Abstract
Interstitial cells of Cajal (ICC) are important cells which coordinate gastrointestinal motility. ICC express Kit receptor tyrosine kinase, and Kit immunohistochemistry reveals ICC morphology and distribution in the gastrointestinal musculature. ICC show a highly branched morphology and form unique networks. Myenteric ICC (ICC-MY) are located at the layer of the myenteric plexus and serve as electrical pacemakers. Intramuscular ICC (ICC-IM) and ICC in the deep muscular plexus (ICC-DMP) are distributed within the muscular layers, and are densely innervated by excitatory and inhibitory enteric motor neurons and in close contact with nerve terminals. Recent studies combined with morphological and functional techniques directly revealed that ICC-IM and ICC-DMP are mediators of enteric motor neurotransmission. These types of ICC express several receptors for neurotransmitters such as acetylcholine and substance P and show responses to excitatory nerve stimulations. ICC also express receptive mechanisms for nitric oxide, which is an inhibitory neurotransmitter in the gastrointestinal tract. They can respond to nitrergic nerve stimulation by cyclic GMP production. Kit mutant mice lack ICC-IM and show attenuated postsynaptic responses after intrinsic nerve stimulation. These findings indicate the importance for ICC in neurotransmission in the gastrointestinal tract.
Keywords: interstitial cells of Cajal, enteric nervous system, neurotransmission, smooth muscle, gastrointestinal tract
I. Introduction
The musculature of the gastrointestinal tract is organized into two layers separated by a neural network known as Auerbach’s myenteric plexus. In the outer layer, smooth muscle cells are arranged along the length of the intestinal segments (longitudinal muscle), whereas in the inner layer, smooth muscle cells are arranged transversally to the length of the intestine (circular muscle); these are the basic elements involved in gastrointestinal motility. Several control mechanisms cooperate in the regulation of the normal transit of contents through the gastrointestinal tract, carried out by organized patterns of contraction of the gastrointestinal musculature. The central nervous system, enteric nervous system and control systems within the musculature are activated by distension or chemical stimuli and influence transit. These control systems do not act independently, but are closely intertwined. Recent studies have highlighted that the interstitial cells of Cajal (ICC) are the most important cells coordinating gastrointestinal motility, both as pacemakers and as intermediates between nerves and smooth muscle cells [33, 35].
ICC show a highly branched morphology and form networks associated with the enteric nervous system in the gastrointestinal musculature. In general, ICC can be divided into two groups by their functions [13, 33]. In most regions of the gastrointestinal tract, a thin layer of ICC forms a network of cells lying in the myenteric region, which act as pacemakers. Electrical recordings from gastrointestinal musculature reveal spontaneous rhythmic oscillations in the membrane potential, which are called “slow waves”. Now, ICC are known to be pacemaker cells, which are the source of slow waves [14, 36, 42]. This type of ICC also regulates the spread of slow waves from pacemaker ICC to smooth muscle cells [15]. The second group of ICC has an intramuscular location with individual ICC being distributed amongst the smooth muscle cells and acts as mediators of neurotransmission [14, 53, 54]. Although classical morphological studies proposed this role, recent studies have shown functional evidence about the role of ICC as mediators of neurotransmission.
II. Classification and Distribution of ICC (Fig. 1)
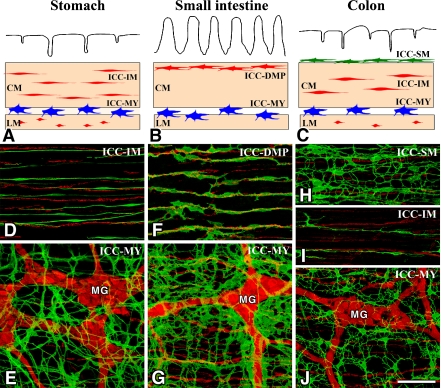
Fig. 1.
Overview of the types of ICC in the gastrointestinal tract. A–C: Schematic representations of ICC in the stomach (A), small intestine (B) and colon (C). ICC-MY (blue) are located between circular (CM) and longitudinal (LM) muscle layers. ICC-IM (red) and ICC-DMP (red) are located within circular and longitudinal muscles. ICC-SM (green) are located at the submucosal surface of the circular muscle layer in the colon. D–J: ICC and nerve networks in the murine stomach (D, E), small intestine (F, G) and colon (H–J). ICC and nerves in whole mount preparations are demonstrated by immunohistochemistry for Kit (ICC marker, green) and PGP9.5 (pan-neuronal marker, red), respectively. ICC-MY with a multipolar shape are associated with myenteric ganglia (Auerbach’s ganglia) (MG). ICC-IM in the stomach and colon have a bipolar shape and are associated with intramuscular nerve fibers. ICC-DMP in the small intestine are associated with deep muscular plexus. ICC-SM are multipolar cells having many thin processes. Bar=100 µm.
