Abstract
We examined the long-term retention of a learned automatic cognitive process in 17 severe TBI participants and 10 controls. Participants had initially received extensive consistent-mapping (CM) training (i.e., 3600 trials) in a semantic category visual search task (Schmitter-Edgecombe & Beglinger, 2001). Following CM training, TBI and control groups demonstrated dramatic performance improvements and the development of an automatic attention response (AAR), indicating task-specific and stimulus-specific skill learning. After a 5- or 10-month retention interval, participants in this study performed a New CM task and the originally trained CM task to assess for retention of task-specific and stimulus-specific visual search skills, respectively. No significant group differences were found in the level of retention for either skill type, indicating that individuals with severe TBI were able to retain the learned skills over a long-term retention interval at a level comparable to controls. Exploratory analyses revealed that TBI participants who returned at the 5-month retention interval showed nearly complete skill retention, and greater skill retention than TBI participants who returned at the 10-month interval, suggesting that “booster” or retraining sessions may be needed when a skill is not continuously in use.
Keywords: Closed-head injury, Diffuse axonal injury, Automatic processes, Automatic cognitive skills, Rehabilitation, Cognitive skill retention
INTRODUCTION
Individuals with traumatic brain injuries (TBIs) constitute a large proportion of those who require extensive post-hospitalization rehabilitation. Central to successful post-injury rehabilitation is the ability to develop and retain new complex cognitive skills. Because automatic component processes serve as fundamental building blocks for complex cognitive skills (Fisk & Rogers, 1992; Logan, 1985), a better understanding of the development and retention of automatic processes following a TBI could have important implications for rehabilitation.
Automatic processing can be described as rapid processing that requires minimal conscious control or effort. In contrast, controlled processing tends to be slow, serial, and under the conscious control of the individual (Schneider & Shiffrin, 1977). The key differentiation between controlled and automatic processing is that of attention; controlled processing relies heavily on conscious attention to the given task, whereas automatic processing occurs with little or no conscious attention. Therefore, unlike automatic processes, multiple controlled processes generally cannot be performed efficiently under situations with a high workload (Schneider & Chein, 2003).
Most of the previous work examining automatic processes in the TBI population supports the notion that processes automatized prior to injury are intact in TBI participants by one-year post-injury (e.g., Schmitter-Edgecombe et al., 1993; Schmitter-Edgecombe & Nissley, 2000; Schmitter-Edgecombe & Rogers, 1997; Vakil et al., 1991). Two recent studies conducted in our laboratory also demonstrate that severe TBI participants (>1 year post-injury) can successfully learn to automatize cognitive components of complex tasks post-injury (Schmitter-Edgecombe & Beglinger, 2001; Schmitter-Edgecombe & Rogers, 1997). In both of these studies, the consistency of practice was manipulated to create a situation where automatic processing could develop (consistent mapping training), and one where controlled processing was continually required (varied mapping training). During a consistent mapping (CM) search situation, participants always respond the same way to a specific class of stimuli across a large number of trials (e.g., >1,800). Such extensive and consistent training on a task leads from performance being under controlled processing to performance becoming automatic (Schneider & Chein, 2003). That is, the participant no longer needs to consciously attend to the stimuli because searching the items has now become an automatic process in which a parallel, rather than a serial, search strategy is being utilized. In contrast, in a varied mapping (VM) situation, responses to the same stimuli can vary from one trial to the next. Because of this inconsistency, the individual must continue to utilize a serial search and, therefore, the task continues to rely on controlled processing.
In the skill learning studies completed by Schmitter-Edgecombe and colleagues (Schmitter-Edgecombe & Beglinger, 2001; Schmitter-Edgecombe & Rogers, 1997), following extended practice in VM training conditions (>1800 trials), TBI participants continued to exhibit slower memory and visual search rates compared to control participants. However, after extensive CM training on the tasks, both groups showed performance characteristics indicative of automatic process development. Whereas the major locus of learning for CM memory search is believed to be the unitization of the memory set (i.e., the memory set of a given number of items becomes one unitized, representative set; Fisk et al., 1995; Schneider & Fisk, 1984), learning for CM visual search is believed to benefit most from the development of an automatic attention response (AAR) and optimal search strategies (Fisk et al., 1995; Shiffrin, 1988).
An AAR refers to a concept wherein the target stimuli automatically attract the participant’s attention rather than requiring a controlled process to direct attention. This is because of an increase in the attention-calling strength of the target stimuli and a decrease in the attention-calling strength of the distractor stimuli. This type of learning represents stimulus-specific learning because an AAR is contingent on the specific stimuli utilized in the given task (Schneider & Shiffrin, 1977; Shiffrin & Dumias, 1981; Shiffrin & Schneider, 1977). The development of optimal search strategies represents task-specific learning because the general demands of the task (e.g., using the keypad, pressing the appropriate keys, knowing where in the visual display to look for the stimuli, etc.) are learned (Batsakes & Fisk, 2000; Fisk et al., 1995). Changing the specific task stimuli will greatly affect performance for stimulus-specific but not task-specific skills. Following extended practice with a CM visual search task, Schmitter-Edgecombe and Beglinger (2001) showed that TBI participants successfully developed a stimulus-specific AAR and task-specific optimal search strategies.
