Abstract
The purpose of this study was to describe the frequency–current (f–I) relationships of hindlimb α-motoneurones (MNs) in both anaesthetized and decerebrate rats in situ. Sprague–Dawley rats (250–350 g) were anaesthetized with ketamine and xylazine (KX) or subjected to a precollicular decerebration prior to recording electrophysiological properties from sciatic nerve MNs. Motoneurones from KX-anaesthetized rats had a significantly (P < 0.01) hyperpolarized resting membrane potential and voltage threshold (Vth), increased rheobase current, and a trend (P = 0.06) for a smaller after-hyperpolarization (AHP) amplitude compared to MNs from decerebrate rats. In response to 5 s ramp current injections, MNs could be categorized into four f–I relationship types: (1) linear; (2) adapting; (3) linear + sustained; and (4) late acceleration. Types 3 and 4 demonstrated self-sustained firing owing to activation of persistent inward current (PIC). We estimated the PIC amplitude by subtracting the current at spike derecruitment from the current at spike recruitment. Neither estimated PIC nor f–I slopes differed between fast and slow MNs (slow MNs exhibited AHP half-decay times > 20 ms) or between MNs from KX-anaesthetized and decerebrate rats. Motoneurones from KX-anaesthetized rats had significantly (P < 0.02) hyperpolarized ramp Vth values and smaller and shorter AHP amplitudes and decay times compared to MNs from decerebrate rats. Pentobarbitone decreased the estimated PIC amplitude and almost converted the f–I relationship from type 3 to type 1. In summary, MNs of animals subjected to KX anaesthesia required more current for spike initiation and rhythmic discharge but retained large PICs and self-sustained firing. The KX-anaesthestized preparation enables direct recording of PICs in MNs from intact animals.
The frequency–current (f–I) relationships of motoneurones (MNs) demonstrate several nonlinearities, which reflect their active properties in response to suprathreshold current injections. The f–I relationship derived from short duration square-wave intracellular current injection can be described by several ranges: (1) primary; (2) secondary; and (3) tertiary (Kernell, 1965b,c; Schwindt, 1973). Longer duration current pulses are accompanied by a decrease in MN firing frequency (FF) known as spike-frequency adaptation, which can be described in three stages: (1) initial; (2) early; and (3) late (Granit et al. 1963; Kernell, 1965a,b; Kernell & Monster, 1981; Sawczuk et al. 1995; Powers et al. 1999; Powers & Binder, 2001; Miles et al. 2005). More recently, square-wave and triangular current pulses have been used to determine MN f–I relationships and the effect of persistent inward currents (PICs) on these relationships (Hounsgaard et al. 1988; Lee & Heckman, 1998a; Bennett et al. 2001).
Persistent inward currents in MNs are depolarizing currents generated by non-inactivating or slowly inactivating voltage-gated Na+ and Ca2+ channels (Lee & Heckman, 1999; Li & Bennett, 2003), when the membrane potential is depolarized above their activation threshold. These currents can mediate a plateau potential that in turn allows the MN to express self-sustained rhythmic firing. A plateau potential can be initiated by a short depolarizing current pulse and remain for a long period of time before being terminated either spontaneously or by brief hyperpolarizing current (Kiehn & Eken, 1998). This property allows a MN to fire for a long period of time. Thus, a MN can behave in a ‘bistable’ manner, in that it can be toggled between an active and a quiescent state. Furthermore, a MN PIC can be amplified by a host of neurotransmitters and neuromodulators (Heckman et al. 2004). Neuromodulatory input to a MN via axons originating in the ralphe nucleus and locus coeruleus of the brainstem releases the monoamines serotonin (5-HT; Hounsgaard et al. 1988; Skydsgaard & Hounsgaard, 1996) and noradrenaline (NA; Lee & Heckman, 2000), which can enhance the PIC and render the motoneurone more excitable (Gilmore & Fedirchuk, 2004).
A plateau potential and its underlying PIC that conduct through voltage-gated ion channels have been examined in both in vivo and in vitro animal models through the use of voltage-clamp (Schwindt & Crill, 1977, 1981, 1982; Bennett et al. 2001) and current-clamp techniques (Hounsgaard et al. 1988; Lee & Heckman, 1998a; Bennett et al. 2001) with a concurrent addition of specific voltage-gated ion channel agonists, antagonists and neuromodulators. The current-clamp technique, in the form of a triangular current pulse (referred to as ramp current hereafter), has been used as a tool to determine f–I relationships and to estimate the associated PIC (ePIC) of hindlimb α-MNs in cats in vivo (Hounsgaard et al. 1988; Lee & Heckman, 1998a) and rat tail MNs in vitro (Bennett et al. 2001). However, this technique has not been used to determine the ePIC or describe f–I relationships in rat hindlimb α-MNs in situ. The first purpose of this paper is to determine whether hindlimb α-MNs of our in situ rat preparation demonstrate ePICs and f–I relationship patterns that are comparable with those reported by Bennett et al. (2001).
In previous studies, the ePIC was defined by subtracting the current at which MN rhythmic firing spike derecruitment occurs from the current at which MN rhythmic firing spike recruitment occurs. The plateau potential or underlying PIC appears as a steep rise in firing frequency in the f–I relationship. In other words, a MN firing pattern elicited by a ramp current illustrates the plateau current as an counter-clockwise f–I hysteresis (Hultborn, 1999). Furthermore, it has been shown that bistable MNs have lower rheobase currents, suggesting that smaller MNs are influenced by PIC in a different manner from bigger MNs (Lee & Heckman, 1998a). Although this technique has been described as a meaningful way to estimate the size of the PIC and to illustrate MN f–I relationships as well as the PIC relationship to passive properties, other important information remains to be uncovered. Some further questions include the following. (1) Does the ePIC correlate with other passive MN properties? (2) Does MN ePIC change owing to the history (e.g. blood pressure, time of day, CO2 levels) of the experimental preparation? (3) Will the ePIC depend on the duration of the glass microelectrode impalement of the MN? (4) Do voltage threshold (Vth), after-hyperpolarization (AHP) amplitude and AHP ¾ decay time change from spike to spike throughout the ramp? Thus, the second purpose of this paper is to attempt to answer this series of questions pertaining to ePIC.
It has been suggested (Hultborn & Kiehn, 1992; Hultborn, 1999) that, owing to the masking effects of anaesthetics, PICs were not evident in previous experiments in which animals were anaesthetized with barbiturates. More recently, it has been shown that in decerebrate animals compared to animals deeply anaesthetized with pentobarbitone, current generated by stimulation of muscle spindle Ia afferents was amplified four times by active dendritic currents (Lee & Heckman, 2000). Furthermore, when pentobarbitone was added to an in vitro turtle spinal cord slice preparation, plateau potentials were no longer seen in MNs (Guertin & Hounsgaard, 1999). We wished to determine how the addition of pentobarbitone to an in situ rat preparation would affect the MN ePIC and the f–I relationship.
Finally, we anaesthetized the rat with a ketamine–xylazine (KX) mixture. Xylazine is an α2-adrenoceptor agonist, which should not influence the amplitude or activation of PIC. In contrast, ketamine is an N-methyl-d-aspartate (NMDA) receptor antagonist (Liu et al. 2001). These receptors are present in adult turtle MNs (Guertin & Hounsgaard, 1998) and neonatal rat MNs (Hochman et al. 1994; Palecek et al. 1999; Hsiao et al. 2002). Hochman et al. (1994) demonstrated that NMDA-induced rhythmic membrane voltage oscillations revealed the presence of bistable membrane properties. Little is known about the role of NMDA receptors in MNs of adult mammalian preparations, however, and it has been shown (see above) that, in adult mammalian preparations, PICs conduct through Na+ and Ca2+ channels. Thus, the final purpose of the present paper is to determine whether KX anaesthesia influences rat hindlimb α-MN ePIC and f–I relationship. A portion of these results has been presented elsewhere in abstract form (Button et al. 2005a,b).
Methods
Treatment of animals
Female Sprague–Dawley rats weighing 275–325 g were obtained from the University of Manitoba (Winnipeg, Manitoba, Canada), and initially housed in groups of two in plastic cages situated in an environmentally controlled room maintained at 23°C and kept on a 12 h–12 h light–dark cycle. The rats were provided with water and food ad libitum throughout the experiment. Rats (n = 20) underwent experimental procedures within 7 days of receipt. All procedures were approved by the animal ethics committee of the University of Manitoba and were in accordance with the guidelines of the Canadian Council of Animal Care.
