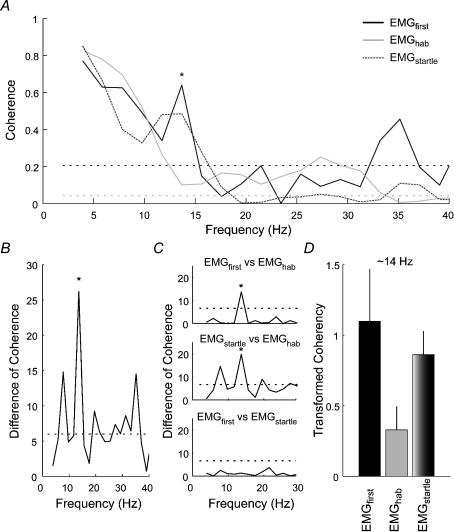
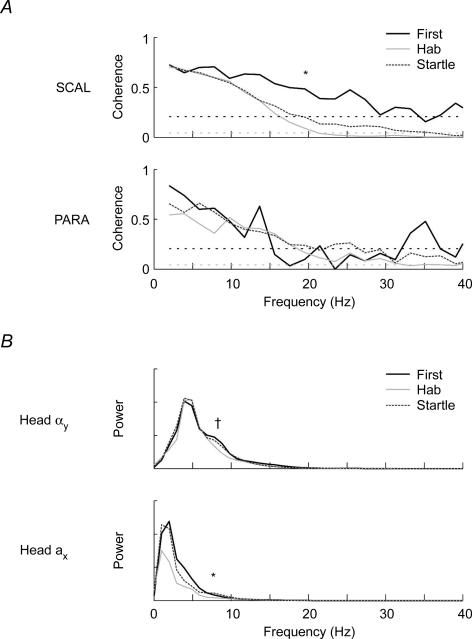
The data from experiment 3 confirmed and extended the results of experiments 1 and 2. For the first exposure to a whiplash-like perturbation, significant bilaterally synchronous activity was again observed in all three muscles between 4 and 20 Hz (Figs 5 and 6) with a secondary peak in coherence between 10 and 20 Hz. For the SCM muscles, an omnibus DoC test at 13.7 Hz revealed a significant difference between the coherence observed in the first trial (EMGfirst), habituated trials (EMGhab) and perturbation + auditory tone (EMGstartle) conditions (P < 0.001). Post hoc comparisons revealed that habituation to the forward perturbation reduced this secondary peak in coherence (from 0.64 to 0.10; P < 0.001). The addition of an acoustic startle to the perturbation increased the coherence from 0.10 to 0.49 (P < 0.001), and this combined response was not different from that of the first perturbation (P > 0.05). For the SCAL muscles, the omnibus DoC revealed a significant difference between the coherence observed at 19.5 Hz for the three conditions (EMGfirst, EMGhab and EMGstartle; P = 0.002). Decomposition of the omnibus DoC showed a significant decrease in coherence with habituation (from 0.48 to 0.09; P = 0.008), but a non-significant increase with the superposition of the loud auditory tone (from 0.09 to 0.20; P = 0.14). Although the PARA muscles exhibited significant coherence for the first trial, no significant changes in coherence magnitude were observed following habituation to the perturbation or superposition of the loud acoustic tone over the perturbation.
Figure 5. Coherence between the left and right SCM muscles recorded during exposure to 1.8 km h−1 forward perturbations (experiment 3).
A, estimated averaged coherence between the left and right SCM muscles for the first perturbation (EMGfirst; black line), habituated perturbations (EMGhab; grey line) and combined perturbation and acoustic startle (EMGstartle; dotted dark grey line). The horizontal dotted lines represents the 95% confidence limit for the coherence estimates (black line: EMGfirst; grey line: EMGhab and EMGstartle). B, distribution of the omnibus DoC test between the EMGfirst; EMGhab and EMGstartle conditions for all frequencies. The dotted line indicates the P = 0.05 threshold for a χ2 distribution with 2 degrees of freedom. C, distribution of the pair-wise post hoc DoC tests for the frequencies between 0 and 30 Hz. The dotted line indicates the P = 0.01 threshold for a χ2 distribution with 1 degree of freedom. D, bar graphs showing the transformed coherency (tanh−1) at the ∼14 Hz frequency for the various conditions. Note the significant difference (depicted by * in panels A, B and C) in coherence at ∼14 Hz associated with a peak in coherence for the EMGfirst and EMGstartle conditions and reduction in coherence for the habituated postural responses.
