Abstract
The subthalamic nucleus (STN) plays an important role in movement control by exerting its excitatory influence on the substantia nigra pars reticulata (SNR), a major output structure of the basal ganglia. Moreover, excessive burst firing of SNR neurons seen in Parkinson's disease has been attributed to excessive transmission in the subthalamonigral pathway. Using the ‘blind’ whole-cell patch clamp recording technique in rat brain slices, we found that focal electrical stimulation of the STN evoked complex, long-duration excitatory postsynaptic currents (EPSCs) in SNR neurons. Complex EPSCs lasted 200–500 ms and consisted of an initial monosynaptic EPSC followed by a series of late EPSCs superimposed on a slow inward shift in holding current. Focal stimulation of regions outside the STN failed to evoke complex EPSCs. The late component of complex EPSCs was markedly reduced by ionotropic glutamate receptor antagonists (2-amino-5-phosphonopentanoic acid and 6-cyano-7-nitro-quinoxalone) and by a GABAA receptor agonist (isoguvacine) when these agents were applied directly to the STN using a fast-flow microapplicator. Moreover, the complex EPSC was greatly enhanced by bath application of the GABAA receptor antagonists picrotoxin or bicuculline. These data suggest that recurrent glutamate synapses in the STN generate polysynaptic, complex EPSCs that are under tonic inhibition by GABA. Because complex EPSCs are expected to generate bursts of action potentials in SNR neurons, we suggest that complex EPSCs may contribute to the pathological burst firing that is associated with the symptoms of Parkinson's disease.
The substantia nigra zona reticulata (SNR) is a midbrain structure that serves as a major output nucleus for the basal ganglia. Striatum and globus pallidus externa (GPE) send substantial GABA-containing inputs to SNR neurons (Ribak et al. 1980), whereas the SNR receives a significant excitatory glutamate-containing input from the subthalamic nucleus (STN) (Kita & Kitai, 1987). Composed of GABA-containing output neurons, the SNR sends inhibitory connections to ventral thalamus, superior colliculus, and pedunculopontine nucleus (Parent, 1990; Richards et al. 1997). Although SNR neurons fire regularly spaced action potentials in isolated tissue preparations (Shen & Johnson, 1997), these neurons have a wide range of firing pattern in the intact, behaving animal. For example, extracellular recordings in monkeys showed that the rate and pattern of SNR neuronal firing can be altered dramatically by eye movement or visual stimuli (Hikosaka & Wurtz, 1983) and during active or passive limb movements (Schultz, 1986). These alterations in rate and pattern of firing are undoubtedly due to the influence of synaptic inputs to SNR neurons.
In animal models of Parkinson's disease, previous studies have shown that the loss of nigrostriatal dopamine neurons results in an increased incidence of burst firing in SNR neurons (Sanderson et al. 1986; Wichmann et al. 1999). Moreover, others have shown that treatment with anti-parkinsonian dopaminergic drugs reduces the incidence of burst firing in SNR neurons (Murer et al. 1997; Tseng et al. 2000). Furthermore, Wichmann et al. (2001) showed that parkinsonian signs are significantly improved in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys by chemically lesioning the SNR with ibotenic acid or by suppressing neuronal activity by local application of the GABAA receptor agonist muscimol to the SNR. These studies are consistent with the hypothesis that excessive burst firing in SNR neurons is abnormal and may contribute to symptoms of parkinsonism. This hypothesis has gathered more support from studies showing that lesions to the STN reduce burst firing of SNR neurons recorded in parkinsonian rats (Ryan & Sanders, 1993; Burbaud et al. 1995). The findings that parkinsonism can be alleviated by lesioning the STN (Bergman et al. 1990) and by deep brain stimulation of the STN (Starr et al. 1998) have further emphasized the importance of the STN in clinical manifestations of Parkinson's disease. Thus, there is growing appreciation that many of the motor symptoms of Parkinson's disease may be mediated by burst firing caused by overactive output from STN to GABA-containing neurons in the SNR (Blandini et al. 2000).
We undertook the present study in order to characterize the physiology of the subthalamonigral pathway in horizontal slices of rat brain. Using standard whole-cell recording techniques, we found that electrical stimulation of the STN evokes polysynaptic, complex EPSCs in SNR neurons. Our findings provide useful information on how the subthalamonigral pathway may promote burst firing in the SNR.
Methods
Tissue preparation
Horizontal slices containing diencephalon and rostral midbrain (300 μm thick) were prepared from young Sprague-Dawley rats (postnatal days 8–21; Bantin and Kingman, Seattle, WA, USA) as previously described (Shen & Johnson, 1997). According to a protocol approved by the Portland Veterans Affairs Medical Center Institutional Animal Care and Use Committee, rats were anaesthetized by breathing air saturated with isoflurane and killed by severing major thoracic vessels. The brain was removed rapidly and slices were cut in cold physiological saline with a vibratome. A slice containing the STN and SNR was then placed on a supporting net and submerged in a continuously flowing solution (2 ml min−1) of the following composition (mm): NaCl, 126; KCl, 2.5; CaCl2, 2.4; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 19; glucose, 11, gassed with 95% O2 and 5% CO2 (pH 7.4) at 36°C. Using a dissection microscope for visual guidance, the SNR was identified 1–2 mm lateral to the substantia nigra zona compacta, whereas the STN was located as grey matter approximately 2.7 mm lateral to the midline and 2 mm rostral to the centre of the SNR.
Electrophysiological recordings
Whole-cell recordings were made with pipettes containing (mm): potassium gluconate, 130; MgCl2, 2; CaCl2, 1; EGTA, 11; Hepes, 10; ATP, 1.5; GTP, 0.3 (pH 7.3). Membrane currents were recorded under voltage clamp (−70 mV) and amplified with an Axopatch-1D amplifier. Data were acquired using a personal computer with a Digidata analog/digital interface and analysed using pCLAMP software (Molecular Devices, Axon Instruments, Foster City, CA, USA). Holding currents were recorded continuously using a MacLab analog/digital interface, Chart software (ADInstruments, Castle Hill, Australia) and a Macintosh computer. Membrane potentials have been corrected for the liquid junction potential (10 mV).
Synaptic currents
Synaptic currents were evoked in SNR neurons using a bipolar stimulation electrode (2–4 MΩ impedance at 1000 Hz, 10 nA; Frederick Haer & Co., USA) that was placed within the brain slice. Rectangular pulses (0.1 ms duration) of constant current (20–400 μA) were used to evoke excitatory postsynaptic currents (EPSCs) every 10 s. A complex EPSC, which could only be evoked when the stimulation electrode was placed in the STN, were identified as a short-latency EPSC followed by a series of notches and inflections that were superimposed on a long-lasting inward current. In most cases, we successfully avoided the appearance of inhibitory postsynaptic currents (IPSCs) by repositioning the stimulation electrodes, reversing polarity, or by adjusting the stimulation intensity. In some experiments, multiple electrical stimuli (2–5 pulses, 10 ms interval) or superfusion with picrotoxin (100 μm) or bicuculline (30 μm) were used to increase the amplitude of complex EPSCs. Complex EPSCs were quantified by measuring their integrated area using pCLAMP 9 software (Molecular Devices) run on a personal computer.