An important question that remains unanswered by previous studies is that of skill retention. Specifically, do individuals with a TBI retain stimulus-specific and task-specific skills at a level comparable to controls when such processes are not recurrently in use, or is there a difference in the level of skill decay? To answer this question, TBI and control participants who had successfully developed stimulus-specific and task-specific skills in an earlier visual search study (Schmitter-Edgecombe & Beglinger, 2001) were retested in the present study following a long interval (i.e., 5- or 10-months). We hypothesized that TBI participants would show no more decay of stimulus-specific skills than control participants. This prediction was based on the previously reviewed findings, which showed that automatic processes developed prior to sustaining a severe TBI are typically intact at one year post-injury, and new automatic processes can be developed post-injury at a level comparable to normal controls. Generating a solid hypothesis for the retention of general, task-specific skills was more difficult because, to date, research on the long-term rate of forgetting in TBI populations remains sparse. In general, previous studies that have examined this issue have utilized shorter retention intervals ranging from 30 minutes to six weeks and more controlled, rather than automatic, processes. These studies have found that significant differences between TBI and control groups can be curtailed if differences in initial learning and acquisition for the material were controlled (e.g., Carlesimo et al., 1997; DeLuca et al., 2000; Hillary et al., 2003; Kapur et al., 1996). We hypothesized that if this is the case, then the TBI and control participants would display comparable levels of general, task-specific skill retention as well.
METHOD
Participants
Participants were recruited from a sample of 18 individuals with a severe TBI resulting from a closed-head injury (15 male, 3 female participants) and from 18 matched controls who participated in a previous visual search skill acquisition study (see Schmitter-Edgecombe & Beglinger, 2001). Eight TBI and six control participants took part in a retention testing session five months following initial training, whereas nine TBI and four control participants took part 10 months following initial training. This resulted in an overall sample of 17 TBI and 10 control participants. The one TBI individual that did not return initially agreed to participate, but missed his appointment and could not be rescheduled. The non-returning control participants either could not be located (n = 2), did not respond to attempted solicitations (n =4), or failed to attend scheduled appointments (n = 2).
For detailed information concerning injury characteristics, exclusionary criteria, and review of medical records, readers should refer to the original skill-learning study (Schmitter-Edgecombe & Beglinger, 2001). A severe TBI was defined by a review of medical records indicating that (a) duration of coma was >48 hours or (b) depth of coma, as assessed by the Glasgow Coma Scale (Teasdale & Jennett, 1974), was ≤8 or less. All TBI participants were more than one year post-injury at initial testing. Analyses re-examining demographic and cognitive variables replicated the original skill-learning study (see Table 1; a detailed narrative can be found in Schmitter-Edgecombe & Beglinger, 2001). All data included in this manuscript were obtained in compliance with the regulations of the Washington State University Institutional Review Board, and all participants received monetary compensation and parking expenses.
Table 1.
Demographic and neuropsychological data for the TBI and control groups
| TBIs
|
Controls
|
||||||
|---|---|---|---|---|---|---|---|
| Variables or test | N | M | SD | N | M | SD | d |
| Age (years) | 17 | 34.12 | 9.41 | 10 | 32.18 | 9.43 | .21 |
| Education (years) | 17 | 13.82 | 1.91 | 10 | 13.20 | 1.23 | .37 |
| Occupational statusa | |||||||
| Mother | 16 | 3.12 | 1.99 | 9 | 2.11 | 1.76 | .51 |
| Father | 14 | 2.14 | 1.35 | 9 | 2.22 | 1.30 | −.06 |
| Coma duration | 17 | 29.71 | 27.14 | ||||
| PTAb | 17 | 83.88 | 83.56 | ||||
| TSIc | 17 | 10.92 | 8.65 | ||||
| Barona FSIQ estimate | 17 | 104.86 | 3.90 | 10 | 106.48 | 8.99 | −.26 |
| Learning and memory | |||||||
| CVLT Trials 1–5d | 16 | 49.68 | 10.31 | 10 | 59.80 | 6.44 | −1.11* |
| WMS-Rd | |||||||
| Visual reproduction I | 17 | 34.82 | 3.97 | 10 | 37.70 | 2.31 | −.83* |
| Visual reproduction II | 17 | 30.82 | 6.97 | 10 | 36.00 | 3.23 | −.88* |
| Logical memory I | 16 | 19.68 | 7.83 | 10 | 31.70 | 8.24 | −1.49** |
| Logical memory II | 16 | 15.06 | 7.97 | 10 | 29.70 | 7.74 | −1.84** |
| Category fluency (animals)d | 16 | 18.31 | 5.47 | 10 | 23.40 | 5.03 | −.95* |
| Processing speed | |||||||
| SDMT writtend | 17 | 41.59 | 11.57 | 10 | 57.00 | 7.33 | −1.51** |
| SDMT orald | 16 | 50.19 | 15.12 | 10 | 63.20 | 7.60 | −1.01* |
| Memory span | |||||||
| WAIS-R digit spand | 16 | 15.12 | 3.15 | 10 | 17.60 | 3.71 | −.73 |
| Alphabet span teste | 16 | 3.90 | .82 | 10 | 4.50 | .52 | −.81 |
Note. TBI = traumatic brain injury; CVLT = California Verbal Learning Test; WMS-R =Wechsler Memory Scale-Revised; WAIS-R =Wechsler Adult Intelligence Scale-Revised; PTA = Post-traumatic Amnesia duration; TSI =Time since injury; d = Effect size
Scored on 6-pt Occupational Scale (WAIS-R; Wechsler, 1981; 1 = professional & technical workers; 6 = not in labor force).