Surgery
For these terminal experiments, rats were taken from their cages and anaesthetized with ketamine and xylazine (80 and 10 mg kg−1, respectively, i.p.). Each rat also received an intraperitoneal injection (volume 6.6 ml kg−1) of saline containing 5% dextrose and 0.05 mg kg−1 atropine. Briefly, the anaesthetized rat was surgically prepared for electrophysiological recording via impalement of spinal motoneurones following: (1) a tracheotomy; (2) catherization of the femoral artery; (3) an incision to allow stimulation of the sciatic nerve of the left hindlimb; (4) exposure, removal and cleaning of the spinal vertebrae in preparation for the laminectomy; and (5) a laminectomy from T12 to S1. Following the tracheotomy, the rat was ventilated (Harvard Apparatus, Canada) with pure oxygen-enriched room air, at a tidal volume of approximately 2 ml, and a ventilation rate of approximately 60–80 strokes min−1. Expired carbon dioxide levels were measured via a CAPSTAR 100 CO2 analyser (CWE Inc. Ardmore, PA, USA) and were maintained between 3.0 and 4.0%. Mean arterial pressure (MAP; Pressure Monitor BP-1; World Precision Instruments, Sarasota, FL, USA) was also measured and maintained between 80 and 110 mmHg. Anaesthesia was maintained by constant infusion of a physiological saline solution containing ketamine and xylazine (9 and 1 mg h−1, respectively) via the femoral artery catheter (Pump 11, Harvard Apparatus). The depth of anaesthesia was verified frequently via MAP and CO2 levels and toe pinch and eye blink reflexes.
Following exposure and cleaning of the spinal vertebrae, the rat was transferred to a stereotaxic unit in preparation for the laminectomy. Rectal temperature was monitored and maintained near 37°C using a Homeothermic Blanket Control Unit (Harvard Apparatus). The head, thoracic and lumbar vertebrae, hips and left foot were immobilized with clamps, and the open leg and back incisions were filled with mineral oil. The dura mater covering the spinal cord was incised, and the large dorsal roots comprising afferents from the left hindlimb were cut and reflected over the right side of the cord. An opening was made in the pia mater just lateral to the entry zone of these roots into the cord, in preparation for introduction of the glass microelectrode. Immediately before beginning the search for motoneurones, a pneumothorax was performed by making a 5 mm incision between ribs T5 and T6 on the left side of the thorax.
In another series of experiments, rats were initially anaesthetized with isofluorane and the surgical procedures stated above were performed. In addition to these procedures, the carotid arteries were separated from vagal, aortic and sympathetic nerves and ligated. The carotid arteries were then cannulated towards the head with short saline-filled tubing. The other end of the tubing was connected to a 20 gauge syringe needle used to inject 0.06–0.07 ml of mixed polyvinylsiloxane (PVS; extrude from Kerr or Reprosil from Dentsply Caulk, medium and heavy body) into each carotid artery via a 1 ml syringe. The PVS entered into the external and internal carotid arteries, filling all arteries that these vessels supplied. Once the vessels were filled, a precollicular decerebration was performed (for details of the decerebration procedure see Fouad & Bennett, 1998). Upon the decerebration, anaesthesia was terminated and the rat was allowed to stabilize for 1 h before electrophysiological recordings began.
Drugs and solutions
To reduce blood pressure and respiration-related movement artifacts and to stabilize the rat for proper electrophysiological recordings, several solutions were injected intravenously, as follows: (1) a solution of 100 mm of NaHCO3 (Fisher scientific) and 5% dextrose (Fisher Scientific) dissolved in 25 ml of distilled deionized H2O; (2) 300 mosmol l−1 of Ficoll 70 (Sigma) dissolved in normal saline, which was used as a plasma expander; and (3) pancuronium bromide (0.2 mg kg−1), as a muscle relaxant. The pancuronium bromide was injected intravenously immediately before the start of electrophysiological recordings and was re-administered upon the reappearance of muscle contraction in response to sciatic nerve stimulation. Upon the addition of pancuronium bromide, depth of anaesthesia was monitored by MAP. In one experiment, sodium pentobarbitone (50 mg kg−1) was administered intravenously to determine the effect, if any, on the PIC (Guertin & Hounsgaard, 1999; Lee & Heckman, 2000).
Measurement of motoneurone properties
Thin-walled glass microelectodes (1.0 mm, World Precision Instruments, Sarasota, FL, USA) were pulled (Kopf Vertical Pipette Puller, David Kopf Instruments, Tujurga, CA, USA), and filled with 2 m potassium citrate. Electrode impedances were approximately 10 MΩ. The tip of the electrode was positioned at a hole in the pia mater and was lowered with an inchworm microdrive system (Burleigh Instruments Inc., NY, USA) into the cord in steps of 10 μm. The sciatic nerve was stimulated with a bipolar silver electrode at a frequency of 1 s−1, while the microelectrode was advanced through the cord and the field potential was continuously monitored. In some experiments, a fine-wire tungsten electrode (World Precision Instruments, 5 MΩ) was used to identify the location and depth in the spinal cord where the field potential was the largest. Evidence of successful impalement of an α-MN was a sudden increase in potential to at least −50 mV, and an antidromic action potential with a spike amplitude of > 55 mV and a reproducible latency of less than 2.5 ms from the stimulation artifact. When recording had stabilized for at least 2 min, resting membrane potential was recorded. During recording, an axoclamp intracellular amplifier system (Axoclamp 2B, Axon Instruments Inc., Union City, CA, USA) was used, either in bridge or discontinuous current-clamp modes (DCC; 2–3 kHz switching), with capacitance maximally compensated. The following passive MN properties were recorded in bridge mode: antidromic action potential (from an average of 10 spikes), and orthodromic action potential in response to a 0.5 ms current pulse of supramaximal intensity (from an average of 40 spikes). Rheobase current (50 ms square-wave current amplitude resulting in spikes 50% of the time) and cell input resistance (from an average of 60, 1 nA hyperpolarizing pulses each lasting 100 ms) were recorded in DCC mode. From these recordings we determined antidromic spike height and time course, amplitude and half-decay time of the AHP following an action potential evoked by a 0.5 ms current pulse, resting membrane potential (RMP), rheobase current, voltage threshold and cell input resistance (IR). The half-decay time of the AHP was used to separate ‘slow’ from ‘fast’ MNs, as we have done previously (Beaumont & Gardiner, 2002, 2003).
Measurement of motoneurone f–I relationship
After measuring MN passive properties, cells were then challenged with slow triangular current ramps (range: 0.5–6.0 nA s−1), and the voltage response was measured in DCC mode. Peak amplitude of the ramp depended entirely on the rhythmic threshold of the MNs, and an attempt was made to evoke trains of impulses containing 10–75 spikes over a 0.5–2.5 s duration. Ramps were used to determine the MN f–I relationship, to evoke voltage-dependent plateaus and to measure the underlying PIC as previously described (Hounsgaard et al. 1988; Lee & Heckman, 1998a; Bennett et al. 2001). During the current ramps, the ePIC producing the plateau and sustaining firing was estimated from the difference in injected current at spike recruitment compared with spike derecruitment (see Fig. 1; Lee & Heckman, 1998a; Bennett et al. 2001). For computing the average f–I relationship type and ePIC for each MN, we used a series of three to six current ramps. Voltage thresholds of the spikes elicited during the ramp were measured at the potential where there was an acceleration in the rate of depolarization to > 10 mV (2 ms)−1 (modified from Brownstone et al. 1992).
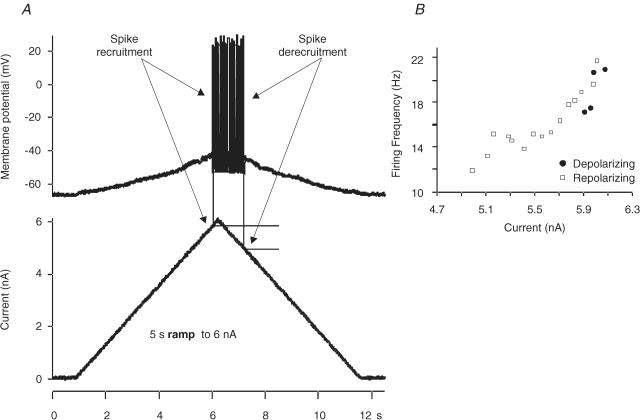
Figure 1. f–I relationships determined by using 5 s up–down ramp injections.
A, the top trace is the rhythmic discharge of the MN in response to a ramp current (bottom trace). Arrows point to the current at spike recruitment and spike derecruitment. Estimated PIC (ePIC) was calculated by subtracting the current at spike derecruitment from the current at spike recruitment. B is a plot of the instantaneous firing frequency and current from traces in A. In this example, the f–I relationship was plotted as an counter-clockwise hysteresis.
