Abstract
Plasmodium yoelii is a rodent parasite commonly used as a model to study malaria infection. It is the preferred model parasite for liver-stage immunological studies and is also widely used to study hepatocyte, erythrocyte and mosquito infection. We have generated a P. yoelii yoelii 17XNL line that is stably transfected with the green fluorescent protein (gfp) gene. This parasite line constitutively expresses high levels of GFP during the complete parasite life cycle including liver, blood and mosquito stages. These fluorescent parasites can be used in combination with fluorescence activated cell sorting or live microscopy for a wide range of experimental applications.
Keywords: Plasmodium yoelii, transfection, malaria, green fluorescent protein
Malaria is caused by infection with a parasitic protozoa of the genus Plasmodium which causes millions of deaths each year. Currently, efforts in understanding the basic biology of this parasite have been greatly facilitated by the availability of the genome sequences of different parasite species that in combination with targeted gene disruption have allowed the identification of many individual gene functions (de Koning-Ward et al., 2000).
Four species of rodent malaria (Plasmodium yoelii, Plasmodium berghei, Plasmodium chabaudi and Plasmodium vinckei) have been adapted to grow in laboratory rodents (Carter and Diggs, 1977). These species reproduce many of the biological characteristics of the human malaria parasite and have allowed the development of many experimental procedures later adapted for use with P. falciparum, a prime example being stable genetic transformation (van Dijk et al., 1995).
Gene targeting in rodent Plasmodium parasites was first developed for P. berghei (Menard et al., 1997), and has allowed the rapid identification of protein functions in different stages of the life cycle of the parasite. P. yoelii is another commonly used rodent malaria model parasite. It presents numerous similarities to human P. falciparum and P. vivax and is extensively used to study on the biology of liver stage and blood stage antigens and their role in immunity and vaccine development (Carlton et al., 2005). P. yoelii sporozoites are presumed to be a better experimental model for the human infection at this stage (Silvie et al., 2003).
The generation of GFP-expressing P. berghei parasite lines (Natarajan et al., 2001; Franke-Fayard et al., 2004) has made possible the understanding of parasite basic biological processes and facilitated difficult experimental approaches. In recent years, numerous studies have used these parasites to understand Plasmodium infection of liver, blood and mosquito (Frischknecht et al., 2004; Vanderberg and Frevert, 2004; Vlachou et al., 2004; Franke-Fayard et al., 2005; Frevert et al., 2005). Since P. yoelii gene targeting is now available (Mota et al., 2001), we report the generation of a GFP-expressing P. yoelii yoelii 17X non-lethal (NL) parasite line (PyGFP) that is highly fluorescent during the complete life cycle of the parasite and constitutes a useful tool for diverse types of studies dealing with all life stages of this parasite.
The generation of PyGFP parasites was performed by transfection of an uncloned population of P. yoelii yoelii 17XNL parasites with a vector (pL0016 (Franke-Fayard et al., 2004), provided by Dr. Waters, Leiden University, The Netherlands) containing a genome fragment of the small subunit of ribosomal RNA genes (c-ssurrna) of P. berghei (Franke-Fayard et al., 2004). Since the sequence of the c-ssurrna is 96% identical to the one of P. yoelii, this vector can integrate into the c-rrna gene unit of P. yoelii. This vector also contains the pyrimethamine-resistant form of T. gondii (tgdhfr/ts) selection cassette and the gfp gene under the control of the pbef1αa promoter. The detailed structure of this transformation vector and the targeting strategy have been described before for P. berghei and results in the integration of one copy of the gfp gene by single cross over into the parasite genome (Franke-Fayard et al., 2004).
Linearized vector containing the gfp gene was introduced into the blood stages of P. yoelii using the recently developed non-viral Nucleofector technology (Janse et al., 2006). Blood was collected from five P. yoelii infected Swiss Webster mice and incubated for 8 h before purification of schizonts as described (Mota et al., 2001). 107 schizonts were mixed with 100μl of Nucleofector Solutions containing 7 μg of ScaI/SacII digested vector. After transfection, 150 μl of complete medium (Waters et al., 1997) was added and the solution is injected intravenously into a single mouse. Starting 26 h post-inoculation, mice were treated with 5 mg/kg of pyrimethamine intraperitoneally for 3 days. Pyrimethamine stock solution was made in DMSO (25 mg/ml) and subsequently diluted 20 times in PBS before injection of 100 μl into mice (final DMSO concentration injected was 5%, which is not toxic to mice). Parasitemia was still detected at this time (0.01%). A pyrimethamine-resistant, fluorescent population expanded in the following days without drug treatment. This parasite population was transferred to new mice and collected for further analysis. We obtained a P. yoelii population containing both wild type and transgenic parasites with integrated vector, as determined by PCR analysis (Fig. 1). This population was cloned as described (Franke-Fayard et al., 2004) and 8 clones were analyzed for correct integration of the vector by PCR. One clone with correct vector integration as determined by PCR was selected (Fig. 1).