Drugs
All drugs were dissolved into aqueous stock solutions or dimethyl sulfoxide. Stock solutions of drugs were diluted at least 1: 1000 to the desired concentration in superfusate immediately prior to use. Dimethyl sulfoxide, diluted 1: 1000 in artificial spinal fluid, had no effect on either holding current or synaptic currents. Approximately 30 s were required for the drug solution to enter the recording chamber; this delay was due to passage of the perfusate through a heat exchanger. In some experiments, drugs were applied locally by fast flow through a microapplicator (tip diameter, 500 μm). The microapplicator was positioned immediately above the brain slice in the region of interest. In order to minimize spread of drug-containing solution from the microapplicator to the recording pipette, we arranged the brain slice in the tissue chamber such that flow from the microapplicator was always parallel to the bath flow and away from (perpendicular to) the SNR. (RS)-α-Methyl-4-carboxyphenylglycine (MCPG) and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) were obtained from Tocris Cookson Inc. (Ellisville, MO, USA). Bicuculline methiodide, picrotoxin, isoguvacine, scopolamine, l-glutamate (±)-2-amino-5-phosphonopentanoic acid (AP5) and 6-cyano-7-nitro-quinoxalone (CNQX) were obtained from Sigma Chemical Co. (St Louis, MO, USA).
Data analysis
Numerical data in the text and error bars in figures are expressed as mean ± s.e.m. Student's paired two-tailed t tests were used to test for significant differences using SigmaStat software (Jandel Scientific, San Rafael, CA, USA) run on a personal computer. A significant difference was accepted when P < 0.05. Prior to analysing data for significance, a Kolmogorov-Smirnov test was performed to ensure that data were distributed normally.
Results
General observations
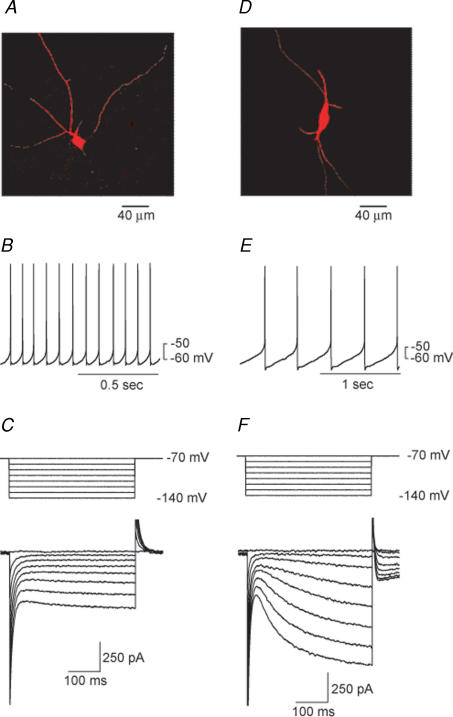
The most common type of neuron in the SNR is shown in Fig. 1A. This neuronal type (type I) has extensive local dendritic arborization, and has been shown by immunocytochemistry to contain glutamic acid decarboxylase (Richards et al. 1997). This GABA-containing neuron is considered the major output neuron from the SNR (Parent, 1990). As we (Shen & Johnson, 1997) and others (Nakanishi et al. 1987) have described, this type of neuron has a relatively fast spontaneous firing rate with narrow action potentials (Fig. 1B), exhibits prominent inward rectification and has little or no hyperpolarization-activated time-dependent H current (Ih) (Fig. 1C). A less common type of SNR neuron (type II) is shown in Fig. 1D. This neuronal type has sparse local dendritic branching and has been shown to contain tyrosine hydroxylase (Richards et al. 1997). The type II neuron, which is presumed to contain dopamine, has a relatively slow spontaneous firing rate with broad action potentials (Fig. 1E), has a small amount of inward rectification, and has a large Ih (Fig. 1F) (Nakanishi et al. 1987; Richards et al. 1997). Because we wanted to study the effects of STN stimulation on the major output neuron of the SNR, the present study focused on type I neurons. Therefore, all subsequent data were obtained from these presumed GABA-containing neurons as identified by the neurophysiological characteristics illustrated in Fig. 1B and C.
Figure 1. Two types of neuron in the SNR.
A, B and C show characteristics of presumed GABA-containing neurons. D, E and F show characteristics of presumed dopamine-containing neurons. A and D, photomicrographs of biocytin-filled neurons in 200 μm thick brain slices. B and E, current-clamp recordings from the two types of SNR neuron (0 pA holding current). Note that both cells fire action potentials spontaneously and regularly (12 Hz versus 2.5 Hz). C and F, voltage-clamp recordings showing responses to voltage steps (400 ms duration) from −70 mV to −140 mV (10 mV increments). Note that the presumed GABA neuron had a small Ih, whereas the presumed dopamine neuron had a pronounced Ih. All subsequent data were obtained from presumed GABA-containing neurons as identified by the electrophysiological characteristics illustrated in B and C.
Synaptic currents in SNR neurons evoked by STN stimulation
A single bipolar electrical stimulus delivered to the STN evoked a complex EPSC in 26 out of 38 SNR neurons. A typical complex EPSC, illustrated in Fig. 2A, is characterized by a small, short-latency EPSC (early component) followed by multiple notches and inflections (late component) that are superimposed on a slow inward current that lasted 200–500 ms. The early EPSC occurred after a latency of 4.6 ± 0.3 ms, and was typically quite small, with an average amplitude of 33.5 ± 6.4 pA (n = 26). EPSCs that comprised the late component typically had progressively larger amplitudes (Fig. 2B), and their long duration contributed to the summation of inward currents (Fig. 2C). When complex EPSCs were evoked repeatedly, as shown in Fig. 2C, one can see that the latency and amplitude of the early EPSC remained relatively constant. In contrast, EPSCs that comprise the late component had variable latencies and amplitudes even though complex EPSCs were evoked at the same stimulus strength. Variable latencies and amplitudes of EPSCs in the late component suggest polysynaptic transmission, whereas the constant latency and amplitude of the early EPSC suggest monosynaptic transmission.
Figure 2. Monosynaptic and polysynaptic components of complex EPSCs.
A, a single electrical stimulus delivered to the STN evoked a complex EPSC with early (indicated by an arrow) and late components. Note the notches and inflections on the rising and decay phases in the late component. B, a complex EPSC evoked in the same neuron shown in A with lower stimulus intensity. Note the different time scale for this trace. C, three consecutive traces are superimposed that illustrate early and late components of complex EPSCs. The early component (indicated by arrow) had a constant latency and amplitude, suggesting that it was monosynaptic. EPSCs in the late component had variable latencies, which suggests a polysynaptic aetiology. Note that the late component EPSC amplitudes summate, which contributes to a long-lasting inward current that contains many notches and inflections. Numbers above traces are stimulation strengths 0.04 and 0.02 mA.
