Abstract
We have previously demonstrated an increase in the excitability of the leg motor cortical area in relation to acquisition of a visuo-motor task in healthy humans. It remains unknown whether the interaction between corticospinal drive and spinal motoneurones is also modulated following motor skill learning. Here we investigated the effect of visuo-motor skill training involving the ankle muscles on the coupling between electroencephalographic (EEG) activity recorded from the motor cortex (Cz) and electromyographic (EMG) activity recorded from the left tibialis anterior (TA) muscle in 11 volunteers. Coupling in the time (cumulant density function) and frequency domains (coherence) between EEG–EMG and EMG–EMG activity were calculated during tonic isometric dorsiflexion before and after 32 min of training a visuo-motor tracking task involving the ankle muscles or performing alternating dorsi- and plantarflexion movements without visual feedback. A significant increase in EEG–EMG coherence around 15–35 Hz was observed following the visuo-motor skill session in nine subjects and in only one subject after the control task. Changes in coherence were specific to the trained muscle as coherence for the untrained contralateral TA muscle was unchanged. EEG and EMG power were unchanged following the training. Our results suggest that visuo-motor skill training is associated with changes in the corticospinal drive to spinal motorneurones. Possibly these changes reflect sensorimotor integration processes between cortex and muscle as part of the motor learning process.
The primary motor cortex is highly involved in acquisition of motor skills in animals and humans (Kleim et al. 1996, 1998, 2002; Nudo et al. 1996; Remple et al. 2001; VandenBerg et al. 2002). Several studies using non-invasive transcranial magnetic stimulation (TMS) have demonstrated an increase in excitability and an expansion of the cortical representation of specific muscles involved in the motor learning task (Pascual-Leone et al. 1994, 1995; Elbert et al. 1995; Classen et al. 1998, 1999; Lotze et al. 2003; Perez et al. 2004; Jensen et al. 2005). These changes in cortical excitability are partly associated with changes in corticospinal activity (review by Doyon & Benali, 2005). Adaptations in corticospinal input to spinal motoneurones have been shown to take place during a motor task that requires a high degree of attention and precision (Murthy & Fetz, 1996; Schmied et al. 2000; Kilner et al. 2002; Kristeva-Feige et al. 2002), but, so far, the interaction between corticospinal inputs and spinal motorneurons has not been investigated in relation to motor skill learning in humans.
It is possible to obtain information about the synaptic drive to spinal motoneurones during voluntary movement from coherence analysis in human subjects (Farmer et al. 1993; Conway et al. 1995; Salenius et al. 1997). The coherence spectrum between two signals provides an estimate of the magnitude of correlation (or coupling) between specific frequency components in the two signals (Farmer et al. 1993; Halliday et al. 1995; Farmer et al. 1997). In humans, it was first suggested that coherence between magnetoencephalography (MEG) and electromyography (EMG) is probably mediated by fast corticospinal axons and their monosynaptic connections to spinal motorneurones (Conway et al. 1995). This indicated that coupling between cortical activity recorded by electroencephalography (EEG) and muscle activity recorded by EMG in the 15–35 Hz frequency may reflect discharge of corticospinal cells in this frequency range (Farmer et al. 1993; Conway et al. 1995). Corticomuscular coupling in the 15–35 Hz frequency is abolished during movement and is greater during periods of steady contraction (Kilner et al. 1999, 2003). Furthermore, cortex and muscle coupling is modulated in a task-dependent manner in primates and humans (Baker et al. 1997; Kilner et al. 1999, 2003). Such findings suggest that corticomuscular coupling plays a role in sensorimotor integration processes within the motor system, but, although several hypotheses have been proposed, the functional significance of the coupling still remains unclear (Baker et al. 1999; Baker & Baker, 2003; Kilner et al. 2003; Salenius & Hari, 2003; Riddle & Baker, 2005).
In this study we tested whether the changes in cortical excitability documented in previous studies in relation to motor skill learning (Perez et al. 2004) also result in changes in the interaction between corticospinal drive and spinal motorneurones. We evaluated corticomuscular coherence in the 15–35 Hz frequency band following visuo-motor skill training involving the ankle muscles. We observed that corticomuscular coherence in this frequency band was significantly increased following the training session for the trained muscle suggesting that the training induced changes in the corticospinal drive to the muscle.
Methods
Subjects
Eleven healthy volunteers (aged of 27 ± 4 years; 7 female, 4 male) participated in the study. All of them gave their informed consent to the experimental procedure, which was approved by the local ethics committee. The study was performed in accordance with the Declaration of Helsinki. Only subjects who showed significant coherence between the leg motor cortex and the tibialis anterior (TA) muscle before the training session were included in the study. Inclusion of these subjects allowed us to compare changes in the magnitude of corticomuscular coherence before and after training. Coherence between electroencephalography (EEG) and electromyography (EMG, TA) and between a pair of TA EMG recordings was assessed before and after each session (Fig. 1A). Corticomuscular coherence is abolished during movement and is greater during steady periods of contraction (Baker et al. 1997; Kilner et al. 1999). Therefore, measurements were taken while the subjects performed a tonic dorsiflexion corresponding to approximately 10–15% of the maximal voluntary contraction (MVC). During these measurements an integrated TA EMG signal was provided as visual feedback on an oscilloscope to ensure that a constant level of contraction was maintained during measurements. Eleven subjects participated in the visuo-motor skill training. Only eight of them were able to participate in the control session. The two sessions were separated by 1–3 weeks. Four subjects showed coherence between cortex and left and right TA muscle before the training session. To test the specificity of the training effect, corticomuscular coherence was tested following the training session on both sides in these four subjects.
