Summary
Functional optical imaging showed that odor or electric shock stimuli presented to the fly causes transient calcium influx into the two major axon branches of α/β mushroom body (MB) neurons. One pairing of odor and electric shock stimuli or multiple pairings in a massed configuration did not alter odor-evoked calcium influx. In contrast, animals that received multiple pairings in a spaced configuration exhibited a robust increase in calcium influx into the MB axons when tested at 9 or 24 hr after training but not at 3 hr post-training. This modification occurred only in the α/β branch of the neurons and was blocked by mutation at the amnesiac gene, inhibition of protein synthesis, or the expression of a protein blocker of the transcription factor Creb. Thus, behavioral long-term olfactory memory appears to be encoded as a branch-specific modification of calcium influx into the α/β MB neurons that occurs after spaced training in a protein synthesis-, Creb-, and amnesiac-dependent way.
Introduction
Memories are formed from the modifications that occur within neurons due to learning. These modifications, or cellular memory traces, can be molecular, biophysical, or cellular in nature. They may involve changes in ion channel properties and distribution which govern the excitability of neurons, growth or retraction of neuronal processes to facilitate the establishment or abolishment of neural connectivity, modifications of downstream cell signaling molecules that alter a neuron’s response to sensory information, and morphological and functional synaptic changes that affect a neuron’s ability to stimulate its synaptic partners. A major goal in neuroscience is to understand the nature of cellular memory traces, the neurons in which they develop, the mechanisms by which they form, their duration, and how the complete set of cellular memory traces within different areas of the nervous system guide behavior after learning.
In Drosophila, olfactory classical conditioning is a robust and well-studied type of learning in which olfactory cues are usually paired with electric shock, such that conditioning leads to learned avoidance behavior of the odor used for conditioning (Tully and Quinn, 1985). Behavioral studies have revealed that different conditioning protocols generate different forms of memory. For instance, a conditioning protocol consisting of a 1 min presentation of an odor (conditioned stimulus, CS+) along with 12 electric shock pulses (unconditioned stimulus, US), followed by a 1 min presentation of a counter odor (conditioned stimulus, CS-) without shock, generates robust initial memory that decays over the period of about 1 day (Tully et al., 1994). A protocol consisting of 5-10 such conditioning cycles with no rest between each cycle (massed conditioning) generates robust initial memory that decays over a few days. A protocol consisting of 5-10 conditioning cycles with a rest between cycles (spaced conditioning) generates a longer-lasting memory of 4-7 days that is dependent upon protein synthesis at the time of conditioning (Pascual and Preat, 2001; Isabel et al., 2004; Perazzona et al., 2004). Since different conditioning protocols generate different forms of memory, they must also generate distinct cellular memory traces that underlie the conditioned behavior.
Some of the cellular memory traces that potentially underlie conditioned behavior have recently been discovered. A single cycle of olfactory classical conditioning generates an immediate but short-lived (7 min) memory trace in the antennal lobe (AL) that is registered as the recruitment of additional sets of projection neurons into the representation of the CS+ odor (Yu et al., 2004). A second memory trace forms after conditioning in the dorsal paired medial (DPM) neurons (Yu et al., 2005). This trace is displayed as increased calcium influx and synaptic transmission in response to the CS+ odor in one branch of the DPM neurons that innervates the vertical lobes of the mushroom bodies (MB). It forms with a delay, appearing 30 min after training and persisting for at least 1 hr, and requires the function of the amnesiac (amn) gene. Dopaminergic neurons that innervate the axons of the MB neurons have been reported to form a calcium-based memory trace immediately after conditioning (Riemensperger et al., 2005) and there is an increase in synaptic protein synthesis in regions of the AL one day after spaced olfactory conditioning (Ashraf et al., 2006). Although it remains unclear whether these memory traces form independently of one another and to what extent that they guide post-conditioning behavior, such studies of olfactory conditioning using cellular functional optical imaging have begun to reveal the nature, duration, and location of candidate cellular memory traces that may influence post-conditioning, olfactory behavior.
Conspicuously missing from the above list of neurons reported to form memory traces after olfactory conditioning are the MB neurons themselves. This is surprising, since a great deal of evidence has accumulated suggesting that these neurons are a major site for the formation and storage of olfactory memory (McGuire et al., 2001; Dubnau et al., 2001; Davis, 2004; Davis, 2005). Here, we present data that defines the elusive MB memory trace. The cellular memory trace is expressed as an increased calcium influx into one class of MB neurons, the α/β neurons, and is generated only by a spaced conditioning protocol, not by single cycle or massed conditioning. The trace is delayed, forming between 3 and 9 hr after conditioning, and intriguingly is axon branch specific, forming only in the αaxon branch of the α/β MB neurons and not in the β branch. In addition, the data suggest that the memory trace is dependent on protein synthesis at the time of conditioning, the activity of the transcription factor, Creb, and the activity of the amnesiac (amn) gene. Thus, the data reveal a delayed, branch specific memory trace that is protein synthesis-, amn-, and Creb-dependent, which is formed by a conditioning protocol that also generates protein synthesis-, amn-, and Creb-dependent long-term behavioral memory.
Results
The α/β MB neurons respond to odor and shock stimuli with calcium influx
To search for a cellular memory trace in the MB neurons, we first tested whether MB axons respond with calcium influx to odors delivered to the antennae and electric shock pulses delivered to the abdomen of Drosophila. Flies carrying the calcium reporter transgene Uas-G-CaMP expressed by c739-Gal4 were prepared for in vivo imaging of brain by mounting them stably under a laser-scanning confocal microscope to detect basal fluorescence and the resulting change in fluorescence following the application of odor or electric shock. The c739-Gal4 transgene is an enhancer detector element that drives expression from Uas-transgenes specifically in the α/β MB neurons using anonymous enhancers in the vicinity of the hr39 gene (unpublished data). This Gal4 driver was chosen because it impairs memory retrieval at 3 and 24 hr after conditioning when used in combination with the conditional blocker of synaptic transmission, Uas-Shits (McGuire et al., 2001; Isabel et al., 2004). The cell bodies of the α/β MB neurons reside in the dorsal and posterior cellular cortex. Each neuron extends a single axon that splits into two branches - the αand β branches - near the anterior edge of the brain (Figure 1A). We collected imaging data across time from thick optical sections at two different depths in the brain in order to visualize calcium influx separately into the α or β branches of the α/β MB neurons (Figure 1A).
Figure 1.