ICC express Kit receptor tyrosine kinase, which is a product of the KIT gene in laboratory animals and humans [4, 27]. The location of different types of ICC and their morphological features obtained by Kit immunohistochemistry are shown in Figure 1. Electron microscopic studies have revealed the ultrastructural characterizations of ICC, such as numerous mitochondria, abundant intermediate filaments and gap junctions (Fig. 2) [13, 23, 24]. Immunohistochemical and ultrastructural studies using small laboratory animals such as mice, rats and guinea pigs have revealed ICC characteristics and distribution as follows.
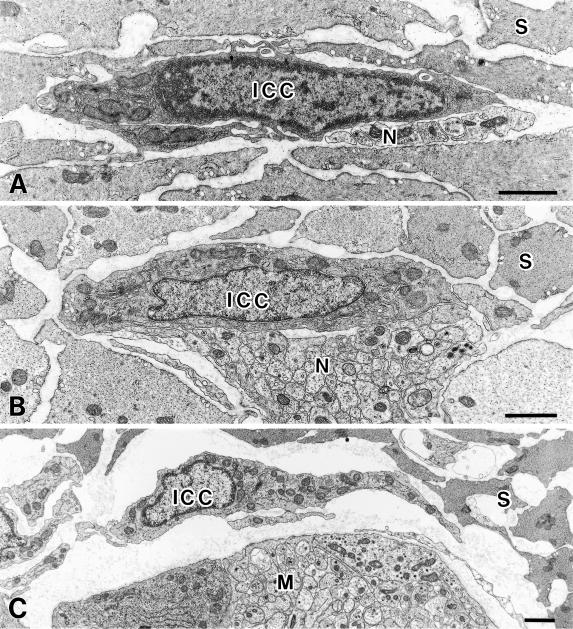
Fig. 2.
Ultrastructural characteristics of ICC. A: ICC-IM (ICC) in the rat stomach circular muscle layer have an electron-dense cytoplasm, many mitochondria and endoplasmic reticulum. Nerve terminals (N) are in close contact with ICC-IM. S shows smooth muscle cells in the circular layer. B: ICC-DMP (ICC) in the rat jejunum have caveolae, many mitochondria and well developed Golgi apparatus and endoplasmic reticulum. Nerve fiber bundles (N) are in close contact with ICC-DMP. S shows smooth muscle cell in the circular layer. C: ICC-MY (ICC) in the rat jejunum have caveolae, many mitochondria and well developed Golgi apparatus and endoplasmic reticulum. Myenteric ganglion (M) is located near ICC-MY. S shows smooth muscle cells in the circular layer. Bars=1 µm.
1. ICC-MY (ICC-MP, ICC-AP): ICC at the layer of the myenteric plexus (Auerbach’s plexus)
ICC-MY are located within the intermuscular space between the circular and longitudinal muscle layers throughout the stomach, small intestine and large intestine. ICC-MY have a multipolar shape with several cytoplasmic processes that project a few branching processes, and form gap junctions with each other. Although ICC-MY form an independent network from the myenteric plexus in the space of the myenteric layer, a cellular network of ICC-MY surrounds the myenteric ganglia and connecting strands of the myenteric plexus. ICC-MY are pacemaker cells in the stomach and small intestine that generate slow waves in the musculature [15, 35, 42].
2. ICC-IM (ICC-CM, ICC-LM): ICC at the intramusculature in the circular and longitudinal muscle layers
ICC-IM are distributed within the muscle layers of the esophagus, stomach and large intestine. ICC-IM have a bipolar shape with few branching processes. Their axes are oriented parallel to the muscle fibers in the circular and longitudinal layers. ICC-IM have gap junctions with each other or with smooth muscle cells. They make a coarse network in the muscle layers and associate with nerve fibers and terminals. ICC-IM are innervated preferentially by enteric motor nerves and act as mediators of neurotransmission [14, 53, 54]. A full description is presented below.
3. ICC-DMP: ICC in the deep muscular plexus layer of the small intestine
ICC-DMP are present in the small intestine, and distributed between the inner and outer parts of the circular muscle layer at the level of the deep muscular plexus (DMP). ICC-DMP are multipolar cells with thin processes along the nerve fibers in DMP. ICC-DMP have well-developed gap junctions with each other or with smooth muscle cells. Although ICC-DMP make a thin network in the narrow space of the DMP layer, those in the proximal duodenum make a three-dimensional complex with nerve fibers [18]. ICC-DMP receive preferential innervation and recent observations revealed ICC-DMP as one of the ICC-IM [55].
4. ICC-SM (ICC-SMP): ICC on the submucosal surface of the circular muscle layer
ICC-SM is distributed along the submucosal surface of the circular muscle layer of the gastric antrum and the colon. These cells have a multipolar shape with several processes and form their own network. ICC-SM have gap junctions with each other or with smooth muscle cells, and are thought to be pacemaker cells in the colon [30, 35, 42].