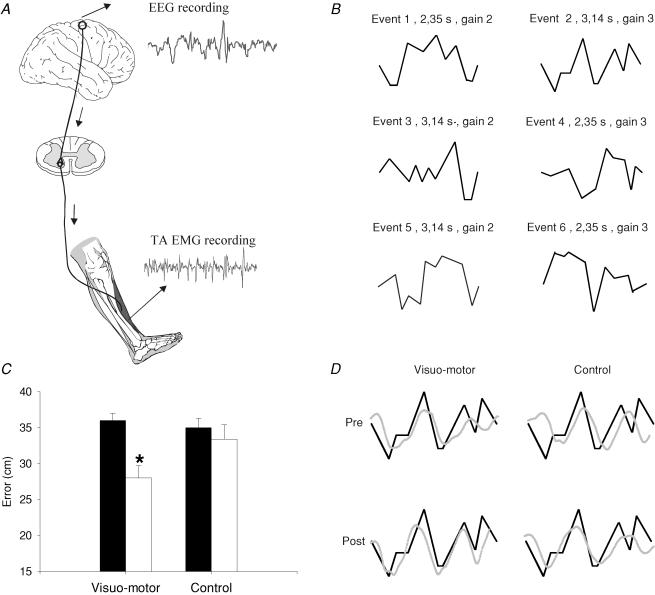
Figure 1. Effect of visuo-motor skill training and a control session on motor performance.
A, experimental set-up. Electroencephalographic (EEG) activity was recorded from the scalp over the leg area of the motor cortex. Electromyographic (EMG) activity was recorded by surface electrodes over the tibialis anterior muscle (TA). Coherence and cumulant density functions were calculated for paired EEG–EMG and EMG–EMG recordings during tonic dorsiflexion before and after the visuo-motor skill training and control session. B, the six randomized figures presented during the visuo-motor skill training session. Event no. 2 was used to assess motor performance before and after each training session. C, bar graph demonstrating the average motor performance before and after visuo-motor skill and control session (n = 7). The ordinate shows the mean error value from all subjects (cm). The abscissa shows the time at which measurements were taken (before, filled bar and after, open bar). Error bars indicate standard errors of the mean (*P < 0.05). D, traces showing the event selected for analysis (thick line) and the subject performance (thin line). Each thin line represents the average of the subject performance for 4 min. The traces shown are from a single subject.
Visuo-motor skill training
Each subject participated in a 32 min session of visuo-motor skill training involving the ankle muscles. The angles of the hip, knee and ankle joints were 120, 160 and 110 deg, respectively. The foot was attached to a foot-plate, which was connected to a goniometer. For this session we used a purpose written PC program. Subjects were instructed to perform voluntary ankle dorsi-plantarflexion movements, while observing a computer screen positioned in front of them. On the computer screen a series of six randomized figures were presented in random order. Each figure illustrated a different series of combinations of dorsi- and plantarflexion movements. The position of the ankle joint (measured by the goniometer) was displayed as a circular cursor on the computer screen. The cursor moved automatically from the left to the right at a velocity that was determined for each screen protocol. Subjects were able to control the movement of the cursor by performing ankle dorsi- and plantarflexions. During dorsiflexion the cursor moved downward, while during plantarflexion the cursor moved upward. The training consisted of eight 4 min periods of continuous ankle dorsi- and plantarflexion movements with 2 min of rest in between. To measure motor performance, one of the six randomized figures that the subject had to follow by moving the ankle joint was divided in 10 equal parts by vertical lines. The error was measured as the difference between the target and the actual position of the ankle joint at each of these points. A sum error was obtained for each subject during the first 4 min of training and compared to the error during the last 4 min of training. Motor performance was recorded during the first and the last 4 min of visuo-motor skill training session. Signals were recorded by Spike2 software (CED, Cambridge, UK) and stored for later analysis.
Control session (without visual feedback)
For the control session, subjects were instructed to perform alternating voluntary ankle dorsi- and plantarflexion movement at a comfortable speed for eight 4 min periods with a 2 min rest interval. The starting position was neutral (90 deg) and the amplitude of the movements ranged between 25 deg of dorsi- and 30 deg plantarflexion. This amplitude of movement was similar to the movements performed during the visuo-motor skill training session. Subjects were instructed to perform a single dorsi- and plantarflexion movement in 1 s, which corresponded to the average frequency of movement in the visuo-motor skill training session. The speed of movement was approximately 48–64 deg s−1. Motor performance was assessed by instructing the subjects to perform 4 min of the visuo-motor skill task before and after the session (see visuo-motor skill session).
EMG recordings
Surface electrodes were used for stimulation and recording EMG activity. EMG activity was recorded from the TA muscle during all experiments. Two pairs of bipolar surface electrodes were positioned on the trained leg and one pair on the contralateral or untrained leg (interelectrode distance, 2 cm). The amplified EMG signals were filtered (band-pass, 5 Hz to 1 kHz), sampled at 2 kHz, and stored on a PC for off-line analysis.
EEG recordings
EEG activity was recorded through a pair of bipolar silver electrodes. One electrode was placed at the vertex (Cz) and the other one 5 cm frontal to Cz. EEG signals were amplified (50 000 times), filtered (1–1000 Hz) and stored for later analysis.
Data analysis
The procedures for calculation of coherence and cumulant density functions between two signals have been described in detail in previous publications (Halliday et al. 1995).
Briefly, fxx(λ) and fyy(λ) are used to represent the power spectra of processes x and y, respectively.
In the frequency domain, the correlation between the EEG and EMG signals is assessed through coherence functions (Halliday et al. 1995). The coherence function between the two signals is defined at frequency l as:
| (1) |
In the time domain the cumulant density function, denoted by qxy(u), is defined as the inverse Fourier transform of the cross spectrum
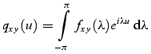 |
(2) |
In order to summarize the correlation structure across subjects we added the values for coherence and cumulant density functions for all subjects. The spectra and cumulant density functions were compared before and after each session. For analysis, we used the EEG signal as a reference. At baseline, all subjects showed a significant level of coherence (above 95% confidence interval) between the EEG and TA EMG recordings around 15–35 Hz frequency during tonic dorsiflexion.
EEG–EMG and EMG–EMG coherence estimates were compared for statistically significant differences before and after the visuo-motor skill training and control session using the difference of coherence test described in Rosenberg et al. (1989). This test is applied to the coherency estimates (where the coherence is the magnitude squared of the coherence), which are first transformed using Tanh−1 to stabilize the variance. The difference of these transformed coherency estimates were tested for significance using a standardized normal variate, with variance 1/L, at a 5% significance level, where L is the number of segments averaged in the spectral estimates; (see Rosenberg et al. 1989 for further details).
