Abstract
It is widely presumed that odor quality is a direct outcome of odorant structure, but human studies indicate that molecular knowledge of an odorant is not always sufficient to predict odor quality. Indeed, the same olfactory input may generate different odor percepts depending on prior learning and experience. Combining functional magnetic resonance imaging with an olfactory paradigm of perceptual learning, we examined how sensory experience modifies odor perception and odor quality coding in the human brain. Prolonged exposure to a target odorant enhanced perceptual differentiation for odorants related in odor quality or functional group, an effect that was paralleled by learning-induced response increases in piriform cortex and orbitofrontal cortex (OFC). Critically, the magnitude of OFC activation predicted subsequent improvement in behavioral differentiation. Our findings suggest that neural representations of odor quality can be rapidly updated through mere perceptual experience, a mechanism that may underlie the development of odor perception.
Introduction
How the brain transforms sensory events at the nose into discrete odor percepts is a central question in olfactory neuroscience. Evidence from animal models suggests that neural representations of odor quality (e.g., “mint” or “rose”) naturally follow from an odorant’s molecular composition. Via systematic projections from olfactory receptor neurons (Buck and Axel, 1991; Araneda et al., 2000), complex configurations of molecular features are assembled into odor-specific spatial maps in the olfactory bulb (Malnic et al., 1999; Meister and Bonhoeffer, 2001; Johnson et al., 2002; Xu et al., 2003), where each pattern is thought to reflect a unique neural signature of odor quality. However, this “bottom-up” view of odor coding is at odds with human data suggesting that higher-order cognitive processes can profoundly alter odor quality perception for an odorant that remains invariant.
Verbal context strongly influences the perception of odor quality – a rose by any other name would not smell as sweet. For example, the same odorant smells entirely different depending on whether it is labeled as fresh cucumber or mildew (Herz and von Clef, 2001). Learning also changes odor quality. A cherry odor becomes smokier in quality after being experienced together with a smoky odor (Stevenson, 2001). Thus, a given set of olfactory receptors activated by an odorant may not map directly onto a given odor percept (Shepherd, 2004). Rather, odor perception may rely on more synthetic, or integrative, mechanisms subserved by higher-order brain regions (Wilson and Stevenson, 2003). Along these lines, recent work in our laboratory (Gottfried et al., 2006) indicates that odor quality coding involves a network of olfactory areas, including posterior piriform cortex, orbitofrontal cortex (OFC), and hippocampus, and that these codes are independent of molecular functional group (as one critical attribute of odorant structure). In the present study, we continue this investigation by examining how learning, specifically perceptual learning, modifies odor perception and odor quality coding in the human brain.
Perceptual learning refers to a phenomenon whereby sensory experience induces changes in behavior and brain function (Gibson, 1991; Goldstone, 1998; Gilbert et al., 2001; Fahle and Poggio, 2002). In the olfactory domain, repeated presentations of an odor reduce olfactory detection thresholds (Dalton et al., 2002; Stevens and O’Connell, 1995) and can even boost olfactory sensitivity in seemingly anosmic subjects (Mainland et al., 2002; Wysocki et al., 1989). Exposure to wine (Owen and Machamer, 1979) or beer (Peron and Allen, 1988) is sufficient to improve sensitivity towards stimuli whose chief sensory property is olfactory. Experience and familiarity significantly enhance odor quality discrimination (Jehl et al., 1995; Rabin, 1988), while exposure to odor mixtures alters the perceived quality of the individual components (Stevenson, 2001). Many of these studies provide examples of stimulus “differentiation,” an important mechanism of perceptual learning in which experience refines sensory perception through differentiation of stimulus features, dimensions, or categories (Gibson, 1991; Goldstone, 1998; Schyns et al., 1998). Notably, despite growing behavioral evidence for olfactory perceptual learning, how this form of learning updates odor quality codes in the human brain is unknown.
Recent electrophysiological studies in anesthetized rats suggest that perceptual learning modifies odor-evoked activity in piriform cortex, independently of responses in the olfactory bulb. As a result of prolonged odorant exposure (habituation), single-unit firing in anterior piriform neurons showed increasingly differentiated responses to different odorants (Wilson, 2000; Wilson, 2003), in the absence of corresponding changes in olfactory bulb neurons. This enhancement of neural discrimination requires sufficient odor experience: after only brief (10 s) exposure to a binary odorant mixture, piriform responses were markedly reduced to both the mixture and its components, but after prolonged exposure (50 s), piriform neurons continued firing to the components (Wilson, 2003). These data suggest that adequate sensory experience favors the formation of novel odor representations in piriform cortex, which could promote olfactory differentiation at both the behavioral (Cleland et al., 2002; Fletcher and Wilson, 2002; Johnson et al., 2002) and neural (Wilson, 2000; Wilson, 2003) levels.
In the present study, we combined functional magnetic resonance imaging (fMRI) techniques with an olfactory habituation paradigm (Hall, 1991; Wilson, 2000; Wilson, 2003) to test the following questions: Does prolonged olfactory exposure (as a simple form of perceptual learning) lead to sensory plasticity within the human brain? To what extent are neural representations of odor quality and odorant structure (functional group) modified by olfactory experience? Our main hypothesis was that prolonged sensory experience would modulate neural representations of odor quality and odorant structure (group) in human piriform cortex, an area previously implicated in coding of these particular features (Gottfried et al., 2006). In addition, we predicted that learning-induced neural plasticity would be expressed in olfactory projection areas of OFC, given its prominent role across a variety of olfactory learning and memory paradigms in animals (Schoenbaum and Eichenbaum, 1995) and humans (Zatorre and Jones-Gotman, 1991; Savic et al., 2000; Dade et al., 2002; Gottfried et al., 2003). Finally, in parallel to the neural effects, we hypothesized that odor experience would facilitate perceptual differentiation between odorants sharing critical qualitative or structural attributes.
During fMRI scanning, human volunteers smelled a target odorant (TG) destined for habituation, a quality-related odorant (QR; either ‘floral’ or ‘mint’), a functional group-related odorant (GR; either ketone or alcohol), and a control odorant (CT) unrelated to TG either in quality or group (Fig. 1), both before and after 3.5-min continuous exposure to the TG stimulus (Fig. 2). Inclusion of the QR and GR conditions enabled us to probe the specificity of learning-induced changes across the dimensions of odor quality and odorant group independently, while the CT condition provided a baseline to adjust for non-specific effects. Importantly, the selection of odorants made it possible to assign the stimuli to each of the four conditions (TG, QR, GR, CT), counterbalanced across subjects, to minimize odorant-specific confounds. Pairwise similarity ratings of odor quality (Peron and Allen, 1988; Stevenson, 2001; Case et al., 2004), collected thirty minutes before and thirty minutes after prolonged TG exposure, provided a behavioral index of perceptual learning. Our findings indicate that olfactory experience augments perceptual expertise for odorants similar in quality or structure, and that this learning effect persists for as long as 24 hours. Neural response enhancement in piriform cortex and OFC corresponded to these behavioral findings, suggesting that sensory-specific information about an odorant is not static or fixed within these cortical regions, but is highly malleable and can be rapidly updated by perceptual experience.
Figure 1.
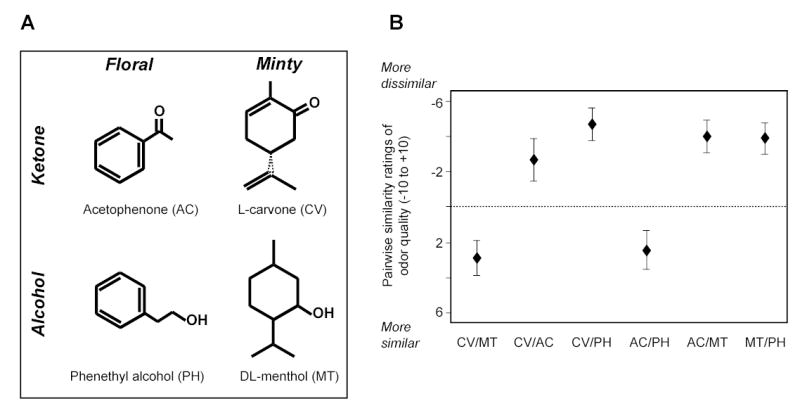
Odor stimuli. (A) Chemical structures of odorants used in the study. These cyclic molecules were chosen to vary systematically in perceptual quality (floral or minty) and functional group (ketone or alcohol). (B) Initial psychophysical characterization of the odorants indicated that floral (AC/PH) and minty (CV/MT) pairs were each rated more similar in perceptual quality, compared to the other pairs.
