Abstract
Förster's resonance energy transfer (FRET) can be used to study protein-protein interactions in living cells. Numerous methods to measure FRET have been devised and implemented; however, the accuracy of these methods is unknown, which makes interpretation of FRET efficiency values difficult if not impossible. This problem exists due to the lack of standards with known FRET efficiencies that can be used to validate FRET measurements. The advent of spectral variants of green fluorescent protein and easy access to cell transfection technology suggests a simple solution to this problem: the development of genetic constructs with known FRET efficiencies that can be replicated with high fidelity and freely distributed. In this study, fluorescent protein constructs with progressively larger separation distances between donors and acceptors were generated and FRET efficiencies were measured using fluorescence lifetime spectroscopy, sensitized acceptor emission, and spectral imaging. Since the results from each method were in good agreement, the FRET efficiency value of each construct could be determined with high accuracy and precision, thereby justifying their use as standards.
Förster's resonance energy transfer (FRET) is a process in which a donor fluorophore in the excited state nonradiatively transfers energy to an acceptor molecule (1). FRET efficiency, defined as the fraction of donor excitation events that result in energy transfer to an acceptor, can be used to calculate the separation distance between a donor and acceptor inside living cells. Numerous methods have been developed to measure FRET, yet their accuracy is currently unknown (2), thus interpretation and comparison of FRET measurements are problematic. A simple solution to this problem is the development of “standards” in the form of genetic constructs encoding fluorescent proteins (FPs) with known FRET efficiencies that can be freely distributed and used to calibrate and validate FRET imaging systems.
A major obstacle in the use of FPs as FRET standards is determining an absolute FRET efficiency for a given construct. After genetically engineering a set of FP constructs containing a single donor and acceptor separated by progressively larger linkers, our strategy was to measure the FRET efficiency of each construct using three different methods; one based on fluorescence lifetime microscopy (3,4) (FLIM-FRET), one based on sensitized acceptor emission (5,6) (E-FRET) and one based on differences in emission spectra (7) (sRET). Since valid FRET methods will yield the same FRET efficiency for a given sample, a consensus of several different FRET methods, each monitoring different manifestations of FRET, could serve as the basis for concluding that a measured FRET efficiency is accurate and would therefore qualify a construct for use as a standard.
Accordingly, constructs were generated in which Cerulean (8) (C, a blue fluorescent protein variant serving as the donor) is attached to Venus (9) (V, a yellow variant serving as the acceptor) with either 5, 17, or 32 amino acid linkers in between them termed C5V, C17V, and C32V, respectively (Supplementary Material). Constructs with shorter linkers should have higher FRET efficiencies. Thus, C5V is expected to have the highest FRET efficiency of the group, followed by C17V and C32V. Our initial screen of these constructs involved an examination of the emission spectra with two-photon excitation. Cerulean is preferentially excited at 820 nm and has an emission peak at 475 nm, whereas Venus, which is preferentially excited at 940 nm, has an emission maximum at 528 nm. Since all constructs are comprised of Cerulean and Venus at a 1:1 stoichiometry, the emission spectra for the constructs should always appear as a complex spectra of the two fluorophores. The relative intensity of the two emission peaks should be a function of the FRET efficiency of the construct. Specifically, the 475 nm Cerulean peak should decrease relative to the 528 nm peak of Venus with increasing FRET efficiency.
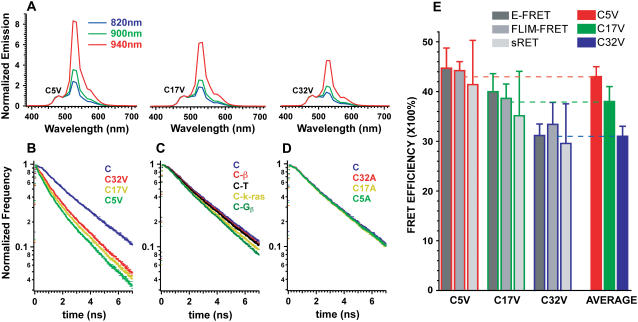
HEK 293 cells transfected with the different FRET constructs were subjected to spectral imaging in which an emission spectrum is acquired for every pixel in the field of view (7,10). The emission spectra of each construct was obtained using 820, 900, or 940 nm excitation and normalized to the 475 nm peak of Cerulean (Fig. 1 A). As expected, the emission of each construct had peaks at 475 nm and 528 nm. The magnitude of the 528 nm emission peak was always higher than the 475 nm emission, suggesting that the Cerulean in these constructs transfer energy to Venus by FRET. The 528 nm peak, for all constructs examined, increased with excitation wavelength relative to Cerulean's 475 nm emission peak due to increased direct excitation of Venus. C5V had the highest 528 nm peak followed by C17V and C32V, which is consistent with the prediction that C5V should have the highest FRET efficiency and C32V the lowest.