A two-way ANOVA was used to determine the effect of the visuo-motor skill task and the control session (with time of measurement as a factor: pre and post) on EEG and EMG power (with frequency as a factor: 0–10, 11–20, 21–30, 31–40 and 41–50 Hz). A repeated measures ANOVA was performed to compare the effect of training on motor performance (with training (skill versus control) and time (pre versus post) as factors). Bonferroni's post hoc test was performed on significant comparisons. Pearson's correlation analysis was performed to test correlation between motor performance and the magnitude of coherence after each session.
Results
Motor performance
In order to compare motor performance in the same subjects who participated in both sessions data from only seven subjects are reported. A repeated measures ANOVA showed a significant interaction between the factors training (skill versus control) and time (pre and post) (F = 7.08, P = 0.03), indicating that the performance of the visuo-motor task was improved following the visuo-motor skill training session, but not following the control session (Fig. 1C). The error (cm) between the target and the actual movement exerted by the subject decreased by 22.2% following the training session (36 cm before and 28 cm after training) and by only 6% following the control session (35 cm before and 33 cm after the session). Traces from a single subject are shown in Fig. 1D.
EEG–EMG coupling
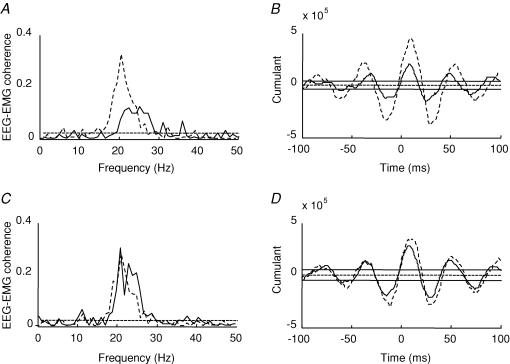
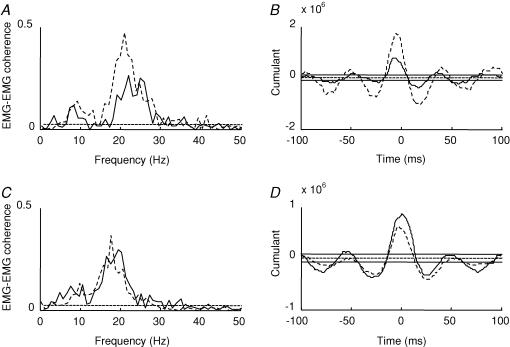
The difference of coherence test described in Rosenberg et al. (1989) showed that following the visuo-motor skill training session, 9 of the 11 subjects showed a significant increase in the magnitude of coherence in the 15–35 Hz frequency range (before: 0.12 ± 0.05 (range 0.05–0.2) and after: 0.22 ± 0.1 (range 0.09–0.4); mean ± s.d.; see Table 1). Figure 2A illustrates the changes in corticomuscular coherence following the visuo-motor skill training in a single subject (dashed lines: after training, continous lines: before training). The peak of coherence before the training session was observed between 20 and 27 Hz and shifted to lower frequencies around 18 Hz following the training session. In contrast, following the control session coherence around 15–35 Hz was increased in only 1 out of 8 subjects (difference of coherence test described in Rosenberg et al. 1989; Fig. 2C, data from a single subject) and there was no change in the range of frequencies in which significant coherence was observed (19–28 Hz before and 18–29 Hz after the control session).
Table 1.
Coherence estimates in individual subjects before and after the visuo-motor skill training and a control session
| Visuo-motor skill Control session | ||||||||
|---|---|---|---|---|---|---|---|---|
| Peak coh (pre) | Peak coh. (post) | Range (pre) | Range (post) | Peak coh. (pre) | Peak coh. (post) | Range (pre) | Range (post) | |
| 1 | 0.13 | 0.33 | 19–29 | 16–28 | 0.19 | 0.2 | 21–25 | 20–27 |
| 2 | 0.16 | 0.4 | 20–32 | 18.5–30 | 0.31 | 0.28 | 18–30.5 | 19–30 |
| 3 | 0.05 | 0.09 | 24–27 | 18–28 | 0.05 | 0.07 | 22–27 | 18–28 |
| 4 | 0.2 | 0.32 | 17–24 | 14–24 | 0.4 | 0.4 | 16–20 | 14–22 |
| 5 | 0.2 | 0.26 | 21–32 | 15–28.5 | 0.19 | 0.1 | 22.5–35 | 24–34 |
| 6 | 0.15 | 0.1 | 21–31 | 21–30 | 0.12 | 0.07 | 16.5–34 | 14–32 |
| 7 | 0.05 | 0.09 | 20–27 | 18–28 | 0.1 | 0.13 | 22–33 | 23–32 |
| 8 | 0.1 | 0.12 | 15–21 | 14–20 | 0.1 | 0.1 | 14–26 | 13–27 |
| 9 | 0.07 | 0.16 | 20–30 | 13–31 | — | — | — | — |
| 10 | 0.12 | 0.17 | 26–30 | 22.5–32 | — | — | — | — |
| 11 | 0.1 | 0.4 | 18.5–25 | 15–25 | — | — | — | — |
Peak coherence values (peak coh.) and frequency range (range; Hz) at which coherence estimates were measured in all subjects who participated in the visuo-motor skill training and control session. The peak coherence corresponds to the highest peak of the coherence estimate for each subject measured before (pre) and after (post) the visuo-motor skill training and control session. The range correspond to the frequency domain at which the coherence peak was significantly different as measured by the difference of coherence test described in Rosenberg et al. (1989), P < 0.05.
Figure 2. Effect of visuo-motor skill training and control session on EEG–EMG coherence.
A and C, traces showing EEG–EMG coherence before (continuous line) and after (dashed line) the visuo-motor skill (A) and control session (C) from two different subjects. B and D, the cumulant density function from the same subject before (continuous line) and after (dashed line) the visuo-motor skill training (B) and control session (D). The ordinate shows the size of the cumulant density function. The abscissa shows the time domain (milliseconds). The 95% confidence limit is given by the horizontal dashed line in A and C and by the horizontal continuous lines on either side of zero in band D.
Four subjects showed significant corticomuscular coherence around 5–15 Hz before the visuo-motor skill training. After training, only one of these subjects showed an increase in corticomuscular coherence in this frequency range. Before the control session three subjects showed a significant coherence around 5–15 Hz frequency. None of them showed any changes after the session.