Figure 6. Coherence for the SCAL and PARA muscles and power for the kinematic data recorded during 1.8 km h−1 forward perturbations (experiment 3).
A, estimated averaged coherence between the left and right SCAL and PARA muscles for the first perturbation (black line), habituated perturbations (grey line) and combined perturbation and acoustic startle (dotted dark grey line). The horizontal dotted lines represents the 95% confidence limit for the coherence estimates (black line: EMGfirst; grey line: EMGhab and EMGstartle). Note the significant coherence levels between 10 and 20 Hz for the first trial in both muscles. * denotes the significant difference in coherence observed in the SCAL muscles between the first postural response and the habituated postural responses at 19.5 Hz. B, estimated averaged power spectra for the head angular acceleration (Head αy) and head forward acceleration (Head ax) for the first perturbation (black line), habituated perturbations (grey line) and combined perturbation and acoustic startle (dotted dark grey line). † and *, respectively, denote the differences in the 85 percentile frequency (head angular acceleration) and power (head linear acceleration) observed between conditions.
Spectral analysis of the head acceleration signals revealed that most of the power was below 20 Hz (Fig. 3). In experiment 1, the power of the head angular acceleration signal peaked at ∼5 Hz with a secondary peak between 5 and 10 Hz. The power of the head linear acceleration peaked at ∼2 Hz with no secondary peak. For the first trial of experiment 3, the frequency content of the angular and linear acceleration signals showed similar features to that described for experiment 1 (Fig. 6). The secondary peak in the power of the head angular acceleration signal appeared to decrease following repeated exposures to the same perturbation, though this reduction was not significant. The 85th percentile frequency of the power spectral density of the head angular acceleration decreased with repeated exposures to the perturbation (from 10.0 to 8.5 Hz; P = 0.03), but did not increase significantly with the subsequent addition of the startle. On the other hand, the power of the head linear acceleration signal between 5 and 10 Hz decreased with repeated exposures to the perturbation (from 4.8 to 4.0; P = 0.02) and increased with the superposition of an acoustic startle to the perturbation (from 4.0 to 4.8; P = 0.02) to a level that was not different from the first perturbation (P > 0.05). No significant changes were observed in the 85th percentile frequency for the head linear acceleration.
Discussion
This study revealed increased synchrony between the left and right neck muscles in the 10–20 Hz bandwidth during a subject's first exposure to a real or simulated rear-end impact accompanied by a secondary peak between 5 and 10 Hz in the power spectrum of the head angular acceleration signal. This localized increase in synchrony was observed in three independent groups of subjects using two different perturbation protocols, and appears similar to the synchronous activity previously observed in the upper limb muscles during acoustic startle (Grosse & Brown, 2003). Grosse & Brown (2003) attributed their increased synchrony to increased reticulospinal activity induced by startle. We cannot use their findings, however, to conclude that our observed increase in reticulospinal drive is necessarily due to startle because reticulospinal drive is also important in the control and regulation of posture (Drew et al. 1986; Mori et al. 1995; Schepens & Drew, 2004). Neck muscles in particular have many connections in the reticular formation (Wilson & Peterson, 1988) and the latency of the neck postural responses suggest polysynaptic (and possibly reticular) connections rather than monosynaptic spinal connections (Abrahams & Rose, 1975; Forssberg & Hirschfield, 1994; Vibert et al. 2001; Blouin et al. 2003; Siegmund et al. 2003). Thus the postural responses-rather than a startle response-during the first exposure could be responsible for the increased bilateral synchrony observed between 10 and 20 Hz.
The second and third parts of experiments 2 and 3 provide the data needed to distinguish between these two possible explanations for this bilateral synchrony. First, the increased synchrony between 10 and 20 Hz seen in the first trial decreased significantly in the habituated trials of both latter experiments. Hence, reticulospinal control of the head and neck during postural corrections was not responsible for the local peak in synchronized EMG activity between 10 and 20 Hz. Second, the local peak in synchronized EMG activity reappeared in habituated subjects who were simultaneously exposed to a loud acoustic stimulus. Thus, the local peak in coherence between 10 and 20 Hz is related to startle and its presence in the first-trial data suggests that a startle response forms part of an individual's neuromuscular response to a novel or unexpected perturbation.