Figure 2.

Experimental design. (A) The four odorants were evenly assigned to four conditions: target (TG), quality-related (QR), structure/functional group-related (GR), and unrelated control (CT). The diagram shows that each odorant was assigned to each condition equal numbers of times, to minimize odorant-specific confounds. N, number of subjects. (B) Overview of paradigm. Pre-experiment (I) and post-experiment (III) behavioral assessments were conducted 15 min before and 15 min after the imaging experiment (II), which was divided into three sessions. (C) In pre- and post-habituation sessions, odorants (or air) were delivered for 2 s, followed by a 9-s period during which time subjects made an odor intensity rating (noted by “*”). In the habituation session, the TG odorant was continuously delivered for 3.5 min, and subjects rated odor intensity at 30-s intervals.
Results
Prolonged TG exposure elicits behavioral and neural habituation
We first examined the behavioral impact of habituation on intensity ratings during the 3.5-min period of prolonged exposure to the TG odorant. It is evident in Fig. 3A that intensity ratings rapidly decreased during the first 30-sec block, and then reached an asymptotic level at 50% of the initial rating within the next 60–90 sec. Trend analysis revealed that this behavioral profile declined across the seven successive 30-sec blocks, significantly conforming to an exponential decay function with a time-constant of 1/8 session length (F1,15 = 55.60; p < 0.001). The pattern of decline in intensity ratings closely corresponds to numerous prior psychophysical studies in humans (Ekman et al., 1967; Cain, 1974; Berglund et al., 1978), validating the efficacy of our paradigm in inducing behavioral habituation.
Figure 3.

Behavioral and neural effects during within-session habituation to the TG odorant. (A) Over the 3.5 min period of habituation, odor intensity ratings progressively declined, conforming to an exponential decay with a time-constant of 1/8 (yellow curve). (B and C) Neural responses in right posterior piriform cortex (B) and left olfactory OFC (C) both decreased from the first to the last time block, paralleling the intensity rating profile. (D and E) Group statistical parametric maps (SPMs) show robust neural habituation over the 7 blocks (as fitted by the exponential decay function) in bilateral areas of piriform cortex and left olfactory OFC. Habituating activity is shown overlaid on coronal (D) and sagittal (E) sections of the mean T1-weighted anatomical scan (threshold for display, p < 0.001). In (D), the circled areas indicate posterior piriform cortex (at y = 0) and left olfactory OFC (at y = 28). (E) The group SPM is superimposed on the sagittal section of the averaged T1 image. A/H, amygdala/hippocampus; C, cerebellum; P, piriform cortex.
Our next analysis focused on the neural correlates of within-session habituation to the TG odorant. Significant response decline over the seven 30-sec blocks (as predicted by a 1/8 exponential decay function; see Experimental Procedures) was present throughout much of piriform cortex (Figs. 3B, D, E). This included left anterior piriform (x, y, z coordinates = −30, 4, −16; Z = 3.38; and −20, 4, −12; Z = 3.13; p’s < 0.05 corrected for small-volume [SVC]), left posterior piriform (−30, 2, −16; Z = 3.99; and −18, 0, −16; Z = 3.29; p’s < 0.05 SVC), and right posterior piriform (18, 2, −16; Z = 3.19; and 18, 6, −18; Z = 3.17; p’s < 0.05 SVC). As illustrated in Fig. 3E, the habituating responses extended posteriorly within the medial temporal lobe to encompass amygdala and anterior hippocampus. These findings closely concur with prior fMRI studies showing robust odor habituation in olfactory cortex (Sobel et al., 2000; Poellinger et al., 2001). However, in contrast to these studies, we also observed habituating activity in left olfactory OFC (−24, 28, −12; Z = 3.27; p < 0.05 SVC, Figs. 3C-E), which has not been previously described. The above effects were not due to systematic respiratory differences over the seven 30-sec blocks, as there were no significant differences in inspiratory volume, amplitude, or latency (all p’s > 0.1; one-way ANOVA).
Olfactory experience promotes perceptual differentiation of odor quality
The above results show that our paradigm effectively induced within-session habituation at the behavioral and neural levels. Therefore, we next explored how prolonged TG exposure influenced the corresponding determinants of olfactory perceptual learning.
Similarity ratings of odor quality provided a critical behavioral index of perceptual learning. The central prediction was that from pre- to post-habituation, prolonged exposure to the TG odorant would enhance perceptual differentiation between TG-related pairs similar in odor quality (TG/QR) and odorant molecular group (TG/GR), in comparison to corresponding non-TG pairs (see Experimental Procedures). This analysis revealed that similarity ratings of odor quality decreased (became more dissimilar) for quality- and group-related pairs containing the TG odorant (Figs. 4A &S1). Similarity changes (pre – post) significantly differed across the six pairs of odorants (χ2 = 15.13; df = 5; p < 0.01; Friedman Test). Planned follow-up contrasts between the TG pairs and the corresponding non-TG pairs confirmed that ratings of TG/QR and TG/GR pairs became significantly more dissimilar than the non-TG counterparts (p’s < 0.05; Wilcoxon test, one-tailed), whereas rating changes for the unrelated (TG/CT) pair did not differ from the unrelated non-TG pair (p = 0.40). Together these results indicate that following experience with the TG odorant, related odorants (in quality or functional group) became easier to differentiate, provided that such differentiation involved the TG odorant.
Figure 4.