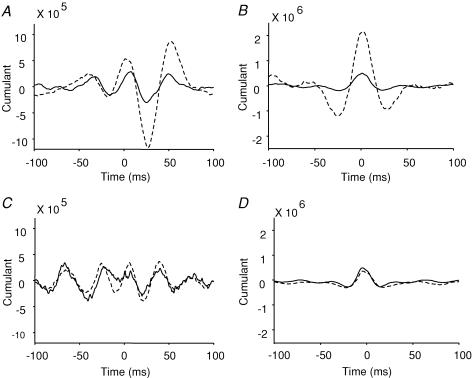
Figure 2B–D illustrates the cumulant density function from a single subject calculated for the EEG and EMG before and after either the control or the visuo-motor skill training session. In all subjects, it is seen that following the visuo-motor skill training session the largest negative peak observed at a lag around 28 ms increased significantly (P < 0.01, Fig. 3A). This lag is consistent with transmission in corticospinal pathways (Farmer et al. 2004). No changes were observed in this peak following the control session (P = 0.6, Fig. 3C).
Figure 3. Effect of visuo-motor skill training and control session on EEG–EMG and EMG–EMG cumulant density function.
A and C, the EEG–EMG mean cumulant density function from all subjects before (continuous line) and after (dashed line) the visuo-motor skill training (A) and control session (C). The ordinate shows the size of the cumulant density function. The abscissa shows the time domain (milliseconds). B and D, the EMG–EMG mean cumulant density function from all subjects before (continuous line) and after (dashed line) the visuo-motor skill training (B) and control session (D). The ordinate shows the size of the cumulant density function. The abscissa shows the time domain (milliseconds).
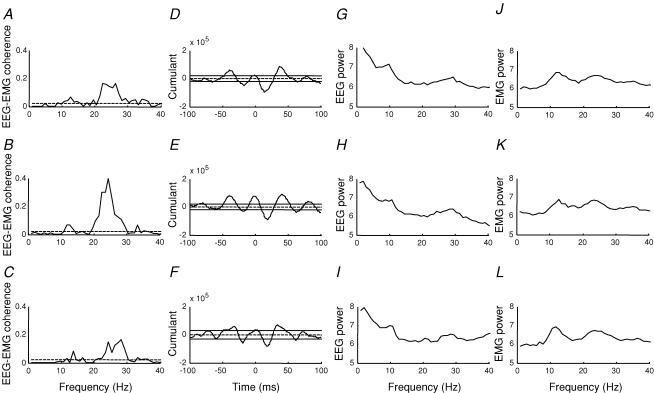
Several recordings of 2 min duration were obtained for up to 40 min after the training session in all subjects. As shown for a single subject in Fig. 4A–C the increase in coherence could last for up to 15 min. However, on average it lasted for 11.5 min in the 11 subjects (range 5–30 min). The large negative peak at a lag around 28 ms in the cumulant density function was increased for a similar duration (Fig. 4D–F). These changes in coherence and the cumulant density function were not accompanied by any changes in the EEG and EMG power as illustrated in Fig. 4G–L. In both EEG and EMG power spectra characteristic peaks around 10 and 20 Hz were observed. In all subjects an ANOVA showed no differences in either EEG or EMG power following either the visuo-motor skill training or control session.
Figure 4. Time course of the effect of visuo-motor skill training on EEG–EMG coherence.
Traces showing the duration of the effect of visuo-motor skill training on EEG–EMG coherence around 15–35 Hz in a single subject. Measurements are shown at baseline (before training, A), immediately after (B), and up to 15 min after the visuo-motor skill session was stopped (C). The cumulant density function at each corresponding time is shown in the right side (D–F). The ordinate shows the size of the cumulant density function. The abscissa shows the time domain (milliseconds). The EEG power (G–I) and EMG power (J–L) is shown at the same time points. The 95% confidence limit is given by the dashed horizontal line in A, B and C and by the continuous lines on either side of zero in D, E and F.
Pearson correlation analysis showed a weak, but not significant correlation between performance of the task and the magnitude of corticomuscular coherence after the visuo-motor skill training session (r = 0.47, P = 0.2). We also found no significant correlation between performance of the task and the magnitude of the cumulant density function after the visuo-motor skill training session (r = 0.36, P = 0.4).
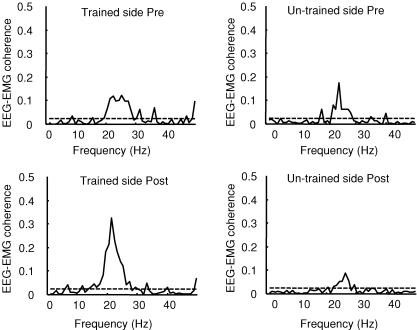
In a control experiment we tested if changes in corticomuscular coherence were specific to the trained muscle. Four subjects showed significant coherence in the 15–35 Hz frequency range between EEG and EMG recorded from the contralateral (untrained) TA during dorsiflexion prior to the visuo-motor skill training session. Figure 5 illustrates the size of the corticomuscular coherence in the trained and untrained muscle before and after the visuo-motor skill training session in a single subject. In this as well as the other subjects no changes in the magnitude of coherence were observed following the training session.
Figure 5. Effect of visuo-motor skill training on EEG–EMG coherence on the trained and untrained muscle.
Traces showing the size of corticomuscular coherence on the trained and untrained limb, before and after the visuo-motor skill training session in a single subject. On each graph, the ordinate shows the size of EEG–EMG coherence and the abscissa shows the frequency domain (Hz). The 95% confidence limit is given by horizontal dashed line.