Stimulus-dependent aspects of the complex EPSC
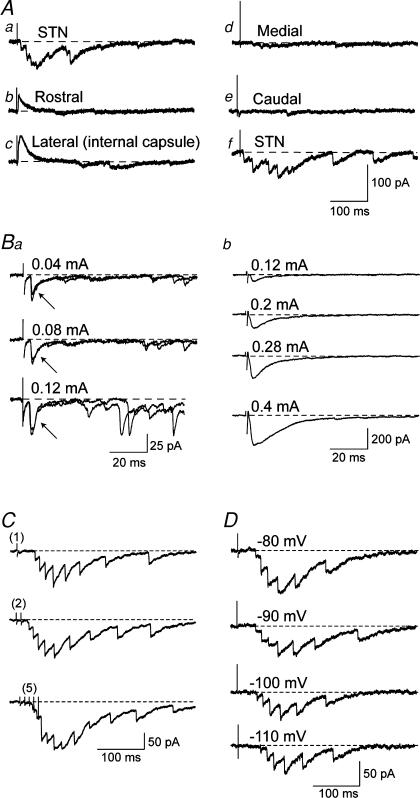
Complex EPSCs could be evoked only when the stimulation electrode was placed within the STN. As seen in recordings from the SNR neuron shown in Fig. 3A, stimulation of the STN evoked a complex EPSC that was replaced by a simple IPSC when the stimulation electrode was moved to a position just rostral to the STN or lateral (to the internal capsule). Also, no significant response was evoked when the stimulation electrode was moved medial to the STN (in the lateral hypothalamic area) or to a position caudal to the STN. It should be noted that the ‘caudal’ position lies between the STN and SNR, and yet complex EPSCs were never observed at this stimulus intensity. Finally, the complex EPSC could again be evoked in the SNR neuron when the stimulation electrode was returned to the STN (see Fig. 3A, traces a–f). Similar results were obtained in four other neurons. These findings suggest that the circuitry within the STN is necessary for generating the complex EPSC as recorded in SNR neurons.
Figure 3. Stimulus-dependent characteristics of complex EPSCs.
A, electrical stimulation of the slice evoked complex EPSCs only when the stimulation electrodes were placed in the STN (a). Electrodes placed rostral (b) or lateral (c) to the STN only evoked monosynaptic IPSCs. Moving electrodes medial (d) or caudal (e) to the STN failed to evoke complex EPSCs. Returning electrodes to the STN (f) evoked the complex EPSC. All traces in A are from the same neuron. Ba, effects of increasing stimulus intensities on EPSCs evoked by stimulation of the STN. Two responses are superimposed for each stimulation intensity. Note that the amplitude and latency of the early component (indicated by arrows) are relatively constant despite increasing stimulation strengths. However, the amplitudes of EPSCs in the late, polysynaptic component markedly increased with higher stimulation strengths. Recordings are from the same SNR neuron; stimulus intensity was 0.1 mA. Bb, local stimulation of the SNR near the recording site evoked only short latency, monosynaptic EPSCs in an SNR neuron. Note that the EPSC amplitude increased markedly at higher stimulation strengths. C, the amplitude of the complex EPSC increased with increasing number of electrical stimuli delivered to the STN. Numbers in parentheses indicate number of stimuli delivered. D, the complex EPSC is voltage dependent. The amplitude of the complex EPSC was larger at less hyperpolarized test potentials.
Figure 3B shows the effects of varied stimulus intensities delivered to either STN (Fig. 3Ba) or the SNR near the recording site (Fig. 3Bb) on EPSCs evoked in SNR neurons. As seen in Fig. 3Ba, higher stimulation strengths delivered to the STN increased both the amplitude and frequency of EPSCs in the late component of the complex EPSC. In contrast, the amplitude of the early, short-latency EPSC increased to a much smaller extent in response to higher stimulus intensities (40–120 μA). For comparison, Fig. 3Bb shows that focal stimulation of SNR near the recording site fails to evoke complex EPSCs despite relatively high stimulus intensities (from 120 to 400 μA). Stimulation near the recording site evoked monophasic EPSCs with short latencies (1.4 ± 0.1 ms; n = 7), and relatively large amplitudes. Moreover, amplitudes of these monophasic EPSCs increased markedly with increasing stimulation strengths (see Fig. 3Bb). These results are consistent with the idea that increasing the stimulus intensity delivered to the SNR near the recording pipette increases the amplitude of monophasic EPSCs by recruiting greater numbers of input fibres. In contrast, we hypothesize that increasing the stimulus intensity delivered to the STN causes a greater number of late-appearing EPSCs due to the activation of progressively larger numbers of polysynaptic connections.
We next examined the effects of number of stimuli and different test voltages on the amplitude of complex EPSCs evoked by STN stimulation. Figure 3C shows that increasing the number of stimuli (1–5 stimuli at 100 Hz) greatly increased the amplitude of the late component of the complex EPSC. This figure also shows that the increased amplitude of the late component is due, in part, to summation of multiple late EPSCs. Figure 3D shows that the amplitude of the complex EPSC is reduced at more hyperpolarized test voltages (−80 to −100 mV). As seen below (Fig. 6), this voltage dependence of the late component may be due to hyperpolarization-induced block of NMDA receptor-gated currents (Mayer et al. 1984). In order to record complex EPSCs with good amplitudes, we routinely recorded at a less hyperpolarized voltage (−70 mV) and sometimes used double stimuli.
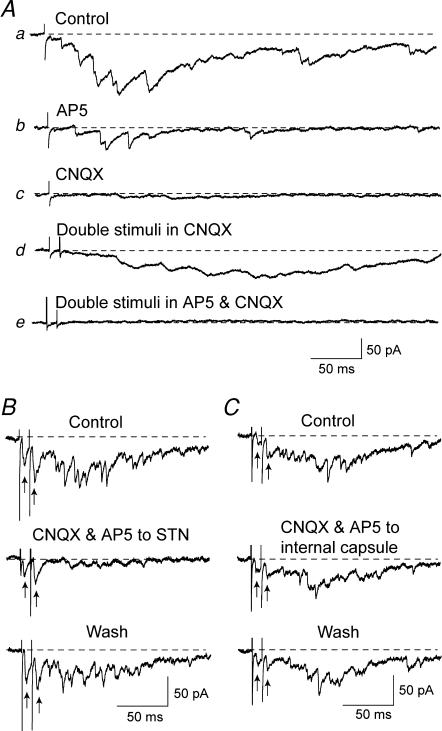
Figure 6. Pharmacology of complex EPSCs.
A, bath application of AP5 (50 μm) and CNQX (10 μm) blocked complex EPSCs. AP5, applied alone, markedly attenuated the complex EPSC (b). Although CNQX nearly abolished the complex EPSC (c), increasing the stimulus number to two (10 ms interval) caused an increase in amplitude of the complex EPSC despite continued application of CNQX (d). However, the combined application of AP5 and CNQX completely abolished the complex EPSC despite double stimuli. B, local, fast-flow application of AP5 (50 μm) and CNQX (10 μm) to the STN significantly reduced the late, polysynaptic component of the complex EPSC, whereas the early, monosynaptic component (indicated by arrows) was much less affected. Note that pairs of stimuli were used to evoke complex EPSCs. C, local application of AP5 and CNQX to the internal capsule did not affect the early or late component of the complex EPSC.