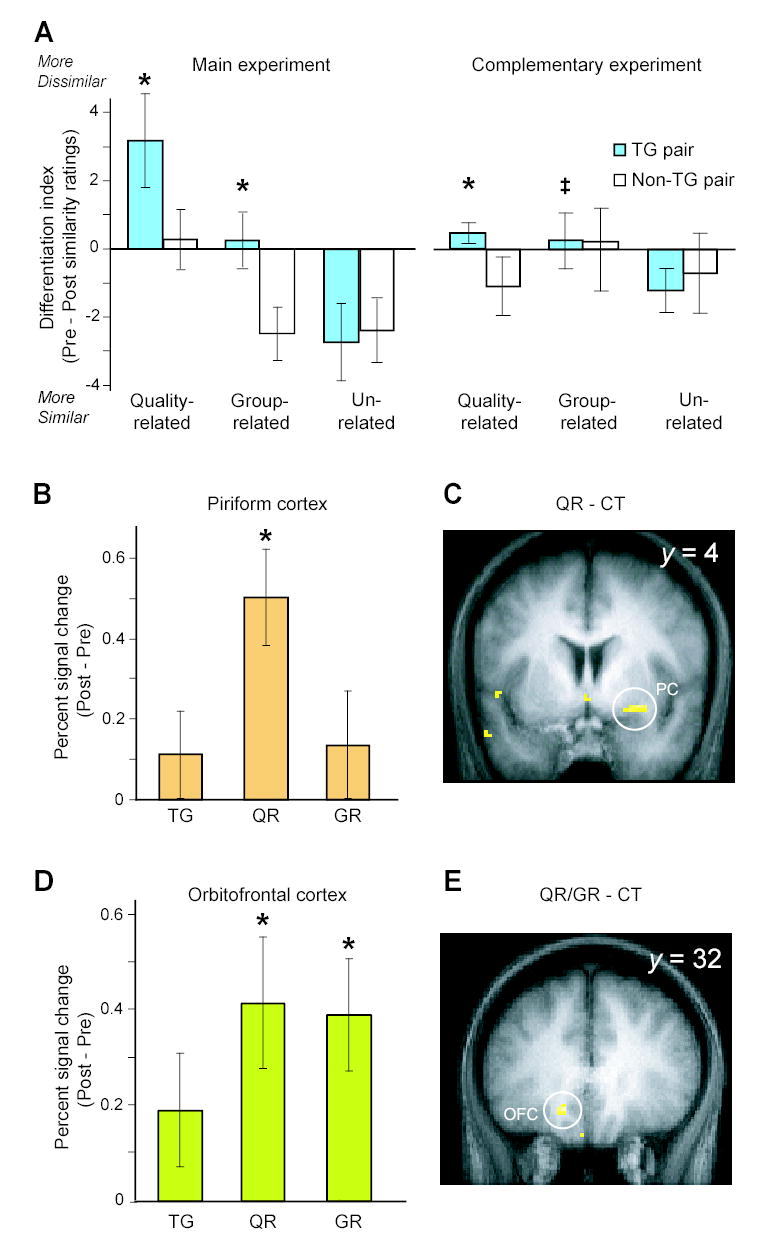
Odor experience-induced perceptual learning. (A) Prolonged exposure to the TG odorant enhanced perceptual differentiation for quality-related (TG/QR) and group-related (TG/GR) pairs, relative to the non-TG counterparts (see text for details). Left, main experiment; right, complementary experiment (collapsed across three time-points). *, significant compared to non-TG pairs; p < 0.05. ‡, significant compared to the unrelated control pair; p < 0.05. (B) Condition-specific plots of percent signal change from pre- to post-habituation (adjusted for the CT baseline) reveal a significant increase in activation to the QR odorant in right posterior piriform cortex (*, p < 0.05 SVC). (C) The group SPM highlights the QR-related neural plasticity in piriform cortex (image overlaid on a coronal T1-weighted section; threshold, p < 0.001). (D) In olfactory OFC, condition-specific signal plots indicate significant signal enhancement for both QR and GR conditions in left olfactory OFC (*, p < 0.05 SVC). (E) The group SPM (coronal slice) shows learning-induced activity in left olfactory OFC in response to the QR and GR odorants collapsed.
Interestingly, there was a general tendency for odorants to smell more alike at the end of the study (Fig. 4A), which may have been caused by olfactory fatigue due to the prolonged duration of the experiment. We note that while the group related TG pair became only slightly more dissimilar in absolute terms, there was a statistically significant increase in relative dissimilarity, when compared to its non-TG baseline pair.
Olfactory experience induces neural plasticity in piriform cortex and OFC
The preceding results indicate that odor experience selectively enhanced perceptual differentiation for quality-related (TG/QR) and group-related (TG/GR) odorant pairs, but not for the unrelated pair (TG/CT). The demonstration of olfactory perceptual learning raises the distinct possibility that neural plasticity in olfactory brain regions may mediate these behavioral changes. An omnibus ANOVA containing the first-level contrasts (pre- vs post-habituation) of the four odorant conditions identified two regions that exhibited significant differences in response change across conditions (Figs. 4B–E): right posterior piriform cortex (28, 6, −10; Z = 3.01; and 22, 4, −12; Z = 2.97; both p’s = 0.001 uncorrected) and left olfactory OFC (−12, 32, −10; Z = 3.31; p < 0.001 uncorrected). These activity patterns could not be attributed to sniffing differences across conditions, as there were no significant differences in inspiratory volume, peak, or latency (p’s > 0.1; repeated-measures ANOVA).
Condition-specific plots of the signal change (post – pre) in right posterior piriform cortex, adjusted for the change to the CT condition, show that prolonged TG exposure elicited substantial response enhancement to the QR condition, which was not evident for the TG and GR conditions (Figs. 4B & S2A). A follow-up contrast between QR and CT confirmed that QR-evoked activity was significantly increased (from pre- to post-habituation) in this region of piriform cortex (26, 6, −10; Z = 3.54; p < 0.05 SVC; Fig. 4C). Furthermore, the specificity of this effect for odor quality was demonstrated in the direct comparison between QR and GR conditions, which highlighted a significant difference in activation change in the same piriform region, albeit at a slightly reduced threshold (Z = 2.71; p = 0.003 uncorrected).
In contrast, the plots of signal change in left olfactory OFC (derived from the ANOVA) revealed a somewhat different response profile (Figs. 4D &S2B). Here, the response enhancement was clearly evident to both the QR and GR conditions, whereas little change was observed in response to the TG condition (relative to CT). Follow-up contrasts to the CT baseline confirmed enhanced OFC activation for both the QR condition (−14, 30, −14; Z = 3.32; p < 0.05 SVC; Fig. 4E) and the GR condition (−14, 30, −14; Z = 3.23; p < 0.05 SVC; Fig. 4E), but not for the TG condition (even at reduced thresholds of p < 0.01 uncorrected). Thus, neural representations of both odor quality and odorant structure exhibited plasticity in this region.
While the present findings provide evidence for learning-related plasticity at the behavioral and neural levels, it remains unclear whether the response enhancement in piriform cortex or OFC actually predicted the extent of perceptual differentiation. Hence, we performed a correlation analysis by regressing the subject-specific changes in neural activation (post- minus pre-habituation) against the changes in similarity ratings of odor quality (pre- minus post-habituation). In right piriform cortex there was no significant correlation between the magnitude of QR-evoked activity and the decrease in similarity ratings (even at p < 0.1 uncorrected). However, in left olfactory OFC, the correlation between neural activity and behavioral indices of learning (pooled across QR and GR conditions) was significant (at −14, 34, −8; Z = 3.32; p < 0.05 SVC; R = 0.75) and within 3.5 mm of the main learning effect in OFC (Fig. 5). Therefore, these findings support the idea that the experience-induced increments in human OFC activity may underlie the subsequent behavioral enhancement of olfactory perception.
Figure 5.

Experience-induced neural plasticity in OFC predicts olfactory perceptual learning. (A) The scatterplot demonstrates a strong correlation between the level of learning-induced OFC signal (mean of QR and GR) and the behavioral magnitude of perceptual learning (change in odor quality similarity, mean of TG/QR and TG/GR effects). (B) The group SPM is superimposed on a mean T1-weighted coronal section (p < 0.001) and displays the area in OFC (circled) exhibiting this correlation.
Rapid recovery of TG habituation (post-habituation session)
The previous findings are notable for learning-induced response enhancement to the QR and GR conditions, but not to the TG condition (even at a reduced threshold of p < 0.05 uncorrected; cf. Figs. 4B, 4D &S2). We suspect that the absence of a TG effect was due to concomitant neural suppression as a result of persisting TG habituation. Indeed, we observed that there was an early reduction in intensity ratings for the TG odorant (i.e., self-habituation) during post-habituation, which progressively recovered toward the end of this last session (Fig. 6). This behavioral profile closely accords with prior studies showing that recovery from self-habituation (as indexed by intensity ratings) occurs within 3–4 minutes following cessation of continuous odorant exposure (Ekman et al., 1967; Pierce et al., 1996). By comparison, there was no evidence for cross-habituation of odor intensity to the QR or GR odorant. A repeated-measures ANOVA confirmed a significant condition-by-time interaction in the degree of intensity change (F4.34, 65.06 = 2.45; p = 0.05). Follow-up comparisons between the CT condition and each of the other odorant conditions verified significant self-habituation to the TG odorant during the first quarter-session (p < 0.01; Wilcoxon test), but not during the three subsequent quartiles (p’s > 0.1). There was also no evidence for cross-habituation to the QR or GR odorant conditions during any quartile (p’s > 0.1). Importantly, by the end of the post-habituation session (last quartile), there were no significant differences in intensity across the four odorant conditions (p > 0.1).
Figure 6.