5. ICC-SS: ICC in the subserosal layer
ICC-SS are distributed in the subserosal layer of the colon [44, 45]. These cells have a multipolar shape with several processes and form their own network. The function of these cells is obscure.
III. Structural Relationship between ICC and Nerves
As mentioned above, ICC are distributed near nerve elements. Numerous ultrastructural studies have shown that enteric motor neurons are closely associated with both ICC-IM and ICC-DMP (Figs. 2, 3). In the stomach, nerve terminals which contain numerous small clear vesicles and large dense-cored vesicles often are in contact with ICC-IM. The close contact areas between the nerve terminals and ICC-IM often lack basal lamina and show 20 nm or less space. There are specialized cell membrane differentiations similar to the active zone of the presynaptic membrane and postsynaptic density of the postsynaptic membrane [1, 2, 16, 28, 47]. ICC-DMP also show close contact with nerve terminals containing accumulated synaptic vesicles [37].
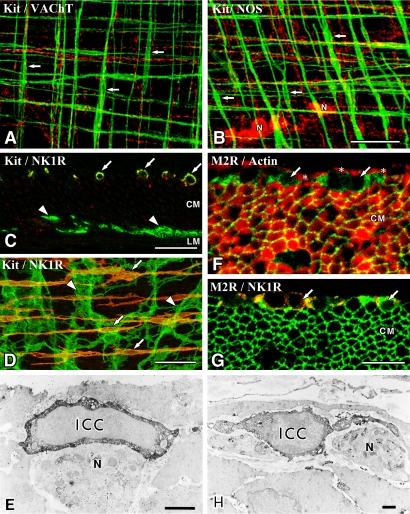
Fig. 3.
ICC are associated with nerve fibers and express receptors. A, B: ICC-IM (Kit immunoreactivity, green) in the murine fundus are associated with cholinergic nerve fibers containing VAChT (A, red) and nitrergic nerve fibers containing NOS (B, red). ICC-IM are aligned horizontally in the circular muscle layer and vertically in the longitudinal muscle layer (arrows). N shows NOS-containing nerve cell bodies in the myenteric ganglia. Whole mount preparations were imaged by confocal microscope. C–E: Neurokinin 1 receptor (NK1R) distribution in the murine small intestine. ICC-DMP (arrows in C and D) labeled with Kit antibody (green) specifically express NK1R (red) in the circular muscle layer (CM), whereas ICC-MY (arrowheads) are immunonegative for NK1R between circular and longitudinal (LM) muscle layers. Immunoelectron microscopy (E) reveals ICC-DMP (ICC) expressing NK1R and associated with nerve fibers (N) in the DMP. F–H: Muscarinic M2 receptors (M2R) distribution in the guinea pig small intestine. M2R (green, arrows) positive cells are observed between the inner circular (asterisks) and outer circular muscles (CM) shown by actin immunoreactivity (F, red). Smooth muscle cells in the outer circular muscle also showed M2R. ICC-DMP (arrows in G) contain both M2R (green) and NK1R (red) immunoreactivity in the musculature. Immunoelectron microscopy (H) shows that M2R-immunopositive cells (ICC) are typical ICC-DMP and associated with DMP (N). Bars=50 µm (A, B, D), 20 µm (C), 10 µm (F, G), 1 µm (E, H).
Double-labeling immunohistochemistry for antibodies against Kit and antibodies against vesicular acetylcholine transporter (VAChT), substance P or nitric oxide synthase (NOS) have been used to analyze the interaction between ICC and motor neurons. The primary transmitters of excitatory motor neurons which innervate circular and longitudinal muscles are acetylcholine and tachykinins. Therefore, most enteric excitatory nerves are detected by VAChT and substance P immunoreactivity [11]. Nerve fibers with VAChT and substance P are closely associated with the cell bodies and processes of ICC-IM in the stomach (Fig. 3) [1, 16, 39, 47, 52]. In the small intestine, ICC-DMP are heavily associated with nerve fibers containing VAChT and substance P (Fig. 3) [7, 19, 25, 47]. Inhibitory neurons have multiple transmitters, including nitric oxide (NO), vasoactive intestinal polypeptide (VIP) and adenosine triphosphate (ATP). Many inhibitory motor neurons contain NOS immunoreactivity and release NO as a relaxant to smooth muscle [11]. Nerve fibers containing NOS are closely associated with both cell bodies and processes of ICC-IM (Fig. 3) [1, 16, 39, 47, 52] and ICC-DMP [43, 47]. Transmission electron microscope studies clearly showed close relationships between ICC and enteric motor nerve terminals which contain VAChT, substance P or NOS [43, 47, 48, 52]. These data demonstrate that ICC-IM and ICC-DMP are densely innervated by excitatory and inhibitory enteric motor neurons.