Fig. 1.
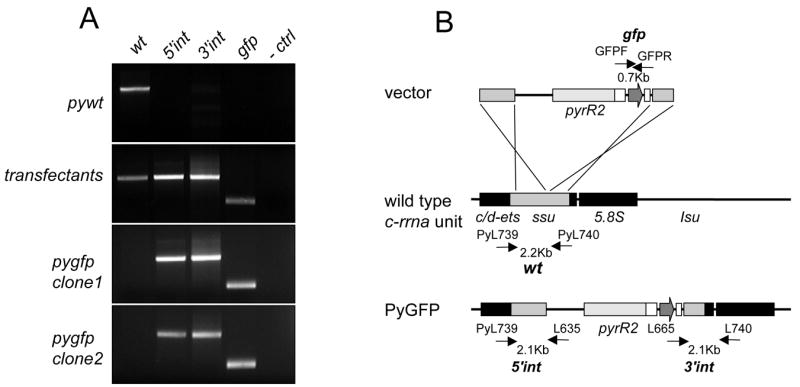
Generation of P. yoelii parasite mutant lines that express GFP. (A) Correct integration of the vector in PyGFP as shown by PCR. Lane 1: Detection of DNA of wild type (Pywt) parasites (primers PyL739 (5'-ATGTAATATTTGGATATTTC) and PyL740 (5'-TCACCTACGGAAACCTTGTTAC)); lane 2: verification of PyGFP 5' integration site (primers PyL739/L635(de Koning-Ward et al., 1998)); lane 3: verification of PyGFP 3' integration site (primers L665(de Koning-Ward et al., 1998)/PyL740); lane 4: amplification of gfpmut3 (primers GFPFNh (5'-GCTAGCAGTAAAGGAGAAGAACTTTTCA)/GFPRNh (5'-TCGCTAGCTTTGTATAGTTCATCCATGC); lane 5: negative control. (B) Schematic representation of integrated pL0016 vector into the c-rrna unit (Franke-Fayard et al., 2004).
Swiss Webster mice were infected with the cloned parasite, which developed blood stage infection with growth characteristics comparable to wild type parasites (parasitemia of 18% was reached after 12 days after infection with 1x106 infected erythrocytes). Microscopic analysis of the blood from these mice showed a number of brightly green fluorescent erythrocytes (Fig. 2A). We found co-localization of green fluorescence with P. yoelii-infected erythrocytes as determined using a DNA dye (Fig. 2A). Analysis of these parasites by flow cytometry showed that infected erythrocytes can be easily differentiated using this technique (Fig. 3). Also the percentage of GFP-positive fraction was correlated to parasitemia (data not shown).
Fig. 2.
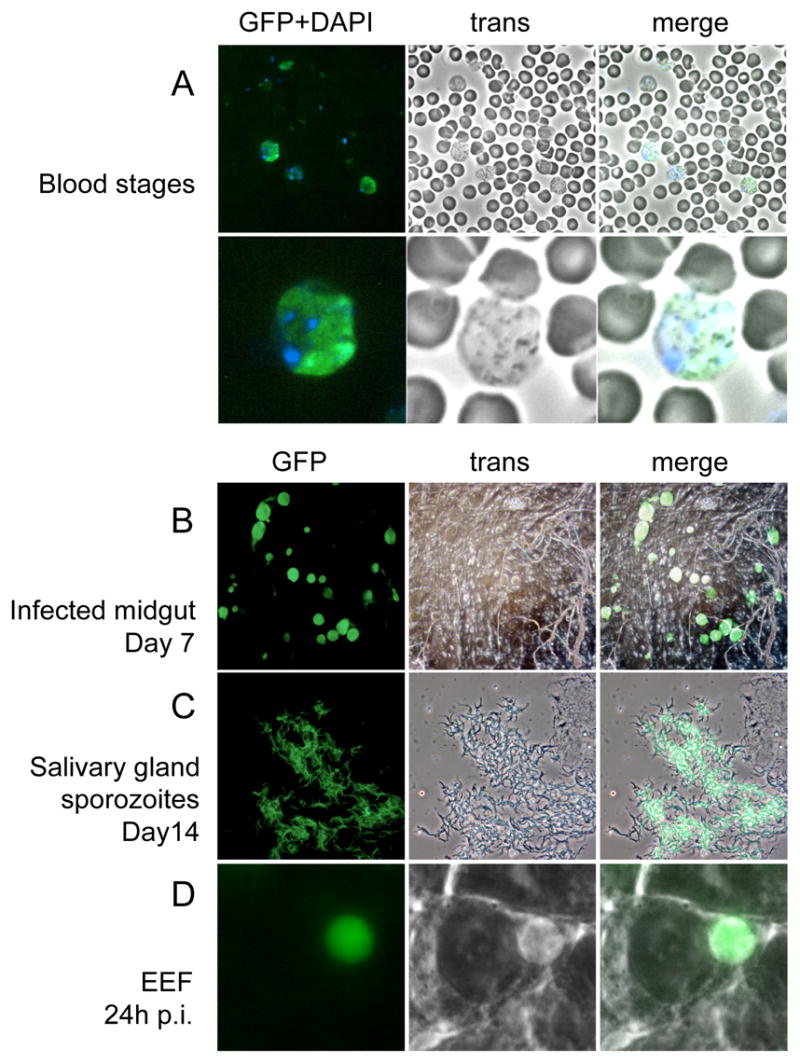
Fluorescence of PyGFP in different life stages. (A) blood stage parasites stained with DAPI, lower panel shows higher magnification; (B) infected midgut at day 7 post feeding; (C) sporozoites in salivary gland; (D) mature liver stage 24 h post hepatocyte invasion. Fluorescence, bright field and merged images of the same microscopic field are shown in each line.