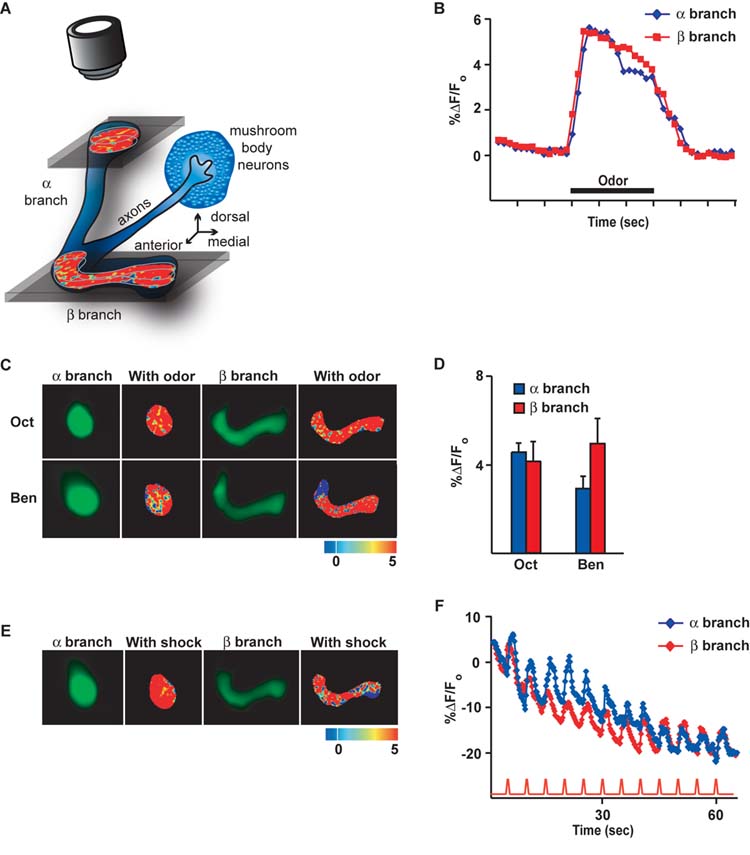
The α/β mushroom body (MB) neurons respond with calcium influx into their αand β axon branches when odors or electric shock stimuli are delivered to Drosophila. (A) Cartoon illustrating the Drosophila α/β MB neurons in isolation along with their processes and the locations from which functional images were obtained. The cell bodies of the MB neurons are clustered in the posterior and dorsal part of the brain. Each α/β MB neuron extends a single axon in a common nerve towards the anterior face of the brain, where each axon splits into a vertically oriented branch (αbranch) and a horizontally oriented branch (β branch). The branches of the α/β MB neurons remain clustered in brain neuropil regions known as the αlobe and the β lobe, which contain the αbranches and the β branches of the neurons, respectively. For functional imaging, a small portion of cuticle was removed from the dorsal head and the fly was stably mounted under a confocal microscope. Functional images from living flies were collected from a dorsal and slightly frontal perspective of the fly at two depths, one to visualize calcium influx into the αbranches of the α/β neurons and one to visualize calcium influx into the β branches. The pinhole of the confocal was open during imaging so that fluorescence was collected from a thick optical section encompassing the structures of interest. (B) Representative time course for the fluorescence response to the odor 3-octanol (Oct) in the αand β branches of MB neurons. The response was calculated as the percent increase in fluorescence over baseline (%ΔF/Fo) as a function of time. For subsequent bar graphs, the %ΔF/Fo was calculated as the % difference between the maximum average intensity over 5 successive imaging frames during the 3 sec odor application and the average intensity over 5 successive frames just prior to odor application. (C) Images of the basal fluorescence of Uas-G-CaMP expressed with c739-Gal4 in the αand β branches of the axons of α/β MB neurons (1st and 3rd columns). The change in fluorescence (%ΔF/Fo), calculated as the percent change in fluorescence (ΔF) relative to baseline (Fo), that occurs following exposure to the odor 3-octanol (Oct) or benzaldehyde (Ben) is illustrated as a false color image (2nd and 4th columns) to the right of each panel showing the basal fluorescence. Each pseudocolor image shown here and in other figures is a single frame snapshot of the response during stimulation. Since the spatial response pattern fluctuates between frames during the stimulation on a pixel-by-pixel basis, the group data (D) better represent the average peak response across the flies that were imaged. (D) The amplitude of the response to odor from group data for both the αand β branches (n = 6 to 8) is shown. The ratio ΔF/Fo was typically close to 4% and proved to be statistically significant (t-test) compared to zero for both odors. (E) Images of the basal fluorescence of Uas-G-CaMP expressed with c739-Gal4 in the αand β branches of the axons of α/β MB neurons (1st and 3rd columns). The response (%ΔF/Fo) of the αand β branches to 90V electric shock pulses is illustrated as a false color image to the right of each panel showing the basal fluorescence. (F) Calcium influx into the αand β branches of the axons of α/β MB neurons that occurs with 90V, 1.25 sec electric shock pulses every 5 sec. The traces represent the average %ΔF/Fo across the region of interest in both αand β branches. An obvious calcium response was observed with each shock pulse riding on top of a decaying background due to bleaching over a 60 sec scanning period.
Stimulation of the flies with the odors 3-octanol (Oct) or benzaldehyde (Ben) elicited significant and reproducible changes in calcium content in both the αand β branches of the α/β MB neurons. Figure 1B illustrates the time course of the percent change in fluorescence (%ΔF/Fo) during odor stimulation. An increase in the %ΔF/Fo was observed shortly after odor onset. The response peaked within one sec and persisted over the course of odor presentation. A representative false color image of the percent change in fluorescence that occurs with odor stimulation for these odors at the peak of the response in both branches of the neurons is illustrated in Figure 1C, with group data for the averages of the peak responses shown in Figure 1D. The group data show a measurable and reproducible change in fluorescence of about 4% in both the αand β branches. Similarly, application to the abdomen of the fly of electric shock pulses of the same intensity, duration, and frequency as those used for behavioral conditioning (Roman and Davis, 2001) produced significant calcium responses in both the αand β branches (Figures 1E and F). These data show that calcium responses to odor and electric shock can readily be detected in the αand β branches of the α/β MB neurons and that the magnitude of the responses between axon branches is similar. It is important to note that the response of brain neurons to electric shock delivered to the abdomen has specificity. Neither olfactory receptor neurons nor GABAergic neurons in the antennal lobe respond to electric shock pulses to the abdomen (Yu et al., 2004), whereas projection neurons in the antennal lobe (Yu et al., 2004), DPM neurons (Yu et al., 2005), and MB neurons all respond to this stimulation. These data therefore show that the α/β MB neurons are activated by odor or electric shock stimulation to the fly and that their responsiveness is registered by calcium influx into the αand β branches using G-CaMP as a reporter.
The α/β MB neurons form a branch-specific, long-term memory trace
The accumulated evidence supporting a role for the MB neurons in olfactory conditioning and the calcium responses of the α/β MB neurons to both odor and electric shock led us to examine the possibility that a cellular memory trace might form in the axon branches of the α/β MB neurons after olfactory classical conditioning. Flies were trained to associate an odor with electric shock in standard behavioral training tubes using three experimental protocols (Figure 2A): (a) a single cycle conditioning protocol (1x forward), (b) a massed conditioning protocol of 5 consecutive single conditioning cycles (5x massed forward), and (c) a spaced conditioning protocol consisting of 5 single conditioning cycles with a 15 min intertrial interval (5x spaced forward). Each conditioning protocol was discriminative using both a CS+ odor paired with electric shock and a CS-odor without electric shock. In addition, we performed the conditioning in a backward fashion, wherein the CS+ odor was presented after the onset of the electric shock stimuli. Backward conditioning protocols generally fail to produce the robust excitatory behavioral conditioning that occurs with forward conditioning (Rescorla, 1988). For each group of flies that received olfactory conditioning, we tested a group of naïve flies that were passed through the same manipulations except that they did not receive the associative conditioning of odor with electric shock stimuli. The performance of each naïve group was subtracted from the performance of the conditioned group with which it was paired in order to obtain a measure (ΔPerformance Index, ΔPI, see Experimental Procedures) of the performance gains due to associative conditioning with the CS+ odor.
Figure 2.