The altered muscle response observed in the perturbation + auditory tone trials of experiment 3 could potentially be related to an auditory-evoked saccular reflex. Although vestibular-evoked myogenic potentials have been evoked by 0.1–2 ms, 140 dB (SPL) stimuli, or 7 ms, 120 dB tone bursts (Colebatch & Halmagyi, 1992; Colebatch et al. 1998; Cheng & Murofushi, 2001; Colebatch, 2001; Welgampola & Colebatch, 2001, 2005), we believe the saccular reflex played either a minor role or no role for three reasons. First, Welgampola & Colebatch (2001) and Cheng & Murofushi (2001) observed that tone bursts longer than 10 ms yielded attenuation of the saccular reflex presumably due to the stapedial reflex. Here, a 40 ms auditory tone burst was used to evoke a generalized startle response in the subjects. Second, the click-evoked saccular reflex consists of an initial positive or negative response peaking at 13 ms after the acoustic stimulus followed by a second response of the opposite polarity peaking at 23 ms. Subjects submitted to forward linear accelerations in combination with a loud acoustic stimulus showed onset times of 69 ± 6 ms for the SCM and 71 ± 9 ms for the PARA muscles, and no sign of EMG activity could be detected on individual or averaged traces around the 13–23 ms interval. Finally, background activity in the muscle of interest is required to evoke the auditory saccular reflex in the neck muscles. Here, subjects were asked to sit comfortably in the car seat and relax their neck muscles prior to the perturbation (see the absence of preimpact muscle activity in Fig. 2). Based on these reasons, we believe that the neck muscle responses observed in perturbation + auditory tone trials of experiment 3 are better explained by a startle response than a saccular reflex.
Startle responses can be triggered by tactile, vestibular and auditory stimuli and yield a protective response by stiffening the neck and trunk muscles (Yeomans et al. 2002). Since all three sensory pathways are probably stimulated in a car collision, it appears that any or all of these sensory modalities could trigger a startle response during a collision and could affect the genesis of whiplash injuries – particularly at the low severity levels responsible for some whiplash injuries (Jakobsson et al. 2000). The presence of a startle response induced by these low intensity collisions may play a significant role in producing whiplash injuries by generating injurious strains in the muscles. In addition, a startle-induced contraction of the deep multifidus muscles could potentially increase capsular ligament strain and exacerbate whiplash injury potential due to the insertion of these muscles onto the cervical facet capsular ligaments (Siegmund et al. 2001; Winkelstein et al. 2001; Anderson et al. 2005). This latter mechanism of injury may help explain why the cervical facet joints have been implicated as the source of pain in about half of chronic whiplash patients (Lord et al. 1996).
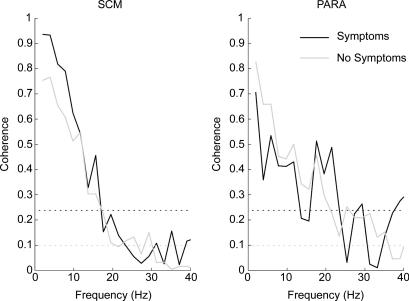
Individuals in experiment 1 were subjected to a real rear-end collision causing a speed change of 4 km h−1. In the days following the test, 29% of these subjects reported transient Grade I whiplash-associated-disorder symptoms, with cervical symptoms and headaches predominating (Brault et al. 1998). This allowed us to compare the neck muscle coherence levels between symptomatic and asymptomatic individuals to assess whether startled individuals are more likely than non-startled individuals to develop symptoms following a car crash. When we compared the coherence levels between subjects with and without symptoms, it appeared that subjects with symptoms had larger (not significant) coherence levels in the 10–20 Hz bandwidth than subjects without symptoms (Fig. 7). The non-significant difference may be a consequence of the low number of symptomatic subjects (n = 12), but this line of inquiry may be interesting to pursue in the future. The presence of symptoms was not recorded in volunteers exposed to the lower acceleration pulses used in experiments 2 and 3.
Figure 7. Comparison of the coherence levels observed in the neck muscles of symptomatic (M =12) and asymptomatic (M = 30) subjects following the single 4 km h−1 forward perturbation (experiment 1).
The increase in coherence seen in the SCM (∼15 Hz) and PARA (∼20 Hz) muscles for subjects reporting symptoms following the single 4 km h−1 collision did not reach significance.
