Abstract
In order to promote preattenive grouping of two sets of tones, one set of tones with a combination of frequency and ear of delivery was intermixed with another set of tones with a different combination of frequency and ear of delivery. The ERPs elicited by tones delivered to one ear that were preceded by three or four tones delivered in a row to the other ear were associated with an enhanced N1, due to the changes in frequency and ear of delivery with respect to the immediately preceding tones. However, no mismatch negativity (MMN) was obtained, even though these tones differed from the previious tones on the two dimensions of frequency and ear of delivery. The data were interpreted to signify that preattentively grouped sets of tones do not elicit MMN with respect to one another. This implies that once acoustic input has been preattentively grouped, the MMN system is dedicated to detecting changes that occur within but not between preattentively grouped stimuli
Keywords: Preattentive processes, scene analysis, event-related potentials, mismatch negativity
Investigations that employ the mismatch negativity (MMN) of event-related potentials (ERPs) have established a system that detects changes in the acoustic environment in a preattentive, automatic manner (Näätänen, 1992). Knowledge about this system is based on the circumstance that it emits a signal, termed the MMN, when one or more changes in previously repeating stimuli are detected. Various studies showed that the MMN is elicited by changes in simple acoustic features, such as when the intensity, frequency or duration of repeating tones (standards) is altered in a subsequent (deviant) tone (Näätänen, 1992). Cowan, Winkler, Teder and Näätänen (1993) and Winkler, Cowan, Csépe, Czigler and Näätänen (1996) found that at least two or three standards must be presented in order for a deviant tone to elicit a MMN. A main theoretical implication of these experiments is that the MMN system operates by extracting invariances or regularities in recent stimuli (e.g., that tonal frequency is constant) and that the MMN is elicited by subsequent stimuli that do not match a representation of regularity (Schröger, 1997; Ritter, Gomes, Cowan, Sussman & Vaughan, 1998; Winkler, Schröger & Cowan, 2001). Since a deviant tone can elicit a MMN by a change in any of a variety of features, this implies that representations of each of these features can be simultaneously maintained. It has also been shown that the MMN system can maintain at least two representations of regularity for the same acoustic feature. Winkler, Paavilainen and Näätänen (1992), for example, used two frequently occurring standards each of which had a different tonal frequency. Delivering a deviant tone with a tonal frequency midway between the tonal frequencies of the two standards elicited two MMNs, one with respect to the frequency of each standard. Similarly, Winkler, Karmos and Näätänen (1996) used two standards each with a different stimulus duration. A deviant midway in duration elicited two MMNs, one with respect to the duration of each standard.
Ritter, Sussman and Molholm (2000) presented two sets of tones, each with a different combination of features, with the intent that the two sets of tones would be separately grouped preattentively. The stimuli were alternated between one tone delivered to the left ear with a high tonal frequency and a short duration (100 ms) and another tone delivered to the right ear with a low tonal frequency and a long duration (300 ms). Thus, there were two sets of standard tones each of which always had the same combination of tonal frequency, perceived location and duration. Infrequent deviant tones of 200 ms were randomly delivered to either ear. When presented to the left ear, the deviants had the perceived location and high tonal frequency of the standards delivered to that ear. When presented to the right ear, the deviants had the perceived location and low tonal frequency of the standards delivered to that ear. The 200 ms deviant tones were deviant with respect to the standard duration of both sets of tones. It was found that a given deviant elicited only one MMN, even though it differed from the representation of the regularity for stimulus duration in both sets of tones. When deviants occurred in the left ear, the peak latency of the MMN was appropriate for a discrimination between stimulus durations of 100 and 200 ms, whereas when deviants occurred in the right ear the peak latency of the MMN was appropriate for a discrimination between stimulus durations of 200 and 300 ms. By contrast to the results of Winkler et al. (1992 and 1996), who found two MMNs when a deviant tone differed from two different standard values of a given feature, the deviants elicited one MMN and only with respect to the standard duration of the set of tones in which it was embedded. (These results have been repliclated by McKenzie and Barry, 2005). The data were interpreted to indicate that the two sets of tones had been separately grouped preattentively and that deviant tones are only compared with the representations of regularities of the group of tones in which they are embedded.