FIGURE 1.
Characterization of FRET standards (A) Normalized emission spectra relative to the peak of the Cerulean emission at 475 nm of C5V, C17V, and C32V with 820, 900, and 940 nm excitation as indicated. Spectra are an average of three different cells. (B) Fluorescence donor lifetime decay curves of Cerulean, C5V, C17V, and C32V. All traces are an average of decay curves from at least five different cells. Note that the lifetime curves become progressively faster as linker size decreases. (C) Fluorescence lifetime decay curves of Cerulean, Cerulean-β (the β3 subunit of voltage-gated calcium channels), Cerulean-TRAF (TNF-associated factor 2aTRAF domain), Cerulean-K-Ras (the membrane-targeting sequence of K-Ras) and Cerulean-Gβ (the β-subunit of the heterotrimeric G-protein complex). The Cerulean fluorescence decays faster with the attachment of various protein adducts. (D) A comparison of the decay curves of Cerulean, C5A, C17A, and C32A. Minor changes in Cerulean's fluorescent lifetime were observed with the attachment of Amber with different sized linkers. (E) FRET efficiencies of C5V (red border), C17V (green border), and C32V (blue border) determined using E-FRET (dark gray; n of at least 10 cells per construct), FLIM-FRET (medium gray; n of 10 cells per construct), and sRET (light gray; n of at least 60 cells per construct) are shown (mean ± SD).The FRET efficiency values generated by each method for any given clone were not statistically different based on the results of an ANOVA (p > 0.05); therefore, FRET efficiency values for each method were combined and an average was calculated. Average FRET efficiency values for C5V (red bar), C17V (green bar), and C32V (blue bar) are shown.
Another predicted manifestation of FRET is a decrease in the fluorescence lifetime of Cerulean (11). Upon excitation by a short pulse of light, a fluorophore has a characteristic probability of emitting a photon resulting in an exponential decay of fluorescence with time (11). Environmental factors such as FRET provide new pathways through which energy can be dissipated resulting in a decrease in a donor's lifetime. After calibrating the time correlated single photon counting (TCSPC) instrumentation (12) with fluorescein (Fig. S1, Supplementary Material), lifetime decay curves for all of the constructs were collected. As predicted, lifetimes decreased as linker size diminished (Fig. 1 B), consistent with the results obtained through spectral imaging.
A comparison of a donor's fluorescence lifetime in the presence and absence of acceptors is required to obtain a FRET efficiency from lifetime data. A decrease in the lifetime of a donor in the presence of an acceptor is attributed to FRET (11). Because Cerulean's chromophore is encapsulated within a β-barrel, its lifetime is thought to be insensitive to most environmental factors and should not change when attached to a protein. As a result, the lifetime of a Cerulean-Venus complex is typically compared to the lifetime of Cerulean alone. To test if Cerulean's lifetime does not change when attached to a protein, it was tagged with four different proteins, and decay curves were acquired (Fig. 1 C). Cerulean's lifetime was altered, suggesting that the use of Cerulean alone in FLIM-FRET calculations is inappropriate. To construct a more suitable donor alone control, we tagged Cerulean to Amber (A, Y67C mutation in Venus) that folds correctly but is incapable of acting as a FRET acceptor (S. Koushik, unpublished results). These Cerulean-Amber constructs, termed C5A, C17A, and C32A, decayed slightly faster than Cerulean alone (Fig. 1 D), and were used in FLIM-FRET calculations for C5V, C17V, and C32V, respectively.
Calculation of accurate FRET efficiencies using FLIM requires not only appropriate donor-alone constructs, but precise lifetime values (Fig. S2, Supplementary Material). Three methods of fitting lifetime curves of the constructs were evaluated (Table S1, Supplementary Material). Based on results obtained for fitting lifetime standards (Fig. S2), we reasoned that lifetimes generated using global fitting (13) of the Venus and Amber-tagged Cerulean constructs produced the best estimates of FLIM-FRET efficiencies and therefore this method was used for this study.