IV. Functional Investigations of ICC and Neurotransmission
The structural proximity of ICC with enteric nerve terminals does not necessarily imply functional interaction between ICC and nerves. Structural interaction of smooth muscle cells and nerve terminals is also frequently observed in the gastrointestinal musculature [28]. To elucidate the functional relationship between ICC and nerves, we need to show evidence that ICC actually receive neural inputs via appropriate receptors and transduce transmitter signals to intracellular signals and events.
Molecular studies using reverse transcription-polymerase chain reaction (RT-PCR) from isolated murine ICC confirm the expression of neurotransmitter receptors on ICC. Both ICC-IM from the gastric fundus and ICC-MY from the small intestine expressed muscarinic acetylcholine receptors (M2 and M3 subtypes), neurokinin receptors (NK1 and NK3 subtypes) and VIP receptor (VPAC1 subtype) [6]. Immunohistochemical studies have clearly shown the expression of receptors in ICC subtypes. A well-studied receptor is neurokinin 1 (NK1) receptor (substance P receptor), expressed in the ICC-DMP of the small intestine (see review [7]). As far as we examined, all ICC-DMP in the murine small intestine showed NK1 receptor (Fig. 3) [19]. It has also been shown that ICC-DMP express muscarinic M2 receptor (Fig. 3) [21] and somatostatin 2A receptor [41]. Serotonin 5-HT3 receptor was expressed in both ICC-DMP and ICC-MY in the rat ileum [12]. Serotonin 5-HT4 receptor was detected in both ICC-DMP and ICC-MY in the murine small intestine [26]. Purinergic P2Y4 receptor was expressed in guinea pig ICC-IM, ICC-DMP and ICC-MY [46]. Purinergic P2X2 and P2X5 receptors were detected in ICC-MY of the guinea pig ileum [5]. Cholecystokinin A receptor was expressed in both ICC and smooth muscle cells in the rat pylorus [29]. Bombesin receptor subtype-3 was expressed in almost all ICC in the rat gastrointestinal tract [32]. G protein-coupled receptors, including neurotransmitter receptors, act via several protein kinases such as protein kinase A (PKA) and C (PKC). Indeed, ICC express PKA, PKCγ and PKCθ subtypes [31, 40].
Recent morphofunctional studies have revealed excitatory neurotransmission on ICC-DMP. In the murine ileum, almost all ICC-DMP were closely apposed to substance P containing nerve fibers and all ICC-DMP expressed NK1 receptor on their surface (Fig. 4) [19]. Electrical field stimulation (EFS) causes nerve activation and release neurotransmitters such as substance P in situ (Fig. 4). One minute EFS (10 Hz, 0.5 ms pulse duration) of the ileal musculature caused NK1 receptor internalization (receptor aggregation in the cytoplasm) in ICC-DMP (Fig. 4) [19], and the same responses could be induced by the administration of exogenous substance P to small intestinal muscles [19, 25]. NK1 receptor internalization was blocked by a specific NK1 receptor antagonist and by tetrodotoxin before nerve stimulation, suggesting that internalization resulted from stimulation of NK1 receptors with neurally released neurokinins such as substance P. Morphofunctional studies also confirmed that ICC were functionally innervated by cholinergic neurons in the small intestine. Molecular and immunohistochemical studies showed muscarinic receptors expression in ICC (Fig. 3) [6, 21]. EFS or acetylcholine exposure caused PKC-ɛ expression in ICC-DMP [49]. PKC-ɛ expression after stimulation was blocked by tetrodotoxin or the acetylcholine receptor antagonist atropine, suggesting that these responses were caused by the activation of muscarinic receptors on ICC-DMP.
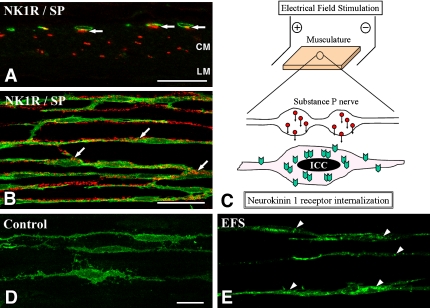
Fig. 4.
Neurokinin 1 receptor (NK1R) internalization of ICC-DMP in the murine small intestine. A, B: ICC-DMP expressing NK1R (green) are in close contact with substance P (red) containing nerve terminals (arrows). CM and LM indicate circular muscle and longitudinal muscle layers, respectively. C: Experimental scheme of electrical field stimulation (EFS). Musculature between the parallel platinum electrodes is stimulated by EFS (10 Hz, 0.5 ms duration, 15V) for 1 min. Stimulated nerve terminals release neurotransmitters such as substance P. ICC-DMP near the nerve terminals receive substance P and substance P-NK1R complexes are internalized into the cell bodies. D, E: NK1R (green) distribution in ICC-DMP before and after nerve stimulation. Before stimulation (D), NK1R are distributed around the peripheries of ICC-DMP. After EFS (E), NK1R are concentrated into granular structures (arrowheads) in ICC-DMP. Bars=20 µm (A, D, E), 50 µm (B).