At the end of these measurements, the microelectrode was backed out of the MN in 5 μm steps, and the voltage outside the cell was recorded. Typically, experiments yielded two MNs with complete complements of data. At the end of the experiment, the rat was killed by an overdose of KCl which was injected intravenously.
Statistics
Only MNs that responded rhythmically to ramp currents and passed all electrophysiological requirements were used in the analysis. The MN passive properties and rhythmic properties were subjected to a one-way analysis of variance (ANOVA) for MN type and group (since all of the decerebration MNs which met the criteria for analysis were of the fast type). Motoneurones were designated as fast or slow based on the half-decay time of the AHP (fast, < 20 ms; slow, > 20 ms). This was done in order to subgroup MNs based on an intrinsic property that covaries highly with muscle fibre type and excitability, and which remains relatively stable in a variety of conditions that evoke changes in other properties (Zengel et al. 1985; Gardiner, 1993; Gardiner & Seburn, 1997; Munson et al. 1997; Cormery et al. 2000). To determine whether significant differences were present in MN f–I relationship type, X2 analysis was used. Some MN rhythmic properties were subjected to a two-way ANOVA procedure on factors of group and spike numbers (see Results for details). Where a significant interaction term was present, data were subjected to a Tukey post hoc comparisons test, to determine significant differences among individual means. All data are expressed as means ± 1 s.d.
Results
We recorded data from 29 MNs in 14 rats that were anaesthetized with ketamine and xylazine. The data set is summarized in Table 1. We separated MNs into ‘fast’ and ‘slow’, using the half-decay time of the AHP. This is based on previous reports (Gardiner, 1993; Cormery et al. 2000, 2005) that rat hindlimb MNs with AHP half-decay times equal to or greater than 20 ms innervate slow-twitch muscle fibres.
Table 1.
Passive and active motoneurone properties
| Motoneurone property | KX fast cells | KX slow cells | Decerebration fast cells | P value |
|---|---|---|---|---|
| (1) RMP (mV) | −70.9 ± 6.3 | −64.2 ± 5.9 | −61.7 ± 7.8 | † 0.005 |
| (2) Vth (mV) | −51.6 ± 6.6 | −46.1 ± 11.7 | −45.6 ± 7.2 | † 0.02 |
| (3) Rheobase current (nA) | 9.8 ± 3.0 | 5.9 ± 4.7 | 6.4 ± 1.8 | * 0.01 |
| n = 21 | n = 7 | n = 14 | † 0.01 | |
| (4) Input resistance (MΩ) | 1.8 ± 0.6 | 2.7 ± 1.2 | 2.2 ± 0.9 | * 0.01 |
| n = 21 | n = 7 | n = 14 | ||
| (5) AHP amplitude (mV) | 1.5 ± 1.1 | 2.7 ± 2.1 | 2.6 ± 2.2 | 0.06 |
| (6) AHP ½ decay time (ms) | 13.8 ± 1.9 | 27.3 ± 6.6 | 12.7 ± 2.9 | * 0.00 |
| n = 21 | n = 7 | n = 14 | ||
| (7) Current at spike recruitment (nA) | 11.0 ± 3.1 | 7.4 ± 3.9 | 6.9 ± 4.0 | * 0.004 |
| n = 21 | n = 7 | n = 14 | † 0.001 | |
| (8) Current at spike derecruitment (nA) | 10.9 ± 3.1 | 7.0 ± 4.0 | 6.6 ± 4.2 | * 0.01 |
| n = 21 | n = 7 | n = 14 | † 0.001 | |
| (9) Instantaneous frequency at spike recruitment (Hz) | 26.5 ± 7.7 | 16.7 ± 7.2 | 19.0 ± 6.7 | * 0.003 |
| n = 21 | n = 7 | n = 14 | † 0.003 | |
| (10) Instantaneous frequency at spike derecruitment (Hz) | 24.7 ± 6.8 | 14.1 ± 5.6 | 16.3 ± 4.6 | * 0.000 |
| n = 21 | n = 7 | n = 14 | † 0.000 | |
| (11) Slope (Hz nA−1) depolarizing | 17.8 ± 9.7 | 15.3 ± 8.5 | 15.1 ± 11.0 | 0.6 |
| n = 21 | n = 7 | n = 14 | ||
| (12) ePIC (nA) | 0.2 ± 0.6 | 0.4 ± 0.3 | 0.3 ± 0.5 | 0.6 |
| n = 21 | n = 7 | n = 14 |
Summary of passive (numbers 1–6 in chart) and active MN properties (numbers 7–12 in chart). Data are presented as means ± 1 s.d. for MNs in each group and include all f–I relationship types. KX, ketamine–xylazine anaesthetized; RMP, resting membrane potential; Vth, voltage threshold; AHP, after-hyperpolarization; and ePIC, estimated persistent inward current.
Significant difference between KX fast and slow motoneurones
significant difference between KX fast and decerebration fast motoneurones.
Motoneurone properties of KX-anaesthetized rats
The one-way ANOVA test revealed significant main effects of MN type (fast versus slow) for several electrophysiological properties. Motoneurone passive and f–I relationship properties are summarized in Table 1 (passive properties are listed from 1 to 6 and f–I relationship properties are from 7 to 12). Fast MNs required 35% more current for spike generation in response to 50 ms square-wave current pulses compared to slow MNs. Input resistance and AHP amplitude were 34 and 45% smaller, respectively, for fast MNs compared to slow MNs. During a ramp current, fast MNs required 32% more current to initiate firing and 35% more current to terminate rhythmic firing compared to slow MNs. Furthermore, the minimum instantaneous firing frequencies of fast MNs were significantly higher at spike recruitment (36%) and derecruitment (42%) compared to slow MNs. The average ePIC was not significantly different between fast and slow MNs.
Types of f–I relationships
We injected ramps of current into the MNs and determined their f–I relationship type (see Table 2 for f–I relationship distributions). This method has been used previously (Hounsgaard et al. 1988; Lee & Heckman, 1998a; Bennett et al. 2001) to determine f–I relationships and as a way to estimate the PIC (see Fig. 1). Similar to the four f–I relationship types demonstrated in rat sacral motoneurones in vitro (Bennett et al. 2001), in situ rat hindlimb α-MNs could also be categorized into the same four f–I relationship types. The f–I relationship types are shown in Fig. 2A–D. Type 1 (n = 7, 17%) MN f–I relationship demonstrated a firing frequency slope (average 24.3 ± 9.5 Hz nA−1) that overlaps on the ascending and descending portions of the ramp current (Fig. 2A). Type 2 (n = 12, 28%) MN f–I relationship demonstrated a clockwise hysteresis (i.e. MN firing rate adaptation) where the firing frequencies were greater during the ascending versus the descending portion of the ramp at any given current and the average slope values of the up and down portions of the ramp current were 12.6 ± 8.4 and 18.9 ± 10.4 Hz nA−1, respectively (Fig. 2B). Type 3 (n = 19, 43%) MN f–I relationship demonstrated a linear regression line with some self-sustained firing corresponding to the tertiary range of firing (Li et al. 2004; i.e. activation of a PIC) and an average slope of 15.8 ± 6.8 Hz nA−1 (Fig. 2C). Type 4 (n = 5, 12%) MN f–I relationship demonstrated a counter-clockwise hysteresis or an acceleration of firing just after spike recruitment and below the linear regression line (i.e. activation of a PIC) corresponding to the secondary range of firing. None of these MNs demonstrated a primary range of firing but rather started firing in the secondary range followed by shallower sloped tertiary range (see Fig. 5 of Li et al. (2001) for further description of these three firing ranges during a ramp). The type 4 f–I relationship firing frequencies were greater during the descending versus the ascending portion of the ramp at any given current, and the average slope values of the up and down portions of the ramp current were 16.7 ± 18.6 (secondary range) and 10.1 ± 2.6 Hz nA−1 (tertiary range), respectively. There were no significant f–I slope differences among f–I relationships or between MN types. However, there was a trend (P = 0.08) for a decreased slope during the down portion of the ramp in the f–I relationship type 4 compared to the other f–I relationship types.
Table 2.