Rapid recovery of TG habituation. As indexed by intensity ratings, self-habituation to the TG odorant persisted into the first quarter of the post-habituation session (as compared to CT), but subsequently recovered by the end of the session, at which point odor intensity did not significantly differ across the four odorant conditions. There was no evidence for cross-habituation to the QR or GR odorants. *, p < 0.05.
As behavioral self-habituation was evident mostly in the first quarter of the post-habituation session (cf. Fig. 6), we reasoned that the neural correlates for self-habituation might be more readily detected in the first half of post-habituation. Indeed, a contrast restricted to the first half of post-habituation, specifically, [(CT1st half post – CTpre) – (TG1st half post – TGpre)], suggested a decline of TG-evoked neural activity, but only at lenient statistical thresholds (piriform cortex: −8, 4, −18; Z = 2.80; p = 0.003 uncorrected; right olfactory OFC: 32, 30, −20; Z = 2.76; p = 0.003 uncorrected). Finally, consistent with the absence of behavioral evidence for cross-habituation, the contrasts of (CTpost-pre – QRpost-pre) and (CTpost-pre – GRpost-pre) did not identify response decreases to the QR and GR conditions (i.e., neural cross-habituation) in either piriform cortex or OFC, even when surveyed at a threshold of p < 0.1 uncorrected. It is worth re-emphasizing that the absence of cross-habituation to the QR and GR odorants ultimately permitted a more straightforward interpretation of learning-induced neural changes in response to odorants related in quality or functional group.
Complementary behavioral experiment
The preceding behavioral results suggest that prolonged odor exposure induces olfactory perceptual learning for quality-related and group-related compounds. However, because these effects were observed at a single time-point approximately 30 minutes following the exposure session, it remains possible that a transient sensory “aftereffect” (Wade, 1994; Fahle and Poggio, 2002) could have contributed to the findings, irrespective of learning per se. To explore this issue more carefully, we conducted a follow-up study to examine the time-course of olfactory perceptual learning (see Supplemental Material). An independent group of 16 subjects provided pairwise similarity ratings at three time points following habituation: 30 (or 10) min, 4 hr, and 24 hr. We also included two novel odorants, geraniol (“floral”) and methyl salicylate (“minty”). Depending on the identity of the TG odorant, one of these served as a new quality-related odorant (QR-New), and the other served as a “filler” odorant. The QR-New stimulus enabled us to determine whether the perceptual learning effect could generalize to odor stimuli beyond the original test set.
This complementary study confirmed our original findings and demonstrated the persistence of perceptual learning for up to 24 hr following habituation (Figs. 4A &S1). Increased dissimilarity for TG quality-related pairs (TG/QR and TG/QR-New), relative to the non-TG quality-related pair, was present at all three time points (p’s < 0.05 at 30 min and 24 hr and p < 0.1 at 4 hr; Wilcoxon test; one-tailed). Comparable learning effects for TG/QR and TG/QR-New pairs across all assessments (no significant difference; p = 0.61; Friedman test) suggested that this effect can generalize to a novel odorant. While rating changes for the TG/GR pair did not differ from the non-TG/GR pair (p = 0.30), two alternative post hoc analyses suggested that perceptual differentiation was also evident for the TG/GR pair across all time-points, compared to the control pair (TG/GR vs. TG/CT, p < 0.05; non-TG/GR vs. non-TG/CT, p = 0.42; Wilcoxon test; one-tailed). Critically, the control pairs in the TG and the non-TG set did not differ in similarity change at any assessment point (p’s > 0.32), underscoring the specificity of this learning effect. Thus, the long-term (24-hr) behavioral enhancement of olfactory perception is compatible with a mechanism of perceptual learning (Fahle and Poggio, 2002). Nevertheless, since certain sensory aftereffects, such as the McCollough effect in color vision, are known to persist over prolonged time intervals (Jones and Holding, 1975, though see Bedford, 1995), we cannot completely exclude the possibility that habituation-induced olfactory aftereffects have partially contributed to the results observed here. Further discussion of these findings is available in the Supplemental Material.
Discussion
In the present study we used an fMRI version of odor habituation to investigate how olfactory experience alters the behavioral and neural correlates of odor quality perception. Our findings demonstrate that exposure to an odorant leads to enhanced differentiation of odor quality. These effects were selective for odorant pairs similar in quality or molecular functional group, but were not observed for unrelated pairs. The persistence of this phenomenon for as long as 24 hrs post-habituation, and its generalization to novel odor stimuli (complementary study), further reinforces the idea that the observed changes in perceptual differentiation are a consequence of olfactory perceptual learning, rather than transient by-products of odor sensitization or sensory aftereffects.
The sensory-specific improvement in olfactory perception was paralleled (and preceded) by response enhancement in piriform cortex and olfactory OFC. Notably, the magnitude of learning-induced activation in OFC directly predicted the degree of perceptual enhancement on the similarity judgment task, on a subject-by-subject basis, suggesting a critical role of the olfactory OFC in perceptual learning. It is interesting to note that fMRI signal increases are commonly observed in non-olfactory fMRI studies of perceptual learning (Gauthier et al., 1999; Gauthier et al., 2000; Schwartz et al., 2002; Furmanski et al., 2004; Sigman et al., 2005). We speculate that the learning-induced plasticity in the fMRI response may reflect either of two neuronal mechanisms, in line with animal studies of perceptual learning (Kossut, 1988; Recanzone et al., 1993; Yang and Maunsell, 2004). One possibility is that perceptual learning induces an enlargement in cortical receptive fields, resulting in recruitment of more neurons and an increase in the fMRI signal (spatial summation). Alternatively, perceptual learning may facilitate synchronization of neuronal activity (temporal summation), eliciting a larger fMRI response. These mechanisms are not mutually exclusive, and either or both of them could form the neural basis for enhanced sensory differentiation or discrimination.
The general idea that sensory experience can induce perceptual expertise has been extensively investigated in visual, auditory, and somatosensory modalities (Goldstone, 1998; Schyns et al., 1998). This effect is even observed in the absence of explicit training or feedback: mere exposure to scribbled pictures (“doodles”) results in subjects being better able to differentiate among related pictures, generating doodle “expertise” (Gibson and Walk, 1956). Likewise, in the present study, prolonged exposure to one odorant resulted in improved differentiation among related odorants (and even among novel related odorants). Thus, with exposure to a floral-smelling alcohol (i.e., phenethyl alcohol), subjects effectively became floral “experts” and simultaneously became experts for the underlying molecular group. Such learning did not generalize to odorants outside of the experienced dimensions (that is, floral experts did not become mint experts, and alcohol experts did not become ketone experts), highlighting a psychological specificity that is a common characteristic of perceptual learning (Gilbert et al., 2001; Fahle and Poggio, 2002). Furthermore, these effects were obtained despite the fact that subjects were completely unaware of the purpose of the study and were engaged in an ongoing intensity rating task. As such, it is unlikely that procedural learning (performance improvements due to task rehearsal and training), which often confounds interpretations of perceptual learning (Hawkey et al., 2004), contributed to the effects seen here.
One important theoretical mechanism of perceptual learning is known as stimulus differentiation, in which features that were psychologically fused become increasingly differentiated (Schyns et al., 1998). This experience-dependent process appears to figure prominently in the acquisition of perceptual expertise (Goldstone, 1998) and is consistent with the current findings. In our study, subjects were better able to differentiate among floral (or mint) smells, and among alcohol (or ketone) groups, perhaps by developing more refined, or differentiated, subcategories of these olfactory features. We speculate that the process of odor feature differentiation, via sensory exposure, may underlie much of the way that humans naturally learn to identify odors in the environment, with progressive and ever more refined differentiation, to the point where we are able to recognize thousands, if not hundreds of thousands, of different smells.