Selective block of polysynaptic EPSCs
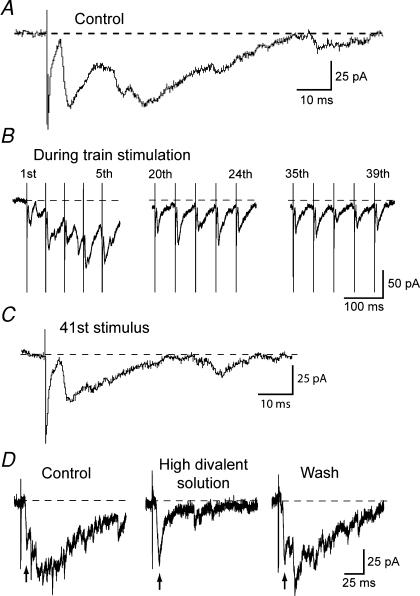
In an effort to further test the hypothesis that complex EPSCs are mediated by polysynaptic transmission, we examined the effects of repetitive electrical stimulation. Figure 4A, B and C shows the ability of a complex EPSC, recorded in a SNR neuron, to follow a 20 Hz train of stimuli (duration 2 s) delivered to the STN. Although the early EPSC could be evoked by each stimulus in the train (Fig. 4B), the EPSC evoked by the last (41st) stimulus in the train clearly shows that the late component of the complex EPSC is severely reduced compared to the early component (Fig. 4C). Because monoysynaptic connections can more faithfully respond to repetitive stimuli compared to polysynaptic connections (Berry & Pentreath, 1976), this result supports the conclusion that the early component is monosynaptic, whereas the late component is polysynaptic. Similar results were observed in five other SNR neurons.
Figure 4. Polysynaptic characteristics of complex EPSCs.
A, complex EPSC evoked in a SNR neuron under control conditions. B, EPSCs recorded during a 2 s train of stimuli delivered to the STN at 20 Hz. C, final EPSC evoked at the end of the 20 Hz train of stimuli. Note that the initial EPSC was relatively intact, whereas the late component of the complex EPSC was much reduced. Recordings in A, B and C are from the same neuron. D, superfusate containing high concentrations of divalent cations (4.2 mm Mg2+ and 7.0 mm Ca2+) reversibly reduced the late component of the complex EPSC. Note that the early, monosynaptic component, which is indicated by arrows, was relatively resistant to inhibition by the high divalent solution.
We next tested the effect of perfusing the slice with elevated concentrations of divalent cations (4.2 mm Mg2+ and 7.0 mm Ca2+), which has been shown to preferentially block polysynaptic as opposed to monosynaptic transmission (Rose & Metherate, 2005). As shown in Fig. 4D, the high divalent cation solution markedly reduced the late component of the complex EPSC, whereas the early component was relatively unaffected. Inhibition of the late component was evident within 5 min of starting the high divalent solution, and recovery was complete within 15 min of washout. This result further supports our conclusion that the late component of complex EPSCs is mediated by polysynaptic transmission. Similar results were obtained in six other SNR neurons.
Effects of local glutamate application
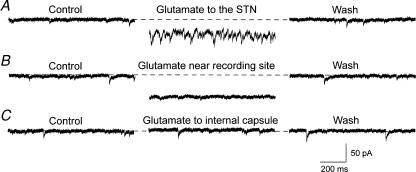
We used a fast-flow microapplicator to apply glutamate to local areas of the brain slice to investigate the effects of chemical activation of the STN on membrane currents recorded in SNR neurons. Local application of glutamate mimicked some aspects of the effect of STN stimulation. As shown in Fig. 5A, local application of glutamate (100 μm) to the STN evoked a dramatic increase in the frequency of spontaneous EPSCs that was accompanied by an inward shift in holding current (n = 5). As a control experiment, we applied glutamate near the recording site in the SNR, but this produced an inward current with no increase in spontaneous EPSCs (n = 5) (Fig. 5B). As a second control experiment, we applied glutamate laterally to the internal capsule; this produced no change in either holding current or frequency of spontaneous EPSCs (n = 4) (Fig. 5C). These results are consistent with our hypothesis that stimulation of the STN, and not adjacent areas in the brain slice, is responsible for the complex EPSC recorded in SNR neurons.
Figure 5. Local application of glutamate to the STN evokes EPSCs in SNR neurons.
A, local fast-flow application of glutamate (100 μm) to the STN caused an inward shift in holding current and a significant increase in EPSC frequency in an SNR neuron. B, local application of glutamate near the recording site (SNR) produced an inward shift in holding current but failed to evoke spontaneous EPSCs. C, local application of glutamate to the internal capsule evoked no change in holding current or EPSC frequency. All recordings were made in the same SNR neuron.
Effects of glutamate receptor antagonists on the complex EPSC
We used selective receptor antagonists to characterize the synaptic transmission that mediates complex EPSCs evoked in SNR neurons. When added to the superfusate, the NMDA receptor antagonist AP5 (50 μm) reduced the integrated area of the complex EPSC by 82 ± 4%(n = 5), as illustrated in Fig. 6Ab. This figure also shows that AP5 preferentially reduced the long-lasting inward shift of the membrane current (presumably the NMDA receptor-mediated component), whereas the frequency of notches and inflections in the late component were less severely reduced. In comparison, the AMPA receptor antagonist CNQX (10 μm) also reduced the complex
EPSC by 83 ± 3%(n = 5). However, Fig. 6Ac shows that CNQX preferentially blocked the notches and inflections that represent fast EPSCs, while leaving the slow late component less affected. Doubling the number of stimuli (10 ms interval) in the presence of CNQX accentuated the amplitude of the slow, late component of the complex EPSC, whereas the fast notches and inflections remained blocked (Fig. 6Ad). This suggests that the sustained inward current of the late component is largely mediated by NMDA receptor stimulation, whereas the fast notches and inflections that represent fast EPSCs are mediated by AMPA receptors. Figure 6Ae shows that superfusion with CNQX plus AP5 completely blocks all components of the complex EPSC, despite double stimuli delivered to the STN. In contrast to the effects of ionotropic glutamate antagonists, the integrated amplitudes of complex EPSCs were not affected by the non-selective metabotropic glutamate receptor antagonist MCPG (300 μm; n = 6), the adenosine A1 receptor antagonist DPCPX (1 μm; n = 5), or by the muscarinic antagonist scopolamine (10 μm; n = 5).
Although the above experiments clearly show that the complex EPSC is mediated by ionotropic glutamate receptors, bath application of glutamate antagonists does not provide information on site of action. Therefore, we used a fast-flow microapplicator to apply CNQX (10 μm) and AP5 (50 μm) to discrete areas of the brain slice while recording complex EPSCs in SNR neurons. As illustrated in Fig. 6B, fast application of AP5 plus CNQX to the STN reduced the late component of complex EPSCs by 86 ± 7%(n = 5). However, this figure also shows that the early, short-latency EPSCs were much less affected by local application of AP5 plus CNQX to the STN. As a control experiment, Fig. 6C shows that local application of AP5 plus CNQX to the internal capsule has no effect on the complex EPSC (n = 3). These results show that the complex EPSC requires activation of ionotropic glutamate receptors within the STN. Moreover, our finding that the early and late components are differentially affected by CNQX and AP5 provides additional evidence that the late component is polysynaptic, because polysynaptic transmission is known to be more sensitive to pharmacological inhibition than is monosynaptic transmission (Berry & Pentreath, 1976).
