Abstract
In Alzheimer’s disease (AD), atrophy negatively impacts cognition while in healthy adults, inverse relationships between brain volume and cognition may occur. We investigated correlations between gray matter volume and cognition in elderly controls, AD and mild cognitive impairment (MCI) patients with memory and executive deficits. AD demonstrated substantial loss in temporal, parietal and frontal regions while MCI exhibited moderate volume loss in temporal and frontal regions. In controls, memory and executive function were negatively correlated with frontal regions, while in AD, memory was positively correlated with temporal and frontal gyri, and executive function with frontal regions. The combination of the two patterns may explain the lack of correlations in MCI. Developmental versus pathological contributions to these relationships are discussed.
Keywords: Mild Cognitive Impairment, Alzheimer’s, Correlation, Volumetry, Memory, Executive
INTRODUCTION
In vivo neuroimaging studies suggest that normal aging [5] as well as Alzheimer’s disease (AD) [12] are associated with gray matter loss. Normal aging is associated with atrophy primarily in frontal [23] and to a lesser degree in parietal [11] and temporal cortices [27] while AD patients have substantial losses in the temporal [see 16 for review] and parietal [12,18] cortices with less atrophy in frontal regions [14]. Most previous studies have used the region of interest (ROI) approach but more recently studies have applied whole brain morphometric techniques, such as voxel-based morphometry (VBM), and have identified atrophy in these same regions [2,11,12]. Whole brain techniques allow for exploration of brain areas that are not easily assessed with ROI approaches [11].
Normal aging is associated with impairments in high level cognitive operations or “executive functions” as well as episodic memory for events [see 17 for review ]. In contrast, AD patients have predominant impairments in episodic memory, in addition to deficits in executive function [see 4 for review]. Based on studies showing that damage to the medial temporal lobe (MTL) impairs memory [28] while frontal damage affects both memory and executive function [25], it seems likely that these cognitive changes may be related to the atrophy described above. For example, studies have shown that in AD, volumes of the hippocampi and entorhinal cortex (ERC) are directly correlated with memory [8,20] while frontal volumes are related to executive function [14]. In contrast, similar correlations in healthy elderly have reported mixed results [26 for review]. Furthermore, studies of young adults have shown inverse correlations between volumes and cognition [10,24]. One proposed explanation is that pruning of ineffective synapses during early development results in smaller volumes and improved cognition [10]. In normal elderly with minimal volume loss, these negative relationships may remain whereas adults with more advanced atrophy may demonstrate positive associations [27].
Patients with Mild Cognitive Impairment (MCI) are at a higher risk than the general population of developing dementia and as such, MCI is sometimes referred to as a “transitional state” [19]. Thus, these patients, with an intermediate level of atrophy between healthy aging and AD, might exhibit relationships between cerebral volume and cognition reflective of their transitional state. In the present study, we wanted to determine whether regional atrophy could explain the cognitive deficits associated with normal aging, MCI and AD. To this end, we examined correlations between gray matter volumes, using VBM, and both episodic memory and executive functioning in controls, MCI, and AD. We hypothesized that in AD, frontal volumes would be positively correlated with executive functioning and frontal and temporal volumes with episodic memory, while similar relationships would be in the negative direction for healthy elderly. Finally, to assess transitional effects from normal aging to AD, we examined these same relationships in MCI subjects.
METHODS
Subjects
Fourteen healthy controls (CN), 32 MCI and 14 probable AD patients participated. Subjects were paid for participation and signed consent statements approved by the Institutional Review Board of the San Francisco VA Medical Center. All subjects were administered neurological examinations and neuropsychological tests. Exclusion criteria were presence and/or history of neurological (e.g. stroke, dementia) or psychiatric (e.g. major depression) disorder or any medical condition that might produce cognitive impairment (e.g. uncontrolled diabetes, moderate cerebrovascular disease (including the presence of white matter lesions)). All MCI patients had cognitive complaints and objective impairments in either episodic memory and/or executive functioning at least 1.5 standard deviations below the age-adjusted mean for at least one of the neuropsychological tests. Final classification of subjects was based on the above factors and consensus of a team of clinicians. A diagnosis of probable AD was made using National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorder’s Association criteria [1]. Group characteristics are shown in Table 1.