A similar conclusion was reached by Winkler et al. (2001) and Gaeta, Friedman, Ritter and Cheng (2001). In these experiments, short trains of tones were used that had identical short SOAs, and the duration of silence between trains was varied. By placing a tone in position 1 of a train with a frequency deviant different from that of the standard of the preceding train, it was expected that the duration of the memory upon which the MMN system depends could be ascertained on the basis of the maximum period of silence between trains where the deviant still elicited a MMN. It was found in both studies that longer periods of silence between trains yielded fewer subjects who exhibited MMNs, with only about half of the subjects exhibiting MMNs for silences of 7 (Winkler et al.) and 8 (Gaeta et al) sec. However, Winkler et al. found that when the same deviant tone was employed in a condition where a 7 sec period of silence was maintained between all tones throughout a run, all of the subjects exhibited a MMN. Therefore it could not be concluded that the absence of the MMN indicated that the 7 sec period of silence between short trains exceeded the duration of the memory. Gaeta et al. found that when there were 8 sec of silence between trains, near 100% behavioral accuracy for detecting the deviant was obtained. Hence, the behavioral data also did not support the view that the duration of the memory was a relevant factor in the absence of the MMN for the longer periods of silence between short trains. Winkler et al. (2001) inferred, and Gaeta et al. (2001) concurred, that increases in the period of silence between trains led the trains to be treated as not belonging together. That is, as the silence between trains increased, the first tone of a given train did not elicit a MMN even though it differed in frequency from the standards of the previous train because the tones of the new train were not processed as belonging to the group of tones in the preceding train. In other words, “One may assume that MMN is only elicited if the deviant stimulus is grouped together with the preceding regular stimuli; that is, that each regularity is relevant only within its own memory group” (Winkler et al., 2001).
Whereas the papers by Ritter et al. (2000), Winkler et al. (2001) and Gaeta et al. (2001) indicated that deviant tones only elicit MMNs with respect to the representations of regularities associated with the group within which the deviant is embedded, the present study was designed to determine whether when tones are preattentively processed as two separate groups, the two groups will elicit MMNs with respect to each other. Put another way, when a switch occurs between two set of tones with different feature values, are the feature values of the new set of tones compared to the regularities of the feature values of the preceding set of tones and the MMN emitted?
To this end, the stimulus paradigm of Ritter et al. (2000) was modified so that the two sets of stimuli were delivered in a quasi-random manner. It was thought that in order to induce preattentive grouping it would be important that the two sets of stimuli still be presented equiprobably. It is not necessary to use globally low probability deviants as in the oddball paradigm to elicit the MMN. This was shown in Sams, Alho and Näätänen (1983), where the MMN was elicited by two equiprobable stimuli. An analysis of local sequential effects, patterned after the pioneering study of Squires, Wickens, Squires and Donchin (1976) investigating local effects on P3, found that the MMN was elicited when one of the tones followed several instances of the other tone. Accordingly, both tones elicited the MMN depending on the immediately preceding sequence of tones. As with P3 (and N2 -- see for example Sams et al., 1983), the enhanced amplitude of the MMN for deviant over standard tones in the oddball paradigm can be mainly attributed to the relative distribution of local instances where a given tone is preceded by several instances of the other tone. In the oddball paradigm, in other words, the deviant stimuli elicit larger MMNs than standards because deviants are much more often preceded by several instances of the standard than are the standards preceded by several instances of the deviant. Using a local sequential analysis, Giese-Davis, Miller and Knight (1991) found similar effects for the MMN as did Sams et al. (1983) using two equiprobable tones in three separate groups of subjects. Accordingly, in the present study the two sets of tones were delivered in an equiprobable manner where the tones of a given set of tones were presented in sequences of one, two, three or four instances in a row followed by one, two, three or four tones in a row of the other set of tones, etc. The MMN was assessed by subtracting the ERPs elicited by the third and fourth identical tones in a row of a given set of tones from the ERPs elicited by a switch to the other set of tones.
Switches between the two sets of tones should elicit enhanced N1 both on the basis of the large change in tonal frequency and spatial location employed (Näätänen & Picton, 1987). If these large changes elicit MMN, it is possible that the latency of MMN could occur in the vicinity of N1. Since N1 is larger contralateral to the eliciting stimulus, whereas MMNs based on acoustic features are larger over the right hemisphere independent of the ear stimulated (Paavilainen , Alho, Reinikainen, Sams & Näätänen, 1991), the hemispheric differences of the ERPs were analysed.
The main difference between the manner in which the two sets of were delivered in Ritter et al. (2000) and the current study is that previously the two sets of tones were alternated between ears whereas in the present experiment 1, 2, 3 or 4 tones of one set occurred in a row before a switch to the other ear occurred. To ascertain that comparable results indicating preattentive grouping of the two sets of tones as in Ritter et al. occurred in the present study, the effects of duration deviants embedded in the two sets of tones was examined in several subjects.
Methods
Participants
Twenty adult volunteers (nine males) between the ages of 18 and 35 participated in the main experiment. Three additional subjects (two females) between the ages of 24 and 29 participated in a study where the effects of duration deviants were analyzed. The participants reported that they had normal hearing and had no known neurological deficits. They were paid for participating in the study. All participants provided written consent, and the institutional Review Board of the Nathan Kline Institute approved the procedures.