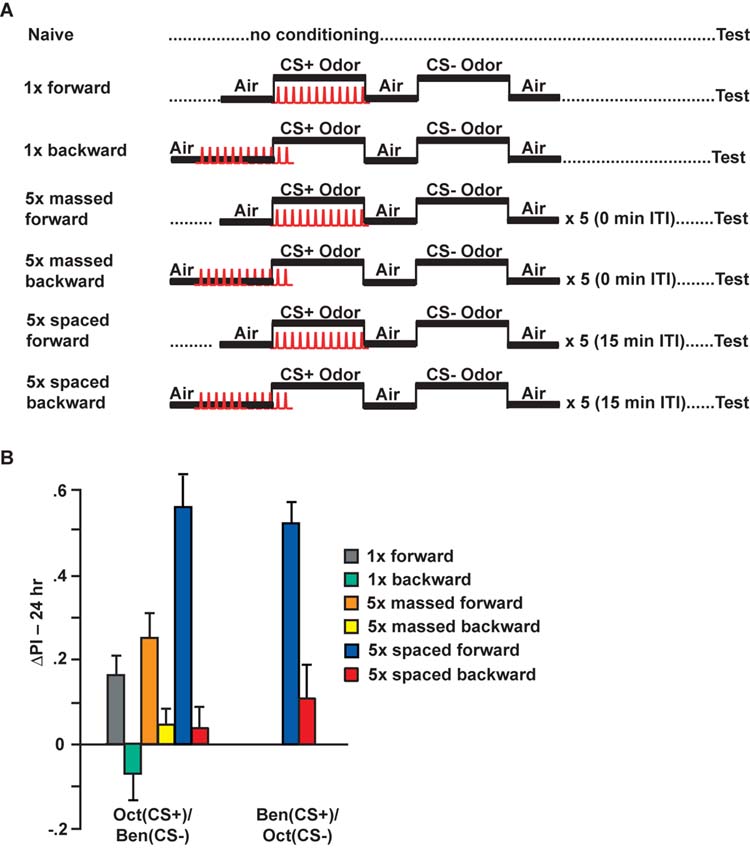
Conditioning protocols: Spaced conditioning produces robust 24 hr performance. (A) Diagram illustrating the seven conditioning protocols that were used for these experiments. Flies carried one copy of c739-Gal4 and one copy of Uas-G-CaMP. Naïve flies were carried through the same procedures as the conditioned animals except that they were not exposed to odor and electric shock. For 1x training, flies received forward or backward conditioning (45 sec offset) with 1 min exposure to the CS+ odor with 12 electric shock pulses (90 V), followed by 1 min exposure to the CS-odor without electric shock. The CS-odor was applied after a 30-sec exposure to fresh air. For massed and spaced conditioning, the 1x training protocol was performed a total of 5 times with either a 0 or 15 min inter-trial interval (ITI), respectively. Flies were transferred to a T-maze at 24 hrs after conditioning and tested for behavioral memory. Some flies were separated prior to behavioral testing and analyzed for cellular memory by functional imaging. (B) The performance gains of flies trained to associate Oct (left) or Ben (right) as the CS+ are shown. The change in Performance Index (ΔPI) was computed by subtracting the scores of each naïve group from the corresponding conditioned group. In all cases, the scores of naïve animals were not statistically significant (t-test) from zero (see Supplemental Figure). 5x spaced forward training had a significant effect on the ΔPI scores compared to the other forward and backward trained groups. For Oct (CS+) and Ben (CS-), p<0.0002; for Ben (CS+) and Oct (CS-), p=0.0002. None of the ΔPI scores of the backward trained groups were statistically significant (t-test) from zero, p>0.2003. n=12 to14 for all groups. Only 5x spaced forward and 5x spaced backward conditioning protocols were performed for the Ben (CS+)/Oct (CS-) odor combination.
Flies conditioned using the spaced forward conditioning protocol exhibited a significantly higher level of associative memory when tested at 24 hr after conditioning than flies conditioned using the 1x forward or 5x massed forward conditioning protocols (Figure 2B). This was observed using either Oct or Ben as the CS+ odor. In addition, flies conditioned using the 1x forward conditioning protocol exhibited associative memory indistinguishable from flies conditioned using the 5x massed forward conditioning protocol. In each case, the corresponding backward conditioned group failed to show any significant performance gains. These data are consistent with prior studies that established these conditioning protocols and indicate that spaced forward conditioning induces more robust 24 hr memory than either 5x massed forward or 1x forward conditioning (Tully et al., 1994).
Some of the flies conditioned using the various conditioning protocols (Figure 2A) using Oct as the CS+ were removed immediately prior to behavioral testing at 24 hr and mounted for imaging the calcium responses to odor. All of the groups, save one, exhibited CS+ odor-responses in the αbranch that quantitatively are typical of naïve flies (Figure 1D) and these responses were indistinguishable from each other for the CS+ odor of Oct (Figures 3A and D). These included the 1x (forward and backward), 5x massed (forward and backward), and 5x spaced backward groups. The only exception was the 5x spaced forward group, which exhibited an odor-elicited calcium response nearly twice as large as any other group. This modification in odor response properties resulted from an increased response magnitude during the odor stimulation without altering the length of the response, as illustrated by the response time course to odor for the representative 5x spaced forward and 5x spaced backward groups (Figure 3B). This modification in odor response properties was specific for the αbranch of the α/β MB neurons and to the CS+ of Oct, since it was not detectable in the β branch with the CS+ odor, or with the CS-of Ben in either the αor β branch. Similar experiments using Ben as the CS+ and Oct as the CS-confirmed that spaced forward conditioning significantly modifies the CS+-elicited calcium response at 24 hr after conditioning only in the α branch of these neurons (Figures 3C and D).
Figure 3.