Summary of f–I relationship types
| f–I relationship type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 1: overlapping | Type 2: adapting | Type 3: linear + sustained | Type 4: acceleration | ||||||||
| Number (n) | n | % | n | % | n | % | n | % | |||
| All MNs | 43 | 7 | 17 | 12 | 28 | 19 | 43 | 5 | 12 | ||
| KX fast MNs | 22 | 4 | 18 | 7 | 32 | 11 | 50 | 0 | 0 | ||
| KX slow MNs | 7 | 1 | 15 | 0 | 0 | 4 | 57 | 2 | 28 | ||
| Decerebration fast MNs | 14 | 2 | 14 | 5 | 36 | 4 | 28 | 3 | 22 | ||
Figure 2. A, B, C and D represent four different ramp-induced f–I relationship types; left is the raw ramp data and right is the plotted f–I relationship.
A, type 1 MN f–I relationship demonstrates a firing frequency slope that overlaps on the ascending and descending current ramps. B, type 2 MN f–I relationship demonstrates a clockwise hysteresis (MN firing rate adaptation). C, type 3 MN f–I relationship demonstrates a linear regression line (upper portion of graph) with some self-sustained firing. D, type 4 MN f–I relationship demonstrates a counter-clockwise hysteresis or an acceleration of firing just after spike recruitment and below the linear regression line.
ePIC amplitudes of f–I relationship types 3 and 4
Frequency–current relationship types 3 and 4 were shown in approximately 60% of MNs from which we recorded. These relationship types demonstrate activation of a PIC. During the ePIC, firing frequencies were decreased by approximately 15% in both fast (30 Hz at spike recruitment versus 25.5 Hz at spike derecruitment) and slow MNs (15.2 Hz at spike recruitment versus 12.9 Hz at spike derecruitment). Figure 3 illustrates a distribution of average ePIC measured from f–I relationship types 3 and 4. For any given cell, if the initial ramp elicited a PIC, all subsequent ramps did so as well. In most of the motoneurones the ePIC amplitude was consistent from ramp to ramp even when the ramp current was increased or decreased. Although ePIC amplitude did not differ among fast and slow MNs, χ2 analysis revealed a tendency (P = 0.09) for a greater proportion of slow MNs (87%) to demonstrate types 3 and 4 f–I relationships compared to fast MNs (50%).
Figure 3. Recordings from MNs demonstrating type 3 and 4 f–I relationships.
Each data point represents the ePIC amplitude of a MN subjected to a series of ramps (minimum of at least three). The grey and white squares represent fast and slow MNs, respectively. The dashed line represents the mean ePIC for all cells. Data points are presented as means ± 1 s.d.
Lastly, the ePIC was not correlated with the animal's vital signs (blood pressure or CO2 levels) throughout the experiment. There was, however, a trend (P = 0.08) for the ePIC to be negatively correlated (−0.44) with time of day during the experiment.
Effect of sodium pentobarbitone on MN ePIC amplitude in a KX-anaesthetized rat
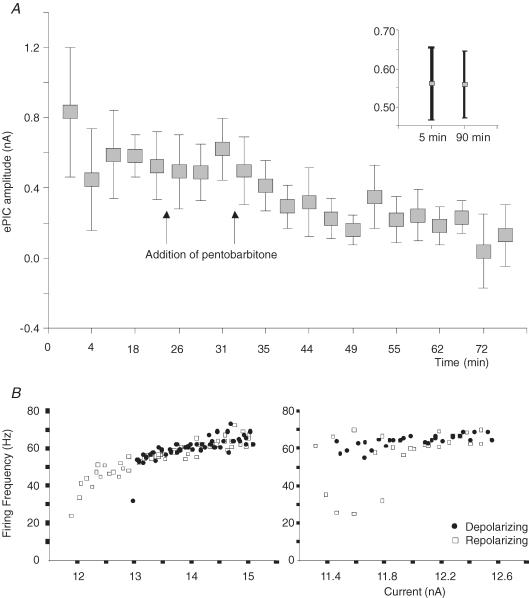
In one experiment, we applied a series of ramps to a MN before and after the intravenous administration of sodium pentobarbitone. In total, the MN was impaled for 80 min. Before the addition of pentobarbitone, the ePIC amplitude average over five ramps was 0.52 nA. This was significantly (P < 0.001) higher than the ePIC amplitude average over the last five ramps recorded 40–60 min following the first injection of pentobarbitone (0.16; see Fig. 4A). Furthermore, the f–I relationship type was converting from type 3 to type 1 (see Fig. 4B). Although ePIC amplitudes decreased, the passive properties RMP, Vth, rheobase, AHP amplitude and duration) remained similar (not shown) before and after the addition of pentobarbitone. Because the MN had been impaled for a long time before the administration of pentobarbitone, we wished to demonstrate that the changes in ePIC were due to the drug rather than impalement time. Therefore, in another experiment we impaled a MN for 90 min without the addition of pentobarbitone and subjected it to a series of ramps. The ePIC amplitude for this neurone was virtually the same at minute 1 (0.55 ± 0.09 nA) and minute 90 (0.56 ± 0.08 nA; see inset in Fig. 4A).
Figure 4. The effect of pentobarbitone on MN ePIC amplitude and f–I relationship.
A, data were recorded from one MN. Each data point represents the ePIC amplitude average from of a series of ramps (minimum of at least three) at each time period before and after the addition of pentobarbitone. Arrows indicate when pentobarbitone was injected into the rat. The inset indicates that the ePIC does not depend on the time course of the glass microelectrode impalement of the motoneurone. B, left is a graph showing the MN f–I relationship (type 3). Its response to a 5 s ramp current injection was measured before the addition of pentobarbitone. Right is a graph demonstrating that upon injection of pentobarbitone, the f–I relationship of the MN is almost converted (within 1 h of motoneurone impalement) from f–I relationship type 3 to type 1.
Voltage threshold and AHP properties of ramp spikes in KX-anaesthetized rats
We also analysed Vth, AHP amplitude and ¾ decay time of the AHP for the initial three spikes during the depolarizing current phase of the ramp, the three spikes at the same current during the repolarizing current phase of the ramp and, when applicable, the last three spikes triggered during the ePIC (see Fig. 5A and B for details) from nine fast and six slow MNs (which demonstrated all 4 types of f–I relationships). Similar to a short orthodromic spike, the AHP amplitudes and durations of spikes during current ramps were smaller and shorter in fast MNs (see Table 3).
Figure 5. Voltage thresholds, AHP amplitudes and AHP ¾ durations measured during the ramp.
A, voltages and currents at which rhythmic firing occurred. The horizontal bars and corresponding numbers below the spikes indicate the spikes that were analysed for the Vth and AHP properties. Only f–I relationship types 3 and 4 (i.e. ePIC) would include a third horizontal bar and corresponding spike numbers. B, the first two spikes from the ramp in A are shown amplified. The voltage threshold was measured at the point indicated by arrow 1, the amplitude of the AHP is indicated by arrow 2 (the difference between Vth and AHP peak amplitude), and the time it takes for the AHP amplitude to return to ¾ of its baseline valueis indicated by arrow 3. C, illustration of the first and last two spikes (spikes 1, 18 and 19) in the ramp from A. Y axis is the membrane potential, and the spikes are truncated. Spike voltage threshold and after-hyperpolarization values are listed in Table 4.
Table 3.
Voltage threshold, AHP amplitude and AHP duration of ramp spikes
| Ramp motoneurone property | KX fast cells | KX slow cells | Decerebration fast cells | P value |
|---|---|---|---|---|
| Ramp Vth (mV) | −45.1 ± 6.1 | −45.5 ± 10.6 | −40.5 ± 5.2 | † 0.007 |
| n = 9 | n = 7 | n = 6 | ||
| Ramp AHP amplitude (mV) | 8.6 ± 2.0 | 10.6 ± 2.7 | 9.4 ± 2.3 | * 0.000 |
| n = 9 | n = 7 | n = 6 | † 0.02 | |
| Ramp AHP ¾ duration time (ms) | 19.9 ± 3.3 | 41.8 ± 19.7 | 35.9 ± 14.9 | * 0.000 |
| n = 9 | n = 7 | n = 6 | † 0.000 |
Data are presented as means ± 1 s.d. of all MNs in each group and include all f–I relationship types.
Significant difference between KX fast and slow motoneurones
significant difference between KX and decerebration fast motoneurones.