Table 1.
Group characteristics
| Controls (CN) (n = 14) | Mild Cognitive Impairment (MCI) (n = 32) | Alzheimer’s Disease (AD) (n = 14) | ||||
|---|---|---|---|---|---|---|
| Characteristic | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range |
| Age | 69.5 (7.4) | 61 – 87 | 74.1 (6.0) | 63 – 86 | 74.6 (11.4) | 55 – 94 |
| Gender | 8/14 F | — | 11/32 F | — | 5/14 F | — |
| Education | 16.4 (2.0) | 12 – 20 | 15.3 (3.5) | 8 – 20 | 14.9 (4.7) | 5 – 22 |
| MMSE | 29.5 (.69) | 28 – 30 | 28.0 (1.8) | 24 – 30 | 21.3 (2.3) | 18 – 26 |
| Memory Score | 0.80 (.94) | −0.5 – 2.5 | −0.42 (1.1) | −2.75 – 1.50 | −1.55 (.73) | −3.25 – (−.25) |
| CVLT II Long Delay Free Recall Discriminability | 1.0 (1.1) | −0.66 – 3 | −0.25 (1.23) | −2.50 – 2 | −2.1 (1.2) | −4.5 – 0 |
| CVLT II Recognition Discriminability | 0.57 (.93) | −0.66 – 2 | −0.59 (1.13) | −3 – 1 | −1 (.73) | −2 – 0 |
| †Executive Score | 0.82 (0.60) | 0 – 1.67 | −0.27 (1.04) | −2.33 – 2.16 | −1.16 (.50) | −1.66 – (−.33) |
| WAIS-III Digit Symbol Correct | 0.90 (0.79) | −0.33 – 2.0 | −.042 (0.94) | −1.66 – 2 | — | — |
| D-KEFS Number-Letter Switching (secs) | 0.79 (0.66) | −0.33 – 1.66 | −0.44 (1.46) | −3 – 1.66 | — | — |
MMSE = Mini Mental State Exam. CVLT = California Verbal Learning Test. WAIS = Wechsler Adult Intelligence Scale. DKEFS = Delis Kaplan Executive Function System.
Executive score in AD patients based on z score of WAIS Digit Span.
Neuropsychological assessment
All subjects were administered a large battery of neuropsychological tests assessing multiple cognitive domains. In order to limit the number of statistical tests, two composite scores were created based on tests that we felt best represented the memory and executive domains.
For the episodic memory score, referred to hereafter as the Memory Score, the z-scores (standard deviations from mean) for the Long Delay Free Recall Discriminability and Recognition Discriminability indices from the standard form of the California Verbal Learning Test (CVLT-II) were averaged together. The composite score for executive function, or the Executive Score, was based on the average of the z-scores for the number of seconds for number-letter switching on the Delis Kaplan Executive Function System (D-KEFS) Trail Making test and the number correct from the Digit Symbol substitution test of the Wechsler Adult Intelligence Scale (WAIS-III). For AD, the Executive Score was based on the z-score of the WAIS-III Digit Span test. The means and ranges for these scores and subtests are shown in Table 1.
Image acquisition and processing
All subjects were scanned on a Siemens 1.5T MR scanner. T1-weighted structural images were acquired with an MPRAGE (magnetization prepared rapid acquisition gradient-echo) sequence (TR 9.7 ms, TE 4 ms, FOV 256 mm, matrix 256 x 256 mm, 154 contiguous slices, slice thickness 1.5 mm). A study-specific T1 template for all subjects was created for use in all processing procedures using SPM2 (Wellcome Department of Imaging Neuroscience; London, UK). Individual T1 images were segmented, normalized and modulated according to the optimized VBM protocol [11]. The modulated gray matter (GM) images were smoothed with a 10 mm Gaussian kernel.
Statistical analyses
GM contrasts were corrected for age and total intracranial volume (TIV). Results were assessed under an α = .05, Bonferroni corrected for the number of resels. Results of group comparisons are displayed on the group average T1 image, created by VBM. Corrected voxel-level statistics are reported in all cases.