Time in days
Time in years
Raw scores.
Simple span score.
p < .05;
p < .01
Equipment
Psychological Software Tools’ Micro Experimental Laboratory (MEL) was used to program the experiment, which was presented on IBM-compatible portable computers.
Design
There were 10 initial training sessions in the original study (Schmitter-Edgecombe & Beglinger, 2001). Important to this study, sessions 2 through 4 of the original study consisted of 1,200 experimental CM training trials per day. In the CM condition, the targets (e.g., Trees) never appeared as distractors, and the distractors (e.g., Vehicles) never appeared as targets. Session 5 was a transfer session in which participants first received 300 Trained CM trials, followed by 300 CM Reversal trials, 300 New CM trials, and finally another 300 trials in the Trained CM condition. In the CM Reversal trials, the roles of the previously Trained CM condition targets and distractors were reversed. In the New CM condition, two new categories were combined into a New CM condition. Assessment of the development of an AAR was conducted by comparing performances in the transfer conditions to the Trained CM condition.
Stimuli
Stimulus-set items were selected from a group of 12 non-overlapping semantic categories (Battig & Montague, 1969; Collen et al., 1975). Each category contained six high association words that were four to six letters in length. Ten of the 12 categories were presented during the initial skill learning study (Schmitter-Edgecombe & Beglinger, 2001). The remaining two categories were used in this retention study along with the original two categories used for each participant during the Trained CM condition (see Retention Testing Procedure below).
General Procedure
A detailed visual layout of the task is provided in Figure 1. For narrative information, see the original skill learning study (Schmitter-Edgecombe & Beglinger, 2001).
Fig. 1.
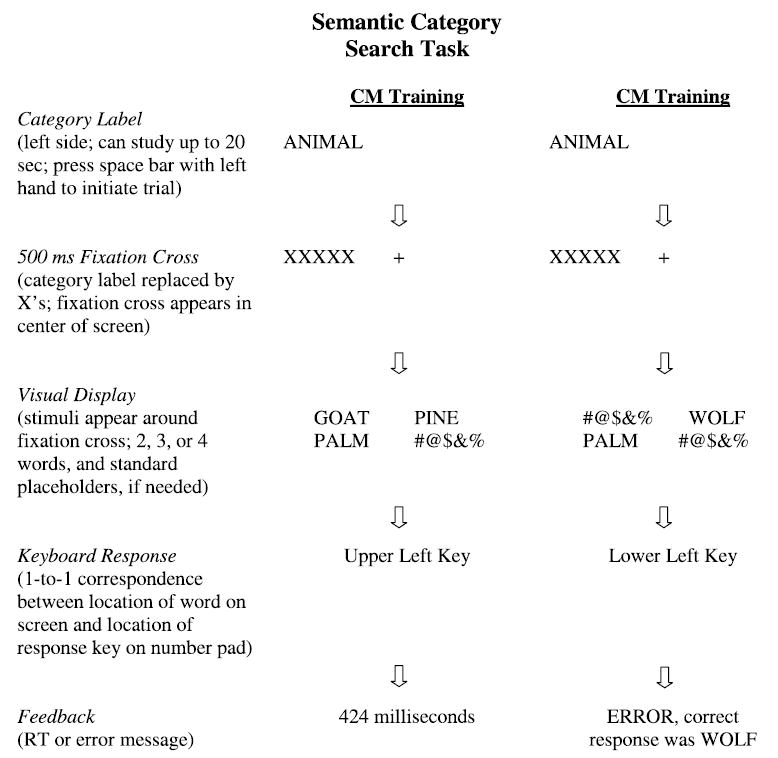
Example of the visual search task experimental trials for a correct CM training response and an incorrect CM training response.
Retention Testing Procedure
To examine both task-specific and stimulus-specific skill retention, all participants completed 300 trials in a Retention New CM condition, followed by 900 trials in the original Trained (Retention) CM condition. The Retention New CM condition was administered prior to the Retention CM condition to allow for the examination of task-specific skills without contamination of stimulus-specific skills. In the Retention New CM condition, participants were required to perform the same task as the Trained CM condition, but with new categories, thus revealing the extent to which general, task-specific skills were retained. In the Retention CM condition, the same categories and their respective category exemplars as the Trained CM condition were utilized to examine stimulus-specific skills.
RESULTS
Because few long-term skill retention studies have been conducted in the TBI population, we initially planned to not only examine differences in overall skill retention between the TBI and control samples, but also to explore skill retention at a five-month interval for approximately half of the participants and a 10-month interval for the other half. Because only a total of 10 control participants returned for retention testing, we were unable to statistically analyze potential differences between the retention intervals for this group. For this reason, as well as the fact that our primary question related to group differences in overall skill retention, data were collapsed across the retention intervals for our initial set of analyses.