Most importantly, irrespective of MN or f–I relationship type, Vth and AHP properties during a ramp were no different from spike to spike (see Fig. 6). Figure 6 (which includes all spikes as opposed to the 9 we measured) shows Vth values and AHPs for all of the ramp spikes shown in Fig. 5A. For this cell, spike recruitment was induced at 17.4 nA and spike derecruitment occurred at 16.1 nA. During this current, the cell generated a total of 19 spikes (12 of which occurred below the current at which spike recruitment was initiated). Voltage threshold at each spike ranged from −42.7 to −46.5 mV, AHP amplitude ranged from 12 to 16 mV, and the AHP ¾ decay time ranged from 25 to 42 ms. Voltage threshold was consistent throughout. However, the AHP amplitude and duration increased and decreased as ramp current increased and decreased, producing a greater range in the AHP properties. The voltage threshold and AHP properties measured at the first and last spikes were very similar in almost every ramp regardless of the MN f–I relationship type (see Fig. 5C). In Fig. 5C, spikes 1, 18, and 19 are at a higher gain then shown in Fig. 5A, and Table 4 summarizes Vth and AHP values recorded from the spikes.
Figure 6. Each data point represents the current and voltage threshold at each spike and its AHP amplitude and AHP ¾ decay time during the subsequent interspike interval (spikes are not shown here) from the example in Fig. 5A.
In this ramp, spike recruitment was induced at 17.4 nA and spike derecruitment occurred at 16.1 nA. During this ramp, the MN discharged a total of 19 spikes (12 of which occurred below the current at which spike recruitment was initiated). Voltage threshold at each spike ranged from −42.7 to −46.5 mV, AHP amplitude ranged from 12 to 16 mV, and the AHP ¾ decay time ranged from 25 to 42 ms.
Table 4.
Voltage threshold, AHP properties and ramp current of first and last two ramp spikes
| Spike 1 | Spike 18 | Spike 19 | |
|---|---|---|---|
| Current required for spike generation (nA) | 17.4 | 16.4 | 16.1 |
| Vth prior to spike generation (mV) | −42.7 | −45.6 | −44.0 |
| AHP amplitude (mV) | 16.0 | 13.4 | 14.3 |
| AHP ¾ duration time (ms) | 35.0 | 34.6 | 42.0 |
Data represent the Vth and AHP properties of the ramp spikes in Fig. 5a and c.
The effect of KX versus decerebration on motoneurone properties
During another set of experiments, we recorded from 14 MNs in six rats that were subjected to precollicular decerebration. All decerebration MNs that met the criteria for analysis were of the fast type. This was due to sampling error, since we did record from some decerebrated MNs with AHP ½ decay times greater than 20 ms, and therefore not because of any effect of the decerebration on MN AHP time course. One limitation in this study is that we compared MNs from an intact anaesthetized animal to a non-intact non-anaesthetized animal. This limitation could be alleviated in the future by comparing MNs from decerebrate animals to KX-treated decerebrate animals. Nonetheless, the one-way ANOVA revealed significant main effects of group (KX versus decerebration) for several electrophysiological properties of fast MNs (see Table 1 for details). Motoneurones from KX-anaesthetized rats had significantly hyperpolarized (14%) RMP and Vth, required 40% more current for spike triggering and had 45% smaller AHP amplitudes compared to MNs from decerebrate animals. During a ramp the MNs from KX-anaesthetized rats required 37 and 38% more current and their instantaneous firing frequencies were 28 and 34% higher at spike recruitment and derecruitment, respectively, compared to MNs from decerebrate animals.
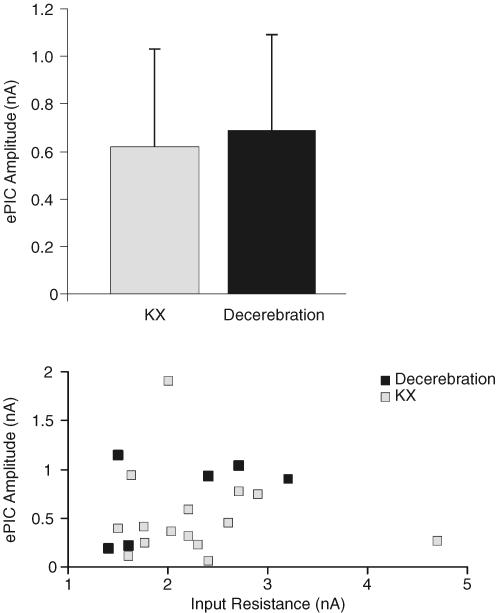
Motoneurones from KX-anaethetized and decerebrate rats demonstrated similar f–I relationship types (see Fig. 2) and distributions (see Table 2). Furthermore, decerebration (0.7 ± 0.4 nA) and KX (0.6 ± 0.4 nA) motoneurone mean ePIC amplitudes measured from the MNs demonstrating type 3 and 4 f–I relationships were also similar (see Fig. 7A). Furthermore, ePIC amplitude did not correlate with MN input resistance in KX-anaesthetized (P = 0.9) or decerebrate rats (P = 0.3; Fig. 7B).
Figure 7. Illustration of average ePIC and its relation to input resistance.
A, comparison of average ePIC amplitude between MNs of KX-anaesthetized and decerebrate rats. Columns represents an average of all ePICs (type 3 and 4 f–I relationships only). Data points are presented as means ± 1 s.d. B, relationship between input resistance and ePIC amplitude. Each data point represents one motoneurone. Correlation coefficients were 0.5 for MNs from decerebrate rats and 0.01 for MNs from KX-anaesthetized rats.
For MNs from KX-anaethetized rats, the Vth values of spikes during current ramps were 11% hyperpolarized, had 9% smaller AHP amplitudes and 45% longer AHP ¾ decay times compared to MNs from decerebrate rats (see Table 3 for details). Similar to MNs from KX-anaethetized rats, for MNs from decerebrate rats the Vth values and AHP properties during a ramp were no different from spike to spike (see Fig. 6).
Discussion
The most important finding of this study is that rat hindlimb α-motoneurones injected with ramp currents resulted in four types of f–I relationships, two of which demonstrated a presence of persistent inward current. The four f–I relationship types are similar to those noted previously in vitro for rat sacral MNs (Bennett et al. 2001). Moreover, it appears that the ePIC amplitude is not dependent on MN type, which has been indirectly addressed previously in cat lumbar α-MNs (Lee & Heckman, 1998a) and rat sacral–caudal MNs (Bennett et al. 2001). However, f–I relationship types 3 and 4 tend to be more frequent in slow compared to fast MNs. The presence of this ramp-induced PIC and its amplitude were not altered when the MNs were subjected to the anaesthetic mixture of ketamine and xylazine compared to the decerebrate preparation, which is used to alleviate the effects of anaesthetic. This finding is very significant because KX allows us to estimate PICs in an intact animal (i.e. not decerebrated).
Prior to injecting ramps of current into the MN, we recorded passive properties that have been reported previously (Beaumont & Gardiner, 2002, 2003). Similar to the previous data that were recorded from MNs in anaesthetized animals, no significant differences existed between the passive properties of fast and slow MNs (RMP and Vth), whereas slow MNs had a significantly lower rheobase current. Furthermore, input resistance, AHP amplitude and AHP ½ decay time were approximately double in slow versus fast MNs. Thus, our findings concerning MN passive properties are comparable to those reported in the literature. In addition, during a ramp, fast MNs required more current to initiate and stop rhythmic firing compared to the slow MNs. Furthermore, fast MNs had higher instantaneous firing frequencies at spike recruitment and derecruitment than slow MNs, which is comparable to rhythmic firing for fast and slow MNs in response to square-wave pulses of current (Cormery et al. 2005). Similar to cat MNs (Lee & Heckman, 1998b), rheobase was correlated with instantaneous firing frequency at spike recruitment and derecruitment as well as the ramp currents at which spike recruitment and derecruitment ocurred.
Motoneurones could be categorized into four f–I relationship types: (1) linear; (2) adapting; (3) linear + self-sustained firing; and (4) acceleration. These results are consistent with those previously reported in rat sacral MNs (Bennett et al. 2001). In the present experiment, f–I relationship types 1 and 2 did not demonstrate the presence of a PIC. This may be explained by the fact that the PIC amplitude of motoneurones tends to be muscle dependent, in that motoneurones innervating the postural muscles (triceps surae) are more likely to demonstrate PICs (Hounsgaard et al. 1988; Conway et al. 1988), which would be advantageous for maintaining force output during postural tasks (Alaburda et al. 2002). Since sciatic nerve MNs in the rat innervate several muscle groups, the MNs demonstrating f–I relationship types 1 and 2 in the present study may have been MNs innervating muscles that are not required to maintain postural tasks or low forces over long periods of time.