Voxel-by-voxel correlations between Memory and Executive scores and GM volume were performed separately for each group. For the Memory Score-GM correlations, age, TIV and the Executive Score were entered as nuisance variables while for the Executive Score-GM correlations, age, TIV and the Memory Score were entered as nuisance variables. Results were assessed under an α = .05, Bonferroni corrected for the number of resels. Results from all significant correlations are displayed on the group average T1 image. Corrected voxel-level statistics are reported in all cases.
RESULTS
Group demographics and neuropsychological test scores are shown in Table 1. No significant differences were found between the groups for age, gender and education [all F(2, 59)’s < 1.98, p’s > .15] while the groups differed in MMSE scores [F(2, 59) = 91.0, p < .000001], memory [F(2, 59) = 19.6, p < .000001] and executive function [F(2, 59) = 18.9, p < .000001]. Follow up t-tests confirmed that MCI were slightly older than CN [t(44) = 2.23, p = .03] but there were no age differences between CN and AD or AD and MCI [all p’s > .1]. MMSE, memory and executive composite scores were higher in CN than MCI or AD [all p’s < .005] and in MCI relative to AD [all p’s < .001]. Memory subtests were greater in CN than in either patient group [all p’s < .002] while recall [t(44) = 4.7, p = .0001] but not recognition [t(44) = 1.2, p = .2] discriminability was greater in MCI than AD. Executive subtests were greater in CN than MCI [all t(44)’s > 2.9, p’s < .006].
Group contrasts
The results of the group contrasts are shown in Table 2 and displayed on the group average brain in Figure 1. A few regions of GM atrophy were found in MCI patients relative to controls including the inferior frontal gyrus, entorhinal cortex (ERC) region of the parahippocampal gyrus, temporal and fusiform gyri. Relative to controls, AD patients demonstrated the most significant volume loss in bilateral medial and lateral temporal regions, extending into medial and lateral parietal cortex. AD patients also demonstrated more atrophy than MCI patients in these regions.
Table 2.
Locations of significant gray matter loss.
| Contrast | Anatomical Location | Peak Voxel Coordinates | Peak T value | Cluster Size | P Value |
|---|---|---|---|---|---|
| CN > MCI | |||||
| L Fusiform Gyrus | −43, −44, −27 | 4.31 | 394 | .00005 | |
| R Inferior Frontal Gyrus | 52, 4, 10 | 4.13 | 522 | .00008 | |
| R Inferior Parietal Lobe | 40, −50, 40 | 3.81 | 95 | .0002 | |
| L Inferior Temporal Gyrus | −45, −22, −21 | 3.53 | 60 | .001 | |
| R Middle Temporal Gyrus | 60, 7, −15 | 3.52 | 42 | .001 | |
| R Parahippocampal Gyrus (ERC) | 18, −9, −28 | 3.32 | 6 | .001 | |
| CN > AD | |||||
| L Superior Temporal Gyrus | −65, −17, 5 | 7.24 | 54, 276 | < .00001 | |
| L Middle Temporal Gyrus | −58, −31, −15 | 6.26 | — | < .00001 | |
| R Rolandic Operculum | 52, 3, 6 | 6.11 | 58, 279 | < .00001 | |
| R Hippocampus | 38, −21, −16 | 5.85 | — | < .00001 | |
| L Middle Occipital Gyrus | −32, −86, 10 | 5.39 | 1687 | < .00001 | |
| R Middle Temporal Gyrus | 59, 1, −35 | 5.20 | 341 | .00001 | |
| R Inferior Frontal Gyrus | 44, 21, −15 | 5.11 | 428 | .00001 | |
| L Middle Frontal Gyrus | −21, 34, 33 | 5.01 | 89 | .00002 | |
| R Middle Occipital Gyrus | 47, −83, 15 | 4.49 | 360 | .00007 | |
| L Inferior Occipital Gyrus | −49, −79, −12 | 4.27 | 76 | .0001 | |
| R Precentral Gyrus | 37, −5, 49 | 4.27 | 177 | .0001 | |
| L Inferior Frontal Gyrus | −42, 34, 3 | 4.21 | 574 | .0001 | |
| MCI > AD | |||||
| L Superior Temporal Gyrus | −58, −12, 3 | 5.83 | 46,720 | < .00001 | |
| R Superior Temporal Gyrus | 49, −31, 17 | 5.05 | 16,317 | < .00001 | |
| R Supramarginal Gyrus | 60, −20, 23 | 4.51 | — | .00002 | |
| R Rolandic Operulum | 43, −10, 20 | 3.99 | — | .00003 | |
| R Inferior Frontal Gyrus | 47, 18, 16 | 4.86 | 3987 | < .00001 | |
| L Lingual Gyrus | −9, −54, 4 | 4.66 | 1601 | .00001 | |
| R Middle Temporal Gyrus | 66, −54, −3 | 4.37 | 733 | .00004 | |
| L Inferior Frontal Gyrus | −37, 29, −1 | 4.32 | 1246 | .00004 | |
Voxel-level statistics (Bonferroni, α = .05 resels) are reported. L = L; R = Right. Coordinates in Montreal Neurological Institute (MNI) average brain space. Italicized locations represent subregions within the larger corresponding cluster.