Original CM Training and Transfer Data
To ensure that our returning sample was representative of the original sample, we first compared the original Trained CM data for the returning TBI and control participants. As can be seen in Figure 2, the RT data for the returning participants replicated the original full sample analysis, indicating that both groups demonstrated significant CM learning. To confirm AAR development, we calculated proportional difference scores separately for each participant by subtracting their Trained CM RT (final 5 blocks of original CM training) from their RT in the New CM and CM Reversal conditions, and then divided by the Trained CM RT. Replicating the original study, the CM Reversal condition significantly disrupted performance and led to longer RTs relative to the New CM condition for both groups, F(1,25) = 40.56, MSE = .50, p < .01, with no significant interaction, F < 2, indicating that the returning TBI and control participants did not differ in their level of AAR development (see Fig. 2). Together, these findings indicated that both the returning TBI and control groups demonstrated comparable levels of stimulus-specific and task-specific skill learning.
Fig. 2.
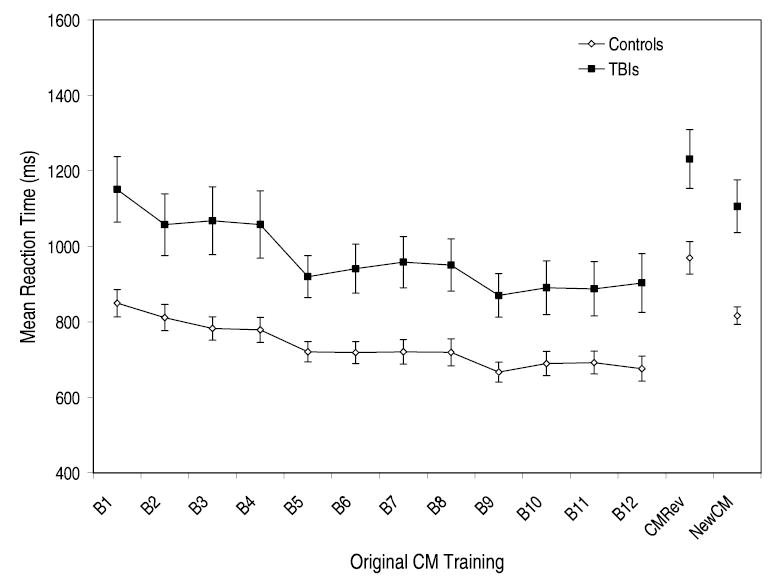
Mean reaction time and standard error data plotted as a function of CM training, CM Reversal training, and New CM Training for the returning controls and the returning TBI groups. Each CM training block represents a total of 300 trials collapsed across set sizes 2, 3, and 4.
Retention Data
Next, we examined potential differences between the returning TBI and control participants in the level of overall skill retention by computing and comparing scores for stimulus-specific savings and task-specific loss. Three hundred Retention New CM trials (5 sets of 60), followed by 900 Retention CM trials (15 blocks of 60), were administered at retention testing. These trials were grouped into blocks (300 trials per block) and averaged across set sizes (set size 2, 3, and 4) to obtain one mean RT per block for each participant. This resulted in one mean RT and accuracy rate per participant for the Retention New CM condition, and three mean RTs and accuracy rates per participant for the Retention CM condition. The analyses that immediately follow utilized only the first mean RT and accuracy rate for the Retention CM condition.
Reaction time data
To determine whether participants’ retention performances were faster than their performances during the first block of initial learning (i.e., first block of initial Trained CM), a Group (returning TBI and returning controls) X Condition (original Trained CM, Retention New CM, and Retention CM) mixed-model ANOVA was conducted on the RT data. Results revealed that control participants (M =810.87, SD = 76.77) exhibited significantly faster overall RT than the TBI participants (M = 1062.41, SD = 58.88), F(1,25) = 6.76, MSE =1195207.12, p < .05. In addition, RT for the Retention CM condition (M = 870.27, SD = 42.83) was also significantly faster than RT for the Retention New CM condition (M =939.28, SD =50.01), t(26) =3.74, p <.01, which, in turn, was significantly faster than RT for the original Trained CM condition (M = 1000.37, SD = 58.59), t(26) =−2.16, p <.05; F(2,50) =11.33, MSE =106705.65, p < .01.
Accuracy data
A 2 (Group) × 3 (Condition) mixed-model ANOVA conducted on the accuracy data revealed no significant main effect of Group, F < 1, or Condition, F < 1, and no significant Group X Condition interaction, F < 1. Accuracy rates were 94%, 95%, and 95% for the TBI group and 94%, 95% and 95% for the control group in the original Trained CM, Retention New CM, and Retention CM conditions, respectively, indicating that the TBI and control participants were uniformly accurate across conditions.
Savings and loss scores
To examine stimulus-specific and task-specific skill retention across groups, we computed savings and loss scores following the procedure used by Fisk and colleagues (1994). A stimulus-specific cost score was computed as the difference between the last block of the original Trained CM condition and the Retention CM condition. A task-specific savings score was computed as the difference between the first block of the original Trained CM condition and the Retention New CM condition. To account for baseline differences between the TBI and control groups, we also computed proportional stimulus specific loss and task-specific savings scores, calculated as the stimulus-specific cost score divided by the last block of the original Trained CM condition and the task-specific savings score divided by the first block of the original Trained CM condition, respectively. The savings and loss scores for the TBI and control groups were then compared using independent-samples t-tests.