Modulation of the complex EPSC by GABAA receptors
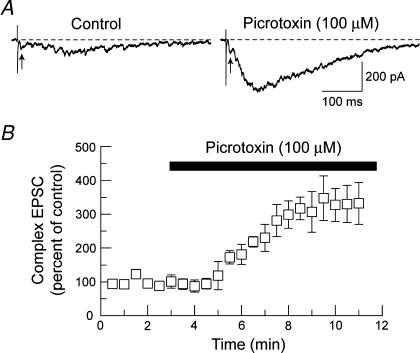
Because the STN receives a large GABA-containing input, mainly from the GPE (Smith et al. 1990; Parent & Hazrati, 1995), we next investigated a possible role for GABA in regulating subthalamonigral transmission. As shown in Fig. 7A, bath application of the GABAA receptor–channel blocker picrotoxin (100 μm) caused a profound potentiation of the complex EPSC. This figure also shows that the late component is potentiated to a much greater extent by picrotoxin compared to the early, monosynaptic component (indicated by arrows in Fig. 7A). The time course of the action of picrotoxin on integrated complex EPSC amplitude is shown in Fig. 7B (n = 5). As can be seen in this figure, the effect of picrotoxin began within 2 min of perfusion and reached its peak within 7 min. Based upon the integrated area of complex EPSCs, picrotoxin potentiated EPSCs by 230 ± 55% compared to control (n = 5; P < 0.001, paired t test). Bicuculline methiodide (30 μm), a GABAA receptor antagonist, increased the integrated area of complex EPSCs by 309 ± 36% (n = 8; P < 0.001). These results show that subthalamonigral transmission is under tonic inhibition by GABA in the brain slice preparation.
Figure 7. GABAA receptor blockade augments complex EPSCs.
A, bath application of picrotoxin (100 μm) greatly increased the amplitude of the complex EPSCs evoked by STN stimulation. Arrows below traces indicate the early, monosynaptic component of the complex EPSC. Note that picrotoxin affects the late component much more than the initial EPSC. B, summary graph showing the time course for potentiation of the integrated area of the complex EPSC by picrotoxin (100 μm). Each data point is the mean ± s.e.m. of 5 SNR neurons.
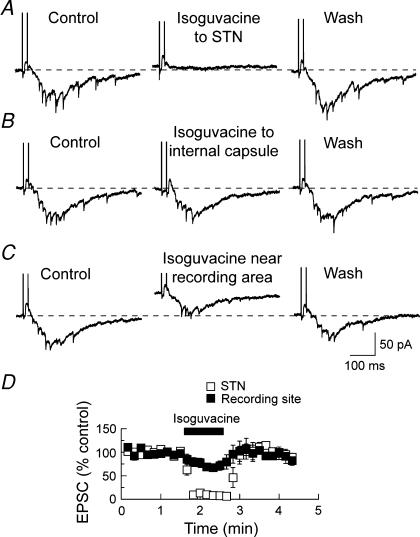
We next investigated the site of action for GABA-mediated inhibition of the complex EPSC. As shown in Fig. 8A, fast-flow application of the GABAA agonist isoguvacine (100 μm) to the STN completely abolished the complex EPSC. It is important to note that local application of isoguvacine did not cause a significant outward shift in holding current that would have been produced if isoguvacine had diffused to the SNR recording area. As a control experiment, Fig. 8B shows that fast-flow application of isoguvacine to the internal capsule produced no change in either the complex EPSC or holding current (n = 4). In contrast, direct, local application of isoguvacine to the SNR near the recording site caused only a modest reduction in the EPSC, despite causing a large outward shift in holding current (Fig. 8C). Figure 8D summarizes these results of local application of isoguvacine. On average, isoguvacine applied locally to the STN caused a 94 ± 3% reduction in the integrated complex EPSC amplitude (n = 3). In contrast, application of isoguvacine to the SNR near the recording site only reduced complex EPSCs by 31 ± 7%(n = 4). Because application of isoguvacine to the SNR also caused an outward shift in holding current, the mild inhibition of complex EPSCs could be explained by an increase in membrane conductance that causes non-selective shunting of membrane currents. Taken together, these results show that the complex EPSC can be selectively inhibited by stimulation of GABAA receptors in the STN. Our results are consistent with the hypothesis that GABA effectively inhibits the complex EPSC by suppressing recurrent polysynaptic transmission in the STN.
Figure 8. Stimulation of GABAA receptors in the STN suppresses complex EPSCs.
A, local, fast-flow application of the GABAA agonist isoguvacine (100 μm) to the STN completely abolished the complex EPSC recorded in an SNR neuron. Note that isoguvacine did not significantly shift holding current. B, local application of isoguvacine to the internal capsule had no effect on complex EPSCs or holding current recorded in the same SNR neuron. C, local application of isoguvacine to the SNR near the recording site caused an outward shift in holding current while only partially reducing the complex EPSC amplitude. D, summary graph showing the time course for suppression of the complex EPSC by isoguvacine. The complex EPSC is abolished when isoguvacine (100 μm) was applied directly to the STN (□), whereas it was only partially reduced when isoguvacine was applied near the recording site (▪). Each data point is the mean ± s.e.m. of 3–4 SNR neurons.
Polysynaptic currents in STN neurons
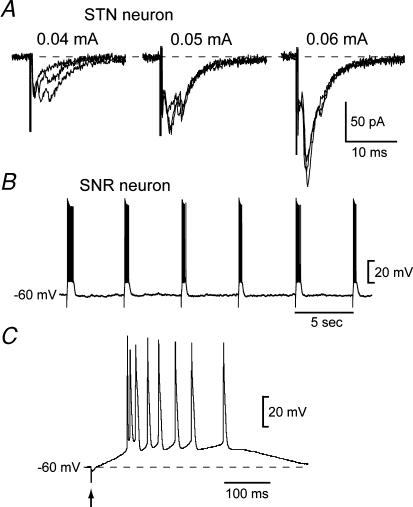
If complex EPSCs in SNR neurons are generated by reverberating circuits in the STN, then one would expect that electrical stimulation of the STN would also evoke complex EPSCs when recording in STN neurons. In order to test this hypothesis, we recorded currents in STN neurons in response to focal electrical stimulation of the STN near the recording site. Figure 9A shows evidence of polysynaptic responses evoked in a STN neuron. In order to evoke complex EPSCs in STN neurons, we found it was necessary to place the stimulation electrode close to the recording pipette (< 100 μm) and use relatively low stimulus intensities (0.02–0.04 mA). Complex EPSCs were observed in five of nine STN neurons. This finding further supports the hypothesis that STN neurons possess recurrent excitatory connections that could provide a basis for complex EPSCs as recorded in SNR.
Figure 9. Polysynaptic currents in STN and burst firing in SNR neurons.
A, low intensity stimulation of the STN near the recording site evoked multiple EPSCs with variable latencies in this STN neuron. Three superimposed responses are shown for each stimulation intensity. B, electrical stimulation of the STN evoked bursts of action potentials in this SNR neuron recorded under current clamp. A burst of 7–12 spikes was evoked by each stimulus. C, a burst of spikes evoked by STN stimulation is shown on an expanded time base. This recording is from the same SNR neuron as shown in ‘B’. The arrow indicates stimulus artifact.