Recent fMRI data from our laboratory indicate that neural representations of odor quality and odorant structure (functional group) are encoded in separable olfactory areas of the human brain (Gottfried et al., 2006). Importantly, the identification of odor quality codes across a network of olfactory regions, including posterior piriform cortex, OFC, and hippocampus (Royet et al., 1999; Savic et al., 2000), was independent of any simple molecular configuration (Gottfried et al., 2006). The present study extends these findings by implying that neural codes of odor quality rely on experience and learning for their formation, rather than simply being a product of structure-based ensembles. To this end, response enhancement to the QR odorant in posterior piriform cortex and OFC provides direct evidence for the role of perceptual experience in shaping neural representations of odor quality.
By comparison to posterior piriform cortex and olfactory OFC, learning-induced neural plasticity was not observed in anterior piriform cortex. The absence of changes in this region would ensure stimulus constancy of the original sensory input, in keeping with the purported role of anterior piriform in encoding odorant structure (functional group) (Gottfried et al., 2006). It is plausible that the observed differences in neural plasticity between anterior and posterior piriform cortex (Wilson and Sullivan, 2003) reflect the underlying anatomical connectivity of these regions: anterior piriform is the principal target of olfactory bulb (Haberly, 1998) and therefore contains a labeled line for odorant structure, whereas posterior piriform receives the bulk of its inputs from associational fiber systems and would be a better candidate for experience-dependent modulation (Haberly, 1998; Wilson and Sullivan, 2003).
Although increasing evidence suggests that ensemble coding of molecular features in the olfactory bulb is necessary for odor perception (e.g., Linster et al., 2001; Cleland et al., 2002), an opposite picture is emerging from recent rodent electrophysiological studies (Wilson, 2000; Wilson, 2003). These data indicate that piriform cortex is acutely sensitive to the effects of sensory experience and training, often irrespective of odorant structure and independent of responses in the olfactory bulb. The current findings, along with recent data from our laboratory (Gottfried et al., 2006), provide further evidence that odor quality coding in olfactory cortex is not a straightforward outcome of odorant structure. In all likelihood, neural representations of odor quality are a dynamic product of lower-level coding from olfactory bulb and higher-level cortical inputs, under the regulation of learning and experience (Wilson and Stevenson, 2003), attention (Zelano et al., 2005), sensory context (Zellner and Kautz, 1990; Gottfried and Dolan, 2003), and language (Herz, 2003; Shepherd, 2004; de Araujo et al., 2005). Here we have shown that sensory exposure, even in the absence of explicit training, is sufficient to modify neural representations in piriform cortex and OFC, which in turn may underlie the observed improvement in perceptual differentiation for odorants related in quality or functional group. It is thus worth considering that the potential for experience-dependent neural plasticity in olfactory cortex governs the general development of human olfactory perception. This mechanism may underlie the acquisition of fine-grained percepts that distinguish, for example, the smell of Rosa damascena (Bulgarian Rose) from that of Rosa centifolia (Rose Maroc), to the point where we would be able to appreciate the immense richness of aromas in everyday life.
Experimental Procedures
Subjects
A total of 18 healthy subjects (mean age, 24.3 years; range, 20–38 years; 11 women) provided informed consent to take part in the study, which was approved by the Northwestern University Institutional Review Board. Prior to enrollment in the study, subjects were screened to ensure that the qualitatively related odorants were perceived to be more similar in quality, compared to other possible pairings. Two female subjects were excluded due to technical malfunctions during scanning (N = 1) or excessive head motion (N = 1), leaving 16 subjects for the remaining analysis.
Stimuli
We selected four cyclic odorants that systematically varied in odor quality and odorant molecular functional group (Fig. 1A). The decision to focus on these two olfactory features was based on recent data from our laboratory suggesting that neural representations of odor quality and odorant functional group are maintained in human olfactory cortex (Gottfried et al., 2006). There were two ‘minty’ smells comprised of one ketone (L-carvone; CV, 5%) and one alcohol (DL-menthol; MT, 10%), and two ‘floral’ smells also comprised of one ketone (acetophenone; AC, 0.1%), and one alcohol (phenethyl alcohol; PH, 5%), all diluted in mineral oil and roughly matched for intensity. An assessment of odor similarity at the beginning of the experiment confirmed that the ‘minty’ pairs and the ‘floral’ pairs smelled more similar than all other possible pairs (Fig. 1B). There was a significant difference across the six odorants (p < 0.001; χ2 = 26.86; Friedman test), and follow-up comparisons verified that the minty pair (CV/MT) and the floral pair (AC/PH) were each rated more similar than the mean of the other four pairs (p’s < 0.01; Wilcoxon test). Note the assignment of odorants to ‘minty’ or ‘floral’ categories is not meant to imply that these stimuli are canonical representations of these odor qualities, but simply that they contain a prominent ‘minty’ or ‘floral’ note (as based on pilot work in our laboratory; also see Amoore, 1969; Arctander, 1994).
In the context of our habituation design, these odorants constituted four conditions: a target (TG) odorant destined for habituation; a quality-related (QR) odorant similar to TG; a functional group-related (GR) odorant similar to TG; and a control (CT) odorant unrelated to TG in either quality or group. Thus, for example, if CV were assigned as TG, then MT would represent the mint-related QR, AC the ketone-related GR, and PH the unrelated CT. Importantly, the assignment of odorants as the TG condition was evenly counterbalanced across subjects, such that odorant-specific attributes could not confound the behavioral and neural effects of habituation (Fig. 2A). Note a fifth no-odor (air only) condition was included during pre- and post-habituation (see below) to help minimize olfactory fatigue and to provide a low-level baseline.
Paradigm
The imaging experiment consisted of 3 sessions: pre-habituation, habituation, and post-habituation (Fig. 2B). During the pre-habituation session (14 min), the four odorants (or air) were delivered 12 times in a pseudo-random order, with the following constraints: no single condition was presented more than twice in a row; and each condition occurred equal numbers of times in each quarter of the session. Trials recurred with a stimulus-onset asynchrony of 14 sec. At the onset of each trial, a visual cue (“Sniff now”) prompted subjects to make a 2-sec sniff, during which time an odorant or air was presented. Approximately 0.5 sec after odor offset, a labeled magnitude scale of odor intensity (Green et al., 1996) appeared on screen for 9 sec, and subjects submitted their rating using a two-button keypad to slide a cursor along the scale. Thus a total of 12 intensity ratings were collected for each condition. Odorants were delivered using an MRI-compatible, 10-channel computer-controlled olfactometer (airflow set at 3 L/min), which permits rapid delivery of odor in the absence of tactile, thermal, or auditory confounds (Gottfried et al., 2002; Gottfried et al., 2006). Sniff cues and rating scales were back-projected onto a mirror affixed to the headbox of the scanner. Stimulus presentation and collection of intensity ratings were controlled using Cogent2000 software (Wellcome Dept., London, UK), as implemented in Matlab (Mathworks, Natick, MA)
In the habituation session (3.5 min), the TG odorant was presented continuously at a 4-fold higher concentration (and via a separate olfactometer line) than during pre- and post-habituation, based on prior psychophysical data suggesting this approach maximizes behavioral habituation (Pierce et al., 1996). Subjects were instructed to breathe through their noses at all times. Seven intensity ratings for the TG odorant were collected at 30-sec intervals during this session, to establish the degree and time-course of habituation. The post-habituation session (14 min) followed immediately after the habituation session and was identical to pre-habituation, permitting a direct comparison of behavioral and fMRI responses between pre- and post-habituation sessions.
Behavior: habituation
Within-session habituation to the TG odor was analyzed using a one-way ANOVA in which the seven online intensity ratings were weighted using coefficients derived from a simple exponential function (time constant, 1/8 session length). Our decision to model the behavioral data in this manner was based on prior human psychophysical studies of constant odor stimulation (Ekman et al., 1967; Cain, 1974; Berglund et al., 1978) suggesting that perceived intensity declines exponentially and rapidly reaches an asymptotic level, with a decay function approximating 1/8. As a similar time-course has been observed in numerous fMRI studies of odor-evoked activity in human piriform cortex (Sobel et al., 2000; Poellinger et al., 2001; Gottfried et al., 2002), we also used this exponential function to model neural habituation (see below).