NO from motor nerves is a potent inhibitory neurotransmitter that activates soluble guanylate cyclase (sGC) in the target cells. sGC is expressed in ICC-DMP of the rat small intestine [34] and almost all ICC in the guinea pig [20]. Activated sGC catalyses the conversion of GTP to cyclic GMP (cGMP), a second messenger in intracellular signaling cascades. Studies showing that cGMP content changes after nitrergic stimulation provide evidence that ICC are innervated functionally by nitrergic nerves. Levels of cGMP, detected with immunohistochemistry, were enhanced in ICC by enteric nerve stimulation using the EFS method and following exogenous application of an NO donor, sodium nitroprusside, in the canine colon [38], guinea-pig small intestine [57] and cecum [20]. Enhanced cGMP levels were blocked with the NO antagonist, L-nitroarginine. cGMP-dependent protein kinase I, which is activated by cGMP, was also expressed highly in ICC-DMP [34] in the rat small intestine and almost all ICC in the guinea pig (unpublished data). These observations show that ICC are functionally innervated by nitrergic inhibitory neurons.
V. ICC-deficient Mice Show Loss of Nerve Responses
Several spontaneous mutations of the Kit gene (W locus) have been characterized in detail in mice. Heterozygous W/Wv mutant mice have reduced Kit signaling and show anemia due to the depletion of erythrocytes, sterility due to lack of germ cells, a white coat due to depletion of melanocytes and depletion of mast cells [22]. These mutant mice have lost specific populations of ICC in the gastrointestinal tract [17, 50]. The absence of ICC-MY in the small intestine shows a loss of pacemaker activity in this tissue [17, 50]. The absence of ICC-IM in the stomach and in the lower esophageal and pyloric sphincters in mutant mice shows a defect of nerve responses [3, 39, 51, 52]. In control mice, stimulation of intrinsic nerves evoked a complex response consisting of cholinergic excitatory (excitatory junction potential, EJP) and nitrergic inhibitory components (inhibitory junction potential, IJP). In W/Wv mice, EJP and IJP after intrinsic nerve stimulation were greatly attenuated in these tissues [3, 39, 51, 52]. Since the muscles of W/Wv mice appear to have normal varicose terminals of enteric neurons at normal density, the absence of neural responses is considered due to the lack of ICC-IM. By inhibition of acetylcholine esterase using neostigmine, cholinergic transmission could be partly restored, leading us to conjecture that acetylcholine could diffuse to and bind directly to muscarinic receptors (Fig. 3) on smooth muscle cells [52]. Sl/Sld mutant mice, which have a mutation of the Steel gene encoding the Kit ligand stem cell factor (SCF), lack the ability to make membrane-bound SCF and to develop ICC-IM in the stomach [22]. As in W/Wv mice, Sl/Sld mice show a reduction of EFS-induced neural responses (EJP and IJP) in gastric muscles [1]. These observations using ICC-deficient mice indicate the functional relationship between ICC-IM and nerve terminals.
VI. ICC-IM and Vagal Afferent Nerves
ICC-IM are closely associated with not only enteric motor nerves but also vagal afferent nerves. Vagal afferent nerve fibers, labeled with the injection of neuronal tracers into the nodose ganglia, can terminate as intramuscular arrays (IMA) within the musculature and as intraganglionic laminar endings (IGLE) within the myenteric ganglia of the stomach and duodenum [8]. These afferent fibers transmit mechanoreceptive information from the muscle wall [8]. Murine IMA consisted of axonal arborization of various lengths and ran parallel to the long axis of and formed close appositions with ICC-IM [8]. In the stomach of W/Wv and Sl/Sld mutants, IMA numbers fell by 50 to 80% compared with wild mice [9, 10]. These data suggest that ICC-IM are important for the normal development and maintenance of IMA, and that they functionally interact with IMA.
VII. Conclusion
Considerable progress has been made in our understanding of the mechanisms that underlie ICC and neurotransmission. It has long been recognized from morphological studies that ICC-IM and ICC-DMP are densely and closely innervated. The importance of these observations became apparent when the responses to nerve stimulation were examined in W/Wv ICC-deficient mice, and recent molecular and morphofunctional studies directly revealed the neural inputs to ICC. ICC-IM and ICC-DMP play an important role in neurotransmission; however, there is also evidence of other functions such as the generation of ongoing spontaneous discharges known as unitary potentials [14] and stretch receptors that can influence pacemaker activity [56]. It is unclear how excitatory and inhibitory nerve responses in ICC are transferred to nearby smooth muscle cells. A simple explanation is the transfer of information through electrical conduction between ICC and smooth muscle cells [13]. ICC are also assumed to be sources of second messengers that diffuse into nearby smooth muscle cells or sources of secretory substances that regulate smooth muscle cells and neurons. Further advances combined with morphological, functional and molecular studies are required to answer these questions.