Procedure
Participants were instructed to ignore the sounds and watched a film with no sound. Pure tones of 75 dB SPL were delivered via headphones at the rate of 1/370 ms with rise and fall times of 20 ms each. Tones presented to the left ear were 1494 Hz and 100 ms in duration. Tones presented to the right ear were 440 Hz and 300 ms in duration. Hence, the two sets of tones differed in ear of delivery, tonal frequency and duration. Throughout each run, the stimuli were delivered in a quasi-random manner with either one, two, three or four identical tones in a row presented to one ear followed by one, two, three or four identical tones in a row presented to the other ear. There were relatively more instances of three and four identical tones in a row than one or two identical tones in a row (about 33% of the tones appeared in positions 1 and 2, and about 66% of the tones appeared in positions 3 and 4). There was a total number of 860 stimuli in a run and 12 runs, with a short break between runs.
In assembling the stimuli for assessing the effects of duration deviants, it was noticed that it was difficult to discriminate a 200 ms deviant from a 300 ms standard. To make this discrimination easier in order to obtain more robust MMNs, the duration of the standards delivered to the left ear was reduced to 75 ms and duration deviants of 125 ms were used. Duration deviants delivered to the left ear had the frequency of the standards of the left ear (i.e., 1494 Hz) and duration deviants delivered to the right ear had the frequency of the standards of the right ear (i.e., 440 Hz). The stimuli were delivered in the same way as above except that duration deviants occurred randomly on about 20% of the trials in positions 3 and 4 of repeating tones of the two ears. There was a total number of 1050 stimuli in a run and 8 runs, with a short break between runs.
ERP Recording
The electrical activity of the brain was recorded from 128 scalp electrodes (impedances <5 kΩ), referenced to the nose, using band passes of 0.05 and 100 Hz, and digitized at 500 Hz. The data were subsequently lowpass digitally filtered off-line at 45 Hz. Epochs of 500 ms were employed including a 100 ms prestimulus baseline, and baseline corrected over the entire epoch. Trials with blinks and eye movements were rejected off-line on the basis of vertical and horizontal EOG. An automatic artifact rejection criterion of 70 μVs was used for all other scalp recordings. In the main experiment, the average number of accepted standards delivered to each ear was about 520. The average number of accepted trials for ERPs based on tones delivered to one ear following standards delivered to the other ear for each ear was about 470. For the assessment of duration deviants, the average number of accepted deviants trials in each ear was about 150, and the average number of accepted standards in each ear was about 500.
Data Analysis
In the main experiment, for display purposes grand mean ERPs were obtained for the sum of the third and fourth identical tones in a row (hereafter called “standards” for simplicity) delivered to one ear, and for the first tone delivered to same ear that followed the third or fourth standard in a row delivered to the other ear, separately for each ear. In this way, the standard and switch ERPs were elicited by stimuli that had the same frequency and duration and were delivered to the same ear. A comparison of these waveforms for a given ear was used to determine whether the switch tones presented to one ear contained a MMN compared to the standards delivered to the same ear. The alpha level employed in all statistical tests was .05. When appropriate, the Greenhouse-Geisser correction was used.
In assessing the effects of duration deviants, the ERPs elicited by the deviants were summed separately for each ear. The ERPs elicited by the standards were also summed separately for each ear. For each subject, the ERPs elicited by the deviants and standards of each ear were displayed along with the subtraction of the standard from the deviant ERPs to delineate the MMN.
Results
Figure 1 depicts the grand mean ERPs at Fz for the standard (gray traces) and the switch trials (blue curves) for tones delivered to the left ear (upper left panel) and the right ear (upper right panel). For the switch tones presented to the right ear (right upper panel), there was an enhanced negativity relative to the standard tones around 115 ms followed by an enhanced P2. For the switch tones delivered to the left ear (left upper panel), there was an unexpected slow positive shift that extended from 30-40 sec prior to stimulus onset to the end of the epoch. This positive shift was both robust and reliable in that it could be observed for the grand mean of the first ten subjects (middle left panel) as well as the grand mean of the last ten subjects (lower left panel) and also readily observed in the data of individual subjects (see below). Note that the grand mean results for the switch trials delivered to the right ear were similarly reliable (middle and lower right panels).
Figure 1.