Coherence analyses require EMG recordings without electrical cross-talk. Although the presence of cross-talk contamination between our muscle recordings cannot be ruled out, we believe the effects of cross-talk was minimal and could not explain the observed results due to various reasons. First, the coherence spectra show well-defined peaks in coherence whereas coherence across the whole spectrum would be expected if cross-talk was present (Hansen et al. 2005). Second, the various conditions (EMGfirst, EMGhab and EMGstartle) yielded specific changes in the coherence spectral estimates that cannot be attributed to cross-talk. Finally, Hansen et al. (2005) reported that cross-talk was minimal between EMG recordings performed in the tibialis anterior when the pairs of recording electrodes were separated by at least 10 cm, which is about the distance between the two pairs of electrodes used to record bilaterally from the SCM and SCAL muscles, though not the PARA muscles.
Another limitation of the techniques used here is the possible bias in the confidence levels introduced by a few individuals with very high coherence levels. To determine whether our confidence intervals were biased by a few subjects, we re-analysed the SCM data using smaller time segments. Data from experiment 2 were divided into eight smaller time segments whereas data for experiment 3 were divided into seven smaller time segments. These re-analyses using the averaged transformed coherency levels between 10 and 20 Hz for both experiments 2 and 3 confirmed the prior results of the DoC tests: habituation decreased transformed coherency levels between 10 and 20 Hz (experiment 2, P = 0.010) and superimposing a loud sound over the forward perturbation increased this value (experiment 3, P = 0.003). Hence, the results of the DoC tests were not sensitive to changes in the statistical method used to explore differences between conditions.
The 1.5–2.1 g acceleration pulses used in the current studies are at the lower end of those reported to cause whiplash injuries (Jakobsson et al. 2000), but are more severe than the 0.5–1.4 g pulses used by other researchers (Magnusson et al. 1999; Kumar et al. 2000, 2002; Vibert et al. 2001). More importantly, our early peak accelerations (16 ms for the sled pulses) produce pulses with onsets that replicate more severe rear-end collisions (see Fig. 2) than the late peaks (> 100 ms (Kumar et al. 2000)) used by other researchers, and thus are more relevant to the study of whiplash injury.
In summary, the results of this experiment show that the postural response to a single, unexpected rear-end impact contains a startle response – a finding that suggests startle could be part of the mechanism responsible for whiplash injuries. Previous work using traditional data analyses led some authors to hypothesize the possible involvement of a startle response in the first exposure to a perturbation (Allum et al. 1992; Bisdorff et al. 1994; Blouin et al. 2003; Siegmund et al. 2003). The spectral analyses, however, provided additional insight and allowed us to test this hypothesis using a physiological variable known to be a surrogate marker of reticulospinal activity (Grosse & Brown, 2003). Further work is needed to determine whether startled individuals are more likely than non-startled individuals to develop symptoms following a rear-end collision.
Acknowledgments
This work was funded by the Natural Sciences and Engineering Research Council of Canada (J.T.I.), BC Neurotrauma Grant (J.T.I., G.P.S., J.S.B.), Canadian Institutes of Health Research (J.S.B.), Michael Smith Foundation for Health Research and BC Workers Compensation Board (J.S.B.), and MEA Forensic Engineers & Scientists (G.P.S.). We thank Mr Jeff Nickel and Mr Mircea Oala-Florescu for their technical help with the various experiments and Mr John Brault for collecting the electromyographic data in experiment one.