EMG–EMG coupling
In all subjects recordings were obtained from two pairs of electrodes placed over the TA muscle at a distance of around 12–15 cm in order to study also the coupling between populations of TA motor units. It is less likely that at this distance between electrodes cross-talk affected our results (Winter et al. 1994). Following the visuo-motor skill training session, 8 of the 11 subjects showed a significant increase in the magnitude of coherence around 15–35 Hz frequency (before, 0.4 ± 0.11 (range 0.27–0.6) and after 0.6 ± 0.17 (range 0.3–0.8); mean ± s.d.). Figure 6A illustrates the changes in corticomuscular coherence following the visuo-motor skill training in a single subject (dashed lines: after training, continuous lines: before training). A similar change in coherence was not observed following the control session (Fig. 6B). Seven of the eight subjects also showed a significant increase in the magnitude of EEG–EMG coherence around 15–35 Hz following the training session. Pearson correlation analysis showed a moderate, but not significant correlation between the magnitude of enhancement of EMG–EMG and EEG–EMG coherence after the visuo-motor skill training (r = 0.52, P = 0.2). Figure 6B–D illustrates the cumulant density function from a single subject calculated for the EMG and EMG before and after either the control or the visuo-motor skill training session. In all subjects, it is seen that following the visuo-motor skill training session the largest peak was increased significantly (P = 0.04, Fig. 3B) while no changes were observed in this peak following the control session (P = 0.3, Fig. 3D). These changes in coherence and the cumulant density function were not accompanied by any changes in the EMG power. ANOVA showed no differences in EMG power following either the visuo-motor skill training or the control session.
Figure 6. Effect of visuo-motor skill training and a control session on EMG–EMG coherence.
A and C, traces showing the size of EMG–EMG coherence around 13–35 Hz before (continuous line) and after (dashed line) the visuo-motor skill (A) and control session (C) in a single subject. B and D, the cumulant density function from the same subject before (continuous line) and after (dashed line) the visuo-motor skill training (B) and control session (D). The ordinate shows the size of the cumulant density function. The abscissa shows the time domain (milliseconds). The 95% confidence limit is given by the dashed horizontal line in A and C and by the continuous lines on either side of zero in band D.
Different levels of EMG
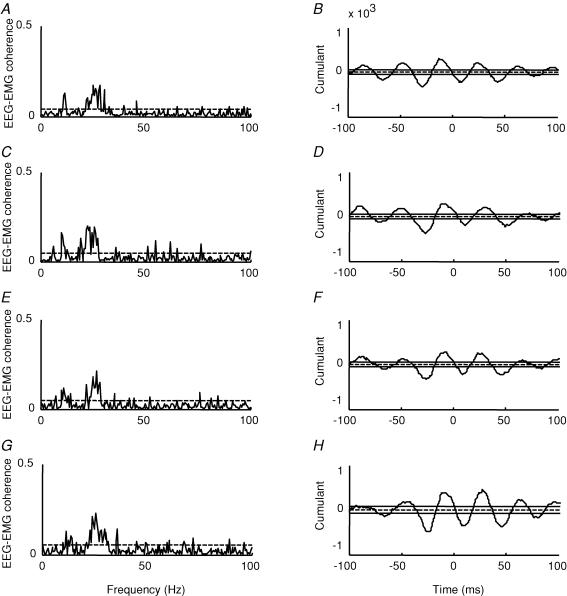
The background TA EMG was continuously monitored during the tonic contraction at which the corticomuscular coherence was tested, to ensure that the contraction level was comparable before and after the training sessions. Indeed, the TA RMS amplitude was not different before and after the visuo-motor skill training (P = 0.5). However, small differences in the level of EMG were unavoidable and it may also be questioned to what extent the EMG level reliably reflects the effort exerted by the subject. Since it is unclear to what extent the amount of corticomuscular coherence is influenced by the contraction level and the exerted effort during ankle dorsiflexion, we recorded the EEG–EMG coherence at several different levels of contraction in five of the subjects. Data from one of these subjects is illustrated in Fig. 7. In this subject as in the four other subjects no significant differences in the magnitude of coherence in the 15–35 Hz frequency band was observed for contraction levels between 5 and 20% of MVC. In 3 out of the 5 subjects, coherence was increased at 30% of MVC. In our study subjects performed between 10 and 15% of MVC before and after training (12.7 ± 2.6, mean ± s.d.). Small changes in the level of MVC are thus unlikely to have contributed to the observed increase of coherence following the training.
Figure 7. Effect of increasing levels of voluntary dorsiflexion on EEG–EMG coherence.
A, C, E and G, traces from a single subject showing the size of corticomuscular coherence around 15–35 Hz at increasing level of voluntary dorsiflexion (A, 5%, C, 10%, E, 15% and G, 20% of the maximum voluntary contraction, MVC). The ordinate shows the size of the corticomuscular coherence. The abscissa shows the frequency domain (Hz). B, D, F and H, the mean cumulant density function from the same subject at each level of MVC. The ordinate shows the size of the cumulant density function. The abscissa shows the time domain (milliseconds). The 95% confidence limit is given by the horizontal dashed lines in A, C, E and G by the continuous lines on either side of zero in B, D, F and H.
Discussion
The main finding in the present study is that corticomuscular coherence in the 15–35 Hz frequency band increased by approximately 50% in 9 out of 11 subjects following visuo-motor skill training, but not after a control session consisting of simple ankle dorsi- and plantarflexion movements. Since this increase was furthermore restricted to the trained leg and did not involve changes in EEG and EMG power, it likely reflects changes in the corticospinal drive to the spinal motorneurones as part of the motor learning process.
Mechanism of increased corticomusclar coherence
Perez et al. (2004) observed an increased excitability of the cortical representation of the TA muscle following the same visuo-motor training task as investigated in the present study. Similar findings have been reported in a number of studies for the hand area of the motor cortex (Pascual-Leone et al. 1994, 1995; Elbert et al. 1995; Classen et al. 1998, 1999; Lotze et al. 2003; Perez et al. 2004; Jensen et al. 2005). It has been suggested that corticomuscular coherence is probably mediated by fast corticospinal axons and their monosynaptic connections to spinal motorneurones (Farmer et al. 1993; Conway et al. 1995). Therefore, it is not surprising that changes in the excitability of the corticospinal cells, which is implied by the observations of Perez et al. (2004), also lead to changes in corticomuscular coherence as observed in the present study. The similar duration of the changes in cortical excitability in the study by Perez et al. (2004) and corticomuscular coherence following the training session (around 10 min) also supports that the two observations are related.