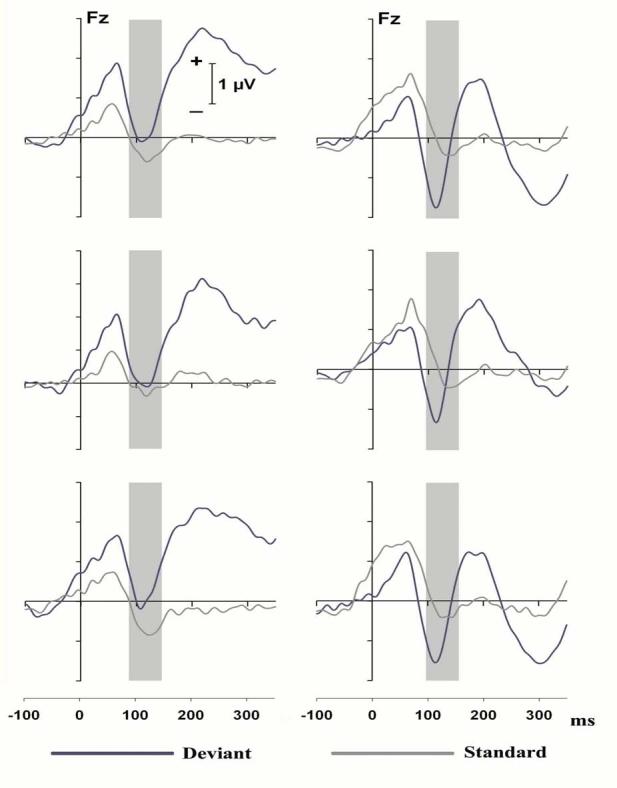
Top panels: grand mean ERPs at Fz for all 20 subjects for switch (blue curves) and standard trials (gray curves) presented to the left (left panel) and right (right panel) ear. Middle and bottom panels: similar grand mean ERPs for the first 10 (middle panels) and the second 10 (bottom panels) subjects. Vertical bars indicate the area of interest.
For the left ear tones there was a greater difference between the peak of P1 and the peak of the subsequent negativity for the switch than the standard tones, however the negativity of the switch tones barely went below baseline. Rather than measure the ERPs elicited by the switch tones from the peak of P1 to the peak of the subsequent negativity, which would not permit determining the relative contribution of the two waves, the ERPs elicited by the switch tones presented to the left ear were digitally high-pass filtered at 1 Hz. This brought the negativity more below baseline . However, for both the left and right ear tones, it appeared that the overlapping positive shift at the beginning of the epoch still reduced the negativity at 115 ms. To achieve the best possible measurement of the negativity, the ERPs elicited by the switch trials were re-baselined for the period from 40 ms prior to stimulus onset to 10 ms poststimulus.
The three panels on the far left of Figure 2 depict, for the left ear stimuli at Fz, the original ERPs (upper panel), the results of using the 1 Hz filter (middle panel) and the results of using a 40 to 10 ms baseline in addition to the 1 Hz filter (lower panel). The two panels on the far right of Figure 2 depict, for the right ear stimuli at Fz, the original ERPs (upper panel) and the result of using the -40 to 10 ms baseline (lower panel). As can be seen by examining the lower left and right panels, the ERPs associated with the left and right ears now had P1 waves of approximately equivalent amplitude for switch and standard stimuli. The peak of the negativity elicited on the switch trials in these waveforms was about 1 μV below baseline for the left ear and 2 μVs below baseline for the right ear. The left and right middle panels of Figure 2 present voltage maps for the peak of the negativity at 115 ms elicited on the switch trials delivered to the left and right ears, respectively. The contralateral nature of the negativity can be clearly seen for the left and right ear stimuli.
Figure 2.
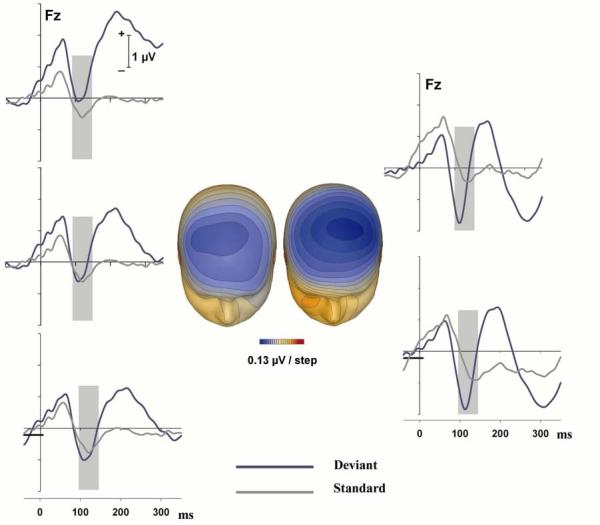
Top panels: Original grand mean ERPs at Fz for switch (blue curves) and standard trials (gray lines) presented to the left (left panel) and right (right panel) ear. Middle left panel: The same ERPs after using a 1 Hz high pass filter for the switch trials delivered to the left ear. Bottom left panel: The same ERPs using a baseline of −40 to 10 ms in addition to a 1 Hz high pass filter for the deviants of the left ear. Bottom right panel: Grand mean ERPs for switch and standard trials delivered to the right ear using a baseline of −40 to 10 ms. The solid horizontal bars at the bottom of the left and right bottom panels indicate the baseline used for the waveforms of the two bottom panels. The two panels in the middle of the figure: Voltage maps for the negativity associated with a switch to the left ear (left panel) and right ear (right panel).