References
- Abrahams VC, Rose PK. Projections of extraocular, neck muscle, and retinal afferents to superior colliculus in the cat: their connections to cells of origin of tectospinal tract. J Neurophysiol. 1975;38:10–18. doi: 10.1152/jn.1975.38.1.10. [DOI] [PubMed] [Google Scholar]
- Allum JH, Honegger F, Keshner EA. Head-trunk coordination in man: Is trunk angular velocity elicited by a support surface movement the only factor influencing head stabilization? In: Berthoz A, Graf W, Vidal PP, editors. The Head-Neck Sensory Motor System. New York: Oxford University Press; 1992. pp. 571–575. [Google Scholar]
- Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Meth. 1997;73:69–79. doi: 10.1016/s0165-0270(96)02214-5. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Hsu AW, Vasavada AN. Morphology, architecture, and biomechanics of human cervical multifidus. Spine. 2005;30:E86–E91. doi: 10.1097/01.brs.0000153700.97830.02. [DOI] [PubMed] [Google Scholar]
- Bisdorff AR, Bronstein AM, Gresty MA. Responses in neck and facial muscles to sudden free fall and a startling auditory stimulus. Electroencephalogr Clin Neurophysiol. 1994;93:409–416. doi: 10.1016/0168-5597(94)90146-5. [DOI] [PubMed] [Google Scholar]
- Blouin JS, Descarreaux M, Belanger-Gravel A, Simoneau M, Teasdale N. Attenuation of human neck muscle activity following repeated imposed trunk-forward linear acceleration. Exp Brain Res. 2003;150:458–464. doi: 10.1007/s00221-003-1466-9. [DOI] [PubMed] [Google Scholar]
- Blouin JS, Inglis JT, Siegmund GP. Auditory startle alters the response of human subjects exposed to a single whiplash-like perturbation. Spine. 2006;31:146–154. doi: 10.1097/01.brs.0000195157.75056.df. [DOI] [PubMed] [Google Scholar]
- Brault JR, Siegmund GP, Wheeler JB. Cervical muscle response during whiplash: evidence of a lengthening muscle contraction. Clin Biomech. 2000;15:426–435. doi: 10.1016/s0268-0033(99)00097-2. [DOI] [PubMed] [Google Scholar]
- Brault JR, Wheeler JB, Siegmund GP, Brault EJ. Clinical response of human subjects to rear-end automobile collisions. Arch Phys Med Rehabil. 1998;79:72–80. doi: 10.1016/s0003-9993(98)90212-x. [DOI] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain. 1991;114:1891–1902. doi: 10.1093/brain/114.4.1891. [DOI] [PubMed] [Google Scholar]
- Cheng PW, Murofushi T. The effects of plateau time on vestibular-evoked myogenic potentials triggered by tone bursts. Acta Otolaryngol. 2001;121:935–938. [PubMed] [Google Scholar]
- Colebatch JG. Vestibular evoked potentials. Curr Opin Neurol. 2001;14:21–26. doi: 10.1097/00019052-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Day BL, Bronstein AM, Davies RA, Gresty MA, Luxon LM, Rothwell JC. Vestibular hypersensitivity to clicks is characteristic of the Tullio phenomenon. J Neurol Neurosurg Psychiatry. 1998;65:670–678. doi: 10.1136/jnnp.65.5.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch JG, Halmagyi GM. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992;42:1635–1636. doi: 10.1212/wnl.42.8.1635. [DOI] [PubMed] [Google Scholar]
- Drew T, Dubuc R, Rossignol S. Discharge patterns of reticulospinal and other reticular neurons in chronic, unrestrained cats walking on a treadmill. J Neurophysiol. 1986;55:375–401. doi: 10.1152/jn.1986.55.2.375. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Hirschfield H. Postural adjustements in sitting humans following external perturbations: muscle activity and kinematics. Exp Brain Res. 1994;97:515–527. doi: 10.1007/BF00241545. [DOI] [PubMed] [Google Scholar]
- Grosse P, Brown P. Acoustic startle evokes bilaterally synchronous oscillatory EMG activity in the healthy human. J Neurophysiol. 2003;90:1654–1661. doi: 10.1152/jn.00125.2003. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241:5–8. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR. On the application, estimation and interpretation of coherence and pooled coherence. J Neurosci Meth. 2000;100:173–174. doi: 10.1016/s0165-0270(00)00267-3. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data – theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hansen NL, Conway BA, Halliday DM, Hansen S, Pyndt HS, Biering-Sorensen F, Nielsen JB. Reduction of common synaptic drive to ankle dorsiflexor motoneurons during walking in patients with spinal cord lesion. J Neurophysiol. 2005;94:934–942. doi: 10.1152/jn.00082.2005. [DOI] [PubMed] [Google Scholar]