The increase in presynaptic inhibition of Ia afferents, which we have reported recently (Perez et al. 2005), is also a possible contributing factor to the increased corticomuscular coherence following the training session. It is well documented that sensory, as well as internally generated, events, may lead to decreased EEG activity in the alpha and beta bands (event-related desynchronization; Neuper & Pfurtscheller, 2001) and reduced corticomuscular coherence in the beta band (Salenius et al. 1997; Feige et al. 2000; Hansen & Nielsen, 2004). Increased presynaptic inhibition might depress the Ia afferent input (at least to the spinal motoneurones; cf. Perez et al. 2005) sufficiently to reduce this desynchronization effect from Ia afferent activity and hence result in increased corticomuscular coherence. However, there are also studies which support the opposite effect. Kilner et al. (2004) thus reported weak corticomuscular coherence in a deafferented subject and suggested that the sensory afferents would normally contribute to the corticomuscular coherence. In line with this, block of cutaneous feedback from the hand also leads to reduced corticomuscular coherence (Fisher et al. 2002). To what extent sensory feedback of different modalities contributes to corticomuscular coherence is thus not fully clarified and any role of changes in this feedback following the training session therefore remains uncertain.
Previous studies have shown that intracortial inhibition decreases in relation to the expansion of the cortical representation of specific muscles during motor learning (Liepert et al. 1998; Classen et al. 1999; Perez et al. 2004), which suggests that changes in the cortical neuronal circuitry may be involved in the changes in the cortical excitability. Such changes may also be involved in the increased corticomuscular coherence, since both inhibitory and facilitatory local circuitries likely influence the oscillatory properties of the corticospinal cells significantly (Baker & Baker, 2003; Hansen & Nielsen, 2004). A previous study demonstrated that diazepam increases the EEG power around 20 Hz with no effect or a small suppression of corticomuscular coherence at this frequency (Baker & Baker, 2003). This may indicate that inhibitory systems have a different effect on cortical oscillations and corticomuscular coherence or that corticomuscular coherence may have a more independent role in sensorimotor integration processes.
Relation of corticomuscular coherence to motor learning and task performance
The increased corticomuscular coherence following the training session is unlikely to be caused by a non-specific ‘after-effect’ such as the increased corticomuscular coherence observed following movements by Baker et al. (1999) and the event-related synchronization in the EEG beta band observed by Pfurtscheller and others (Pfurtscheller & Neuper, 1992, 1997; Salmelin & Hari, 1994; Feige et al. 1996). Firstly, these effects are seen immediately following contractions and are relatively short lasting (up to a couple of seconds at most), whereas the increased coherence in our study lasted several minutes. Secondly, the corticomuscular coherence was only observed following the training session, whereas the control session, which included an equal amount of muscle contraction at similar movement velocities, had no effect.
It is also unlikely that changes in the level of muscle contraction following the training session contributed to the observed changes in corticomuscular coherence, since we, and others (Mima et al. 1999), found no changes in the level of coherence with increasing contraction levels well below and above those maintained by the subjects during the test (Fig. 7).
The variability in the magnitude of coherence between subjects in this and previous studies indicates that corticomuscular coherence in the 15–35 Hz frequency band is not essential for task performance as such (see for instance Kilner et al. 1999; Riddle & Baker, 2005). We also found no significant correlation between the changes in corticomuscular coherence and the improved motor performance. Furthermore, the subjects performed equally well 10–15 min after the training session as immediately after, although the magnitude of the corticomuscular coherence was very different. It is thus unlikely that the increased corticomuscular coherence and the improved performance are directly related to each other.
It therefore seems more likely that the increased corticomuscular coherence is somehow related to the process of learning the motor task. There is good evidence that the primary motor cortex is involved in the early consolidation of motor memory and that it is essential in the early stages of acquisition of novel motor tasks (Müllbacher et al. 2001). The available data also suggest that increased cortical excitability and the increased representation of specific muscles in the early stages of motor learning in all likelihood reflect this early acquisition and consolidation (Müllbacher et al. 2001; Pascual-Leone et al. 1995). It seems likely to us that the changes in corticomuscular coherence that we have observed here are yet another manifestation of this early acquisition.
It has been suggested that the increased cortical excitability and increased representation of specific muscles in relation to motor learning may also be related to the increased attention to the task, which is a characteristic of early acquisition of novel motor tasks (Pascual-Leone et al. 1995; Hazeltine et al. 1997; Müllbacher et al. 2001; Perez et al. 2004). The visual feedback provided during the visuo-motor skill training session may have contributed to increase the attention to the motor task. There is indeed independent evidence that corticomuscular coherence may also be related to the level of attention to the task. Kristeva-Feige et al. (2002) observed that the magnitude of corticomuscular coherence was increased during a motor task that requires a high degree of attention and precision. In their study synchronization within the beta band was shifted to lower frequencies (within the beta band) when low precision in force production was required, suggesting that the frequency of synchronization possibly encodes precision in force production (Kristeva-Feige et al. 2002). We also observed that the coherence within the beta band shifted to lower frequencies following the visuo-motor skill training session. This may indicate that after the training, subjects required less attention to perform the same motor task. This may be a reflection of the early acquisition process.
The level of attention to the motor task may also have contributed to the findings by Kilner et al. (2000), who observed that corticomuscular coherence increases with the compliance of an object held in the hand. More indirect evidence comes from observations of short-term motor unit synchronization, which is enhanced during demanding motor task requiring high levels of attention (Kilner et al. 2002; Schmied et al. 2000). It has been argued that short-term motor unit synchronization reflects the corticospinal drive to the motoneurones and thus may be closely related to corticomuscular coherence (Farmer et al. 1993; Conway et al. 1995; Halliday et al. 1998). In the present study we also observed an increase in EMG: EMG coherence and the central peak in the cumulant density function following the training task.
Our observations could appear to be at variance with the observations by Semmler et al. (2004), who demonstrated that individuals who trained a motor task that requires a high degree of attention and precision (i.e. musicians) for a prolonged period of time showed a less pronounced motor unit coherence around 15–35 Hz than untrained individuals. However, Semmler et al. did not study an active motor learning process as we did in the present study. In addition, musicians may show long-term adaptations in relation to motor skill training, which may be different from the short-term adaptations observed in our study. Another explanation for the difference in the results is that musicians perform much more fractionated tasks than the one performed in our study. A more independent discharge of motor units in the hand muscles of musicians has been observed and suggested to indicate adaptations in the central descending command in these subjects (Semmler & Nordstrom, 1998). Indeed, motor unit synchronization generated by a larger number of independent inputs is less likely to produce significant coherence peaks compared to common branched inputs (Taylor & Enoka, 2004).