No significant difference was found in the amount of loss for stimulus-specific skills between the returning TBI (M =−67.66, SD =93.09) and control participants (M =−93.65, SD =66.10), t(25) =−.77. The task-specific savings analysis also revealed no significant difference between the returning TBI (M =85.75, SD =190.48) and control (M =36.43, SD =99.20) groups, t(25) =−.76. Analyses of the proportional difference scores revealed similar findings, indicating that the returning TBI and control participants did not differ significantly in stimulus-specific skill loss (TBI: M =−.09, SD = .10; control M = −.15, SD = .09), t(25) =−1.36, or in task-specific skill savings (TBI: M =.06, SD = .16; control: M = .03, SD = .12), t(25) = −.46.
The small sample size and large standard deviation values for both skill types could have potentially obscured group differences. However, given that the TBI participants exhibited numerically greater retention of stimulus-specific and task-specific skills compared to the returning controls, an increase in sample size would not influence the interpretation of our findings. TBI participants would likely continue to demonstrate skill retention comparable to, or above, that of controls with a larger sample size. As will be seen in the following section, part of the large variability in skill retention for both groups was a factor of duration of the retention interval.
TBI retention interval exploration
Given that approximately half of the returning TBI participants completed retention testing after 5 months and the other half after 10 months, we explored possible differences between intervals in stimulus-specific and task-specific skill retention. An independent-samples t-test on proportional difference scores revealed a significantly greater loss for stimulus-specific skills after the 10-month retention interval (M =−.15, SD =.07) compared to the 5-month interval (M = −.03, SD = .07), t(15) = 3.63, p < .01. In fact, the data demonstrated nearly complete savings of stimulus-specific skills for the TBI participants retested at the 5-month retention interval. Although the difference in task-specific savings between the retention intervals did not reach statistical significance, t(15) = 1.54, p > .05, the results were also in the direction of decreased retention from the 5-month (M = .12, SD = .10) to the 10-month (M =.01, SD =.19) interval, with the 10-month TBI group exhibiting essentially no task specific savings. A similar decrease in the retention of both skill types from the 5-month (stimulus specific: M =−.12, SD =.11; task-specific: M = .06, SD = .10) to the 10-month (stimulus-specific: M =−.18, SD =.06; task-specific: M =−.003, SD =.14) interval was noted for the control group, although statistical analyses were not conducted because of the small sample size (see Fig. 3).
Fig. 3.
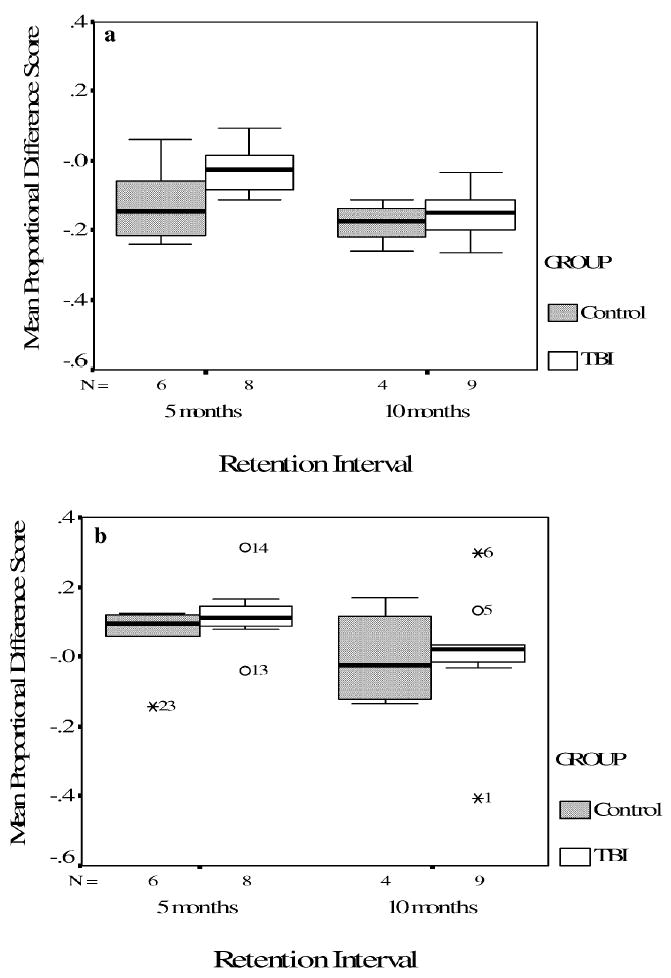
Mean proportional difference score and standard error data for Stimulus-specific loss (3a) and Task specific savings (3b) for TBI and control participants that returned at 5 months and 10 months.