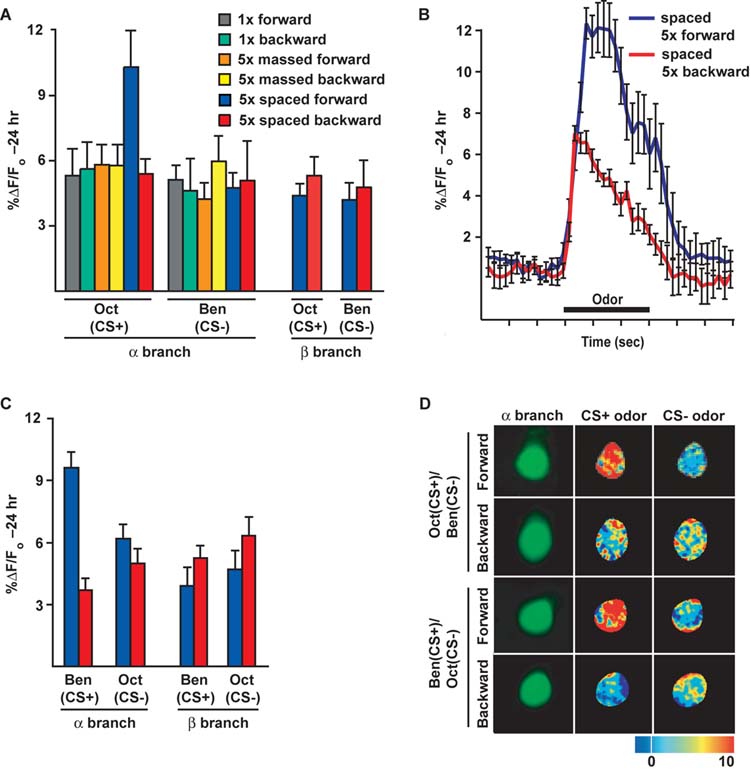
A branch-specific, long-term memory trace forms in the αbranches of the α/β MB neurons after spaced forward conditioning of Drosophila. (A) Response to the CS+ and CS-odors 24 hr after conditioning. A significant increase in %ΔF/Fo was detected in the αbranch using Oct as the test stimulus following 5x spaced forward conditioning with Oct as the CS+ and Ben as the CS-compared to any other group (p backward, 5x massed forward and backward, and 5x spaced backward) were similar to each other and naïve animals presented with odor (Figure 1D; p differences in the %ΔF/Fo response to the CS-(Ben) were detected among any of the conditioning groups (p response magnitude in the β branch after 5x spaced forward compared to 5x spaced backward conditioning [for Oct (CS+), p=0.3595; for Ben (CS-), p=0.6876]. n=6 to11 for all groups]. (B) Group time course for the response to the CS+ of Oct in the αbranch after spaced forward conditioning compared to spaced backward conditioning. The graph was made using the data from the same flies used for the bar graph in panel A. Error bars are the standard error of the mean. (C) Calcium responses in the αand β branches of MB axons in animals conditioned with Ben as the CS+ and Oct as the CS-. There was a significant increase in response to the CS+ in the αbranches after forward spaced conditioning when compared to the corresponding backward conditioned group, but no change in response to the CS-[for Ben (CS+), p<0.0001; for Oct (CS-), p=0.4099, n=9 to 17 for all groups]. There was no significant difference in response to the CS+ or CS-in the β branches after forward spaced conditioning when compared to the corresponding backward conditioned group [for Ben (CS+), p=0.2853; for Oct (CS-), p=0.2203, n=8 to 9 for both groups]. (D) Images of the basal fluorescence of Uas-G-CaMP expressed with c739-Gal4 in the αbranch of α/β MB neurons (1st column). The change in fluorescence (%ΔF/Fo) that occurs following exposure to the CS+ or CS-odor is illustrated as a false color image (2nd and 3rd columns, respectively). A robust increase in calcium influx was detected in the αbranch following CS+ odor stimulation 24 hr after 5x spaced forward conditioning for both odor combinations, while the calcium responses to the CS+ after spaced backward conditioning and to the CS-for both spaced forwards and spaced backward conditioning was similar to the odor responses of naïve animals.
Five considerations contribute to the conclusion that the axon branch specificity observed for the memory trace is not an artifact of the imaging procedures. First, for optical imaging we utilized a wide open pinhole, producing an optical slice calculated to be 32 micrometers with the numerical aperature of the objective lens and at the excitation wavelength used for these experiments. This eliminates the possibility that we inadvertently and consistently sampled a specific optical plane from the β lobe that failed to form the calcium trace, since the depth of the β lobe along the dorsal/ventral axis is approximately 25 micrometers. Second, the basal fluorescence from the β branches was of the same magnitude as that for the αbranches using the same gain and laser power for excitation (Figure 1C and E). Thus, distribution of the G-CaMP reporter between branches was evenly distributed between branches so that responses in both branches could be observed (Figure 1C-F). Third, the value of interest in evaluating the formation of a memory trace is the %ΔF/Fo, the percent change in fluorescence. If basal fluorescence is observed, then significant changes from this baseline may be detectable. Fourth, the quantitative change in %ΔF/Fo after spaced forward conditioning in the αbranch is nearly twice that observed after other conditioning protocols. In contrast, the quantitative change in %ΔF/Fo after spaced forward conditioning in the β branch fails to exhibit even a trend of an increase relative to other conditioning protocols (Figure 3A). Indeed, there is a trend of an increase with spaced backwards conditioning over spaced forward conditioning in the β branch (Figure 3A and C). Fifth, recent experiments have detected a memory trace in the γlobe of the MBs that forms under certain conditions (not shown), indicating that memory traces can be detected in structures residing at the same imaging depth and oriented in the same direction as the β branches. These considerations together strongly argue for branch specificity in memory trace formation, although we cannot eliminate the unlikely possibility that differences in branch orientation between the two lobes relative to the optical axis may impose an observational bias.
The branch-specific cellular memory trace forms between 3 and 9 hr after conditioning
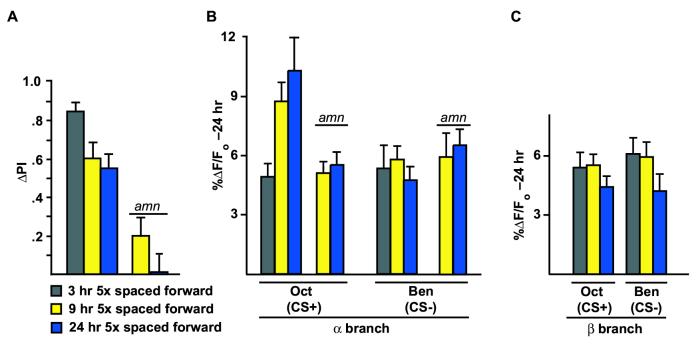
We then assayed flies at various times after spaced forward conditioning to delimit the time during which the cellular memory trace forms. Behavioral memory decays after spaced forward conditioning by 9 hr to the level observed at 24 hr after conditioning (Figure 4A). The cellular memory trace formed in response to the CS+ exhibits the opposite trend (Figure 4B), such that at 3 hr after training the response in the αbranch of the α/β MB neurons approximates that observed in naïve flies (Figure 1D). The enhanced calcium response in the αbranch was observed at 9 hr after conditioning to be indistinguishable from the response observed at 24 hr (Figure 4B). The calcium responses in the β branch remain constant across the measured time points (Figure 4C).
Figure 4.
The branch-specific calcium trace forms between 3 and 9 hr after spaced forward conditioning and requires the function of the memory gene, amnesiac. (A) Memory measured by odor-avoidance response after spaced forward conditioning decreases with time. Flies carrying Uas-G-CaMP and c739-Gal4 in a wild-type or amn mutant (amnchpd) background were trained using the 5x spaced forward conditioning protocol with Oct (CS+) and Ben (CS-) and tested 3, 9, and 24 hr later for behavioral memory (ΔPI). Flies that were wild-type at the amn locus performed significantly better at 3 hr than other time points (p<0.0315; Fisher’s PLSD post-hoc test). The ΔPI scores for these flies tested at 9 and 24 hr were not significantly different from each other (p=0.6844). Flies carrying the amnchpd allele performed significantly worse at both 9 and 24 hr compared to the normal controls (p<0.0007 and p<0.0014, respectively, at the two time points; Fisher’s PLSD post-hoc test). n=10 to 12 for all groups. (B) Time course for the cellular memory trace in the αbranch of the MB neurons in flies from (A). There was a significant difference in the measured calcium trace (%ΔF/Fo) in response to the CS+ (Oct) in the αbranch at 24 hr and 9 hr after spaced forward conditioning compared to 3 hr in flies that were amn+. For 24 hr and 9 hr forward, p=0.2834; for 24 hr and 3 hr, p=0.0011; for 9 hr and 3 hr, p=0.0124. There was no significant difference in response to the CS-at any time point in the αbranch (p≥0.4153). For the amnchpd mutants, there was significantly lower response to CS+ in the αbranch both at 9 hr (p=0.0054) and 24 hr (p=0.0004) compared to amn+ flies. There was no significant difference in response to the CS-at either 9 or 24 hr in the α branch compared to the 3 hr response in amn+ flies (p≥0.1944) for amn+ and amnchpd.n=9 to 11 for all groups. (C) Time course for the cellular memory trace in the β branch of the MB neurons in flies from (A). There was no significant difference in the measured calcium trace (∆F/F ) in response to the CS+ (Oct) at 24 hr, 9 hr and 3hr after spaced conditioning in the β branch (p≥0.1380) of amn+ flies. In addition, there was no significant difference in response to the CS-among the different time points (p groups.