Over 50% of the MNs we recorded from demonstrated type 3 and 4 f–I relationships. It is within these relationship types that the specific PIC voltage-gated ion channels are activated (Lee & Heckman, 1998b; Hounsgaard et al. 1988; Bennett et al. 2001). The difference between type 3 and 4 f–I relationships is probably due to the voltage at which the specific voltage-gated PIC channels are activated. The PIC in types 3 and 4 may be mediated through persistent Na+ and L-type Ca2+ channels activated prior to the start of rhythmic firing, while the PIC in type 4 may also include a L-type Ca2+ channel activated at a voltage threshold after the start of rhythmic firing (Li & Bennett, 2003). Furthermore, differences in the f–I relationship between types 3 and 4 may be due to the saturation level of the L-type Ca2+ channel. Type 3 f–I relationship may activate the calcium PIC in a graded manner, upon which additional increases in input result in minimal changes in the Ca2+ PIC (i.e. it is saturated). However, during a type 4 f–I relationship the calcium PIC is perhaps activated upon MN recruitment, leading to a steep increase in the firing frequency that coincides with the secondary range of the f–I relationship. Once the Ca2+ PIC is saturated, the slope of the f–I relationship is then reduced, corresponding to the tertiary range of firing (Elbasiouny et al. 2005).
Even though f–I relationship types 3 and 4 demonstrated linear and counter-clockwise relationships, respectively, their ePIC magnitude was not different. It has been reported that Na+ and Ca2+ PIC channels contribute approximately equally to the total PIC of the MNs (Li & Bennett, 2003), so it is possible that these channels were activated equally in the type 3 and 4 f–I relationships reported here. In addition, there was no MN type-related ePIC amplitude dominance. This was not surprising, since Lee & Heckman (1998b) demonstrated that MN bistability (a property facilitated by PIC) was more pronounced in low versus high rheobase current (a characteristic of slow versus fast type MNs, respectively; see Beaumont & Gardiner, 2002); however, there was no difference in the initial PIC conductance. The lack of bistability in high rheobase current MNs may be attributed to a faster inactivation of the PIC channels. Bennett et al. (2001) also showed that no difference existed in the amount of PIC between low and high rheobase current sacral–caudal MNs. It also appears that ePIC amplitude is not related to MN type, rheobase current or input resistance in rat hindlimb MNs. Hence, MNs of all sizes have the ability to produce self-sustained firing. Although the previous statement may be of merit, our data lend some support to a MN type difference in the proportions of MNs demonstrating f–I relationship types 3 and 4. There is a tendency for a greater proportion of slow MNs to have a type 3 or 4 f–I relationship, which does indeed support the findings (Lee & Heckman, 1998b) that a greater number of smaller MNs demonstrate bistability.
It has been suggested that the f–I function which uses current injected into the soma to fire the cell does not provide a good measure of the enhancement of synaptic input by the PIC (Heckman et al. 2004). However, the ramp technique which is used to determine the f–I function for a MN has been successfully used as a way to estimate or at least demonstrate activation of its PIC or a lack thereof. We injected a series of ramp currents into each MN and, unlike earlier reports, averaged all ePIC amplitudes as overall PIC. Interestingly, if the first ramp demonstrated f–I relationship type 3 or 4 (i.e. PIC) all subsequent ramps in the series also demonstrated f–I relationship type 3 or 4 (i.e. PIC). These ramp-induced ePIC amplitudes were similar across the series, with low variability. This was also the case for the other two f–I relationship types. To further illustrate the presence of PIC in types 3 and 4 f–I relationships, we injected ramps in a MN before and after the addition of pentobarbitone. Pentobarbitone was chosen because it abolishes MN PICs (Hultborn & Kiehn, 1992; Guertin & Hounsgaard, 1999; Hultborn, 1999). Motoneurones of animals anaesthetized with pentobarbitone still retain the ability to fire rhythmically, since it does not seem to affect the fast persistent inward current (Na2+ PIC channel; Lee & Heckman, 2000). L-Type Ca2+ channels located on MN dendrites do not appear to amplify synaptic Ia currents in pentobarbitone-anaesthetized cats compared to decerebrate cats (Lee & Heckman, 2000). Thus, it is more likely that pentobarbitone may influence the L-type Ca2+ channels and subsequently decrease MN bistability. Similarly, our pentobarbitone-treated MN had a major decrease in ePIC amplitude to a point where its f–I relationship was almost completely converted from type 3 to type 1. The ePIC was not completely reduced to zero, suggesting that perhaps some persistent inward Na+ current may not have been inhibited. The MN impalement duration lasted 80 min for these recordings, but we later showed that impalement time does not influence the ePIC amplitude or f–I relationship type of the MN. These findings reconfirm that ramps evoke PICs which subsequently alter the f–I relationship of the MN.
The possibility of an AHP amplitude and duration changing throughout an individual ramp was investigated. Lower firing frequencies at spike derecruitment where the activated PIC is supposed to help maintain these frequencies might be partly explained by changes in the interspike interval AHP amplitude and duration. Perhaps towards the end of motor-unit discharge, a sustained subthreshold depolarization with the addition of noise may cause the MN to fire one or more times (Matthews, 1996; Kudina, 1999; Gorassini et al. 2002). However, this finding was in human motor units, and perhaps the fluctuation in background noise and spontaneous firing was due to the subject's inadequacy to control their level of voluntary contraction. It has also been suggested in cat spinal MNs that an increased AHP duration contributes to the low firing frequencies at spike derecruitment (Wienecke et al. 2005). Our data indicate that there were no differences between Vth, AHP amplitude or AHP duration from spike to spike during a ramp for any f–I relationship type. Occasionally, the final spike had a longer AHP ¾ decay time compared to the first spike during a ramp which demonstrated a f–I relationship type 3 and 4, but this occurred infrequently. Furthermore, the average number of spikes triggered during the ePIC was 11 (not shown in Results) and, even if the final spike AHP ¾ decay time was prolonged, this did not apply to the other 10 spikes. A potential limitation to this finding was that our ramp currents were selected on the basis of only evoking 10–75 spikes over a 0.5–2.5 s duration (i.e. slow firing rate), and we acknowledge that if we used higher ramp currents the firing frequency would have increased and caused changes to the properties listed above (Schwindt & Crill, 1982; Bennett et al. 2001). However, the main purpose of these measurements was to demonstrate that additional spike generation is probably not attributable to changes in these properties but instead to the activated PIC.
Another purpose for our study was to determine the effects of ketamine and xylazine on motoneurone properties. Therefore, we also recorded MN properties from decerebrate rats. It has been suggested recently that anaesthetic agents may render the MN as ‘sleeping’ and may mask some of its important functional properties (Hultborn & Kiehn, 1992; Guertin & Hounsgaard, 1999; Delgado-Lezama & Hounsgaard, 1999; Button et al. 2005a), which are significant in ‘awake’ MNs. Thus, it was important to determine whether MN properties do indeed differ in an anaesthetized versus an unanaesthetized rat preparation. The RMP and Vth in MNs of decerebrate rats were significantly depolarized compared to MNs from KX-anaesthetized animals and required far less rheobase current to reach that threshold. Ketamine has been shown to increase K+ permeability in synaptosomes (Okun et al. 1986) and to block voltage-gated Na+ and K+ channels in superficial dorsal horn neurones of the lumbar spinal cord, thereby reducing their excitability (Schnoebel et al. 2005). Perhaps this also holds true for the MN. An increase in the permeability of K+ would result in a hyperpolarized RMP in MNs of KX-anaesthetized rats compared to MNs of decerebrate rats. This may suggest that changes in the activation of the ion channels or a change in their conductance that is responsible for these motoneurone properties may occur as a result of KX. Even though the AHP amplitude was different, AHP duration remained the same. Ketamine has been shown inhibit large conductance Ca2+-activated K+ (BK) channels in GH3 cells (Denson & Eaton, 1994). BK channel activation is dominant during the big and fast portion of the AHP and, if inhibited in the MN by KX, it would lead to decreased AHP amplitude compared to MNs of decerebrate rats. Since AHP ½ decay times were similar in MNs of decerebrate and KX-anaesthetized rats, one could speculate that the ion channels remain open for similar periods of time, but that fewer ion channels open. Similarly, MNs of decerebrate rats required less current to initiate and end rhythmic firing and had lower instantaneous firing frequencies at this initiation and end period during rhythmic firing compared to MNs of KX-anaesthetized rats. Overall, based on rheobase current and the amount of current required to initiate rhythmic firing, a KX mixture appears to render the MN as less excitable, and so must affect the ion channels that underlie these basic and rhythmic properties.