Complex EPSCs promote bursts of action potentials in SNR neurons
In order to illustrate the possible functional significance of complex EPSCs, we performed recordings of SNR neurons under current-clamp conditions. After establishing that STN stimulation evoked a complex EPSC in a SNR neuron under voltage clamp, we then switched to current-clamp mode and recorded the effect of STN stimulation on membrane potential. As shown in Fig. 9B, a single electrical stimulus delivered to the STN can evoke a burst of action potentials in the SNR neuron. Bursts consisted of 7–12 spikes and could be evoked reliably every 5 s, as shown in Fig. 9B. A typical burst, shown with an expanded time base in Fig. 9C, was characterized by an increasing spike interval over time, and the first spike in a burst was taller and narrower than the last spike in the burst. This result clearly shows that the complex EPSC recorded under voltage clamp can evoke bursts of action potentials in SNR neurons when recorded under current-clamp conditions.
Discussion
The present study shows that stimulation of the STN evokes complex synaptic responses in SNR neurons consisting of an early monosynaptic current followed by multiple late EPSCs. This complex EPSC is dependent upon direct activation of neurons in the STN, because stimulation elsewhere in the slice is ineffective. Moreover, we show that generation of the complex EPSC is regulated by tonic GABA-mediated inhibition of STN neurons. We conclude that complex EPSCs recorded in SNR neurons result from polysynaptic activation of recurrent collaterals within the STN, on which GABA-containing inputs exert a tonic inhibitory influence.
Complex EPSCs are polysynaptic
The main finding of the present work is that a single electrical stimulus delivered to the STN evokes a complex synaptic response in SNR neurons consisting of an initial short-latency EPSC followed by a series of variable-latency EPSCs. We conclude that the initial EPSC is a monosynaptic response because it exhibited a fixed latency and relatively constant amplitude in response to increasing stimulus intensities. Moreover, repetitive stimuli evoked the initial EPSC more reliably than the late component of the complex EPSC, which is further evidence for the monosynaptic nature of the initial EPSC. In contrast, EPSCs in the late component showed considerable variability in latencies and amplitudes even when evoked at a single stimulation intensity, suggesting a polysynaptic mechanism. Increasing the stimulus intensity also markedly increased the amplitude and shortened the latency of late components of complex EPSCs, which is expected for polysynaptic events (Berry & Pentreath, 1976). Furthermore, AMPA and NMDA receptor antagonists reduced the late component more effectively compared to reducing the initial EPSC, which provides additional evidence that the late component is polysynaptic. Our finding that complex EPSCs are mediated by activation of ionotropic glutamate receptors is similar to the receptor dependence for complex EPSCs reported for neurons in the dorsal horn (Miller & Woolf, 1996), hypothalamus (Boudaba et al. 1997), hippocampus (Crépel et al. 1997), and superior colliculus (Saito & Isa, 2003).
Complex EPSCs are generated in the STN
Our finding that direct electrical stimulation of the STN evokes complex EPSCs, whereas stimulation of adjacent brain regions fails to evoke complex EPSCs, strongly suggests that the complex EPSC requires activation of STN neurons and their connections. However, one could argue that the complex EPSC might have been generated by stimulation of axons that are known to pass through the STN (Iwahori, 1978). If this were the case, then one would have expected that electrical stimulation of the region between SNR and STN would also effectively evoke complex EPSCs, but this was not observed. Our finding that local application of ionotropic glutamate antagonists to the STN effectively blocks complex EPSCs suggests that excitatory synapses within the STN must be activated to evoke complex EPSCs. This conclusion is also supported by our observation that local microapplication of glutamate to the STN, but not to adjacent brain regions, evokes spontaneous EPSCs in SNR neurons. Finally, we showed that microapplication of the GABA agonist isoguvacine can abolish complex EPSCs, but only when applied directly to the STN. These results strongly suggest that polysynaptic excitatory connections within the STN are essential for the generation of complex EPSCs in the subthalamonigral pathway.
Mechanism of complex EPSC generation
Although our data clearly show that participation of circuits within the STN is required for the generation of complex EPSCs in SNR neurons, the precise mechanism can still be debated. Our data are most consistent with the hypothesis that complex EPSCs are generated by activation of recurrent collateral axons that release glutamate onto adjacent STN neurons. Although a histological study by Kita et al. (1983) found that about half of all STN neurons possess recurrent collateral axons that remain in the nucleus, other studies found little or no evidence of recurrent collaterals (Hammond & Yelnik, 1983; Sato et al. 2000). Furthermore, Wilson et al. (2004) performed simultaneous extracellular recordings of pairs of STN neurons and showed no evidence of synchronized action potentials, which would have been expected if recurrent excitatory collaterals were functional. However, it is possible that activation of recurrent collaterals might require a stronger stimulus than that provided by tonic single-spike firing. For example, depolarizing plateau potentials that can be evoked in STN neurons by EPSPs (Otsuka et al. 2001) would be expected to reinforce excitatory transmission and thereby improve the likelihood that recurrent collaterals would be activated. Regardless, our data showing that polysynaptic EPSCs can be recorded in STN neurons provides empirical evidence for the presence of recurrent collaterals. Activation of recurrent collateral connections arising from glutamate-containing neurons is a common mechanism for the generation of complex EPSCs in other brain regions such as hypothalamus (Boudaba et al. 1997) and the CA1 region of the hippocampus (Crépel et al. 1997).
In addition to recurrent collateral activation, other mechanisms may contribute to the generation of complex EPSCs. For example, IPSPs mediated by GABA have been shown to facilitate the de-inactivation of voltage-gated Na+ channels and thereby enhance the excitatory influence of EPSPs in STN neurons (Baufreton et al. 2005). Thus, it is possible that GABA-containing synaptic inputs from GPE might augment reverberating excitatory circuits in the STN and thereby contribute to the generation of complex EPSCs in SNR neurons. Although this may be an important mechanism in vivo, it is unlikely to play a role in our experiments because complex EPSCs can still be evoked when the GPE is absent from the slice (data not shown). The presence of gap junctions could provide an alternate mechanism for recurrent excitation in the STN that might contribute to complex EPSCs. However, it is difficult to imagine how gap junctions could account for the long durations of complex EPSCs. Moreover, at least one ultrastructural study has failed to find evidence of gap junctions in the STN (Chang et al. 1983). Finally, one could consider the possibility that complex EPSCs are generated by excitatory interneurons in the STN. Although glutamate-containing interneurons have been shown to mediate complex EPSCs in several brain regions (Baba et al. 2003; Saito & Isa, 2003), to our knowledge the STN has not been shown to contain this cell type.