The branch-specific and long-term MB memory trace requires the function of the amn gene product
Our previous studies have revealed that DPM neurons form a medium-term memory trace in one branch that extensively ramifies within the αlobe of the MBs, but not in another branch that ramifies within the β lobe (Yu et al., 2005). This memory trace is first detected at 30 min after training and persists for at least one hr. In addition, its formation is dependent on the normal expression of the amn+ gene product. Because the DPM neuron memory trace forms prior to the long-term trace detected in the MBs, and because the DPM neuron trace forms in processes that innervate the αlobe of the MBs and probably synapse with the αbranches of MB neurons, we asked whether the MB long-term memory trace requires the prior formation of the DPM neuron memory trace.
Flies carrying the Uas-G-CaMP and c739-Gal4 transgenes along with the amnchpd mutation were tested for behavioral memory at 9 and 24 hr after 5x spaced forward conditioning. No behavioral memory was observed at 24 hr and only a small amount was present at 9 hr (Figure 4A). Prior studies have revealed that amn mutants acquire (learn) odor:shock associations at a rate that is indistinguishable from wild-type flies, but are impaired in the memory of these learned associations (Yu et al., 2005). Consistent with these measurements showing no behavioral memory at 24 hr after 5x spaced forward conditioning, there was no significant increase in odor-evoked calcium influx into the αbranch of the MB neurons in amn mutants at either 9 or 24 hr after conditioning (Figure 4B). Therefore, the formation of long-term behavioral memory and the long-term memory trace in the αbranch of MB neurons requires the function of the amn gene product, probably through its requirement in the DPM neurons for the formation of the medium-term memory trace. Answering where in the brain that amn+ function is required for long-term memory trace formation and pursuing other possible experiments such a synaptic silencing with UAS-Shits to probe the interrelationships of the various memory traces will require the development of a new series of promoter or driver elements that are independent of the Gal4:Uas system, so that rescuing or inactivating transgenes can be expressed independently of the reporters required for imaging.
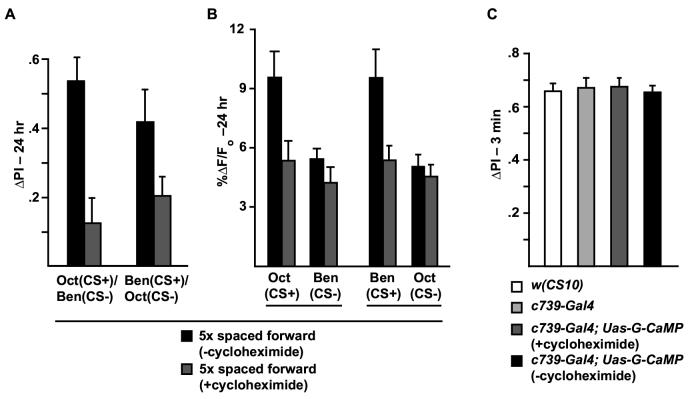
A protein-synthesis inhibitor administered during training and expression of a dCREB repressor molecule blocks the branch-specific cellular memory trace that forms in the α/β MB neurons
Because spaced forward conditioning induces protein-synthesis dependent long-term behavioral memory, we tested whether the branch-specific memory trace induced by this training was also dependent on protein synthesis. Flies were fed on glucose solution with or without the protein synthesis inhibitor cycloheximide (35 mM) prior to spaced forward conditioning and tested 24 hr after conditioning. We observed a significant reduction in 24 hr behavioral performance after spaced forward conditioning of flies fed on cycloheximide compared to flies fed on the vehicle alone (Figure 5A), but this pharmacological treatment had no effect on immediate memory tested after 1x forward conditioning (Figure 5C). We then studied whether cycloheximide treatment alters the formation of the branch-specific memory trace. Functional optical imaging revealed that re-exposure to either Oct or Ben when used as the CS+ odor following spaced forward olfactory conditioning produces a significantly higher calcium response compared to re-exposure to the CS-odor (Figure 5B) confirming earlier results (Figures 3AC-D). This effect was completely blocked by feeding flies on cycloheximide prior to spaced forward conditioning (Figure 5B). Thus, the cellular memory trace that forms in the αbranch of the α/β MB neurons, like long-lasting behavioral memory, is dependent upon active protein synthesis.
Figure 5.
Long-term memory and the αbranch-specific calcium trace are both disrupted by inhibiting protein synthesis. (A) Flies were fed on a 5% glucose solution with or without 35 mM cycloheximide for 12-15 hr prior to 5x spaced forward conditioning. After training, they were transferred to fresh food vials for 24 hr before being tested. The 5x spaced forward performance scores were reduced by feeding on cycloheximide. For Oct (CS+) and Ben (CS-), the decrease in performance was significant, p=0.0006; whereas for Ben (CS+) and Oct (CS-), the decrement approached but did not quite reach significance, p=0.0615. n=12 for all groups. (B) There was a significant difference in the measured calcium trace (%ΔF/Fo) between flies receiving 5x spaced forward conditioning with Oct (CS+) and Ben (CS-) and flies receiving this training but fed on cycloheximide (p=0.0048). A similar significant difference was also observed between cycloheximide-fed and cycloheximide-unfed flies using Ben (CS+) and Oct (CS-) (p=0.0002). Cycloheximide administration had no effect on the response to the CS-odor presented after conditioning with either odor. For Oct (CS+) and Ben (CS-), p=0.2590; for Ben (CS+) and Oct (CS-), p=0.6534. n=12 to 17 for all groups. (C) The 3 min performance scores after 1x forward conditioning of flies expressing Uas-G-CaMP in the MB α/β neurons and fed on cycloheximide for 12-15 hr prior to training and testing were not significantly different from cycloheximide-unfed flies of the same genotype (p=0.6536). The scores were also not significantly different from wild type flies [w(CS10)] or control flies that carried the c739-Gal4 driver by itself (p>6946),n=9 for all groups.