The main critical difference between these grand mean waveforms for the standard and switch tones consisted of an enhanced negativity for the switch ERPs that peaked around 115 msec. In order to determine whether the enhanced negativity was larger contralateral to the stimulated ear, the averaged microvolt values of this negativity were measured in a latency window from 80 to 150 ms at seven electrodes along a coronal line from Fz toward the mastoids and converted to percentages of the microvolt values of Fz, separately for each ear of delivery.
Figure 3 illustrates the amplitude of the negativity at coronal sites from 80 to 150 ms as a percentage of the amplitude at Fz and a depiction of the location of the recording sites measured. As can be seen, switch tones presented to the left ear (blue lines) had a predominance over the right hemisphere and switch tones delivered to the right ear (red lines) had a predominance over the left hemisphere. A 3-way ANOVA based on these percentage values, using the factors ear of delivery, electrode and hemisphere, indicated a significant ear by hemisphere interaction, F(1,19) = 7.48, p = .013. Separate two-way ANOVAs, with factors of ear of delivery and hemisphere, yielded a trend toward a significant interaction for sites L1 and R1, the two electrodes closest to Fz, F(1/19) = 2.83, p = .109, and significant interactions for electrodes L2 and R2, F(1,19) = 4.98, p = .038, electrodes L3 and R3, F (1,19) = 7.15, p = .015, and electrodes L4 and R4, F (1,19) = 5.87, p = .026.
Figure 3.
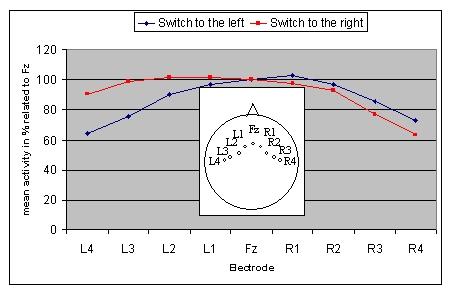
Coronal distribution of the amplitude of the switch trial's negativity peaking at 115 ms delivered to the left (blue curve) and right (red curve) ear as a percentage of the amplitude at Fz, and the location of the electrode sites measured.
In order to determine whether the slow positive shift elicited by switch tones delivered to the left ear was due to hemispheric differences or to the particular stimulus parameters used for the two ears, the headphones were switched between ears for three subjects who had participated in the main experiment. Figure 4 presents the unfiltered data of these subjects at Fz for the main experiment (left half of the figure) and when the headphones were reversed (right half of the figure). For each half of the figure, the left panels are for stimuli delivered to the left ear and the right panels for stimuli delivered to the right ear. The positive shift for switch tones delivered to the left ear in the main experiment can be clearly seen in each subject in the panels on the left half of the figure, as was generally true of the individual subjects in the main experiment. The right two panels of Figure 4 show the data of these same individuals when the earphones were reversed. The data clearly indicate that the slow positive shift now occurred for tones delivered to the right ear. Hence, the presence of this slow positive shift, for which we have no explanation, is not due to hemispheric differences but rather to something about the stimulus parameters used for the two ears interacting with the manner in which the stimuli were delivered.
Figure 4.
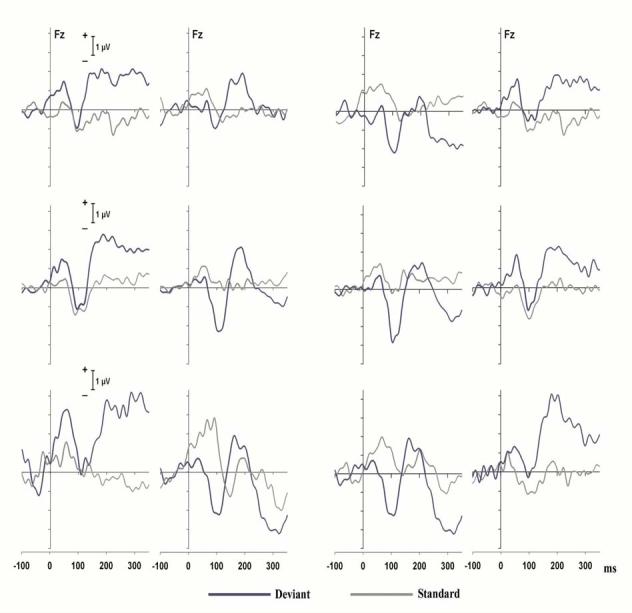
Left two columns: averaged ERPs at Fz for 3 individual subjects for switch (blue curves) and standard trials delivered (gray curves) to the left ear (left column) and the right ear (right column) in the main experiment. Right two columns: same ERPs for the same 3 subjects with the earphones switched.