Regulation of complex EPSCs by GABA
Marked potentiation of complex EPSCs by the GABAA-blocking agents picrotoxin and bicuculline demonstrates that synaptic connections in the STN are under strong, tonic inhibition by endogenous GABA. Potentiation of complex EPSCs could occur by blocking GABAA receptors located postsynaptically at somatodendritic membranes, or by blocking receptors located presynaptically on nerve terminals of recurrent collaterals. Although our studies do not distinguish pre- from postsynaptic sites of action, blockade of postsynaptic GABAA-gated conductances should effectively facilitate recurrent excitation and thereby enhance polysynaptic transmission in the STN. Strong, tonically active inhibition by GABA is characteristic of complex EPSCs in other brain regions, such as the dorsal horn of the spinal cord (Baba et al. 2003) and dentate gyrus (Kneisler & Dingledine, 1995). However, the complex EPSC of the subthalamonigral pathway is somewhat unusual in that it can be evoked without the use of GABAA blockers, which is unlike the findings of some studies of the hippocampus (Crépel et al. 1997; Xiang & Brown, 1998). The large GABA-containing input from GPE is the most likely source of tonic GABA-mediated inhibition in the STN (Smith et al. 1990; Parent & Hazrati, 1995), although these inputs are most likely severed from cell bodies in our slice preparations. However, immunocytochemical studies have demonstrated a small population of glutamic acid decarboxylase-positive neurons in the STN in rats (Oertel & Mugnaini, 1984) and humans (Lévesque & Parent, 2005), which suggests that GABA-containing interneurons might also contribute to tonic GABA-mediated inhibition.
Controversial aspects
The most obvious question that arises from the present work is why have complex EPSCs not been observed by previous investigators? An early study using intracellular recordings showed that STN stimulation evoked monosynaptic EPSPs that were followed by a long-duration depolarization in rat SNR neurons, which might have been consistent with a complex synaptic response (Nakanishi et al. 1987). However, in general, most previous studies using brain slice preparations reported that electrical stimulation evokes monophasic EPSCs in SNR neurons. But in most of these studies, including a report from our own laboratory, the STN was not stimulated directly, but rather EPSCs were evoked by stimulation of regions near the SNR recording site (Shen & Johnson, 1997; Wittmann et al. 2001, 2002). Based upon our present work, we now provide evidence that direct activation of STN neurons is required for generating complex EPSCs. Therefore, it is not surprising that only monophasic EPSCs were observed in previous studies in which the STN was not directly stimulated. However, the studies by Bradley et al. (2000) and Ibañez-Sandoval et al. (2006) are more difficult to reconcile with our work because these investigators found that direct electrical stimulation of the STN only evoked monophasic EPSCs in rat SNR neurons. Several differences in experimental procedure might have contributed to these different results, such as their use of parasagittal (rather than horizontal) slices and recording at room temperature (rather than 36°C). However, we believe the most significant difference was the method of patch recording. Both Bradley et al. and Ibañez-Sandoval et al. used the visualized patch method, which preferentially samples neurons located on the surface of the slice. In contrast, we used the ‘blind’ method of patch-clamp recording, which permitted us to record cells deep in the slice that presumably have a higher likelihood than surface cells of receiving multiple, intact synaptic connections from STN. Thus, this difference in methodology is likely to be the most significant factor in determining whether or not the complex EPSC can be observed.
Functional implications
The present study provides electrophysiological evidence that recurrent excitation in the STN mediates polysynaptic transmission in the subthalamonigral pathway. Complex EPSCs generated by recurrent excitation in the STN will be expected to generate bursts of action potentials in SNR neurons, as we have demonstrated in our in vitro studies. Although burst discharges can be seen in SNR neurons during normal movement (Magarinos-Ascone et al. 1992), excessive burst firing in SNR neurons is associated with rigidity and bradykinesia in experimental models of Parkinson's disease (Murer et al. 1997; Wichmann et al. 1999). Our finding that the generation of complex EPSCs is under strong tonic regulation by GABA in the STN supports the hypothesis that loss of GABA-mediated transmission in the pallidosubthalamic pathway can disinhibit recurrent excitatory connections in the STN (Ryan & Sanders, 1993; Nisbet et al. 1996). Consequently, disinhibition of transmission in the subthalamonigral pathway would be expected to potentiate complex EPSCs and promote burst discharges in the SNR (Ryan & Sanders, 1993). Due to the strong association of burst discharges with symptoms of Parkinson's disease, we suggest that strategies that inhibit complex EPSCs might have therapeutic benefit. For example, deep brain stimulation of the STN might possess therapeutic value in treating parkinsonism because it might interfere with the recurrent excitation that is needed to generate complex EPSCs in the subthalamonigral pathway. Furthermore, pharmacological treatments that increase the stimulation of GABAA receptors in the STN might also be expected to inhibit complex EPSCs and thereby reduce the burst firing of SNR neurons that is associated with parkinsonism. We suggest that the generation of complex EPSCs in the subthalamonigral pathway will have important functional implications in the physiology of normal movement as well as in the pathophysiology of movement disorders.
Acknowledgments
This study was supported by USPHS grant NS 38175 and the Portland Veterans Affairs Parkinson's Disease Research, Education, and Clinical Center.
References
- Baba H, Ji R-R, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Baufreton J, Atherton JF, Surmeier DJ, Bevan MD. Enhancement of excitatory synaptic integration by GABAergic inhibition in the subthalamic nucleus. J Neurosci. 2005;25:8505–8517. doi: 10.1523/JNEUROSCI.1163-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Berry MS, Pentreath VW. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976;105:1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Blandini F, Nappi G, Tassorelli C, Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Schrader LA, Tasker JG. Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol. 1997;77:3396–3400. doi: 10.1152/jn.1997.77.6.3396. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Marino MJ, Wittmann M, Rouse ST, Awad H, Levey AI, Conn PJ. Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia nigra pars reticulata. J Neurosci. 2000;20:3085–3094. doi: 10.1523/JNEUROSCI.20-09-03085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbaud P, Gross C, Benazzouz A, Coussemacq M, Bioulac B. Reduction of apomorphine-induced rotational behaviour by subthalamic lesion in 6-OHDA lesioned rats is associated with a normalization of firing rate and discharge pattern of pars reticulata neurons. Exp Brain Res. 1995;105:48–58. doi: 10.1007/BF00242181. [DOI] [PubMed] [Google Scholar]
- Chang HT, Kita H, Kitai ST. The fine structure of the rat subthalamic nucleus: an electron microscopic study. J Comp Neurol. 1983;221:113–123. doi: 10.1002/cne.902210110. [DOI] [PubMed] [Google Scholar]
- Crépel V, Khazipov R, Ben-Ari Y. Blocking GABAA inhibition reveals AMPA- and NMDA-receptor-mediated polysynaptic responses in the CA1 region of the rat hippocampus. J Neurophysiol. 1997;77:2071–2082. doi: 10.1152/jn.1997.77.4.2071. [DOI] [PubMed] [Google Scholar]