Figure 1.
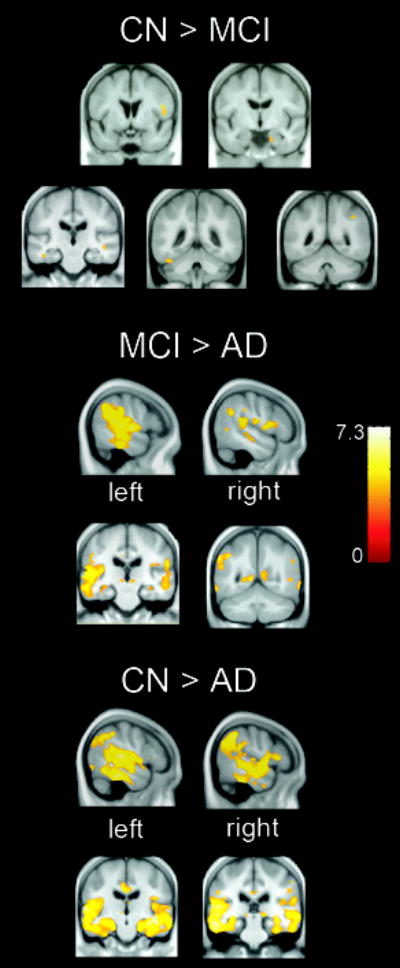
Gray matter atrophy in MCI patients relative to healthy controls (CN), AD relative to MCI patients and AD patients relative to CN, p < .05, corrected for multiple comparisons.
Correlations
The results of the within group voxel-by-voxel correlations are shown in Table 3 and displayed on the group average brain in Figure 2. In controls, all correlations between GM volume and cognition were in the negative direction. Memory was correlated with GM volumes of several frontal gyri while Executive function was correlated with the left middle frontal gyrus. No reliable correlations were observed between GM volume and cognition in MCI patients. In AD patients, all correlations were positive. Memory was correlated with temporal polar, orbital and inferior frontal gyri while Executive functioning was correlated with left frontal gyri.
Table 3.
Locations of significant voxel-by-voxel correlations for gray matter volume.
| Group | Score | Direction of Correlation | Anatomical Location | Peak Voxel Coordinates | Peak T value | Cluster Size | P Value |
|---|---|---|---|---|---|---|---|
| CN | |||||||
| Memory | Negative | L Inferior Frontal Gyrus | −55, 24, 18 | 8.91 | 311 | < .00001 | |
| Memory | Negative | L Middle Frontal Gyrus | −25, 19, 61 | 8.32 | 117 | < .00001 | |
| Memory | Negative | L Superior Frontal Gyrus | −12, −8, 73 | 6.39 | 155 | .00006 | |
| Memory | Negative | L Inferior Parietal Lobe | −36, −56, 56 | 6.03 | 28 | .0001 | |
| Memory | Negative | L Inferior Frontal Gyrus | −39, 20, 30 | 5.39 | 80 | .0002 | |
| Executive | Negative | L Middle Frontal Gyrus | −34, 18, 48 | 5.55 | 84 | .0001 | |
| AD | |||||||
| Executive | Positive | L Inferior Orbital Frontal Gyrus | −25, 15, −28 | 9.71 | 453 | < .00001 | |
| Memory | Positive | L Inferior Orbital Frontal Gyrus | −26, 16, −29 | 7.07 | 118 | .00002 | |
| Executive | Positive | L Medial Frontal Gyrus | −3, 50, 23 | 7.06 | 95 | .00003 | |
| Memory | Positive | R Superior Temporal Pole/Inferior Frontal Gyrus | 58, 12, −6 | 6.85 | 87 | .00003 | |
| Executive | Positive | L Middle Frontal Gyrus | −34, 51, 26 | 5.84 | 99 | .0001 | |
| Memory | Positive | R Anterior Cingulate | 12, 39, 5 | 5.56 | 68 | .0001 | |
Voxel-level statistics (Bonferroni, α = .05 resels) are reported. L = Left; R = Right. Coordinates in Montreal Neurological Institute (MNI) average brain space.