No difference in ePIC amplitude existed between anaesthetized versus unanaesthetized MNs. There is no evidence suggesting that KX affects PIC channels. Xylazine is an α2-adrenoceptor agonist, which presumably should not affect PIC channels, whereas ketamine is an NMDA receptor antagonist (Liu et al. 2001). Persistent inward currents are mediated by L-type Ca2+ channels which are selectively blocked by nimodipine (Li et al. 2004), so it is not expected that KX would influence PIC, which is consistent with the results seen in this study. Although NMDA receptors have a role in membrane bistability (Hochman et al. 1994) and are present in adult turtle MNs (Guertin & Hounsgaard, 1998) and neonatal rat MNs (Hochman et al. 1994; Palecek et al. 1999; Hsiao et al. 2002), they have no influence on PIC. The decerebrate preparation preserves the brainstem and spinal cord in an unanaesthetized state, which does not negatively influence PIC (Kiehn, 1991; Kiehn & Eken, 1998; Hultborn, 1999; Heckman et al. 2004). Thus, ketamine or xylazine does not affect the major PIC channels in these MNs.
We also described Vth and AHP properties from the ramp technique which, to our knowledge, have not been reported in the literature. Similar to the case with short orthodromic spike, the Vth values for ramp spikes were depolarized and AHP amplitudes were bigger in MNs of decerebrate rats. Interestingly, their AHP ¾ decay time was much longer. Ketamine inhibits the small conductance Ca2+-activated K+ channel (SK2; Dreixler et al. 2000), which is responsible for the slow portion of the AHP and practically the whole AHP during rhythmic firing (Miles et al. 2005). Perhaps KX partly blocked the small conductance Ca2+-activated K+ channel and its conductance in the MN, which lead to a decreased AHP amplitude and duration compared to MNs of decerebrate animals. The change in these AHP properties are more than likely to be the result of a ketamine-induced change to the SK2 channel, which behaves somewhat differently during rhythmic firing compared to an orthodromic spike.
Lastly, the change in firing frequency at spike recruitment versus derecruitment (types 3 and 4 f–I relationships only; approximately 4 Hz difference) during ramp currents in our intact anaesthetized animal preparation is comparable to that found via paired motor-unit methods in awake rats (Gorassini et al. 1999) and humans (Gorassini et al. 2002). Motor units can be derecruited at lower frequencies than they are initially recruited at during slow triangular muscle contractions, muscle vibration and sinusoidal muscle stretch. The difference in firing frequency between recruitment and derecruitment is thought to be provided by the intrinsic PIC of the motoneurone. Since these frequencies are comparable, perhaps the ePIC amplitudes found in MNs from our intact anaesthetized rat preparation could be used as a starting point for estimating the size of PICs in motor units of awake rats and humans.
In conclusion, rat hindlimb α-motoneurones injected with ramp currents can be categorized into one of four different f–I relationship types. Two of these f–I relationships, which were more frequent in slow MNs, demonstrated the existence of persistent inward current. The ePIC amplitude did not differ between f–I relationships type 3 and 4 or between fast and slow MNs. Although the anaesthetic mixture of ketamine and xylazine renders the MN less excitable to initiate spike threshold or rhythmic threshold compared to MNs of decerebrate animals, it does not affect the ePIC amplitude, but only the voltage and current required to activate these channels and AHP properties during a short spike or a burst of spikes. Ramp current injections activated the PIC channels in MNs of intact animals anaesthetized by KX and decerebrate animals. This statement is supported by the following findings: (1) once a MN demonstrates an ePIC, all subsequent ramp currents injected into that same MN also evoke ePICs; (2) the MN f–I relationship remains the same from ramp to ramp; (3) the addition of pentobarbitone decreases the ramp ePIC, as previously shown (Guertin & Hounsgaard, 1999) in turtle MNs; (4) during the ramp, the Vth and AHP properties of the spikes remain unchanged; and (5) the ramp technique has been verified as successful in demonstrating the PIC, as in other animal preparations (Hounsgaard et al. 1988; Lee & Heckman, 1998a; Bennett et al. 2001). Finally, the anaesthetic mixture of ketamine and xylazine allows us to measure PIC in intact animals, and this mixture could be administered during future planned experiments to determine how the MN ePIC is influenced by different physiological parameters.
Acknowledgments
This research was supported by grants from NSERC Canada, CIHR, and the Canada Research Chairs program. The authors would like to thank Gilles Detillieux, Matt Ellis and Maria Setterbom at University of Manitoba for technical assistance, Farrell Cahill for assistance in data analysis, and Dr Jayne Kalmar for comments and suggestions during manuscript preparation. P.F.G. is Canada Research Chair in Physical Activity & Health Studies at University of Manitoba.
References
- Alaburda A, Perrier JF, Hounsgaard J. Mechanisms causing plateau potentials in spinal motoneurones. Adv Exp Med Biol. 2002;508:219–226. doi: 10.1007/978-1-4615-0713-0_27. [DOI] [PubMed] [Google Scholar]
- Beaumont E, Gardiner P. Effects of daily spontaneous running on the electrophysiological properties of hindlimb motoneurones in rats. J Physiol. 2002;540:129–138. doi: 10.1113/jphysiol.2001.013084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont E, Gardiner PF. Endurance training alters the biophysical properties of hindlimb motoneurons in rats. Muscle Nerve. 2003;27:228–236. doi: 10.1002/mus.10308. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Jordan LM, Kriellaars DJ, Noga BR, Shefchyk SJ. On the regulation of repetitive firing in lumbar motoneurones during fictive locomotion in the cat. Exp Brain Res. 1992;90:441–455. doi: 10.1007/BF00230927. [DOI] [PubMed] [Google Scholar]
- Button DB, Gardiner KR, Marqueste T, Gardiner PF. Washington, DC: Society for Neuroscience; 2005a. Frequency-current relationships in anesthetized and decerebrated rat hindlimb motoneurones in situ. Program no. 750.9 2005. Abstract Viewer/Itinerary Planner. (Abstract) [Google Scholar]
- Button DC, Gardiner K, Zhong H, Roy RR, Egerton VR, Gardiner PF. In situ frequency-current (f-I) relationships of hindlimb α-motoneurones (α-Mns) in spinal cord transected (st) and spinal cord isolated (si) rats. Can J Appl Physiol. 2005b;30:S15. (Abstract) [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in α-motoneurones induced by intravenous injection of l-DOPA and clonidine in the spinal cat. J Physiol. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormery B, Beaumont E, Csukly K, Gardiner P. Hindlimb unweighting for 2 weeks alters physiological properties of rat hindlimb motoneurones. J Physiol. 2005;568:841–850. doi: 10.1113/jphysiol.2005.091835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormery B, Marini JF, Gardiner PF. Changes in electrophysiological properties of tibial motoneurones in the rat following 4 weeks of tetrodotoxin-induced paralysis. Neurosci Lett. 2000;287:21–24. doi: 10.1016/s0304-3940(00)01110-1. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Hounsgaard J. Adapting motoneurons for motor behavior. In: Binder MD, editor. Peripeheral and Spinal Mechanisms in the Neural Control of Movement. Vol. 123. NY, USA: Elsevier; 1999. pp. 57–63. chap. 5Progress in Brain Research. [DOI] [PubMed] [Google Scholar]
- Denson DD, Eaton DC. Ketamine inhibition of large conductance Ca2+-activated K+ channels is modulated by intracellular Ca2+ Am J Physiol. 1994;267:C1452–C1458. doi: 10.1152/ajpcell.1994.267.5.C1452. [DOI] [PubMed] [Google Scholar]
- Dreixler JC, Jenkins A, Cao YJ, Roizen JD, Houamed KM. Patch-clamp analysis of anesthetic interactions with recombinant SK2 subtype neuronal calcium-activated potassium channels. Anesth Analg. 2000;90:727–732. doi: 10.1097/00000539-200003000-00040. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Bennett DJ, Mushahwar VK. Simulation of Ca2+ persistent inward currents in spinal motoneurons: mode of activation and integration of synaptic inputs. J Physiol. 2005;570:355–374. doi: 10.1113/jphysiol.2005.099119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad K, Bennett DJ. Decerebration by global ischemic stroke in rats. J Neurosci Meth. 1998;84:131–137. doi: 10.1016/s0165-0270(98)00101-0. [DOI] [PubMed] [Google Scholar]