Before concluding that passage of time accounted for the greater retention of stimulus-specific and task-specific skills in the 5-month TBI group, we compared initial skill development between the 5-month and 10-month TBI group. As can be seen in Figure 4, although the 5-month TBI participants showed a larger decrease in RT between block 4 (end of Day 1 of CM training) and block 5 (beginning of Day 2 of CM training) of the original Trained CM condition (M = 222.16, SD =190.82) compared to the 10-month TBI group (M =63.65, SD =63.38), t(15) =2.36, p <.05, F(11,165) = 3.79, MSE =19115.77, p < .01, both groups demonstrated comparable rates of overall improvement from block 1 to block 12 of training (5-month group: 276.28 ms or a 22% decrease in RT; 10-month group: 222.71 ms or a 21% decrease in RT). In terms of AAR development, both groups exhibited longer RTs in the CM Reversal condition (5-month: M = 1334.68, SD = 133.14; 10-month: M = 1139.73, SD = 83.25) compared to the original New CM condition (5-month: M = 1144.39, SD = 129.74; 10-month: M = 1072.57, SD = 70.63), F(1,15) = 26.38, MSE = .22, p < .01, indicating the development of an AAR. However, the 5-month TBI participants showed greater disruption in the original CM Reversal condition than the 10-month TBI participants, F(1,15) =5.73, MSE =.05, p < .05, suggesting that the 5-month group may have initially developed a stronger AAR. To control for initial AAR development, a univariate ANCOVA was conducted with the proportional stimulus-specific loss score as the dependent variable and AAR development (i.e., the original New CM proportional difference score subtracted from the original CM Reversal proportional difference score) as the covariate. The results continued to reveal a highly significant main effect for TBI Group, F(1,14) = 31.53, MSE = .10, p < .001, indicating that the 5-month group exhibited greater stimulus-specific skill retention (M = .002, SD = .02) compared to the 10-month group (M =−.18, SD =.02). Furthermore, correlational analysis revealed no significant relationship between initial AAR development and stimulus-specific skill retention for the TBI participants, r = −.04.
Fig. 4.
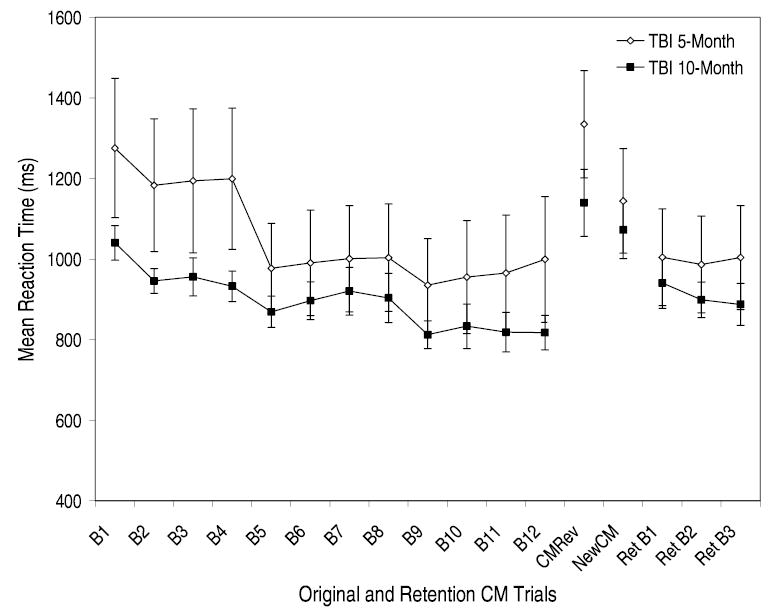
Mean reaction time and standard error data plotted as a function of the initial CM training, transfer conditions, and the first three Retention CM trials for the TBI participants returning after 5 months and 10 months. Each CM training block represents a total of 300 trials collapsed across set sizes 2, 3, and 4.
To evaluate whether differences in injury characteristics, demographic variables, or neuropsychological variables contributed to the findings of differing levels of skill loss between the 5-month and 10-month retention intervals, independent samples t-tests were conducted on the variables reported in Table 1 and injury variables. Results revealed no significant differences between the 5-month and 10-month TBI participants for any injury characteristics, demographic variables, or neuropsychological variables, t’s < 2, p’s > .05. Taken together, these findings indicate that the passage of time is the most likely factor contributing to differences in skill retention between the 5-month and 10-month TBI groups.
Lastly, we examined the 5-month and 10-month TBI groups’ level of re-learning. Consistent with the stimulus-specific savings analysis, a paired-samples t-test revealed no significant difference in performance between the final block of the original Trained CM condition (M = 999.32, SD =442.39) and the first block of the Retention CM condition (M =1004.65, SD =362.88) for the five-month TBI participants, t(7) =.17, p >.05. In contrast, for the 10-month TBI participants, performance during the final block of the original Trained CM condition (M =817.69, SD =128.29) was significantly faster than performance on the first block of the Retention CM condition (M =940.76, SD =133.35), t(8) = 6.61, p < .01. Although the 10-month TBI group demonstrated improvements in performance throughout the retention trials (i.e., over a total of 900 trials), they did not reach their final-level performance of original CM training [final block of original CM training: (M = 817.69, SD = 128.29); final block of Retention CM condition: (M = 887.60, SD =140.17)], t(8) = 4.30, p < .01 (see Fig. 4).
DISCUSSION
We were interested in whether individuals who had sustained a severe TBI (>1 year post-injury) could retain task-specific and stimulus-specific skills over a long-term retention interval (i.e., 5–10 months) at a rate comparable to controls. In an earlier visual search skill learning study (Schmitter-Edgecombe & Beglinger, 2001), we found that individuals with a TBI were able to develop general, task-specific skills, and an AAR (i.e., a stimulus-specific skill) at a level comparable to controls. The TBI and control participants who returned for the retention phase of this study were representative of the original sample, as RT performances for both groups improved with CM training and a comparable level of AAR development was demonstrated. Therefore, we were able to examine the long-term retention of skills initially developed to a similar level by TBI and control participants.