Figure 2.
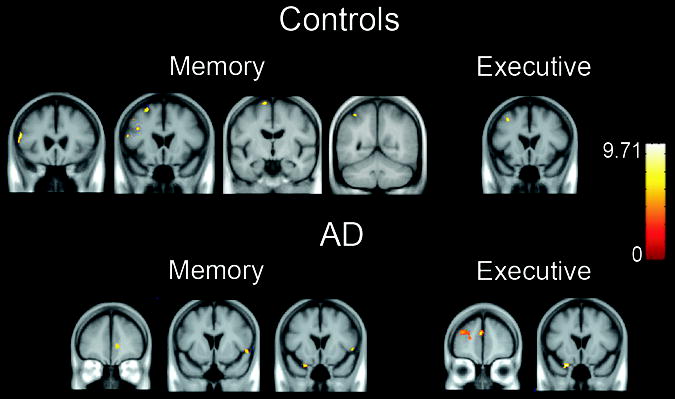
Correlations between gray matter volumes and memory and executive function scores in CN and AD groups, p < .05, corrected for multiple comparisons.
DISCUSSION
In the current study, MCI and AD patients demonstrated impairments in both memory and executive functioning and we aimed to investigate the volumetric substrates of these impairments. As predicted, MCI patients had brain volumes intermediate between normal aging and AD, with moderate loss in temporal, frontal and parietal regions while AD exhibited substantial losses in these areas. One recent study showed that patients with amnestic MCI exhibited greater atrophy in the MTL than those with multi-domain MCI, who exhibited more diffuse neocortical atrophy [3]. This may explain the lack of pronounced atrophy in the MTL in the current MCI sample, which included amenstic and non-amnestic subjects. In this same previous study, all MCI patients, regardless of subtype, exhibited atrophy in the inferior frontal gyrus, similar to what we observed. Although the hippocampus has been the focus of most previous volumetric studies, histological evidence suggests that AD pathology may be widely distributed in MCI [21]. These results suggest that MCI may be associated with diffuse structural changes and highlights the importance of examining patients with various clinical expressions in conjunction with whole brain analyses.
We hypothesized that temporal and/or frontal regions would be associated with episodic memory while frontal regions would be associated with executive function. Consistent with this, in healthy elderly, superior, middle and inferior frontal regions were negatively correlated with memory and the middle frontal gyrus was negatively correlated with executive functioning. Similarly, in AD, orbital and inferior frontal gyri and the temporal pole were positively correlated with memory while several frontal gyri were positively correlated with executive functioning. Lesion studies suggest that the these frontal regions (middle, inferior) may be involved in episodic memory [9], while the temporal poles have been implicated in language processing [6], which likely contributed to memory performance for the verbal materials in the present study. Several frontal regions have been implicated in executive control processes, including initiation, attention switching, monitoring of information and inhibition of irrelevant stimuli [25]. Collectively, these data are consistent with the idea that episodic memory is dependent on frontal and temporal regions while executive functioning is dependent on the frontal cortex. Finally, the volumetric reductions relative to controls in the temporal poles, inferior and middle frontal gyri and the direct relationship between the volumes of these regions and memory and/or executive functioning suggests that pathologically induced atrophy in these regions likely contributes to the cognitive deficits in AD patients
In controls, all correlations were in the negative direction, meaning that smaller volumes reflected better cognition. This contrasts with the consistently positive correlations between cerebral volumes and cognition in AD patients (e.g.[7,15]). Several studies of young adults found negative correlations between cerebral volume and cognition [10,24]. It has been suggested that insufficient synaptic pruning during brain development in adolescence may lead to reduced cognition in young adults, hence the inverse relationships [10]. The negative correlations we observed in this group are consistent with previous reports of negative correlations between frontal and temporal gray matter volumes, memory and executive function in healthy older adults scoring above the age-adjusted mean for various cognitive tests [13,22,27]. One possibility is that some older adults, within or above the age-adjusted level for gray matter volume and cognition, exhibit negative correlations like those of young adults [27]. Consistent with this hypothesis, the older controls in the current study scored above age-adjusted norms on all measures.