- Jakobsson L, Lundell B, Norin H, Isaksson-Hellman I. WHIPS – Volvo's whiplash protection study. Accid Anal Prev. 2000;32:307–319. doi: 10.1016/s0001-4575(99)00107-4. [DOI] [PubMed] [Google Scholar]
- Kumar S, Narayan Y, Amell T. Role of awareness in head-neck acceleration in low velocity rear-end impacts. Accid Anal Prev. 2000;32:233–241. doi: 10.1016/s0001-4575(99)00114-1. [DOI] [PubMed] [Google Scholar]
- Kumar S, Narayan Y, Amell T. An electromyographic study of low-velocity rear-end impacts. Spine. 2002;27:1044–1055. doi: 10.1097/00007632-200205150-00009. [DOI] [PubMed] [Google Scholar]
- Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine. 1996;21:1737–1744. doi: 10.1097/00007632-199608010-00005. discussion 1744–1745. [DOI] [PubMed] [Google Scholar]
- Magnusson ML, Pope MH, Hasselquist L, Bolte KM, Ross M, Goel VK, Lee JS, Spratt K, Clark CR, Wilder DG. Cervical electromyographic activity during low-speed rear impact. Eur Spine J. 1999;8:118–125. doi: 10.1007/s005860050140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Iwakiri H, Homma Y, Yokoyama T, Matsuyama K. Neuroanatomical and neurophysiological bases of postural control. Adv Neurol. 1995;67:289–303. [PubMed] [Google Scholar]
- NASA. Hanover, MD, USA: 1978. Anthropometric Source Book, NASA Reference Publication 1024, vol. 1. National Aeronautics and Space Administration, Scientific and Technical Information Office. [Google Scholar]
- Padgaokar AJ, Krieger KW, King AI. Measurement of angular acceleration of a rigid body using linear accelerometers. Transactions of the American Society of Mechanical Engineers. 1975;75-APMB-3:522–526. [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol. 1989;53:1–31. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol. 2004;92:2217–2238. doi: 10.1152/jn.01189.2003. [DOI] [PubMed] [Google Scholar]
- Siegmund GP, King DJ, Lawrence JM, Wheeler JB, Brault JR, Smith TA. Head/neck kinematic response of human subjects in low-speed rear-end collisions (973341) Proceedings 41st Stapp Car Crash Conf. 1997;41:357–385. [Google Scholar]
- Siegmund GP, Myers BS, Davis MB, Bohnet HF, Winkelstein BA. Mechanical evidence of cervical facet capsule injury during whiplash: a cadaveric study using combined shear, compression, and extension loading. Spine. 2001;26:2095–2101. doi: 10.1097/00007632-200110010-00010. [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Sanderson DJ, Myers BS, Inglis JT. Rapid neck muscle adaptation alters the head kinematics of aware and unaware subjects undergoing multiple whiplash-like perturbations. J Biomech. 2003;36:473–482. doi: 10.1016/s0021-9290(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S, Zeiss E. Scientific monograph of the Quebec Task Force on Whiplash-Associated Disorders: redefining ‘whiplash’ and its management. Spine. 1995;20:1S–73S. [PubMed] [Google Scholar]
- Sturzenegger M, DiStefano G, Radanov BP, Schnidrig A. Presenting symptoms and signs after whiplash injury: the influence of accident mechanisms. Neurology. 1994;44:688–693. doi: 10.1212/wnl.44.4.688. [DOI] [PubMed] [Google Scholar]
- Vibert N, MacDougall HG, de Waele C, Gilchrist DP, Burgess AM, Sidis A, Migliaccio A, Curthoys IS, Vidal PP. Variability in the control of head movements in seated humans: a link with whiplash injuries? J Physiol. 2001;532:851–868. [Google Scholar]
- Welgampola MS, Colebatch JG. Characteristics of tone burst-evoked myogenic potentials in the sternocleidomastoid muscles. Otol Neurotol. 2001;22:796–802. doi: 10.1097/00129492-200111000-00014. [DOI] [PubMed] [Google Scholar]
- Welgampola MS, Colebatch JG. Characteristics and clinical applications of vestibular-evoked myogenic potentials. Neurology. 2005;64:1682–1688. doi: 10.1212/01.WNL.0000161876.20552.AA. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Peterson BW. Vestibular and reticular projections to the neck. In: Peterson BW, Richmond FJ, editors. Control of Head Movement. New York: Oxford University; 1988. pp. 129–140. [Google Scholar]
- Winkelstein BA, McLendon RE, Barbir A, Myers BS. An anatomical investigation of the human cervical facet capsule, quantifying muscle insertion area. J Anat. 2001;198:455–461. doi: 10.1046/j.1469-7580.2001.19840455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev. 2002;26:1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]