VIII. Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from Japan Society for Promotion of Science. We would like to thank Prof. Yoshiaki Nojyo and other members of the Department of Anatomy, University of Fukui, for their help and encouragement with our research, and also Drs Kenton M. Sanders and Sean M. Ward, Department of Physiology and Cell Biology, University of Nevada, for carrying out NK1 receptor research.
IX. References
- 1.Beckett E. A., Horiguchi K., Khoyi M., Sanders K. M., Ward S. M. Loss of enteric motor neurotransmission in the gastric fundus of Sl/Sld mice. J. Physiol. 2002;543:871–887. doi: 10.1113/jphysiol.2002.021915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckett E. A., Takeda Y., Yanase H., Sanders K. M., Ward S. M. Synaptic specializations exist between enteric motor nerves and interstitial cells of Cajal in the murine stomach. J. Comp. Neurol. 2005;493:193–206. doi: 10.1002/cne.20746. [DOI] [PubMed] [Google Scholar]
- 3.Burns A. J., Lomax A. E., Torihashi S., Sanders K. M., Ward S. M. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc. Natl. Acad. Sci. U S A. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns A. J., Herbert T. M., Ward S. M., Sanders K. M. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell Tissue Res. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G., Lavin S. Interstitial cells of Cajal and purinergic signalling. Auton. Neurosci. 2002;97:68–72. doi: 10.1016/s1566-0702(02)00005-x. [DOI] [PubMed] [Google Scholar]
- 6.Epperson A., Hatton W. J., Callaghan B., Doherty P., Walker R. L., Sanders K. M., Ward S. M., Horowitz B. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am. J. Physiol. Cell Physiol. 2000;279:C529–C539. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- 7.Faussone-Pellegrini M. S. Relationships between neurokinin receptor-expressing interstitial cells of Cajal and tachykininergic nerves in the gut. J. Cell Mol. Med. 2006;10:20–32. doi: 10.1111/j.1582-4934.2006.tb00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fox E. A., Phillips R. J., Martinson F. A., Baronowsky E. A., Powley T. L. Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography. J. Comp. Neurol. 2000;428:558–576. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Fox E. A., Phillips R. J., Martinson F. A., Baronowsky E. A., Powley T. L. C-Kit mutant mice have a selective loss of vagal intramuscular mechanoreceptors in the forestomach. Anat. Embryol. (Berl.) 2001;204:11–26. doi: 10.1007/s004290100184. [DOI] [PubMed] [Google Scholar]
- 10.Fox E. A., Phillips R. J., Byerly M. S., Baronowsky E. A., Chi M. M., Powley T. L. Selective loss of vagal intramuscular mechanoreceptors in mice mutant for steel factor, the c-Kit receptor ligand. Anat. Embryol. (Berl.) 2002;205:325–342. doi: 10.1007/s00429-002-0261-x. [DOI] [PubMed] [Google Scholar]
- 11.Furness J. B. The Enteric Nervous System. Blackwell; Malden, MA.: 2006. [Google Scholar]
- 12.Glatzle J., Sternini C., Robin C., Zittel T. T., Wong H., Reeve J. R., Jr, Raybould H. E. Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterology. 2002;123:217–226. doi: 10.1053/gast.2002.34245. [DOI] [PubMed] [Google Scholar]
- 13.Hanani M., Farrugia G., Komuro T. Intercellular coupling of interstitial cells of Cajal in the digestive tract. Int. Rev. Cytol. 2005;242:249–282. doi: 10.1016/S0074-7696(04)42006-3. [DOI] [PubMed] [Google Scholar]
- 14.Hirst G. D. S., Ward S. M. Interstitial cells: involvement in rhythmicity and neural control of gut smooth muscle. J. Physiol. 2003;550:337–346. doi: 10.1113/jphysiol.2003.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirst G. D. S., Edwards F. R. Role of interstitial cells of Cajal in the control of gastric motility. J. Pharmacol. Sci. 2004;96:1–10. doi: 10.1254/jphs.crj04002x. [DOI] [PubMed] [Google Scholar]
- 16.Horiguchi K., Sanders K. M., Ward S. M. Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell Tissue Res. 2003;311:299–313. doi: 10.1007/s00441-002-0657-1. [DOI] [PubMed] [Google Scholar]
- 17.Huizinga J. D., Thuneberg L., Kluppel M., Malysz J., Mikkelsen H. B., Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 18.Iino S. Muscular innervation of the proximal duodenum of the guinea pig. Arch. Histol. Cytol. 2000;63:327–343. doi: 10.1679/aohc.63.327. [DOI] [PubMed] [Google Scholar]