Figure 5 displays the ERP results of the three subjects and their grand means for the duration deviants. The left column contains grand means and each of the 3 columns to the right contains the data for an individual subject. The upper panels display the superimposed ERPs elicited by standards (gray lines) and duration deviants (blue lines) for the left ear. The middle panels show the same ERPs for the right ear. The bottom panels show the superimposed subtractions of the standard ERPs from the deviant ERPs for the left (thick lines) and right (thin lines) ears. For the left ear, an early MMN was elicited with a latency appropriate to a discrimination between the 125 ms deviant and the shorter standard of the left ear, followed by P3a. For the right ear, a later MMN was elicited with a latency appropriate to a discrimination between the 125 ms deviant and the longer standard of the right ear. The difference in latency of the MMN for the two ears was mirrored by a similar difference in the latency of the P3a for the two ears. The important result was that the deviants elicited only one MMN, associated with the standard of the ear to which they were presented.
Figure 5.
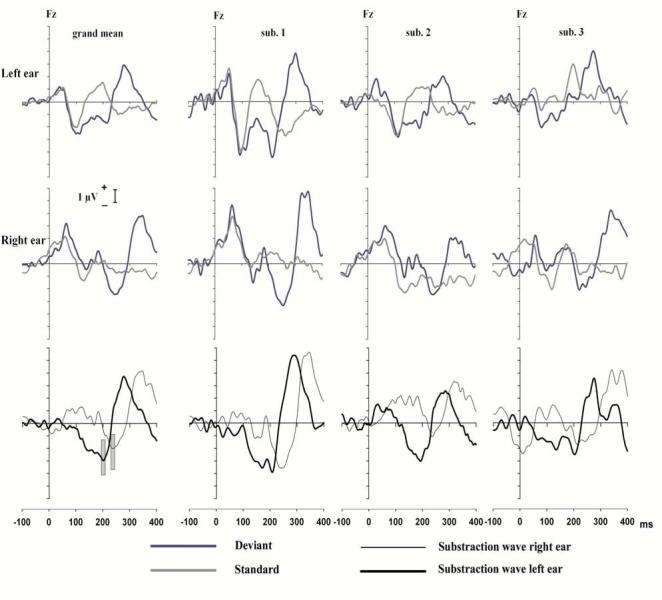
Far left column: The top panel shows the Grand mean ERPs at Fz for standards (gray lines) and duration deviants (blue lines) for tones delivered to the left ear. The middle panel shows the same ERPs for tones delivered to the right ear. The bottom panel shows the subtraction of the grand mean ERPs elicited by the standards shown in the panels above from the grand mean ERPs elicited by the deviants of the panels above for the left (solid lines) and right (dashed lines) ears. Other columns: Each column contains the data for one of the 3 individual subjects in a manner identical to that for the grand means. The vertical bars in the lower left panel indicate the early and late latency MMNs.
Discussion
The data obtained with the duration deviants displayed in Figure 5 replicate and extend the results of Ritter et al. (2000) to the modifications of the present study. As in the previous study, duration deviants delivered to the left ear elicited MMN with respect to the duration of the standards of the left ear and no MMN with respect to the duration of the standards presented to the right ear. On the other hand, duration deviants delivered to the right ear elicited MMN with respect to the duration of the standards of the right ear and no MMN with respect to the duration of the standards presented to the left ear. Given that Winkler et al. (1996) found that an intermediate duration deviant elicited 2 MMNs with regard to 2 standard duration tones (one shorter and one longer in duration), even after 4 presentations of one of the standards in a row, in our design the deviant duration tone following 2 and 3 occurrences of the standard duration tones of one ear had the potential of eliciting 2 MMNs. The data, accordingly, support the interpretation that the two sets of tones were preattentively grouped and that deviants elicit MMNs only with respect to the group in which they are embedded.
A question pertaining to the results of the main experiment concerns the nature of the enhanced negativity associated with the switch trials. It has been found that with monaural stimuli N1 is larger over the hemisphere contralateral to the stimulated ear (Näätänen & Picton, 1987; Paavilainen et al., 1991). The peak latency of 115 ms for the enhanced negativity is consistent with N1 elicited by auditory stimuli. However, given the large separation between the tonal frequencies of the tones delivered to the two ears, had a MMN been elicited on the switch trials it could have had a peak latency in the vicinity 115 ms.
One means of distinguishing between N1 and MMN elicited by tones is their topography. In contrast to N1, the MMN is generally found to be larger over the right hemisphere, especially for monaural stimuli. Paavilainen et al. (1991) found the MMN to be larger over the right hemisphere for frequency, intensity and duration deviants, independent of the ear stimulated. Giard, Perrin, Pernier & Bouchet (1990) and Deouell, Bentin & Giard (1998) obtained the same result for monaurally delivered frequency deviants. In addition, Deouell et al. found a similar asymmetry for binaurally delivered tones. Using binaural stimuli, Liebenthal et al. (2003) examined the MMN and found that simultaneously recorded ERP and fMRI activity were both larger at the right hemisphere, concluding that “the predominant activation underlying frequency deviant detection is centered in the right superior temporal lobe” (p. 1403). The MMN has also been found to be larger over the right hemisphere for deviants delivered to left and right locations (Giard et al., 1990; Deouell & Bentin, 1998; Deouell et al., 1998). The contralateral predominance for the enhanced negativity found in the present study for switch trials associated with the left and right ear, accordingly, is in line with previous findings for N1 rather than what has been found for the MMN.