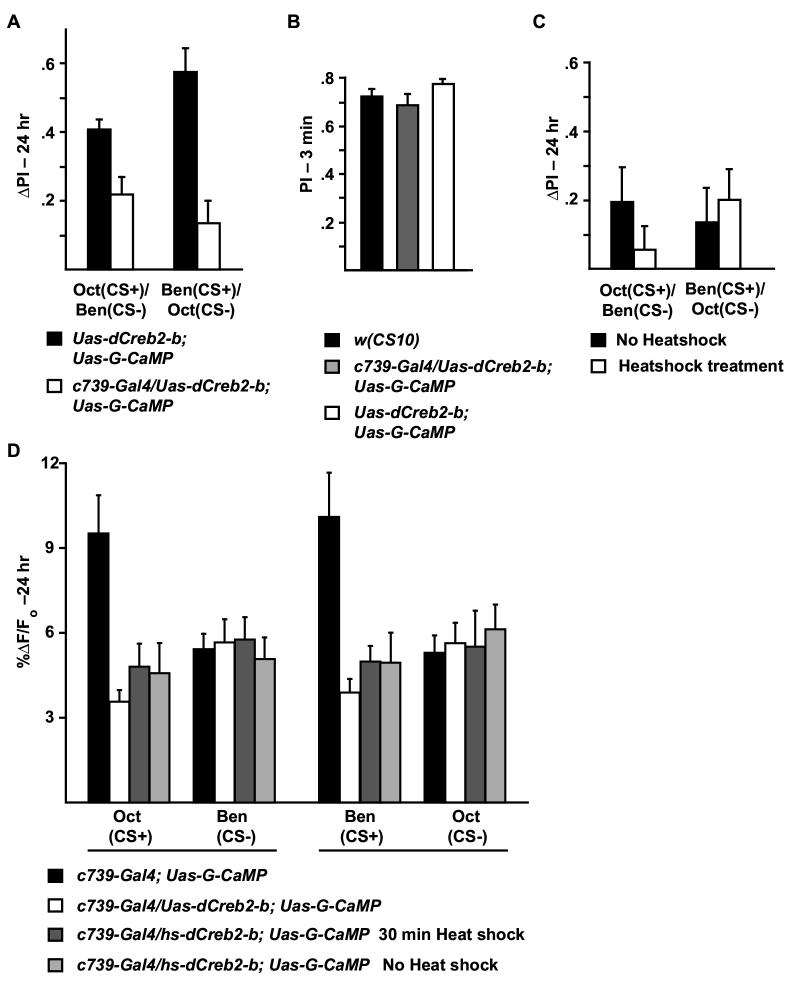
An accumulation of evidence also indicates that long-term memory formation requires the functional participation of the transcription factor Creb, the cAMP response element binding protein (Yin et al., 1994; Kandel, 2001). Expression of a repressing isoform named dCreb2-b in wild-type flies under the control of a heat-shock promoter was reported to produce a heat-shock dependent impairment of long-term memory formation (Yin et al., 1994). Other investigators have observed that the hs-Creb2-b transgene may also impair long-term memory in the absence of heat shock due to basal expression (Perazzona et al., 2004).
We constructed a Uas-dCreb2-b transgene to determine whether expression of the dCreb2-b repressor molecule in the α/β MB neurons blocks the formation of long-term behavioral memory. This transgene was combined with the Uas-G-CaMP transgene to obtain control flies, and with Uas-G-CaMP and c739-Gal4 to obtain flies that express both the reporter and the dCreb-2 repressor in the α/β MB neurons. Behavioral experiments revealed that control flies carrying Uas-elements without the Gal4 driver have 24 hr memory after spaced forward conditioning comparable to wild-type flies (PI of 0.4-0.6), and that flies additionally carrying the c739-Gal4 driver element are impaired in long-term memory (Figure 6A). Expression of the dCreb2-b repressor molecule in the α/β MB neurons had no effect on 3 min memory after 1x forward conditioning (Figure 6B), confirming that expression of the repressor impairs long-but not short-term memory. These results demonstrate that long-term memory is impaired by the expression of a dCreb2-b repressor in the α/β MB neurons, mapping a requirement for Creb activity in long-term memory to these neurons. Flies expressing the dCreb2-b repressor in the α/β MB neurons were also functionally imaged 24 hr after spaced forward conditioning. This expression also abolished the branch specific long-term memory trace that is normally observed with either Oct or Ben as the CS+ odor, but had no effect on responses to the CS-odor (Figure 6D). We conclude that both long-term behavioral memory and the branch-specific long-term memory trace that normally forms following spaced forward conditioning are impaired by the expression of a dCreb2-b repressor in the α/β MB neurons.
Figure 6.
Long-term memory and the branch-specific calcium trace are both disrupted by expression of a Creb repressor. (A) The 24 hr performance scores after 5x spaced forward conditioning were severely reduced by the expression of a dCreb2-b repressor in the MB α/β neurons using c739-Gal4. Control flies carried the Uas-G-CaMP and Uas-dCreb-2b transgenes. The experimental flies carried these two transgenes along with the c739-Gal4 transgene. For Oct (CS+) and Ben (CS-), p=0.0286; for Ben (CS+) and Oct (CS-), p=0.0006. n=6 to 12 for all groups. (B) The 3 min performance scores after 1x forward conditioning of flies expressing the dCreb2-b repressor in the MB α/β neurons were not significantly different from wild type flies [w(CS10)] or control flies that carry the Uas-G-CaMP and Uas-dCreb2-b transgenes without the Gal4 driver; p=0.4654 and p=0.0882, respectively. n=9 for all groups. (C) Global expression of the dCreb2-b repressor using a heat shock promoter-driven transgene (hs-dCreb2-b) significantly depressed behavioral memory. Flies carrying hs-Creb2-b along with c739-Gal4 and Uas-G-CaMP received 30 min heat shock or no heat shock prior to 5x spaced forward conditioning and were tested 24 hours later. Performance scores of the flies were significantly reduced (ΔPI<0.2) compared with other genotypes that received comparable training (ΔPI typically 0.4-0.6 for 5x spaced forward conditioning; see Figures 2B, 4A, 5A). There was no effect of heat shock compared to the non-heat shocked group [for Oct (CS+) and Ben (CS-), p=0.2557 and for Ben (CS+) and Oct (CS-), p=0.6294] indicating a performance disruption due to basal activity of the hs-dCreb2-b transgene (Perazzona et al., 2004). n=12 to14 for all groups. (D) There was a significant difference in the measured calcium trace (%ΔF/Fo) in response to the CS+ 24 hr after spaced forward conditioning between flies carrying only the expressed reporter (c739-Gal4; Uas-G-CaMP) and flies that carried the expressed reporter along with Uas-dCreb2-b or hs-dCreb2-b. For Oct (CS+) and Ben (CS-), p≤0.0003; for Ben (CS+) and Oct (CS -), p in response to the CS-among the genotypes. For Oct (CS+) and Ben (CS-), p for Ben (CS+) and Oct (CS-), p=0.0006. n=6 to 12 for all groups.
We extended these results by testing the effect of the previously generated hs-Creb2-b transgene on both long-term behavioral memory and the long-term memory trace. We conditioned flies containing the hs-dCreb2-b repressor transgene and the transgenic elements used for functional optical imaging (c739-Gal4; Uas-G-CaMP). Flies carrying hs-Creb2-b were impaired in 24 hr memory compared to control flies in the presence or absence of heat shock (Figure 6C), confirming behavioral studies conducted in another laboratory showing that the hs-Creb2-b transgene impairs long-term memory without heat shock (Perazzona et al., 2004). Moreover, expression of the hs-Creb2-b transgene also impaired the formation of the long-term memory trace with or without heat shock (Figure 6D).