- 19.Iino S., Ward S. M., Sanders K. M. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J. Physiol. 2004;556:521–530. doi: 10.1113/jphysiol.2003.058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iino S., Horiguchi K., Nojyo Y. Interstitial cells of Cajal in nitric oxide neurotransmission. Auton. Neurosci. 2005;119:147. [Google Scholar]
- 21.Iino S., Nojyo Y. Muscarinic M2 acetylcholine receptor distribution in the guinea-pig gastrointestinal tract. Neuroscience. 2006;138:549–559. doi: 10.1016/j.neuroscience.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura Y., Hirota S., Nishida T. A loss-of-function mutation of c-kit results in depletion of mast cells and interstitial cells of Cajal, while its gain-of-function mutation results in their oncogenesis. Mutat. Res. 2001;477:165–171. doi: 10.1016/s0027-5107(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 23.Komuro T. Comparative morphology of interstitial cells of Cajal: ultrastructural characterization. Microsc. Res. Tech. 1999;47:267–285. doi: 10.1002/(SICI)1097-0029(19991115)47:4<267::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 24.Komuro T., Seki K., Horiguchi K. Ultrastructural characterization of the interstitial cells of Cajal. Arch. Histol. Cytol. 1999;62:295–316. doi: 10.1679/aohc.62.295. [DOI] [PubMed] [Google Scholar]
- 25.Lavin S. T., Southwell B. R., Murphy R., Jenkinson K. M., Furness J. B. Activation of neurokinin 1 receptors on interstitial cells of Cajal of the guinea-pig small intestine by substance P. Histochem. Cell Biol. 1998;110:263–271. doi: 10.1007/s004180050288. [DOI] [PubMed] [Google Scholar]
- 26.Liu M., Geddis M. S., Wen Y., Setlik W., Gershon M. D. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G1148–G1163. doi: 10.1152/ajpgi.00245.2005. [DOI] [PubMed] [Google Scholar]
- 27.Matsuyama A., Kamoshida S., Mizoguchi Y., Shimomura R., Hirasawa Y., Inada K., Tsutsumi Y. Appropriate epitope retrieval for c-kit protein immunostaining in routinely prepared specimens of gastrointestinal stromal tumor. Acta Histochem. Cytochem. 2004;37:87–93. [Google Scholar]
- 28.Mitsui R., Komuro T. Direct and indirect innervation of smooth muscle cells of rat stomach, with special reference to the interstitial cells of Cajal. Cell Tissue Res. 2002;309:219–227. doi: 10.1007/s00441-002-0592-1. [DOI] [PubMed] [Google Scholar]
- 29.Patterson L. M., Zheng H., Ward S. M., Berthoud H. R. Immunohistochemical identification of cholecystokinin A receptors on interstitial cells of Cajal, smooth muscle, and enteric neurons in rat pylorus. Cell Tissue Res. 2001;305:11–23. doi: 10.1007/s004410100402. [DOI] [PubMed] [Google Scholar]
- 30.Pluja L., Alberti E., Fernandez E., Mikkelsen H. B., Thuneberg L., Jimenez M. Evidence supporting presence of two pacemakers in rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G255–G266. doi: 10.1152/ajpgi.2001.281.1.G255. [DOI] [PubMed] [Google Scholar]
- 31.Poole D. P., Van Nguyen T., Kawai M., Furness J. B. Protein kinases expressed by interstitial cells of Cajal. Histochem. Cell Biol. 2004;121:21–30. doi: 10.1007/s00418-003-0602-8. [DOI] [PubMed] [Google Scholar]
- 32.Porcher C., Juhem A., Peinnequin A., Bonaz B. Bombesin receptor subtype-3 is expressed by the enteric nervous system and by interstitial cells of Cajal in the rat gastrointestinal tract. Cell Tissue Res. 2005;320:21–31. doi: 10.1007/s00441-004-1032-1. [DOI] [PubMed] [Google Scholar]
- 33.Rumessen J. J., Vanderwinden J. M. Interstitial cells in the musculature of the gastrointestinal tract: Cajal and beyond. Int. Rev. Cytol. 2003;229:115–208. doi: 10.1016/s0074-7696(03)29004-5. [DOI] [PubMed] [Google Scholar]
- 34.Salmhofer H., Neuhuber W. L., Ruth P., Huber A., Russwurm M., Allescher H. D. Pivotal role of the interstitial cells of Cajal in the nitric oxide signaling pathway of rat small intestine. Morphological evidence. Cell Tissue Res. 2001;305:331–340. doi: 10.1007/s004410100410. [DOI] [PubMed] [Google Scholar]
- 35.Sanders K. M. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- 36.Sanders K. M., Koh S. D., Ordog T., Ward S. M. Ionic conductances involved in generation and propagation of electrical slow waves in phasic gastrointestinal muscles. Neurogastroenterol. Motil. 2004;16(Suppl 1):100–105. doi: 10.1111/j.1743-3150.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 37.Seki K., Komuro T. Further observations on the gap-junction-rich cells in the deep muscular plexus of the rat small intestine. Anat. Embryol. (Berl.) 1998;197:135–141. doi: 10.1007/s004290050125. [DOI] [PubMed] [Google Scholar]