In AD patients with substantial atrophy and reduced cognition relative to healthy elderly, smaller volumes were associated with lower functioning. From this we conclude that while developmental synaptic pruning dominates the relationship between gray matter volumes and cognition in healthy older adults, pathologically-induced atrophy dominates this association in AD. The lack of correlations in MCI may be explained by the fact that this group likely represents a combination of early AD and normal aging and the combination of the two contrasting correlation patterns results in no observable correlations across this group. Another possibility is that the contrasting patterns are explained by a transitional stage from normal aging to MCI to AD. Specifically, if insufficient pruning during development results in a larger number of low functioning neurons, an inverse relationship between volume and cognition may occur. A transition from negative to positive correlations could evolve as progressive neuron loss reduces the overall number of neurons. That is, assuming that there are more high than low functioning neurons in the healthy brain, the progressive loss would result in a greater contribution of high functioning neurons to cognition, producing the positive correlations. One prediction from these data is that as some MCI patients progress toward dementia and exhibit further volume loss, they will exhibit more positive relationships between cognition and cerebral volumes. Future longitudinal investigation of these patients will be useful in this regard.
CONCLUSION
In summary, as predicted from lesion studies, episodic memory functioning was correlated with frontal and temporal gray matter volumes while executive functioning was correlated with frontal gray matter volumes. While these associations were in the negative direction for healthy elderly, they were in the positive direction for AD patients, who also exhibited substantial temporal and frontal atrophy. The fact that several of these atrophic regions were positively correlated with cognition in these patients yet negatively correlated with cognition in healthy elderly suggests that pathological atrophy negatively impacts cognition. Consistent with the idea that MCI patients represent the transitional state between healthy aging and AD, no correlations were observed in this group. This suggests that MCI patients are a heterogeneous group of subjects, some of whom are affected by AD pathology.
Acknowledgments
This research was supported by NIA grant 2R01AG10897-18.
References
- 1.Criteria for the clinical diagnosis of Alzheimer's disease. Excerpts from the NINCDS-ADRDA Work Group report. J Am Geriatr Soc. 1985;33:2–3. doi: 10.1111/j.1532-5415.1985.tb02850.x. [DOI] [PubMed] [Google Scholar]
- 2.Baron JC, Chetelat G, Desgranges B, Perchey G, Landeau B, de la Sayette V, Eustache F. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer's disease. Neuroimage. 2001;14:298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 3.Bell-McGinty S, Lopez OL, Meltzer CC, Scanlon JM, Whyte EM, Dekosky ST, Becker JT. Differential cortical atrophy in subgroups of mild cognitive impairment. Arch Neurol. 2005;62:1393–1397. doi: 10.1001/archneur.62.9.1393. [DOI] [PubMed] [Google Scholar]
- 4.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 6.Crinion JT, Warburton EA, Lambon-Ralph MA, Howard D, Wise RJ. Listening to Narrative Speech after Aphasic Stroke: the Role of the Left Anterior Temporal Lobe. Cereb Cortex. 2005 doi: 10.1093/cercor/bhj053. [DOI] [PubMed] [Google Scholar]