We found that the returning TBI and control participants demonstrated comparable levels of skill retention when the data were collapsed across the 5- and 10-month retention intervals. Specifically, difference and proportional difference scores revealed that the returning TBI and control participants were comparable in their levels of stimulus-specific skill loss and task-specific skill savings. These findings are unique in suggesting that once an automatic cognitive process has been developed, individuals with a TBI show skill retention at a level comparable to normal controls without continued practice across a 5- to 10-month period.
In a similar visual search skill retention study, Fisk and colleagues (1994) reported a remarkable amount of skill retention over a 16-month interval, with stimulus-specific skill loss of only 28% and 38% for neurologically normal young and older adult participants, respectively. Using a similar method of data interpretation, our findings also demonstrated an impressively small amount of average skill loss (i.e., 9% for TBIs and 15% for controls) across a shorter 5- to 10-month retention interval. It should be noted, however, that Fisk and colleagues’ method of interpretation may be misleading given that stimulus-specific loss was computed without taking into consideration the maximum possible loss based on initial skill development. After taking this into consideration (i.e., skill gain from the beginning of CM training to the end of CM training), our data indicated that the TBI participants demonstrated an average 9% loss of stimulus-specific skills when the maximum possible loss was 27% on average, and the control participants demonstrated an average 15% loss when the maximum possible loss was 26% on average. This interpretation indicates a greater overall loss for stimulus-specific skills than originally believed.
An interesting pattern that emerged in our data was that TBI participants retested at the 5-month retention interval showed almost complete stimulus-specific skill retention, which was significantly greater than that shown by the TBI participants retested at the 10-month interval. Although examination of the original training data revealed that the 5-month TBI group initially developed a stronger AAR than the 10-month TBI group, retention analyses revealed that the 5-month TBI group continued to demonstrate greater stimulus-specific skill retention compared to the 10-month TBI group after controlling for initial AAR development. These results, combined with the findings that no demographic, injury-related, or neuropsychological variables differed between the two returning TBI groups, indicate that the passage of time is likely the most significant contributor to this pattern of data. Consistent with this explanation, the control data showed a similar pattern of decreased stimulus-specific skill retention between the 5- and 10-month retention intervals. These findings suggest that rehabilitation techniques relying on automatic cognitive skill development and involving visual information may benefit from “booster” or re-training sessions following initial training, especially if the skill is not being continuously utilized. Future research will be needed to further examine this issue, including the best time for booster sessions. Future studies with larger sample sizes will also be needed to more closely examine participant and injury related factors (e.g., site of injury) that might influence skill acquisition rate and retention capacity. In addition, future studies that manipulate training related variables (e.g., number of learning trials, level of stimulus-response consistency) will help clarify the parameters necessary to develop and sustain an automatic cognitive skill.
Although not statistically significant, there was a trend for TBI participants retested at the 5-month interval to show greater task-specific skill retention compared to the 10-month interval. The control group also showed a similar pattern of task-specific skill loss, with both the TBI and control groups demonstrating nearly complete loss of task-specific skills by the 10-month retention interval. The TBI literature generally suggests that differences in the rate of forgetting or memory decay between individuals with a TBI and controls are non-significant once differences in the initial learning and acquisition for the material are controlled (Carlesimo et al., 1997; DeLuca et al., 2000; Hillary et al., 2003; Kapur et al., 1996). The current findings support this by showing similar levels of retention across a 5- to 10-month interval for a skill that was initially learned to a comparable level by both groups.
In an earlier study, we demonstrated that TBI participants were able to automatize components of a complex visual search task at a level comparable to controls. In the current study, we found that TBI participants were also able to retain stimulus-specific and task-specific visual search skills at a level comparable to controls over a collapsed 5-to 10-month retention interval without continued practice. Together, these findings have important implications for cognitive rehabilitation techniques following a severe TBI. Specifically, breaking down complex cognitive skills and consistently training individuals on smaller components of the task in order to develop automatic cognitive processes is a worthwhile strategy since such skills are likely to be retained over a long-term interval, perhaps more so with follow-up “booster” or retraining sessions.
Acknowledgments
This research was partially supported by grant #RO3 HD35838 from NICHD and by grant #R01 NS47690 from NINDS. No financial relationships exist that could be interpreted as a conflict of interest affecting this manuscript. This project was completed in partial fulfillment of Shital Pavawalla’s Master of Science degree in psychology at Washington State University. The authors gratefully acknowledge the contributions of Leigh Beglinger, Amy Simpson, Naomi Chaytor, and Heather Nissley for their support in coordinating data collection and the members of the Head Injury Research Team for their help in collecting and scoring the data. Unless otherwise indicated, the information in this manuscript and the manuscript itself is new and original, is not currently under review by any other publication, and has never been published either electronically or in print.