- 38.Shuttleworth C. W., Xue C., Ward S. M., de Vente J., Sanders K. M. Immunohistochemical localization of 3',5'-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience. 1993;56:513–522. doi: 10.1016/0306-4522(93)90350-o. [DOI] [PubMed] [Google Scholar]
- 39.Song G., Hirst G. D. S., Sanders K. M., Ward S. M. Regional variation in ICC distribution, pacemaking activity and neural responses in the longitudinal muscle of the murine stomach. J. Physiol. 2005;564:523–540. doi: 10.1113/jphysiol.2004.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Southwell B. R. Localization of protein kinase C theta immunoreactivity to interstitial cells of Cajal in guinea-pig gastrointestinal tract. Neurogastroenterol. Motil. 2003;15:139–147. doi: 10.1046/j.1365-2982.2003.00394.x. [DOI] [PubMed] [Google Scholar]
- 41.Sternini C., Wong H., Wu S. V., de Giorgio R., Yang M., Reeve J., Jr, Brecha N. C., Walsh J. H. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J. Comp. Neurol. 1997;386:396–408. [PubMed] [Google Scholar]
- 42.Takaki M. Gut pacemaker cells: the interstitial cells of Cajal (ICC) J. Smooth Muscle Res. 2003;39:137–161. doi: 10.1540/jsmr.39.137. [DOI] [PubMed] [Google Scholar]
- 43.Toma H., Nakamura K., Emson P. C., Kawabuchi M. Immunohistochemical distribution of c-Kit-positive cells and nitric oxide synthase-positive nerves in the guinea-pig small intestine. J. Auton. Nerv. Syst. 1999;75:93–99. doi: 10.1016/s0165-1838(98)00167-2. [DOI] [PubMed] [Google Scholar]
- 44.Toma H., Nakamura K., Kuraoka A., Tanaka M., Kawabuchi M. Three-dimensional structures of c-Kit-positive cellular networks in the guinea pig small intestine and colon. Cell Tissue Res. 1999;295:425–436. doi: 10.1007/s004410051249. [DOI] [PubMed] [Google Scholar]
- 45.Vanderwinden J. M., Rumessen J. J., Bernex F., Schiffmann S. N., Panthier J. J. Distribution and ultrastructure of interstitial cells of Cajal in the mouse colon, using antibodies to Kit and KitW-lacZ mice. Cell Tissue Res. 2000;302:155–170. doi: 10.1007/s004419900170. [DOI] [PubMed] [Google Scholar]
- 46.Van Nassauw L., Costagliola A., Van Op den Bosch J., Cecio A., Vanderwinden J. M., Burnstock G., Timmermans J. P. Region-specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton. Neurosci. 2006;126–127:299–306. doi: 10.1016/j.autneu.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 47.Wang X. Y., Sanders K. M., Ward S. M. Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res. 1999;295:247–256. doi: 10.1007/s004410051231. [DOI] [PubMed] [Google Scholar]
- 48.Wang X. Y., Sanders K. M., Ward S. M. Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell Tissue Res. 2000;302:331–342. doi: 10.1007/s004410000272. [DOI] [PubMed] [Google Scholar]
- 49.Wang X. Y., Ward S. M., Gerthoffer W. T., Sanders K. M. PKC-ɛ translocation in enteric neurons and interstitial cells of Cajal in response to muscarinic stimulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G593–G601. doi: 10.1152/ajpgi.00421.2002. [DOI] [PubMed] [Google Scholar]
- 50.Ward S. M., Burns A. J., Torihashi S., Sanders K. M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J. Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward S. M., Morris G., Reese L., Wang X. Y., Sanders K. M. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 52.Ward S. M., Beckett E. A., Wang X., Baker F., Khoyi M., Sanders K. M. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J. Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward S. M., Sanders K. M. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat. Rec. 2001;262:125–135. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 54.Ward S. M., Sanders K. M., Hirst G. D. S. Role of interstitial cells of Cajal in neural control of gastrointestinal smooth muscles. Neurogastroenterol. Motil. 2004;16(Suppl 1):112–117. doi: 10.1111/j.1743-3150.2004.00485.x. [DOI] [PubMed] [Google Scholar]
- 55.Ward S. M., McLaren G. J., Sanders K. M. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J. Physiol. 2006;573:147–159. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Won K. J., Sanders K. M., Ward S. M. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc. Natl. Acad. Sci. U S A. 2005;102:14913–14918. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Young H. M., McConalogue K., Furness J. B., De Vente J. Nitric oxide targets in the guinea-pig intestine identified by induction of cyclic GMP immunoreactivity. Neuroscience. 1993;55:583–596. doi: 10.1016/0306-4522(93)90526-l. [DOI] [PubMed] [Google Scholar]