The effect of TG exposure on self- and cross-habituation during the post-habituation session was examined by comparing the online intensity ratings for the TG, QR, and GR conditions from the pre- and post-habituation sessions, with the CT condition as the baseline. Because previous studies show that habituation rapidly recovers within 3–4 minutes of the cessation of continuous odor stimulation (Ekman et al., 1967; Pierce et al., 1996), we arranged the 12 intensity ratings (from post-habituation) into four successive quartiles, based on the assumption that any sustained habituation effect would be maximal in the first quartile (which ended 3.5 minutes after the conclusion of the habituation session). We conducted a two-way repeated-measures ANOVA with condition (4 odorants) and time (4 quartiles) as independent variables on changes in intensity ratings from pre-habituation (mean of 12 trials) to post-habituation. The condition-by-time interaction, if significant, would protect a series of follow-up pairwise contrasts (Wilcoxon test, p < 0.05 two-tailed) between intensity rating changes for the CT condition (baseline) and rating changes for each of the other three odorant conditions, per quartile.
Behavior: odor similarity, valence, and pungency
Similarity ratings of odor quality were acquired 30 min before and 30 min after the continuous TG exposure (which happened to be 15 min before and after the imaging experiment, cf. Fig. 2C) to index changes in perceptual differentiation from pre- to post-habituation, as a result of prolonged exposure to the TG odorant. Similarity ratings have the advantage of providing continuous scales that are sensitive to detecting subtle changes in differentiation (Nosofsky, 1988; Hahn, et al., 2005). They are commonly used in investigations of perception-based category learning and classification (Estes, 1986; Nosofsky, 1988; Livingston et al., 1998; Pothos and Chater, 2005) and have proven a sensitive measure in previous olfactory studies of perceptual learning (Peron and Allen, 1988; Stevenson, 2001; Case et al., 2004).
Similarity was rated according to a visual analog scale (Gottfried et al., 2006), with anchors “not at all similar” and “identical,” yielding six discrete pairwise similarity ratings, both for pre- and post-habituation. The six pairwise combinations of the odorants were subsequently divided into two sets. One set consisted of 3 pairs involving the TG odorant: a pair related in quality (TG/QR), a pair related in functional group (TG/GR), and an unrelated pair (TG/CT). The other set consisted of the remaining three pairs involving non-TG combinations, which also formed a pair related in quality (GR/CT), a pair related in group (QR/CT) and a pair unrelated (GR/QR). For example, if CV (minty ketone) were the TG odorant, the second (non-TG) set would include floral quality-related AC and PH (GR/CT), alcohol group-related MT and PH (QR/CT), and unrelated AC and MT (GR/QR) (cf. Fig. 2A). To test for changes in odor quality differentiation (from pre- to post-habituation), similarity ratings for TG pairs were contrasted against ratings for the corresponding non-TG pairs, which controlled for non-specific effects unrelated to the prolonged TG exposure. Based on a directional hypothesis of the learning effect (i.e., enhancement of differentiation), we used one-tailed Wilcoxon signed ranks tests (p < 0.05) for these follow-up contrasts.
Ratings of odor valence and pungency were also obtained before and after scanning using visual analog scales, with anchors “extremely unpleasant” and “extremely pleasant” (valence), or “not at all pungent” and “extremely pungent” (pungency). Ratings based on these scales were converted to values between −10 and 10 before being submitted to repeated-measures ANOVAs to test whether there were differential changes in these indices (from pre- to post-habituation) across odorant conditions.
Respiratory monitoring
During scanning subjects were affixed with a pair of breathing belts to monitor respirations on-line (Gottfried et al., 2002). The output from these belts was transmitted to a piezo-resistive differential pressure sensor (0–1 psi, Honeywell), and the resulting analog signal was amplified, digitized, and recorded on a PC computer using a PowerLab 8/30 data acquisition system and accompanying software (ADInstruments Inc., Colorado Springs, CO). In subsequent analysis, the subject-specific sniff waveforms were baseline-adjusted by subtracting the mean activity in the 500 ms preceding sniff onset, and then averaged across each condition. Sniff inspiratory volume, peak amplitude, and latency to peak were computed for each condition and entered into repeated-measures ANOVAs for statistical analysis in Matlab.
Imaging
Gradient-echo T2-weighted echoplanar images (EPI) were acquired with blood-oxygen-level-dependent (BOLD) contrast on a Siemens Trio 3T MRI scanner, using an eight-channel head coil and an integrated parallel acquisition technique known as GRAPPA (GeneRalized Autocalibrating Partially Parallel Acquisition to improve signal recovery in medial temporal and basal frontal regions. Imaging parameters were: TR, 2 s; TE, 20 ms; slice thickness, 2 mm; gap, 1 mm; in-plane resolution, 1.72 × 1.72 mm; field of view, 220 × 220 mm, matrix size, 128 mm. Image acquisition was tilted at 30° to further reduce susceptibility artifact in olfactory areas. A total of 1,100 volumes (24 interleaved slices per volume covering piriform and orbitofrontal cortices) was obtained over the three sessions. High-resolution (1 × 1 × 1 mm) T1-weighted anatomical scans were acquired after functional scanning, coregistered to the mean functional image, normalized and averaged across subjects to aid localization.
fMRI data pre-processing
The fMRI data were pre-processed using SPM2 (www.fil.ion.ucl.ac.uk/spm/). After the first 5 “dummy” volumes were discarded to permit T1 relaxation, images were spatially realigned to the first volume of the first session and temporally adjusted for slice timing. This was followed by spatial normalization to a standard EPI template, resulting in a functional voxel size of 2 × 2 × 2 mm. The normalized images were smoothed using a 6-mm (full-width half-maximum) Gaussian kernel to account for residual intersubject differences, which has the advantage of permitting group comparisons.
fMRI data analysis
Following image pre-processing, the event-related fMRI data were analyzed in SPM2 using the general linear model (GLM) in combination with established temporal convolution procedures. For the pre-habituation session, 5 vectors of onset times were created, corresponding to the four odor conditions and the air condition. The 5 onset vectors were encoded as stick (delta) functions to assemble 5 event-related regressors of interest for inclusion in the GLM. For the habituation session, 7 vectors (epochs) of onset times were created, each corresponding to successive 15.5-sec periods of passive nasal breathing in the absence of intensity rating judgments, i.e., from the offset of the prior rating scale to the onset of the next rating scale (apart from the first epoch which spanned the first 5 sec of the habituation session, up until the onset of the first rating scale). Because this habituation session involved a repeating series of complex visual perceptual and cognitive processes, including (1) viewing a visual analog scale with several semantic markers, (2) rating odor intensity, and (3) pressing a button to adjust the cursor on the scale, we modeled the habituation session by limiting it to those portions when odor was delivered without those potential confounds. This method helped to disambiguate the effects of odor habituation from those irrelevant processes. For the post-habituation session, in order to accommodate temporal changes in the recovery from habituation, each of the 5 conditions was divided into early (1st 6 trials) and late (2nd 6 trials) periods, resulting in 10 vectors of onset times. Subsequently, all of these regressors were convolved with a synthetic hemodynamic response function, along with its temporal and dispersion derivatives to allow for variations in latency and width of the canonical function. Regressors of no interest included 6 movement-related vectors (derived from spatial realignment), vectors corresponding to the display onset times and durations of the rating scales, and 3 trial-specific sniff parameters (volume, peak and latency).