FRET efficiencies as measured by FLIM-FRET, E-FRET, and sRET are shown in Fig. 1 E. FRET efficiency values generated by different methods were in good agreement even though two methods used pulsed two-photon excitation, whereas a third employed sensitized acceptor emission using steady-state excitation. An analysis of variance (ANOVA) revealed that FRET efficiency values for each construct generated by all methods were statistically indistinguishable (p > 0.05). Additionally, an ANOVA comparing the FRET efficiencies generated by any of the specific methods indicated that the three constructs were different (p < 0.01 by Tukey's post hoc multiple comparison test), validating their use as FRET standards. The FRET efficiency for each clone was calculated by averaging the mean values from each of the three methods (Fig. 1 E). Average FRET efficiency values for specific clones were always within 3% of values generated by the individual methods and spanned a range from 31% to 43%. All three methods could differentiate between C17V and C5V (p < 0.01 by Tukey's multiple comparison test), indicating that a 5% change in FRET efficiency can be detected in living cells. Assuming a dipole orientation factor (κ2) of 2/3 (14), this represents a 0.2 nm change in average separation distance. Although knowledge of the value of κ2 is needed to accurately interpret FRET efficiencies in terms of separation distance, it is not needed to accurately measure the FRET efficiency of a sample.
In conclusion, we have produced FRET standards with FRET efficiency values of 43 ± 2, 38 ± 3, and 31 ± 2%. A set of constructs composed of Cerulean and Amber were also built, and their use increased the accuracy of the FLIM-FRET measurements. Expression of C5V (7) or C32V (6) in different cell lines did not significantly alter their efficiencies, suggesting that the behavior of these constructs was not cell-type specific. Because Venus is thought to mature rapidly (9,15), there should always be one adjacent acceptor for each expressed donor in these constructs. Experiments with C5V (7) and with C32V (6) indicated that a 1:1 stoichiometry was present as expected, supporting the conclusion that Cerulean and Venus fold properly and efficiently. Thus, the numerous and disparate methods used to measure FRET can now be calibrated and/or validated with these standards.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ online at http://www.biophysj.org.
Acknowledgments
We thank S. Ikeda for his advice and suggestions.
This work was supported by the intramural program of the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD 20892.
References
- 1.Förster, T. 1948. Intermolecular energy migration and fluorescence. Annalen der Physik. 2:55–75. [Google Scholar]
- 2.Vogel, S. S., C. Thaler, and S. V. Koushik. 2006. Fanciful FRET. Sci. STKE. 2006:re2. [DOI] [PubMed] [Google Scholar]
- 3.Wang, X. F., A. Periasamy, and B. Herman. 1992. Fluorescence lifetime imaging microscopy (FLIM): Instrumentation and applications. Crit. Rev. Anal. Chem. 23:369–395. [Google Scholar]
- 4.Gadella, T. W. J., T. M. Jovin, and R. M. Clegg. 1993. Fluorescence lifetime imaging microscopy (FLIM): spacial resolution of microstructures on the nanosecond time scale. Biophys. Chem. 48:221–239. [Google Scholar]
- 5.Zal, T., and N. R. Gascoigne. 2004. Photobleaching-corrected FRET efficiency imaging of live cells. Biophys. J. 86:3923–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, H., H. L. Puhl 3rd, S. V. Koushik, S. S. Vogel, and S. R. Ikeda. 2006. Measurement of FRET efficiency and ratio of donor to acceptor concentration in living cells. Biophys. J. 91:L39–L41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thaler, C., S. V. Koushik, P. S. Blank, and S. S. Vogel. 2005. Quantitative multiphoton spectral imaging and its use for measuring resonance energy transfer. Biophys. J. 89:2736–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo, M. A., G. H. Springer, B. Granada, and D. W. Piston. 2004. An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 22:445–449. [DOI] [PubMed] [Google Scholar]
- 9.Nagai, T., K. Ibata, E. S. Park, M. Kubota, K. Mikoshiba, and A. Miyawaki. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20:87–90. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann, T., J. Rietdorf, and R. Pepperkok. 2003. Spectral imaging and its applications in live cell microscopy. FEBS Lett. 546:87–92. [DOI] [PubMed] [Google Scholar]
- 11.Lakowicz, J. R. 1999. Principles of Fluorescence Spectroscopy. Kluwer Academic/Plenum Publishers, New York.
- 12.Becker, W. 2005. Advanced Time-Correlated Single Photon Counting Techniques. A. W. Castleman, J. P. Toennies, and W. Zinth, editors. Springer, Berlin, Heidelberg, New York.
- 13.Knutson, J. R., J. M. Beechem, and L. Brand. 1983. Simultaneous analysis of multiple fluorescence decay curves: a global approach. Chem. Phys. Lett. 102:501–507. [Google Scholar]
- 14.van der Meer, B. W. 2002. Kappa-squared: from nuisance to new sense. J. Biotechnol. 82:181–196. [DOI] [PubMed] [Google Scholar]
- 15.Shaner, N. C., P. A. Steinbach, and R. Y. Tsien. 2005. A guide to choosing fluorescent proteins. Nat. Methods. 2:905–909. [DOI] [PubMed] [Google Scholar]