Fig. 3.
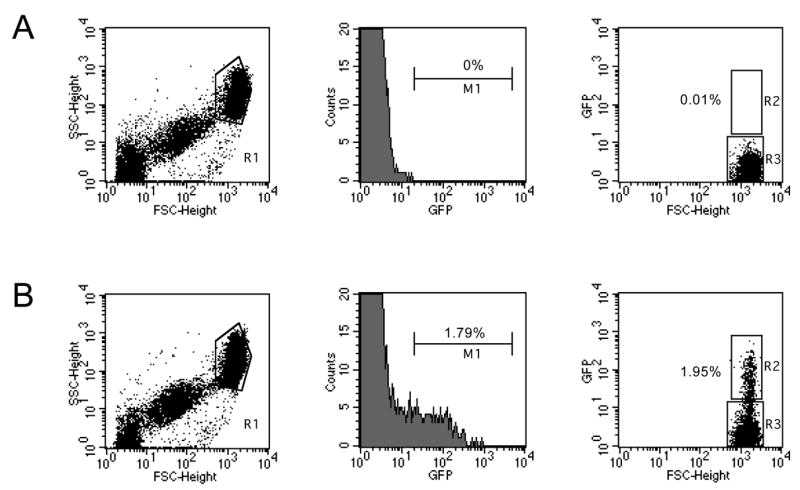
Flow cytometry analysis of fluorescence intensity of live blood stage PyGFP. Blood from infected mice was collected in heparin (400 U/ml) and washed with PBS before resuspension in buffer (PBS 3% FBS). For each sample, 105 cells were analyzed using FACScalibur and Cell Quest software (Becton Dickinson). Dot plot representations of blood cells obtained from wild type (A) or PyGFP (B) infected mice with asynchronous infection. The size analysis (forward (FCS)- and side (SSC)-scatter) shows three populations typical of mouse microplatelets (left population), platelets (central population) and erythrocytes (R1) as described (Chen et al., 2003). The center panel shows the relative GFP-fluorescence intensity of cells in R1. Fluorescent erythrocytes are selected in region M1. The right panel shows the dot plot of fluorescence intensity versus size of cells in R1. Fluorescent erythrocytes are selected in R2, non-fluorescent in R3.
Anopheles stephensi mosquitoes were used to bite mice infected with this cloned parasite to analyze oocyst development. Seven days after the bite, oocyst development in mosquitoes was similar to wt in number and size. The oocysts were brightly fluorescent (Fig. 2B). Analysis of sporozoites, 14 days after mosquito bite, also showed high numbers of fluorescent sporozoites in the salivary glands (Fig. 2C). Microscopic analysis of oocyst and sporozoite forms showed that 100% of the parasites analyzed were brightly fluorescent. Sporozoites were also used to infect hepatoma cells in vitro. We observed normal development of fluorescent exo-erythrocytic forms at 24 (Fig. 2D) and 48 h (not shown).
PyGFP-infected mosquitoes were used to bite uninfected mice. Blood stage infection in these mice developed at the same time as wt parasites, confirming that PyGFP parasites have normal growth properties and complete the life cycle. PyGFP have been deposited at the Malaria Research and Reference Reagent Resource Center (MR4).
Acknowledgments
this work was supported by NIH grant 5R01AI053698 to A.R. We thank Dabeiba Bernal for help with the production of sporozoites.