- Hammond C, Yelnik J. Intracellular labelling of rat subthalamic neurones with horseradish peroxidase: computer analysis of dendrites and characterization of axon arborization. Neuroscience. 1983;8:781–790. doi: 10.1016/0306-4522(83)90009-x. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- Ibañez-Sandoval O, Hernández A, Forán B, Galarraga E, Tapia D, Valdiosera R, Erlij D, Aceves J, Bargas J. Control of the subthalamic innervation of substantia nigra pars reticulata by D1 and D2 dopamine receptors. J Neurophysiol. 2006;95:1800–1811. doi: 10.1152/jn.01074.2005. [DOI] [PubMed] [Google Scholar]
- Iwahori N. A Golgi study on the subthalamic nucleus of the cat. J Comp Neurol. 1978;182:383–397. doi: 10.1002/cne.901820303. [DOI] [PubMed] [Google Scholar]
- Kita H, Chang HT, Kitai ST. The morphology of intracellularly labeled rat subthalamic neurons: a light microscopic analysis. J Comp Neurol. 1983;215:245–257. doi: 10.1002/cne.902150302. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Efferent projections of the subthalamic nucleus in the rat: light and electron microscopic analysis with the PHA-L method. J Comp Neurol. 1987;260:435–452. doi: 10.1002/cne.902600309. [DOI] [PubMed] [Google Scholar]
- Kneisler TB, Dingledine R. Synaptic input from CA3 pyramidal cells to dentate basket cells in rat hippocampus. J Physiol. 1995;487:125–146. doi: 10.1113/jphysiol.1995.sp020866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque JC, Parent A. GABAergic interneurons in human subthalamic nucleus. Mov Disord. 2005;20:574–584. doi: 10.1002/mds.20374. [DOI] [PubMed] [Google Scholar]
- Magarinos-Ascone C, Buno W, Garcia-Austt E. Activity in monkey substantia nigra neurons related to a simple learned movement. Exp Brain Res. 1992;88:283–291. doi: 10.1007/BF02259103. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Miller BA, Woolf CJ. Glutamate-mediated slow synaptic currents in neonatal rat deep dorsal horn neurons in vitro. J Neurophysiol. 1996;76:1465–1476. doi: 10.1152/jn.1996.76.3.1465. [DOI] [PubMed] [Google Scholar]
- Murer MG, Riquelme LA, Tseng KY, Pazo JH. Substantia nigra pars reticulata single unit activity in normal and 6-OHDA-lesioned rats: Effects of intrastriatal apomorphine and subthalamic lesions. Synapse. 1997;27:278–293. doi: 10.1002/(SICI)1098-2396(199712)27:4<278::AID-SYN2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Intracellular study of rat substantia nigra pars reticulata neurons in an in vitro slice preparation: electrical membrane properties and response characteristics to subthalamic stimulation. Brain Res. 1987;437:45–55. doi: 10.1016/0006-8993(87)91525-3. [DOI] [PubMed] [Google Scholar]
- Nisbet AP, Eve DJ, Kingsbury AE, Daniel SE, Marsden CD, Lees AJ, Foster OJF. Glutamate decarboxylase-67 messenger RNA expression in normal human basal ganglia and in Parkinson's disease. Neuroscience. 1996;75:389–406. doi: 10.1016/0306-4522(96)00299-0. [DOI] [PubMed] [Google Scholar]
- Oertel WH, Mugnaini E. Immunocytochemical studies of GABAergic neurons in rat basal ganglia and their relations to other neuronal systems. Neurosci Lett. 1984;47:233–238. doi: 10.1016/0304-3940(84)90519-6. [DOI] [PubMed] [Google Scholar]
- Otsuka T, Murakami F, Song W-J. Excitatory postsynaptic potentials trigger a plateau potential in rat subthalamic neurons at hyperpolarized states. J Neurophysiol. 2001;86:1816–1825. doi: 10.1152/jn.2001.86.4.1816. [DOI] [PubMed] [Google Scholar]
- Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati L-N. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Vaughn JE, Roberts E. GABAergic nerve terminals decrease in the substantia nigra following hemitransections of the striatonigral and pallidonigral pathways. Brain Res. 1980;192:413–420. doi: 10.1016/0006-8993(80)90893-8. [DOI] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience. 1997;80:545–557. doi: 10.1016/s0306-4522(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Rose HJ, Metherate R. Auditory thalamocortical transmission is reliable and temporally precise. J Neurophysiol. 2005;94:2019–2030. doi: 10.1152/jn.00860.2004. [DOI] [PubMed] [Google Scholar]
- Ryan LJ, Sanders DJ. Subthalamic nucleus lesion regularizes firing patterns in globus pallidus and substantia nigra pars reticulata neurons in rats. Brain Res. 1993;626:327–331. doi: 10.1016/0006-8993(93)90596-f. [DOI] [PubMed] [Google Scholar]
- Saito Y, Isa T. Local excitatory network and NMDA receptor activation generate a synchronous and bursting command from the superior colliculus. J Neurosci. 2003;23:5854–5864. doi: 10.1523/JNEUROSCI.23-13-05854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson P, Mavoungou R, Albe-Fessard D. Changes in substantia nigra pars reticulata activity following lesions of the substantia nigra pars compacta. Neurosci Lett. 1986;67:25–30. doi: 10.1016/0304-3940(86)90202-8. [DOI] [PubMed] [Google Scholar]
- Sato F, Parent M, Levesque M, Parent A. Axonal branching pattern of neurons of the subthalamic nucleus in primates. J Comp Neurol. 2000;424:142–152. doi: 10.1002/1096-9861(20000814)424:1<142::aid-cne10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Activity of pars reticulata neurons of monkey substantia nigra in relation to motor, sensory, and complex events. J Neurophysiol. 1986;55:660–677. doi: 10.1152/jn.1986.55.4.660. [DOI] [PubMed] [Google Scholar]
- Shen K-Z, Johnson SW. Presynaptic GABAB and adenosine A1 receptors regulate synaptic transmission to rat substantia nigra reticulata neurones. J Physiol. 1997;505:153–163. doi: 10.1111/j.1469-7793.1997.153bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Bolam JP, von Krosigk M. Topographical and synaptic organization of the GABA-containing pallidosubthalamic projection in the rat. Eur J Neurosci. 1990;2:500–511. doi: 10.1111/j.1460-9568.1990.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Starr PA, Vitek JL, Bakay RAE. Deep brain stimulation for movement disorders. Neurosurg Clin N Am. 1998;9:381–402. [PubMed] [Google Scholar]
- Tseng KY, Riquelme LA, Belforte JE, Pazo JH, Murer MG. Substantia nigra pars reticulata units in 6-hydroxydopamine-lesioned rats: responses to striatal D2 dopamine receptor stimulation and subthalamic lesions. Eur J Neurosci. 2000;12:247–256. doi: 10.1046/j.1460-9568.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR. Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res. 1999;125:397–409. doi: 10.1007/s002210050696. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA, DeLong MR. Antiparkinsonian and behavioral effects of inactivation of the substantia nigra pars reticulata in hemiparkinsonian primates. Exp Neurol. 2001;167:410–424. doi: 10.1006/exnr.2000.7572. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Puntis M, Lacey MG. Overwhelmingly asynchronous firing of rat subthalamic nucleus neurones in brain slices provides little evidence for intrinsic interconnectivity. Neuroscience. 2004;123:187–200. doi: 10.1016/j.neuroscience.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Marino MJ, Bradley SR, Conn PJ. Activation of group III mGluRs inhibits GABAergic and glutamatergic transmission in the substantia nigra pars reticulata. J Neurophysiol. 2001;85:1960–1968. doi: 10.1152/jn.2001.85.5.1960. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Marino MJ, Conn PJ. Dopamine modulates the function of group II and group III metabotropic glutamate receptors in the substantia nigra pars reticulata. J Pharmacol Exp Ther. 2002;302:433–441. doi: 10.1124/jpet.102.033266. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Brown TH. Complex synaptic current waveforms evoked in hippocampal pyramidal neurons by extracellular stimulation of dentate gyrus. J Neurophysiol. 1998;79:2475–2484. doi: 10.1152/jn.1998.79.5.2475. [DOI] [PubMed] [Google Scholar]