Discussion
These data indicate that increases in calcium influx in response to presentation of a conditioned odor occur specifically in the αbranch of the α/β MB neurons only after spaced forward conditioning. Neither 1x forward, 5x massed forward conditioning, nor any variation of backward conditioning is sufficient to promote the post-conditioning increase in calcium influx in this axon branch in response to the CS+. The memory trace was detected as a significant increase in G-CaMP fluorescence in response to the CS+ odor from the three-dimensional region of interest of the brain imaged in flies that received spaced forward conditioning compared to the response from the same area in naïve flies or in flies receiving other types of conditioning that were ineffective at producing long-term behavioral memory. Because the comparison was made for a specific region of interest between flies conditioned by various protocols, we believe that the failure to observe a memory trace in the β branch indicates that the physiological changes producing the memory trace in the αbranch do not occur in the β branch. Nevertheless, we cannot rule out the possibility that some unknown technical constraint, possibly related to the orientation of fibers relative to the angle of light detection, obscured the detection of an authentic trace in the β branch. Furthermore, we find that the formation of the cellular memory trace using functional optical cellular imaging is disrupted by feeding flies an inhibitor of protein synthesis, by the absence of the amn gene product, or by expressing a repressor of the transcription factor, Creb, in the α/β MB neurons. Thus, the evidence together indicates that the calcium-based memory trace discovered from these studies is localized in the α/β MB neurons, formed only in one axon branch of these neurons, formed only after spaced forward conditioning, and is dependent on amn+ activity, normal protein synthesis and Creb activity during training.
The strong correlation between the conditions necessary for the formation of the branch-specific cellular memory trace described here and those that underlie long-term behavioral memory provide an extremely strong argument that the newly discovered memory trace may guide behavior long after the conditioning event. Long-term behavioral memory in Drosophila, like the observed cellular memory trace, is induced by spaced forward conditioning, is disrupted by inhibiting protein synthesis, and requires the activity of dCreb and as shown here, the function of the amn gene. Moreover, behavioral studies of mutant animals with brain structural defects have hinted at a possible tie between long-term behavioral memory and the vertical lobes of the MB neurons. Pascual and Preat (2001) reported that long-term memory induced with spaced forward conditioning is abolished in ala (alpha-lobes absent) flies that are missing the α/α ’ branches of the MB neurons but not the β/β ’ branches, although the low penetrance of the ala mutant weakened this conclusion. Nevertheless, this observation is consistent with the possibility that long-term memory induced by spaced forward training either forms in the α/α ’ branches of the MB neurons or that these branches are required for the circuit-based retrieval of long-term memory formed after spaced forward conditioning. The observation of a long-term cellular memory trace in the αbranch of the α/β MB neurons supports that idea that the memories are formed and stored in this branch of this specific class of MB neuron.
Why is it that a memory trace representing early memories was not discovered during these studies, given that the MBs have been widely implicated in all temporal phases of memory? For instance, ablation of the MBs during development by hydroxyurea poisoning impairs the memory of adult flies immediately after training (de Belle and Heisenberg, 1994), suggesting the importance of the MBs for short-term memory expression. The expression of a wild-type rutabaga transgene specifically in the MB of adult flies rescues the memory impairment of rutabaga mutants that is observed immediately after olfactory conditioning (McGuire et al., 2003; Mao et al., 2004), indicating that normal rutabaga function in the MBs is required for the expression of early memory. Expression of Uas-Shits specifically in MB neurons impairs olfactory memory retrieval at 0.5, 3, and 24 hr after conditioning (McGuire et al., 2001; Dubnau et al., 2001; Isabel et al., 2004), strongly indicating that early memories either form in the MBs or are expressed through these neurons.
There exist at least two explanations for why we detected a trace that is specific to long-term memories. First, it is possible that memory traces that underlie early memories are formed in neurons other than the α/β MB neurons. For instance, early memories may be subserved by the short-term memory trace detected in the antennal lobes (Yu et al., 2004) and/or by memory traces that form in other types of MB neurons such as the α’/β’ or γMB neurons. Second, memory traces produced by cellular mechanisms other than increased calcium influx likely exist and these may subserve early memory. In either case, the expression of both short- and long-term memory appears to be via the output synapses of the MB neurons (McGuire et al., 2001; Dubnau et al., 2001; Isabel et al., 2004), irrespective of where various memory traces may form upstream of these synapses within the olfactory nervous system.
Two other discovered olfactory memory traces - the immediate and short-lived antennal lobe PN memory trace and the medium-term memory trace formed in DPM neurons - occur prior to the long-term memory trace that forms in the MB neurons. This raises the general question of whether the various memory traces are dependent upon one another in a time series for their formation. We have performed an initial query into this question by determining whether the long-term MB trace forms in amn mutants, which fail to form the medium-term memory trace in the DPM neurons (Yu et al., 2005). As shown by the data, amn mutants fail to form long-term behavioral memory measured at 24 hr and they fail to form the long-term memory trace in the αbranch as detected in control flies. These results are therefore consistent with the model that the formation of the medium-term trace in DPM processes that appear to innervate the αbranch of the MB neurons is required for formation of the long-term memory trace in these axon branches. Perhaps the increased calcium influx and synaptic release that occurs in response to an odor CS+ after training from the DPM processes innervating the αlobe are responsible for guiding or permitting the formation of the long-term memory trace in the putative DPM targets - the αbranches of the MB neurons. Further tests of issues regarding the interdependence of the various cellular memory traces require the development of new gene promoter systems that are independent of the Gal4:Uas system to permit expressing the neuronal reporters and transgenes that block synaptic transmission, for instance, in different sets of neurons. Although the mechanisms underlying the branch-specific modifications that result in calcium influx in response to the CS+ after spaced forward conditioning remain to be elucidated, the discovery of multiple memory traces that form at different times after training for different durations in various parts of the olfactory nervous system leads to the general proposition that behavioral memory is guided from multiple and discrete memory trace elements rather than from a single, continuously decaying memory trace.
Experimental Procedures
Transgenic animals and fly culture
Flies were cultured on standard medium at room temperature and transferred overnight to a 25°C incubator before training. Flies carrying Uas transgenes containing Drosophila the Creb2-b repressor coding region (Uas-dCreb2-b, see below) or G-CaMP (Uas-G-CaMP, Wang et al., 2003; Wang et al., 2004) were employed along with the c739-Gal4 driver (c739-Gal4 flies were from K. Kaiser, Division of Molecular Genetics, University of Glasgow, Scotland). For heat shock experiments we used the hs-dCreb2-b repressor line 17-2 (Yin et al., 1994) and crossed these flies with flies containing the c739-Gal4 driver and Uas-G-CaMP. The w(CS10) flies (CS flies carrying the w1118 mutation) served as a wild-type control in certain experiments.
The Uas-dCreb2-b repressor transgene was constructed by Dr. B. Perazzona using a procedure previously described for the generation of a Uas-dCreb2-a transgene (Perazzona et al., 2004). Briefly, genomic DNA from the transgenic fly lines carrying a hs-Creb2 transgene (Yin et al., 1994) was isolated using the Purogene DNA isolation kit (Gentra Systems, Minneapolis, MN). PCR was performed using primers spanning the 5’ and 3’ untranslated and coding regions of the hs-dCreb2 transgene and the PCR products were subcloned into topoisomerase (TOPO) vector pCR 2.1 and sequenced. The dCreb2-b coding cassette was isolated and engineered into the expression vector pPBretU (Roman et al., 1999) followed by germline transformation using standard procedures. For our experiments, we used the Uas-dCreb2-b repressor line T25.2 crossed with c739-Gal4 flies.