- Gardiner PF. Physiological properties of motoneurons innervating different muscle unit types in rat gastrocnemius. J Neurophysiol. 1993;69:1160–1170. doi: 10.1152/jn.1993.69.4.1160. [DOI] [PubMed] [Google Scholar]
- Gardiner PF, Seburn KL. The effects of tetrodotoxin-induced muscle paralysis on the physiological properties of muscle units and their innervating motoneurons in rat. J Physiol. 1997;499:207–216. doi: 10.1113/jphysiol.1997.sp021921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore J, Fedirchuk B. The excitability of lumbar motoneurones in the neonatal rat is increased by a hyperpolarization of their voltage threshold for activation by descending serotonergic fibres. J Physiol. 2004;558:213–224. doi: 10.1113/jphysiol.2004.064717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini M, Bennett DJ, Kiehn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. J Neurophysiol. 1999;82:709–717. doi: 10.1152/jn.1999.82.2.709. [DOI] [PubMed] [Google Scholar]
- Gorassini M, Yang JF, Siu M, Bennett DJ. Intrinsic activation of human motoneurons: possible contribution to motor unit excitation. J Neurophysiol. 2002;87:1850–1858. doi: 10.1152/jn.00024.2001. [DOI] [PubMed] [Google Scholar]
- Granit R, Kernell D, Shortess GK. Quantitative aspects of repetitive firing of mammalian motoneurones, caused by injected currents. J Physiol. 1963;168:911–931. doi: 10.1113/jphysiol.1963.sp007230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. NMDA-Induced intrinsic voltage oscillations depend on L-type calcium channels in spinal motoneurons of adult turtles. J Neurophysiol. 1998;80:3380–3382. doi: 10.1152/jn.1998.80.6.3380. [DOI] [PubMed] [Google Scholar]
- Guertin PA, Hounsgaard J. Non-volatile general anaesthetics reduce spinal activity by suppressing plateau potentials. Neuroscience. 1999;88:353–358. doi: 10.1016/s0306-4522(98)00371-6. [DOI] [PubMed] [Google Scholar]
- Heckman CJ, Gorassini MA, Bennett DJ. Persistent inward currents in motoneuron dendrites: implications for motor output. Muscle Nerve. 2004;31(2):135–156. doi: 10.1002/mus.20261. [DOI] [PubMed] [Google Scholar]
- Hochman S, Jordan LM, Schmidt BJ. TTX-resistant NMDA receptor-mediated voltage oscillations in mammalian lumbar motoneurons. J Neurophysiol. 1994;72:2559–2562. doi: 10.1152/jn.1994.72.5.2559. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao CF, Wu N, Levine MS, Chandler SH. Development and serotonergic modulation of NMDA bursting in rat trigeminal motoneurons. J Neurophysiol. 2002;87:1318–1328. doi: 10.1152/jn.00469.2001. [DOI] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog Brain Res. 1999;123:39–48. doi: 10.1016/s0079-6123(08)62842-3. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Kiehn O. Neuromodulation of vertebrate motor neuron membrane properties. Curr Opin Neurobiol. 1992;2:770–775. doi: 10.1016/0959-4388(92)90132-5. [DOI] [PubMed] [Google Scholar]
- Kernell D. The adaptation and the relation between discharge frequency and current strength of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiol Scand. 1965a;65:65–73. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. High-frequency repetitive firing of cat lumbosacral motoneurones stimulated by long-lasting injected currents. Acta Physiol Scand. 1965b;65:74–86. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- Kernell D. The limits of firing frequency in cat lumbosacral motoneurones possessing different time course of afterhyperpolarization. Acta Physiol Scand. 1965c;65:87–100. [Google Scholar]
- Kernell D, Monster AW. Threshold current for repetitive impulse firing in motoneurones innervating muscle fibres of different fatigue sensitivity in the cat. Brain Res. 1981;229:193–196. doi: 10.1016/0006-8993(81)90756-3. [DOI] [PubMed] [Google Scholar]
- Kiehn O. Plateau potentials and active integration in the ‘final common pathway’ for motor behaviour. Trends Neurosci. 1991;14:68–73. doi: 10.1016/0166-2236(91)90023-n. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Curr Opin Neurobiol. 1998;8:746–752. doi: 10.1016/s0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- Kudina LP. Analysis of firing behaviour of human motoneurones within ‘subprimary range’. J Physiol (Paris) 1999;93:115–123. doi: 10.1016/s0928-4257(99)80142-9. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998a;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998b;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Paradoxical effect of QX-314 on persistent inward currents and bistable behavior in spinal motoneurons in vivo. J Neurophysiol. 1999;82:2518–2527. doi: 10.1152/jn.1999.82.5.2518. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Li Y, Gorassini MA, Bennett DJ. Role of persistent sodium and calcium currents in motoneuron firing and spasticity in chronic spinal rats. J Neurophysiol. 2004;91:767–783. doi: 10.1152/jn.00788.2003. [DOI] [PubMed] [Google Scholar]
- Liu HT, Hollmann MW, Liu WH, Hoenemann CW, Durieux ME. Modulation of NMDA receptor function by ketamine and magnesium: Part I. Anesth Analg. 2001;92:1173–1181. doi: 10.1097/00000539-200105000-00019. [DOI] [PubMed] [Google Scholar]
- Matthews PB. Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J Physiol. 1996;492:597–628. doi: 10.1113/jphysiol.1996.sp021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Dai Y, Brownstone RM. Mechanisms underlying the early phase of spike frequency adaptation in mouse spinal motoneurones. J Physiol. 2005;566:519–532. doi: 10.1113/jphysiol.2005.086033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson JB, Foehring RC, Mendell LM, Gordon T. Fast-to-slow conversion following chronic low-frequency activation of medial gastrocnemius muscle in cats. II. Motoneuron properties. J Neurophysiol. 1997;77:2605–2615. doi: 10.1152/jn.1997.77.5.2605. [DOI] [PubMed] [Google Scholar]
- Okun IM, Aksentsev SL, Konev SV. Effect of the general anesthetic ketamine on the potassium permeability of plasma membranes of brain synaptosomes. [In Russian] Biofizika. 1986;31:917–918. [PubMed] [Google Scholar]
- Palecek JI, Abdrachmanova G, Vlachova V, Vyklick L. Properties of NMDA receptors in rat spinal cord motoneurons. Eur J Neurosci. 1999;11:827–836. doi: 10.1046/j.1460-9568.1999.00489.x. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Powers RK, Sawczuk A, Musick JR, Binder MD. Multiple mechanisms of spike-frequency adaptation in motoneurones. J Physiol (Paris) 1999;93:101–114. doi: 10.1016/s0928-4257(99)80141-7. [DOI] [PubMed] [Google Scholar]
- Sawczuk A, Powers RK, Binder MD. Spike frequency adaptation studied in hypoglossal motoneurons of the rat. J Neurophysiol. 1995;73:1799–1810. doi: 10.1152/jn.1995.73.5.1799. [DOI] [PubMed] [Google Scholar]
- Schnoebel R, Wolff M, Peters SC, Brau ME, Scholz A, Hempelmann G, Olschewski H, Olschewski A. Ketamine impairs excitability in superficial dorsal horn neurones by blocking sodium and voltage-gated potassium currents. Br J Pharmacol. 2005;146:826–833. doi: 10.1038/sj.bjp.0706385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt PC. Membrane-potential trajectories underlying motoneuron rhythmic firing at high rates. J Neurophysiol. 1973;36:434–439. doi: 10.1152/jn.1973.36.3.434. [DOI] [PubMed] [Google Scholar]
- Schwindt P, Crill WE. A persistent negative resistance in cat lumbar motoneurons. Brain Res. 1977;120:173–178. doi: 10.1016/0006-8993(77)90510-8. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Negative slope conductance at large depolarizations in cat spinal motoneurons. Brain Res. 1981;207:471–475. doi: 10.1016/0006-8993(81)90381-4. [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Crill WE. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol. 1982;48:875–890. doi: 10.1152/jn.1982.48.4.875. [DOI] [PubMed] [Google Scholar]
- Skydsgaard M, Hounsgaard J. Multiple actions of iontophoretically applied serotonin on motorneurones in the turtle spinal cord in vitro. Acta Physiol Scand. 1996;158:301–310. doi: 10.1046/j.1365-201X.1996.558326000.x. [DOI] [PubMed] [Google Scholar]
- Wienecke J, Zhang M, Hultborn H. Washington, DC, USA: Society for Neuroscience; 2005. The postspike afterhyperpolarization (AHP) following single spikes is prolonged by a preceding spike-train in cat spinal motneurones. Program no. 750.8. Viewer/Itinerary Planner. (Abstract) [Google Scholar]
- Zengel JE, Reid SA, Sypert GW, Munson JB. Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1985;53:1323–1344. doi: 10.1152/jn.1985.53.5.1323. [DOI] [PubMed] [Google Scholar]