Model estimation based on the GLM yielded condition-specific regression coefficients (beta values) in a voxel-wise manner for each subject. In a second step (random-effects analysis), subject-specific linear contrasts of these beta values were entered into a series of one-sample t-tests or ANOVAs, each constituting a group-level statistical parametric map of the T statistic (SPM). Activations are reported in a limited set of brain areas where we had a priori regional hypotheses, including anterior and posterior piriform cortex and OFC. We defined the “putative” olfactory OFC according to a meta-analysis of human olfactory neuroimaging studies (Gottfried and Zald, 2005) that implicate bilateral regions centered around area 11l, as based on the cytoarchitectonic work of Ongur et al. (2003). Results were corrected for multiple comparisons across small volumes of interest (“small volume correction” [SVC]). SVC for piriform activations was based on the anatomical masks assembled in MRIcro (http://www.mricro.com) and drawn on the mean structural T1 image, with reference to a human brain atlas (Mai et al., 1997). Correction for OFC activations was based on a sphere of 6-mm radius, centered on the coordinates of putative olfactory OFC ([−22, 32, −16], [24, 36, −12]), as described above (Gottfried and Zald, 2005). Significance level was set at p = 0.05 corrected. All reported coordinates correspond to MNI (Montreal Neurological Institute) system.
fMRI contrasts
Within-session habituation
Response habituation to the TG odorant over the 3.5-min period of continuous exposure was analyzed at the random-effects level using SPM2, in which each of the 7 epochs for each subject were entered into a repeated-measures ANOVA with non-sphericity correction (Greenhouse-Geiser correction). Following model estimation, the 7 epochs were weighted by a 1/8-session-length exponential decay function, based on prior data. A T-contrast was used to assess how well the decay function predicted the trend of signal change over the span of this session.
Neural effects of prolonged TG exposure
We hypothesized that prolonged TG exposure would potentially induce two different effects: 1) learning-induced increases in neural activity, as previously shown in non-olfactory versions of perceptual learning (Gauthier et al., 1999, 2000; Schwartz et al., 2002; Furmanski et al., 2004; Sigman et al., 2005); or 2) habituation-induced decreases in neural activity (Sobel et al., 2000; Poellinger et al., 2001). That is, we predicted that olfactory perceptual learning would be associated with response enhancement to the TG, QR, or GR conditions, compared to the CT baseline, whereas neural habituation per se would be paralleled by response suppression to these same conditions.
To examine these neural effects, we conducted an omnibus repeated-measures ANOVA (with non-sphericity correction) to test whether there were any significant differences in response change (from pre- to post-habituation) among all four odorant conditions, in regions of piriform cortex and OFC. We initially conducted four subject-specific T-contrasts that included (TGpost – TGpre), (QRpost – QRpre), (GRpost – GRpre), and (CTpost – CTpre) in a first-level analysis. These contrasts were then entered into an omnibus ANOVA, which guarded against Type I error in a series of follow-up (random-effects) T-contrasts between the CT condition and each of the TG, QR and GR conditions. We restricted the random-effects T-tests to regions that exhibited an above-threshold (at p 0.001 uncorrected) difference across conditions in the ANOVA to provide further safeguard against Type I error. We reported significant responses that survived correction for multiple comparisons using SVC described above (p < 0.05).
Finally, regression analysis was conducted to determine whether the learning-induced changes in olfactory brain areas predicted the subsequent changes in odor similarity ratings. Subject-specific neural activity for the quality-related odorant condition [(QRpost – QRpre) – (CTpost – CTpre)] was regressed on behavioral changes in similarity ratings [(TG/QRpost – TG/QRpre) – (TG/CTpost – TG/CTpre)]. A similar analysis examining the general effects of perceptual learning across quality- and structure-related conditions was performed by collapsing the neural activity across QR and GR conditions, and then regressing these data on the pooled behavioral changes for TG/QR and TG/GR pairs (all adjusted for the CT condition). Regions of activation were corrected for multiple comparisons across small volumes of interest (p < 0.05).
Supplementary Material
Acknowledgments
This material is based upon work supported by the National Institute on Deafness and Other Communication Disorders under Grant No. 1 K08 DC007653-01A1. We thank Ken A. Paller and Jane Plailly for fruitful discussions, and James D. Howard for help with data collection.
References
- Amoore JE. A plan to identify most of the primary odors. In: Pfaffmann C, editor. Olfaction and Taste III. New York: Rockfeller University Press; 1969. [Google Scholar]
- Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. doi: 10.1038/81774. [DOI] [PubMed] [Google Scholar]
- Arctander S. Perfume and Flavor Chemicals (Aroma Chemicals) Carol Stream, IL: Allured Publishing Company; 1994. [Google Scholar]
- Bedford FL. Constraints on perceptual learning: objects and dimensions. Cognition. 1995;54:253–297. doi: 10.1016/0010-0277(94)00637-z. [DOI] [PubMed] [Google Scholar]
- Berglund B, Berglund U, Lindvall T. Olfactory self- and cross-adaptation: effects of time of adaptation on perceived odor intensity. Sens Processes. 1978;2:191–197. [PubMed] [Google Scholar]
- Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Cain WS. Odor intensity after self-adaptation and cross adaption. Perception and Psychophysics. 1974;5:271–275. [Google Scholar]
- Carmichael ST, Clugnet MC, Price JL. Central olfactory connections in the macaque monkey. J Comp Neurol. 1994;346:403–434. doi: 10.1002/cne.903460306. [DOI] [PubMed] [Google Scholar]
- Case TI, Stevenson RJ, Dempsey RA. Reduced discriminability following perceptual learning with odours. Perception. 2004;33:113–119. doi: 10.1068/p5044. [DOI] [PubMed] [Google Scholar]
- Cleland TA, Morse A, Yue EL, Linster C. Behavioral models of odor similarity. Behav Neurosci. 2002;116:222–231. doi: 10.1037//0735-7044.116.2.222. [DOI] [PubMed] [Google Scholar]
- Dalton P, Doolittle N, Breslin PA. Gender-specific induction of enhanced sensitivity to odors. Nat Neurosci. 2002;5:199–200. doi: 10.1038/nn803. [DOI] [PubMed] [Google Scholar]
- Dade LA, Zatorre RJ, Jones-Gotman M. Olfactory learning: convergent findings from lesion and brain imaging studies in humans. Brain. 2002;125:86–101. doi: 10.1093/brain/awf003. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Ekman G, Berglund B, Berglund U, Lindvall T. Perceived intensity of odor as a function of time of adaptation. Scand J Psychol. 1967;8:177–186. doi: 10.1111/j.1467-9450.1967.tb01392.x. [DOI] [PubMed] [Google Scholar]
- Estes WK. Array models for category learning. Cognit Psychol. 1986;18:500–549. doi: 10.1016/0010-0285(86)90008-3. [DOI] [PubMed] [Google Scholar]
- Fahle M, Poggio T. Perceptual learning. Cambrige, MT: MIT Press; 2002. [Google Scholar]
- Fletcher ML, Wilson DA. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 2002;22:RC201. doi: 10.1523/JNEUROSCI.22-02-j0005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furmanski CS, Schluppeck D, Engel SA. Learning strengthens the response of primary visual cortex to simple patterns. Curr Biol. 2004;14:573–578. doi: 10.1016/j.cub.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3:191–197. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nat Neurosci. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Gibson EJ, Walk RD. The effect of prolonged exposure to visually presented patterns on learning to discriminate them. J Comp Physi Psych. 1956;49:239 – 242. doi: 10.1037/h0048274. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/s0896-6273(01)00424-x. [DOI] [PubMed] [Google Scholar]