- 7.Deweer B, Lehericy S, Pillon B, Baulac M, Chiras J, Marsault C, Agid Y, Dubois B. Memory disorders in probable Alzheimer's disease: the role of hippocampal atrophy as shown with MRI. J Neurol Neurosurg Psychiatry. 1995;58:590–597. doi: 10.1136/jnnp.58.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du AT, Schuff N, Kramer JH, Ganzer S, Zhu XP, Jagust WJ, Miller BL, Reed BR, Mungas D, Yaffe K, Chui HC, Weiner MW. Higher atrophy rate of entorhinal cortex than hippocampus in AD. Neurology. 2004;62:422–427. doi: 10.1212/01.wnl.0000106462.72282.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duarte A, Ranganath C, Winward L, Hayward D, Knight RT. Dissociable neural correlates for familiarity and recollection during the encoding and retrieval of pictures. Brain Res Cogn Brain Res. 2004;18:255–272. doi: 10.1016/j.cogbrainres.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Foster JK, Meikle A, Goodson G, Mayes AR, Howard M, Sunram SI, Cezayirli E, Roberts N. The hippocampus and delayed recall: bigger is not necessarily better? Memory. 1999;7:715–732. doi: 10.1080/096582199387823. [DOI] [PubMed] [Google Scholar]
- 11.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 12.Karas GB, Burton EJ, Rombouts SA, van Schijndel RA, O'Brien JT, Scheltens P, McKeith IG, Williams D, Ballard C, Barkhof F. A comprehensive study of gray matter loss in patients with Alzheimer's disease using optimized voxel-based morphometry. Neuroimage. 2003;18:895–907. doi: 10.1016/s1053-8119(03)00041-7. [DOI] [PubMed] [Google Scholar]
- 13.Kohler S, Black SE, Sinden M, Szekely C, Kidron D, Parker JL, Foster JK, Moscovitch M, Winocour G, Szalai JP, Bronskill MJ. Memory impairments associated with hippocampal versus parahippocampalgyrus atrophy: an MR volumetry study in Alzheimer's disease. Neuropsychologia. 1998;36:901–914. doi: 10.1016/s0028-3932(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 14.Kopelman MD, Lasserson D, Kingsley D, Bello F, Rush C, Stanhope N, Stevens T, Goodman G, Heilpern G, Kendall B, Colchester A. Structural MRI volumetric analysis in patients with organic amnesia, 2: correlations with anterograde memory and executive tests in 40 patients. J Neurol Neurosurg Psychiatry. 2001;71:23–28. doi: 10.1136/jnnp.71.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer JH, Schuff N, Reed BR, Mungas D, Du AT, Rosen HJ, Jagust WJ, Miller BL, Weiner MW, Chui HC. Hippocampal volume and retention in Alzheimer's disease. J Int Neuropsychol Soc. 2004;10:639–643. doi: 10.1017/S1355617704104050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BC, Mintun M, Buckner RL, Morris JC. Imaging of Alzheimer's disease. J Neuroimaging. 2003;13:199–214. [PubMed] [Google Scholar]
- 17.Light LL. Memory and aging: four hypotheses in search of data. Annu Rev Psychol. 1991;42:333–376. doi: 10.1146/annurev.ps.42.020191.002001. [DOI] [PubMed] [Google Scholar]
- 18.Pennanen C, Testa C, Laakso MP, Hallikainen M, Helkala EL, Hanninen T, Kivipelto M, Kononen M, Nissinen A, Tervo S, Vanhanen M, Vanninen R, Frisoni GB, Soininen H. A voxel based morphometry study on mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2005;76:11–14. doi: 10.1136/jnnp.2004.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 20.Petersen RC, Jack CR, Jr, Xu YC, Waring SC, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E. Memory and MRI-based hippocampal volumes in aging and AD. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- 21.Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Salat DH, Kaye JA, Janowsky JS. Greater orbital prefrontal volume selectively predicts worse working memory performance in older adults. Cereb Cortex. 2002;12:494–505. doi: 10.1093/cercor/12.5.494. [DOI] [PubMed] [Google Scholar]
- 23.Salat DH, Kaye JA, Janowsky JS. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Arch Neurol. 2001;58:1403–1408. doi: 10.1001/archneur.58.9.1403. [DOI] [PubMed] [Google Scholar]
- 24.Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- 25.Stuss DT, Alexander MP, Floden D, Binns MA, Levine B, McIntosh AR, Rajah N, Hevenor SJ. Fractionation and localization od distinct frontal lobe processes: evidence from focal lesions in humans. In: Knight RT, Stuss DT, editors. Principles of frontal lobe function. Oxford University Press; New York: 2002. pp. 392–407. [Google Scholar]
- 26.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42:1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]