Behavioral assays
Drosophila olfactory learning is frequently assayed using olfactory classical conditioning (Tully and Quinn, 1985). Usually, flies are exposed to two odors in succession, one odor (the conditioned stimulus, CS+) paired with electric shock pulses (unconditioned stimulus; US) followed by a second odor (the CS-) without electric shock. The flies are then presented with the two odors in a T-maze and their avoidance of the CS+ is computed as one-half of the performance index (P.I.). To eliminate naïve odor bias, experiments are performed in a counterbalanced design and averaged, with one group of flies used in the calculation of the performance index being trained to the first odor and a second group to the second odor. Prior test-retest experiments (Tully et al., 1994; Beck et al., 2000) have revealed that the numerical index of P.I. represents the probability that trained flies will make the correct choice of the CS+ odor in the T-maze at testing, rather than representing a probability that an odor:shock association has formed in individual flies. Most or all trained flies probably develop an association during training and the strength of this association at the time of testing affects their choice behavior. Therefore, it is expected that most or all individual flies will develop a cellular memory trace after training, but only a fraction of these will display the correct choice behavior as reflected in the P.I. at testing.
We modified this protocol by adding a corresponding naïve control for each trained group. These flies underwent all of the manipulations as the trained flies in except they were not administered odor or electric shock. After training, the flies were incubated at 25°C for the indicated times (3 min, 3, 9 or 24 hr) before testing in a T-maze. Some conditioned flies were removed prior to behavioral testing and these were analyzed for cellular memory by functional imaging at the indicated times. For each group of flies trained with odor, a naïve group was tested simultaneously. The Performance Indices were then calculated for both the naïve and trained group and the ΔP.I. was obtained by subtracting the naïve score from the score of the corresponding trained group. In all cases, only experiments where the naïve flies exhibited naïve performance scores that were not significantly different from zero (t-test) were used (Figure S1). This modified protocol allowed us to make meaningful comparisons among groups since performance indices were normalized to naïve odor biases rather than counterbalancing with a CS-odor. In addition, the assay allowed us to obtain an index of the performance gains due to conditioning with each specific odor as the CS+, so that these gains could be compared to the results obtained after optically imaging individual flies.
Cycloheximide (CXM) feeding and heat-shock protocol
Flies were collected and placed into feeding tubes (17×100 mm polystyrene test tube Falcon 2017) containing one 1.0 × 2.5 cm Whatmann 3MM filter paper strip soaked with 125 μl of 5 % glucose in 3% ethanol solution or 35 mM CXM in the same solution. Following a feeding period of 12-15 hr at 25°C, the flies fed either CXM or vehicle alone were transferred to fresh food vials and allowed 30 min to recover before training. Previous experiments have shown that 12-14 hr CXM-feeding prior to associative conditioning is sufficient to reduce 24-hr memory following spaced training (Yin et al., 1994).
Heat-shock induction was performed according to previously published protocols (Yin et al., 1994; Perazzona et al., 2004). Briefly, flies were collected and incubated at 25°C overnight before heat-shock treatment. The next day, flies were transferred to empty vials and submerged for 30 min in a 37°C water bath. After heat-shock, the flies were transferred to fresh food vials and allowed a 3 hr recovery period prior to training.
Functional cellular imaging
Functional imaging procedures were similar to those already described (Yu et al., 2004; Yu et al., 2005). Flies containing both a Gal4 driver and Uas-G-CaMP were aspirated from a culture bottle without anesthesia, mounted in pipette tips, and their exposed heads secured to the tip opening with silicon cement. A small area of cuticle was removed from the top of the head capsule and the opening covered with a piece of plastic wrap. The flies were then mounted beneath a 20X objective (NA = 0.7) of a Leica TCS confocal microscope and imaged with a 488 nm excitation line using a completely open pinhole. Under these conditions, the thickness of the optical slice collected was calculated to be 32 micrometers. The emitted light was collected from 520±15 nm. Odors were delivered with pressurized air flowing at a rate of 100 mls per min. Odorants were spread on a small piece of filter paper inside of a syringe barrel and the syringe barrel placed in line with the pressurized air. Concentrated odorants were diluted in mineral oil. The delivery of odorants was accomplished with a 3-way teflon valve under the control of a programmable timer, such that fresh air could be delivered to the animals for a determined period with an instantaneous switch to odor-laced air without altering the overall flow rate. Electric shock pulses were applied to the fly’s abdomen. A total of 12 pulses of electric shock at 90v were delivered with each shock lasting 1.25 sec. To detect the long-term memory trace optically, some conditioned flies were collected after training and tested at 3, 9, and 24 hours after training and tested for calcium influx into the MB axons when the CS+ and CS-odors were delivered with 5-minute intervals.
Data analysis
Images were acquired at 5 frames/per second at a resolution of 256×256 pixels. The image data were analyzed as already described (Yu et al., 2004; Yu et al., 2005). In general, the raw fluorescence images were first smoothed with a 7x7 gaussian convolution filter and then registered. Regions of interest were circumscribed and a pseudo-color image of the %∆F/Fo ratio produced. The value Fo was calculated for each pixel within the region of interest as the fluorescence prior to odor application or the first electric shock as averaged over 5 successive frames. The value was calculated for each pixel within the region of interest as the difference between the maximum average intensity during the 3 sec odor application for 5 successive frames and Fo. The value ∆F for electric shock application was calculated as the average difference between the pixel intensities within each individual time frame and those within Fo.
Supplementary Material
Acknowledgements
Supported by grants NS19904 and NS52352 from the NINDS and the Mathers Charitable Trust. R.L.D. is the R. P. Doherty-Welch Chair in Science at the Baylor College of Science.
References
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Beck CDO, Schroeder B, Davis RL. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J. Neurosci. 2000;20:2944–2953. doi: 10.1523/JNEUROSCI.20-08-02944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Olfactory learning. Neuron. 2004;44:31–48. doi: 10.1016/j.neuron.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: From molecular to systems neuroscience. Ann. Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- de Belle SJ, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Mao Z, Roman G, Zong L, Davis RL. Pharmacogenetic rescue in time and space of the rutabaga memory impairment using Gene-Switch. Proc. Natl. Acad. Sci. USA. 2004;101:198–203. doi: 10.1073/pnas.0306128101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatio-temporal Rescue of Memory Dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- Pascual A, Preat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294:1115–1117. doi: 10.1126/science.1064200. [DOI] [PubMed] [Google Scholar]
- Perazzona B, Isabel G, Preat T, Davis RL. The role of cAMP response element-binding protein in Drosophila long-term memory. J. Neurosci. 2004;24:8823–828. doi: 10.1523/JNEUROSCI.4542-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu. Rev. Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Riemensperger T, Voller T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr. Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- Roman G, Davis RL. Molecular biology and anatomy of Drosophila olfactory associative learning. BioEssays. 2001;23:571–581. doi: 10.1002/bies.1083. [DOI] [PubMed] [Google Scholar]
- Roman G, He J, Davis RL. New series of Drosophila expression vectors suitable for behavioral rescue. BioTechiques. 1999;27:54–56. doi: 10.2144/99271bm09. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J. Comp. Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guo HF, Pologruto TA, Hannan F, Hakker I, Svoboda K, Zhong Y. Stereotyped odor-evoked activity in the mushroom body of Drosophila revealed by green fluorescent protein-based Ca2+ imaging. J. Neurosci. 2004;24:6507–6514. doi: 10.1523/JNEUROSCI.3727-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.