References
- Carlton J, Silva J, Hall N. The genome of model malaria parasites, and comparative genomics. Current Issues in Molecular Biology. 2005;7:23–37. [PubMed] [Google Scholar]
- Carter B, Diggs CL. Parasitic Protozoa. Academic Press; New York: 1977. [Google Scholar]
- Chen Y, Davis-Gorman G, Watson RR, McDonagh PF. Platelet CD62p expression and microparticle in murine acquired immune deficiency syndrome and chronic ethanol consumption. Alcohol and Alcoholism. 2003;38:25–30. doi: 10.1093/alcalc/agg013. [DOI] [PubMed] [Google Scholar]
- de Koning-Ward TF, Thomas AW, Waters AP, Janse CJ. Stable expression of green fluorescent protein in blood and mosquito stages of Plasmodium berghei. Molecular and Biochemical Parasitology. 1998;97:247–252. doi: 10.1016/s0166-6851(98)00134-0. [DOI] [PubMed] [Google Scholar]
- de Koning-Ward TF, Janse CJ, Waters AP. The development of genetic tools for dissecting the biology of malaria parasites. Annual Reviews of Microbiology. 2000;54:157–185. doi: 10.1146/annurev.micro.54.1.157. [DOI] [PubMed] [Google Scholar]
- Franke-Fayard B, Trueman H, Ramesar J, Mendoza J, van der Keur M, van der Linden R, Sinden RE, Waters AP, Janse CJ. A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Molecular and Biochemical Parasitology. 2004;137:23–33. doi: 10.1016/j.molbiopara.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Franke-Fayard B, Janse CJ, Cunha-Rodrigues M, Ramesar J, Buscher P, Que I, Lowik C, Voshol PJ, den Boer MA, van Duinen SG, Febbraio M, Mota MM, Waters AP. Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proceedings of the National Academy of Sciences U S A. 2005;102:11468–11473. doi: 10.1073/pnas.0503386102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frevert U, Engelmann S, Zougbede S, Stange J, Ng B, Matuschewski K, Liebes L, Yee H. Intravital observation of Plasmodium berghei sporozoite infection of the liver. Public Library of Science Biology. 2005;3:e192. doi: 10.1371/journal.pbio.0030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischknecht F, Baldacci P, Martin B, Zimmer C, Thiberge S, Olivo-Marin JC, Shorte SL, Menard R. Imaging movement of malaria parasites during transmission by Anopheles mosquitoes. Cellular Microbiology. 2004;6:687–694. doi: 10.1111/j.1462-5822.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- Janse CJ, Franke-Fayard B, Mair GR, Ramesar J, Thiel C, Engelmann S, Matuschewski K, Gemert GJ, Sauerwein RW, Waters AP. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Molecular and Biochemical Parasitology. 2006;145:60–70. doi: 10.1016/j.molbiopara.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Menard R, Sultan AA, Cortes C, Altszuler R, van Dijk MR, Janse CJ, Waters AP, Nussenzweig RS, Nussenzweig V. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature. 1997;385:336–340. doi: 10.1038/385336a0. [DOI] [PubMed] [Google Scholar]
- Mota MM, Thathy V, Nussenzweig RS, Nussenzweig V. Gene targeting in the rodent malaria parasite Plasmodium yoelii. Molecular and Biochemical Parasitology. 2001;113:271–278. doi: 10.1016/s0166-6851(01)00228-6. [DOI] [PubMed] [Google Scholar]
- Natarajan R, Thathy V, Mota MM, Hafalla JC, Menard R, Vernick KD. Fluorescent Plasmodium berghei sporozoites and pre-erythrocytic stages: a new tool to study mosquito and mammalian host interactions with malaria parasites. Cellular Microbiology. 2001;3:371–379. doi: 10.1046/j.1462-5822.2001.00117.x. [DOI] [PubMed] [Google Scholar]
- Silvie O, Rubinstein E, Franetich JF, Prenant M, Belnoue E, Renia L, Hannoun L, Eling W, Levy S, Boucheix C, Mazier D. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nature Medicine. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- van Dijk MR, Waters AP, Janse CJ. Stable transfection of malaria parasite blood stages. Science. 1995;268:1358–1362. doi: 10.1126/science.7761856. [DOI] [PubMed] [Google Scholar]
- Vanderberg JP, Frevert U. Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes. International Journal of Parasitology. 2004;34:991–996. doi: 10.1016/j.ijpara.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Vlachou D, Zimmermann T, Cantera R, Janse CJ, Waters AP, Kafatos FC. Real-time, in vivo analysis of malaria ookinete locomotion and mosquito midgut invasion. Cellular Microbiology. 2004;6:671–685. doi: 10.1111/j.1462-5822.2004.00394.x. [DOI] [PubMed] [Google Scholar]
- Waters AP, Thomas AW, van Dijk MR, Janse CJ. Transfection of malaria parasites. Methods. 1997;13:134–147. doi: 10.1006/meth.1997.0506. [DOI] [PubMed] [Google Scholar]