- Goldstone RL. Perceptual learning. Annu Rev Psychol. 1998;49:585–612. doi: 10.1146/annurev.psych.49.1.585. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22:10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. The nose smells what the eye sees: crossmodal visual facilitation of human olfactory perception. Neuron. 2003;39:375–386. doi: 10.1016/s0896-6273(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301:1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Winston JS, Dolan RJ. Dissociable Codes of Odor Quality and Odorant Structure in Human Piriform Cortex. Neuron. 2006;49:467–479. doi: 10.1016/j.neuron.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: Meta-analysis and comparison to non-human primates. Brain Res Brain Res Rev. 2005;50:287–304. doi: 10.1016/j.brainresrev.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘labeled magnitude scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Olfactory cortex. New York: Oxford University Press; 1998. [Google Scholar]
- Hahn U, Bailey TM, Elvin LB. Effects of category diversity on learning, memory, and generalization. Mem Cognit. 2005;33:289–302. doi: 10.3758/bf03195318. [DOI] [PubMed] [Google Scholar]
- HALL G. Perceptual and associative learning. Oxford: Oxford University Press; 1991. [Google Scholar]
- Hawkey DJ, Amitay S, Moore DR. Early and rapid perceptual learning. Nat Neurosci. 2004;7:1055–1056. doi: 10.1038/nn1315. [DOI] [PubMed] [Google Scholar]
- Herz RS. The effect of verbal context on olfactory perception. J Exp Psychol Gen. 2003;132:595–606. doi: 10.1037/0096-3445.132.4.595. [DOI] [PubMed] [Google Scholar]
- Herz RS, von Clef J. The influence of verbal labeling on the perception of odors: evidence for olfactory illusions? Perception. 2001;30:381–391. doi: 10.1068/p3179. [DOI] [PubMed] [Google Scholar]
- Jehl C, Royet JP, Holley A. Odor discrimination and recognition memory as a function of familiarization. Percept Psychophys. 1995;57:1002–1011. doi: 10.3758/bf03205459. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ho SL, Xu Z, Yihan JS, Yip S, Hingco EE, Leon M. Functional mapping of the rat olfactory bulb using diverse odorants reveals modular responses to functional groups and hydrocarbon structural features. J Comp Neurol. 2002;449:180–194. doi: 10.1002/cne.10284. [DOI] [PubMed] [Google Scholar]
- Jones PD, Holding DH. Extremely long-term persistence of the McCollough effect. J Exp Psychol Hum Percept Perform. 1975;1:323–327. doi: 10.1037//0096-1523.1.4.323. [DOI] [PubMed] [Google Scholar]
- Kossut M. Modifications of the single cortical vibrissal column. Acta Neurobiol Exp (Wars) 1988;48:83–115. [PubMed] [Google Scholar]
- Linster C, Johnson BA, Yue E, Morse A, Xu Z, Hingco EE, Choi Y, Choi M, Messiha A, Leon M. Perceptual correlates of neural representations evoked by odorant enantiomers. J Neurosci. 2001;21:9837–9843. doi: 10.1523/JNEUROSCI.21-24-09837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston KR, Andrews JK, Harnad S. Categorical perception effects induced by category learning. J Exp Psychol Learn Mem Cogn. 1998;24:732–753. doi: 10.1037//0278-7393.24.3.732. [DOI] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. New York: Thieme; 1997. [Google Scholar]
- Mainland JD, Bremner EA, Young N, Johnson BN, Khan RM, Bensafi M, Sobel N. Olfactory plasticity: one nostril knows what the other learns. Nature. 2002;419:802. doi: 10.1038/419802a. [DOI] [PubMed] [Google Scholar]
- Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell. 1999;96:713–723. doi: 10.1016/s0092-8674(00)80581-4. [DOI] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosofsky RM. Exemplar-based accounts of relations between classification, recognition, and typicality. J Exp Psychol Learn Mem Cogn. 1988;14:700–709. [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Owen DH, Machamer PK. Bias-free improvement in wine discrimination. Perception. 1979;8:199–209. doi: 10.1068/p080199. [DOI] [PubMed] [Google Scholar]
- Peron RM, Allen GL. Attempts to train novices for beer flavor discrimination: a matter of taste. J Gen Psychol. 1988;115:403–418. doi: 10.1080/00221309.1988.9710577. [DOI] [PubMed] [Google Scholar]
- Pierce JD, Jr, Wysocki CJ, Aronov EV, Webb JB, Boden R. The role of perceptual and structural similarity in cross-adaptation. Chem Senses. 1996;21:223–237. doi: 10.1093/chemse/21.2.223. [DOI] [PubMed] [Google Scholar]
- Poellinger A, Thomas R, Lio P, Lee A, Makris N, Rosen BR, Kwong KK. Activation and habituation in olfaction--an fMRI study. Neuroimage. 2001;13:547–560. doi: 10.1006/nimg.2000.0713. [DOI] [PubMed] [Google Scholar]
- Pothos EM, Chater N. Unsupervised categorization and category learning. Q J Exp Psychol A. 2005;58:733–752. doi: 10.1080/02724980443000322. [DOI] [PubMed] [Google Scholar]
- Rabin MD. Experience facilitates olfactory quality discrimination. Percept Psychophys. 1988;44:532–540. doi: 10.3758/bf03207487. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, Costes N, Vigouroux M, Farget V, Sicard G, et al. Functional anatomy of perceptual and semantic processing for odors. J Cogn Neurosci. 1999;11:94–109. doi: 10.1162/089892999563166. [DOI] [PubMed] [Google Scholar]
- Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735–745. doi: 10.1016/s0896-6273(00)81209-x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol. 1995;74:733–750. doi: 10.1152/jn.1995.74.2.733. [DOI] [PubMed] [Google Scholar]
- Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc Natl Acad Sci U S A. 2002;99:17137–17142. doi: 10.1073/pnas.242414599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schyns PG, Goldstone RL, Thibaut JP. The development of features in object concepts. Behav Brain Sci . 1998;21:1–17. 17–54. doi: 10.1017/s0140525x98000107. [DOI] [PubMed] [Google Scholar]
- Shepherd GM. The human sense of smell: are we better than we think? PLoS Biol. 2004;2:E146. doi: 10.1371/journal.pbio.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, Pan H, Yang Y, Stern E, Silbersweig D, Gilbert CD. Top-down reorganization of activity in the visual pathway after learning a shape identification task. Neuron. 2005;46:823–835. doi: 10.1016/j.neuron.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD. Time course of odorant-induced activation in the human primary olfactory cortex. J Neurophysiol. 2000;83:537–551. doi: 10.1152/jn.2000.83.1.537. [DOI] [PubMed] [Google Scholar]
- Stevens DA, O’Connell RJ. Enhanced sensitivity to androstenone following regular exposure to pemenone. Chem Senses. 1995;20:413–419. doi: 10.1093/chemse/20.4.413. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ. The acquisition of odour qualities. Q J Exp Psychol A. 2001;54:561–577. doi: 10.1080/713755972. [DOI] [PubMed] [Google Scholar]
- Wade NJ. A selective history of the study of visual motion aftereffects. Perception. 1994;23:1111–1134. doi: 10.1068/p231111. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Comparison of odor receptive field plasticity in the rat olfactory bulb and anterior piriform cortex. J Neurophysiol. 2000;84:3036–3042. doi: 10.1152/jn.2000.84.6.3036. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons. J Neurophysiol. 2003;90:65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. The fundamental role of memory in olfactory perception. Trends Neurosci. 2003;26:243–247. doi: 10.1016/S0166-2236(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Sensory physiology of central olfactory pathways. New York: Marcel Dekker; 2003. [Google Scholar]
- Wysocki CJ, Dorries KM, Beauchamp GK. Ability to perceive androstenone can be acquired by ostensibly anosmic people. Proc Natl Acad Sci U S A. 1989;86:7976–7978. doi: 10.1073/pnas.86.20.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Liu N, Kida I, Rothman DL, Hyder F, Shepherd GM. Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci U S A. 2003;100:11029–11034. doi: 10.1073/pnas.1832864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M. Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain . 1991;114(Pt 1A):71–84. [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N. Attentional modulation in human primary olfactory cortex. Nat Neurosci. 2005;8:114–120. doi: 10.1038/nn1368. [DOI] [PubMed] [Google Scholar]
- Zellner DA, Kautz MA. Color affects perceived odor intensity. J Exp Psychol Hum Percept Perform. 1990;16:391–397. doi: 10.1037//0096-1523.16.2.391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