There is still a question as to whether, despite the N1 topography of the enhanced negativity, a small MMN could have overlapped the N1. In reviewing the N1 literature, Näätänen & Picton (1987) concluded that whereas the N1 asymmetry is generally small, “there are definite asymmetries in the N1 wave related to ear of stimulation” (p. 395). Given the small asymmetry of N1, it is unlikely that the asymmetry observed in the present data would have been preserved had an overlapping MMN of even moderate size been present. The critical asymmetry was for the switch tones delivered to the right ear. This is because the asymmetry found for tones delivered to the left ear has been found to predominate over the right hemisphere for both N1 and the MMN. Hence, there is no distinction in this regard between the two components. Tones delivered to the right ear, however, have been found to predominate over the left hemisphere for N1 and over the right hemisphere for the MMN.
The negativity elicited by switches delivered to the right ear was about 2 μV at Fz. This reflects an increase of about 1 μV from the negativity elicited by standards to the negativity elicited by the switch trials (Figure 2, right bottom panel). However, it is likely that none of the 1 μV increase could be due to MMN. It has been established that switches from one ear to the other (Butler, 1972) and changes in tonal frequency (Butler, 1968) enhance the amplitude of N1 (reviewed in Näätänen & Picton, 1987). Moreover, there are two reasons why large MMNs, had they been elicited, would be expected with the current design. First, the separations between frequency and location were large. Second, the switch trials differed from the preceding standards in two acoustic characteristics, frequency and location (deviants that differ from standards on two acoustic features are termed double deviants). A number of studies have found that double deviants elicit MMNs with an amplitude that is the sum of the amplitude of each deviant feature when presented alone, i.e., as single deviants (see Schröger, 1997 for a review), including double deviants that consist of changes in frequency and location (Schröger, 1995). It appears, therefore, that the switch trials did not elicit an MMN.
The present study did not find local sequential effects associated with the MMN described in the Introduction as were obtained by Sams et al. (1983) and Gieser-Davis et al. (1993). The absence of the MMN was apparently due to using an experimental design that induced preattentive grouping of the tones. Switching from one group to the other was associated with no change within either group, and the MMN system seems to be focused on changes within but not between groups.
There are two main implications of the results. The data obtained with duration deviants extends the findings of previous studies (Ritter et al., 2000; Winkler et al., 2001; and Gaeta et al., 2001) indicating that when preattentive grouping occurs, deviants only elicit MMN with regard to the group in which they are embedded. The data obtained with switching between preattentively grouped stimuli indicates that once preattentive grouping has taken place, MMNs are not elicited between groups. In Bregman's (1990) view, one of the first preattentive stages of scene analysis is the determination of which aspects of sounds occurring in the same time frame belong together. Once this initial stage of scene analysis is underway, the task of the MMN system seems to be to detect changes within the acoustic events so grouped together.
Acknowledgments
This research was supported by USPHS grant NS30029-26. We are very grateful for the technical assistance and dedication of Ms. Marina Shpaner and Ms. Jeannette Mahoney.