Lack of change in 5–15 Hz corticomuscular coherence
Corticomuscular coherence in the 5–15 Hz frequency band is only rarely observed in healthy subjects (Conway et al. 1995; Salenius et al. 1997; Brown, 2000; Raethjen et al. 2002). In the present study coherence in this frequency band was observed in only four subjects. Our results showed no change in the coherence in three of these four subjects following the visuo-motor skill training session. Since the origin of the 5–15 Hz oscillations is not completely understood (McAuley et al. 1997; Marsden et al. 2000) and we have data from only four subjects it is not possible to reach any conclusions regarding these data.
Functional implications
The functional significance of corticomuscular coherence in the 15–35 Hz frequency band still remains unclear (for reviews see Farmer, 1998; Baker et al. 1999). Human studies have proposed that corticomuscular coherence around 15–35 Hz may act as a component of the descending corticospinal command to recruit motorneurones (Farmer et al. 1993; Conway et al. 1995). Evidence has shown that corticospinal cells are more likely to respond to synchronous input when they are driven at slower rates (Stafstrom et al. 1984; Schwindt et al. 1997). Therefore, it has been argued that more synchronous descending commands might recruit motorneurones more efficiently with a lower input firing rate (Baker et al. 1997, 1999; Kilner et al. 1999). However, what benefit this would have in the relation to motor learning is unclear. We thus tend to believe that the increased corticomuscular coherence simply reflects a tighter cortical control of the muscle activity in relation to the acquisition of the task. This tight cortical control of muscle activity may be the result of a greater binding of visuo-motor information. In the visual system, synchronous oscillations between central networks may be one of the mechanisms that contribute to the binding of related information that is processed by different neuronal networks (Singer & Gray, 1995). Whether this is also the case in the motor system remains unclear.
Acknowledgments
The authors would like to thank Dr Bernie Conway for his helpful comments and Dr David Halliday for writing the scripts for analysis. This study was supported by The Danish Society of Multiple Sclerosis, The Danish Health Research Council, The Danish Ministry of Culture and the Elsass Foundation.
References
- Baker MR, Baker SN. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J Physiol. 2003;546:931–942. doi: 10.1113/jphysiol.2002.029553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Kilner JM, Pinches EM, Lemon RN. The role of synchrony and oscillations in the motor output. Exp Brain Res. 1999;128:109–117. doi: 10.1007/s002210050825. [DOI] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol. 1997;501:225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. Cortical drives to human muscle: the Piper and related rhythms. Progr Neurobiol. 2000;60:97–108. doi: 10.1016/s0301-0082(99)00029-5. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Hallett M, Cohen L. Plasticity of movement representation in the human motor cortex. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:162–173. [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Conway BA, Halliday DM, Farmer SF, Shahani U, Maas P, Weir AI, Rosenberg JR. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J Physiol. 1995;489:917–924. doi: 10.1113/jphysiol.1995.sp021104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Farmer SF. Rhythmicity, synchronization and binding in human and primate motor systems. J Physiol. 1998;509:3–14. doi: 10.1111/j.1469-7793.1998.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Bremner FD, Halliday DM, Rosenberg JR, Stephens JA. The frequency content of common synaptic inputs to motoneurones studied during voluntary isometric contraction in man. J Physiol. 1993;470:127–155. doi: 10.1113/jphysiol.1993.sp019851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SF, Halliday DM, Conway BA, Stephens JA, Rosenberg JR. A review of recent applications of cross-correlation methodologies to human motor unit recording. J Neurosci Meth. 1997;74:175–187. doi: 10.1016/s0165-0270(97)02248-6. [DOI] [PubMed] [Google Scholar]
- Farmer SF, Harrison LM, Mayston MJ, Parekh A, James LM, Stephens JA. Abnormal cortex–muscle interactions in subjects with X-linked Kallmann's syndrome and mirror movements. Brain. 2004;127:385–397. doi: 10.1093/brain/awh047. [DOI] [PubMed] [Google Scholar]
- Feige B, Aertsen A, Kristeva-Feige R. Dynamic synchronization between multiple cortical motor areas and muscle activity in phasic voluntary movements. J Neurophysiol. 2000;84:2622–2629. doi: 10.1152/jn.2000.84.5.2622. [DOI] [PubMed] [Google Scholar]
- Feige B, Kristeva-Feige R, Rossi S, Pizzella V, Rossini PM. Neuromagnetic study of movement-related changes in rhythmic brain activity. Brain Res. 1996;734:252–260. [PubMed] [Google Scholar]
- Fisher RJ, Galea MP, Brown P, Lemon RN. Digital nerve anaesthesia decreases EMG-EMG coherence in a human precision grip task. Exp Brain Res. 2002;145:207–214. doi: 10.1007/s00221-002-1113-x. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Conway BA, Farmer SF, Rosenberg JR. Using electroencephalography to study functional coupling between cortical activity and electromyograms during voluntary contractions in humans. Neurosci Lett. 1998;241:5–8. doi: 10.1016/s0304-3940(97)00964-6. [DOI] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data – theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- Hansen NL, Nielsen JB. The effect of transcranial magnetic stimulation and peripheral nerve stimulation on corticomuscular coherence in humans. J Physiol. 2004;561:295–306. doi: 10.1113/jphysiol.2004.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry R. Attention and stimulus characteristics determine the locus of motor-sequence encoding. A PET study. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol. 2005;99:1558–1568. doi: 10.1152/japplphysiol.01408.2004. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Alonso-Alonso M, Fisher R, Lemon RN. Modulation of synchrony between single motor units during precision grip tasks in humans. J Physiol. 2002;541:937–948. doi: 10.1113/jphysiol.2001.013305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci. 2000;20:8838–8845. doi: 10.1523/JNEUROSCI.20-23-08838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Baker SN, Salenius S, Jousmaki V, Hari R, Lemon RN. Task-dependent modulation of 15–30 Hz coherence between rectified EMGs from human hand and forearm muscles. J Physiol. 