References
- Batsakes PJ, Fisk AD. Age-related differences in dual-task visual search: Are performance gains retained? The Journals of Gerontology. 2000;55B:332–342. doi: 10.1093/geronb/55.6.p332. [DOI] [PubMed] [Google Scholar]
- Battig WF, Montague WE. Category norms for verbal items in 56 categories: A replication and extension of the Connecticut category norms. Journal of Experimental Psychology Monograph. 1969;80(3 Pt 2):1–46. [Google Scholar]
- Carlesimo GA, Sabbadini M, Loasses A, Caltagirone C. Forgetting from long-term memory in severe closed-head injury patients: Effect of retrieval conditions and semantic organization. Cortex. 1997;33:131–142. doi: 10.1016/s0010-9452(97)80009-3. [DOI] [PubMed] [Google Scholar]
- Collen A, Wickens DD, Daniele L. The interrelationship of taxonomic categories. Journal of Experimental Psychology: Human Learning and Memory. 1975;1:629–633. [Google Scholar]
- DeLuca J, Schultheis MT, Madigan NK, Christodoulou C, Averill A. Acquisition versus retrieval deficits in traumatic brain injury: Implications for memory rehabilitation. Archives of Physical Medicine and Rehabilitation. 2000;81:1327–1333. doi: 10.1053/apmr.2000.9390. [DOI] [PubMed] [Google Scholar]
- Fisk AD, Cooper BP, Hertzog C, Anderson-Garlach MM, Lee MD. Understanding performance and learning in consistent memory search: An age-related perspective. Psychology and Aging. 1995;10:255–268. doi: 10.1037//0882-7974.10.2.255. [DOI] [PubMed] [Google Scholar]
- Fisk AD, Hertzog C, Lee MD, Rogers WA, Anderson-Garlach M. Long-term retention of skilled visual search: Do young adults retain more than old adults? Psychology and Aging. 1994;9:206–215. doi: 10.1037//0882-7974.9.2.206. [DOI] [PubMed] [Google Scholar]
- Fisk AD, Rogers WA. The application of consistency principles for the assessment of skill development. In: Regain W, Shute V, editors. Cognitive approaches to automated instruction. Hillsdale, NJ: Erlbaum; 1992. pp. 171–194. [Google Scholar]
- Hillary FG, Schultheis MT, Challis BH, Millis SR, Carnevale GJ, Galshi T, DeLuca J. Spacing of repetitions improves learning and memory after moderate and severe TBI. Journal of Clinical and Experimental Neuropsychology. 2003;25:49–58. doi: 10.1076/jcen.25.1.49.13631. [DOI] [PubMed] [Google Scholar]
- Kapur N, Scholey K, Moore E, Barker S, Brice J, Thompson S, Shiel A, Carn R, Abbott P, Fleming J. Long-term retention deficits in two cases of disproportionate retrograde amnesia. Journal of Cognitive Neuroscience. 1996;8:416–434. doi: 10.1162/jocn.1996.8.5.416. [DOI] [PubMed] [Google Scholar]
- Logan GD. Skill and automaticity: Relations, implications, and future directions. Canadian Journal of Psychology. 1985;39:367–386. [Google Scholar]
- Schmitter-Edgecombe M, Beglinger L. Acquisition of skilled visual search performance following severe closed-head injury. Journal of the International Neuropsychological Society. 2001;7:615–630. doi: 10.1017/s1355617701755099. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Marks W, Fahy JF. Semantic priming following severe closed-head trauma: Automatic and attentional processes. Neuropsychology. 1993;7:136–148. [Google Scholar]
- Schmitter-Edgecombe M, Nissley HM. Effects of divided attention on automatic and controlled components of memory after severe closed-head injury. Neuropsychology. 2000;14:559–569. doi: 10.1037//0894-4105.14.4.559. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Rogers WA. Automatic process development following severe closed-head injury. Neuropsychology. 1997;11:296–308. doi: 10.1037//0894-4105.11.2.296. [DOI] [PubMed] [Google Scholar]
- Schneider W, Chein JM. Controlled and automatic processing: Behavior, theory, and biological mechanisms. Cognitive Science. Special Issue: 2002 Rumelhart Prize Special Issue Honoring Richard Shiffrin. 2003;27:525–559. [Google Scholar]
- Schneider W, Fisk AD. Automatic category search and its transfer. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1984;10:1–15. doi: 10.1037//0278-7393.10.1.1. [DOI] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing: 1. Detection, search, and attention. Psychological Review. 1977;84:1–66. [Google Scholar]
- Shiffrin RM. Attention. In: Atkinson RC, Herrnstein RJ, Lindzey G, Luce RD, editors. Steven’s handbook of experimental psychology: Vol. 1. Learning and cognition. New York: Wiley and Sons; 1988. pp. 739–811. [Google Scholar]
- Shiffrin RM, Dumias ST. The development of automatism. In: Anderson JR, editor. Cognitive skills and their acquisition. Hillsdale, NJ: Erlbaum; 1981. pp. 11–140. [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic information processing: II. Perceptual learning, automatic attending, and a general theory. Psychological Review. 1977;84:127–190. [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness: A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Vakil E, Blachstein H, Hoofien D. Automatic temporal order judgment: The effect of intentionality of retrieval on closed-head injured patients. Journal of Clinical and Experimental Neuropsychology. 1991;13:291–298. doi: 10.1080/01688639108401044. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised. San Antonio. TX: The Psychological Corporation; 1987. [Google Scholar]