REFERENCES
- Bregman AS. Auditory scene analysis. MIT Press; Cambridge, MA: 1990. [Google Scholar]
- Butler RA. Effects of changes in stimulus frequency and intensity on habituation of the human vertex potential. Journal of the Acoustic Society of America. 1968;44:945–950. doi: 10.1121/1.1911233. [DOI] [PubMed] [Google Scholar]
- Butler RA. The influence of spatial separation of sound source on the auditory evoked response. Neuropsychologia. 1972;10:219–225. doi: 10.1016/0028-3932(72)90063-2. [DOI] [PubMed] [Google Scholar]
- Cowan N, Winkler I, Teder W, Näätänen R. Memory prerequisites of mismatch negativity in the auditory event-related potential (ERP) Journal of Experimental Psychology: Learning, Memory and Cognition. 1993;19:909–921. doi: 10.1037//0278-7393.19.4.909. [DOI] [PubMed] [Google Scholar]
- Deacon D, Gomes H, Nousak J-M, Ritter W, Javitt D. Effect of frequency separation and stimulus rate on the mismatch negativity: an examination of the issue of refractoriness in humans. Neuroscience Letters. 2000;287:167–170. doi: 10.1016/s0304-3940(00)01175-7. [DOI] [PubMed] [Google Scholar]
- Deouell LY, Bentin S. Variable cerebral responses to equally distinct deviance in four Auditory dimensions. Psychophysiology. 1998;35:745–754. [PubMed] [Google Scholar]
- Deouell LY, Bentin S, Giard H-H. Mismatch negativity in dichotic listening: Evidence for interhemispheric differences and multiple generators: A mismatch negativity study. Psychophysiology. 1998;35:355–354. [PubMed] [Google Scholar]
- Gaeta H, Friedman D, Ritter W, Cheng J. The effect of perceptual grouping on the mismatch negativity. Psychophysiology. 2001;38:316–324. [PubMed] [Google Scholar]
- Giard M-H, Perrin F, Pernier J, Bouchet B. Brain generators implicated in the processing of auditory stimulus deviance: A topographic event-related potential study. Psychophysiology. 1990;27:627–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Giese-Davis JE, Miller G, Knight RA. Memory template comparison processes in anhedonia and dysthymia. Psychophysiology. 1993;30:646–656. doi: 10.1111/j.1469-8986.1993.tb02090.x. [DOI] [PubMed] [Google Scholar]
- Liebenthal E, Ellingson ML, Spanaki MV, Prieto TE, Ropella KM, Binder JR. Simultaneous ERP and fMRI of the auditory cortex in a passive oddball paradigm. Neuroimage. 2003;19:1395–1404. doi: 10.1016/s1053-8119(03)00228-3. [DOI] [PubMed] [Google Scholar]
- McKenzie DN, Barry RJ. The independence of memory traces of attended and unattended stimuli. Cerebral Cortex. 2005 December 15; doi: 10.1093/cercor/bhj093. advanced access published on line. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Attention and brain function. Erlbaum; Hillsdale, NJ: 1992. [Google Scholar]
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Paavilainen P, Alho K, Reinikainen K, Sams M, Näätänen R. Right hemisphere dominance of different mismatch negativities. Electroencephalography and Clinical Neurophysiology. 1991;78:466–479. doi: 10.1016/0013-4694(91)90064-b. [DOI] [PubMed] [Google Scholar]
- Ritter W, Gomes H, Cowan N, Sussman E, Vaughan HG., Jr. Reactivation of a dormant representation of an auditory stimulus feature. Journal of Cognitive Neuroscience. 1998;10:605–614. doi: 10.1162/089892998563004. [DOI] [PubMed] [Google Scholar]
- Ritter W, Sussman E, Molholm S. Evidence that the mismatch negativity system works on the basis of objects. NeuroReport. 2000;11:61–63. doi: 10.1097/00001756-200001170-00012. [DOI] [PubMed] [Google Scholar]
- Sams M, Alho K, Näätänen R. Sequential effects on the ERP in discriminating two stimuli. Biological Psychology. l983;l7:4l–58. doi: 10.1016/0301-0511(83)90065-0. [DOI] [PubMed] [Google Scholar]
- Schröger E. Processing of auditory deviants with changes in one vs. two stimulus dimensions. Psychophysiology. 1995;34:55–65. doi: 10.1111/j.1469-8986.1995.tb03406.x. [DOI] [PubMed] [Google Scholar]
- Schröger E. On the detection of auditory deviations: A pre-attentive model. Psychophysiology. 1995;34:245–257. doi: 10.1111/j.1469-8986.1997.tb02395.x. [DOI] [PubMed] [Google Scholar]
- Squires KC, Wickens C, Squires NK, Donchin E. The effect of stimulus sequence on the waveform of the cortical event-related potential. Science. 1976;193:1142–1146. doi: 10.1126/science.959831. [DOI] [PubMed] [Google Scholar]
- Winkler I, Cowan N, Csépe V, Czigler I, Näätänen R. Interactions between transient and long-term auditory memory as reflected by the mismatch negativity. Journal of Cognitive Neuroscience. 1996;8:403–415. doi: 10.1162/jocn.1996.8.5.403. [DOI] [PubMed] [Google Scholar]
- Winkler I, Karmos G, Näätänen R. Adaptive modeling of the unattended acoustic environment reflected in the mismatch negativity event-related potential. Brain Research. 1996;742:239–252. doi: 10.1016/s0006-8993(96)01008-6. [DOI] [PubMed] [Google Scholar]
- Winkler I, Paavilainen P, Näätänen R. Can echoic memory store two traces simultaneously? A study of event-related brain potentials. Psychophysiology. l992;29:337–349. doi: 10.1111/j.1469-8986.1992.tb01707.x. [DOI] [PubMed] [Google Scholar]
- Winkler I, Schröger E, Cowan N. The role of large-scale memory organization in the mismatch negativity event-related brain potential. Journal of Cognitive Neuroscience. 2001;13:59–71. doi: 10.1162/089892901564171. [DOI] [PubMed] [Google Scholar]