1999;516:559–570. doi: 10.1111/j.1469-7793.1999.0559v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner JM, Fisher RJ, Lemon RN. Coupling of oscillatory activity between muscles is strikingly reduced in a deafferented subject compared with normal controls. J Neurophysiol. 2004;92:790–796. doi: 10.1152/jn.01247.2003. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Salenius S, Baker SN, Jackson A, Hari R, Lemon RN. Task-dependent modulations of cortical oscillatory activity in human subjects during a bimanual precision grip task. Neuroimage. 2003;18:67–73. doi: 10.1006/nimg.2002.1322. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS, Nudo RJ. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristeva-Feige R, Fritsch C, Timmer J, Lucking CH. Effects of attention and precision of exerted force on beta range EEG-EMG synchronization during a maintained motor contraction task. Clin Neurophysiol. 2002;113:124–131. doi: 10.1016/s1388-2457(01)00722-2. [DOI] [PubMed] [Google Scholar]
- Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Exp Brain Res. 1998;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126:866–872. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- Marsden JF, Ashby P, Limousin-Dowsey P, Rothwell JC, Brown P. Coherence between cerebellar thalamus, cortex and muscle in man: cerebellar thalamus interactions. Brain. 2000;123:1459–1470. doi: 10.1093/brain/123.7.1459. [DOI] [PubMed] [Google Scholar]
- McAuley JH, Rothwell JC, Marsden CD. Frequency peaks of tremor, muscle vibration and electromyographic activity at 10 Hz, 20 Hz and 40 Hz during human finger muscle contraction may reflect rhythmicities of central neural firing. Exp Brain Res. 1997;114:525–541. doi: 10.1007/pl00005662. [DOI] [PubMed] [Google Scholar]
- Mima T, Simpkins N, Oluwatimilehin T, Hallett M. Force level modulates human cortical oscillatory activities. Neurosci Lett. 1999;275:77–80. doi: 10.1016/s0304-3940(99)00734-x. [DOI] [PubMed] [Google Scholar]
- Müllbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136:431–438. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Oscillatory activity in sensorimotor cortex of awake monkeys: synchronization of local field potentials and relation to behavior. J Neurophysiol. 1996;76:3949–3967. doi: 10.1152/jn.1996.76.6.3949. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. Int J Psychophysiol. 2001;43:41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Hallett M. Modulation of cortical motor output maps during development of implicit and explicit knowledge. Science. 1994;263:1287–1289. doi: 10.1126/science.8122113. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nielsen JB. Presynaptic control of group Ia afferents in relation to acquisition of a visuo-motor skill in healthy humans. J Physiol. 2005;568:343–354. doi: 10.1113/jphysiol.2005.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C. Simultaneous EEG 10 Hz desynchronization and 40 Hz synchronization during finger movements. Neuroreport. 1992;3:1057–1060. doi: 10.1097/00001756-199212000-00006. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Edlinger G. On the existence of different types of central beta rhythms below 30 Hz. Electroencephalogr Clin Neurophysiol. 1997;102:316–325. doi: 10.1016/s0013-4694(96)96612-2. [DOI] [PubMed] [Google Scholar]
- Raethjen J, Lindemann M, Dumpelmann M, Wenzelburger R, Stolze H, Pfister G, Elger CE, Timmer J, Deuschl G. Corticomuscular coherence in the 6–15 Hz band: is the cortex involved in the generation of physiologic tremor? Exp Brain Res. 2002;142:32–40. doi: 10.1007/s00221-001-0914-7. [DOI] [PubMed] [Google Scholar]
- Remple MS, Bruneau RM, VandenBerg PM, Goertzen C, Kleim JA. Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behav Brain Res. 2001;123:133–141. doi: 10.1016/s0166-4328(01)00199-1. [DOI] [PubMed] [Google Scholar]
- Riddle CN, Baker SN. Manipulation of peripheral neural feedback loops alters human corticomuscular coherence. J Physiol. 2005;566:625–639. doi: 10.1113/jphysiol.2005.089607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophysics Mol Biol. 1989;53:1–31. doi: 10.1016/0079-6107(89)90004-7. [DOI] [PubMed] [Google Scholar]
- Salenius S, Hari R. Synchronous cortical oscillatory activity during motor action. Curr Opin Neurobiol. 2003;13:678–684. doi: 10.1016/j.conb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol. 1997;77:3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience. 1994;60:537–550. doi: 10.1016/0306-4522(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Schmied A, Pagni S, Sturm H, Vedel JP. Selective enhancement of motoneurone short-term synchrony during an attention-demanding task. Exp Brain Res. 2000;133:377–390. doi: 10.1007/s002210000421. [DOI] [PubMed] [Google Scholar]
- Schwindt P, O'Brien JA, Crill W. Quantitative analysis of firing properties of pyramidal neurons from layer 5 of rat sensorimotor cortex. J Neurophysiol. 1997;77:2484–2498. doi: 10.1152/jn.1997.77.5.2484. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Nordstrom MA. Motor unit discharge and force tremor in skill- and strength-trained individuals. Exp Brain Res. 1998;119:27–38. doi: 10.1007/s002210050316. [DOI] [PubMed] [Google Scholar]
- Semmler JG, Sale MV, Meyer FG, Nordstrom MA. Motor-unit coherence and its relation with synchrony are influenced by training. J Neurophysiol. 2004;92:3320–3331. doi: 10.1152/jn.00316.2004. [DOI] [PubMed] [Google Scholar]
- Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Schwindt PC, Crill WE. Repetitive firing in layer V neurons from cat neocortex in vitro. J Neurophysiol. 1984;52:264–277. doi: 10.1152/jn.1984.52.2.264. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Enoka RM. Quantification of the factors that influence discharge correlation in model motor neurons. J Neurophysiol. 2004;91:796–814. doi: 10.1152/jn.00802.2003. [DOI] [PubMed] [Google Scholar]
- VandenBerg PM, Hogg TM, Kleim JA, Whishaw IQ. Long-Evans rats have a larger cortical topographic representation of movement than Fischer-344 rats: a microstimulation study of motor cortex in naive and skilled reaching-trained rats. Brain Res Bull. 2002;59:197–203. doi: 10.1016/s0361-9230(02)00865-1. [DOI] [PubMed] [Google Scholar]
- Winter DA, Fuglevand AJ, Archer SE. Crosstalk in surface electromyography: theoretical and practical estimates. J Electromyogr Kinesiol. 1994;4:15–26. doi: 10.1016/1050-6411(94)90023-X. [DOI] [PubMed] [Google Scholar]