Abstract
The extracellular signal-regulated kinases (ERK) signalling cascade is a key pathway that mediates the NMDA receptor (NMDAR)-dependent neuronal plasticity and survival. However, it is not clear yet how NMDARs regulate ERK activity. Stimulation of the NMDARs induces a complex modification of ERK that includes both ERK activation and inactivation and depends on particular experimental conditions. Here we show that there exists a differential restriction in the regulation of ERK activity that depends on the pool of NMDAR that was activated. The synaptic pool of NMDARs activates ERK whereas the extrasynaptic pool does not; on the contrary, it triggers a signalling pathway that results in the inactivation of ERK. As a result, simultaneous activation of both extrasynaptic and synaptic NMDAR using bath application of NMDA or glutamate (a typical protocol explored in the majority of studies) produced ERK activation that depended on the concentration of agonists and was always significantly weaker than those mediated by synaptic NMDARs. Since the activation of the extrasynaptic NMDA is attributed mainly to global release of glutamate occurring at pathological conditions including hypoxic/ischaemic insults, traumas and epileptic brain damage, the reported differential regulation of ERK cascade by NMDARs provides a unique mechanism for an early identification of the physiological and/or pathophysiological consequences of NMDAR activation. The negative regulation of the ERK activity might be one of the first signalling events determining brain injury and constitutes a putative target of new pharmacological applications.
The NMDA subtype of glutamate receptors (NMDAR) is a key receptor involved in the regulation of multiple processes related to synaptic plasticity including learning, memory, neuron development, spine formation, long-term potentiation (LTP) and long-term depression (LTD). The mechanisms underlying such diversity of neuronal responses to the activation of a single receptor are not known. At least some of these processes are associated with the NMDA-dependent activation of the extracellular signal-regulated kinases ERK1 and ERK2 (ERK) signalling cascade whose inhibition modifies spine formation, long-term memory, LTP and cell survival (Adams & Sweatt, 2002; Hardingham & Bading, 2003; Goldin & Segal, 2003; Thomas & Huganir, 2004). Stimulation of NMDA receptors by specific agonists (Bading & Greenberg, 1991; Kurino et al. 1995) or via an increase of synaptic activity (Hardingham et al. 2001) results in strong ERK phosphorylation. On the other hand, applications of the high concentrations of NMDA (70–100 μm) to neuronal cultures provokes a complex effect; it induces both activation and inactivation of ERK (Chandler et al. 2001; Kim et al. 2005). Although different suggestions were put forward to explain the complex effects of NMDAR agonists on ERK activity (Chandler et al. 2001; Kim et al. 2005), none of them provided compelling evidence supporting their hypothesis.
Recently we have found that the ERK cascade is brought tightly to the NMDAR by direct interaction between the calcium–calmodulin-regulated guanine-nucleotide exchange factor RasGRF1 and NR2B but not the NR2A subunits of NMDAR (Krapivinsky et al. 2003). Unlike the NR2A subunit that is targeted preferentially to synapses (but see Thomas et al. 2006), the NR2B subunit is expressed in both extrasynaptic and synaptic membrane (Li et al. 1998; Tovar & Westbrook, 1999; Cull-Candy et al. 2001; Thomas et al. 2006). Recent experiments suggest that synaptic and extrasynaptic receptors may have distinct roles in synaptic plasticity, gene regulation and cell death (Lu et al. 2001; Hardingham et al. 2002). Since the ERK signalling cascade is involved in both synaptic plasticity and in the control of transcription factor activity (Thomas & Huganir, 2004), understanding if this signalling cascade is regulated differently by synaptic and extrasynaptic NMDARs is an important step in the characterization of the molecular mechanisms, controlling diverse, often opposite, NMDA-dependent physiological responses.
Here we show that in cultured hippocampal neurons, despite an abundant expression of the NMDAR in the extrasynaptic membrane, the ERK cascade is activated exclusively via synaptic NMDAR. Stimulation of the extrasynaptic NMDAR does not induce activation of the ERK, moreover it evokes ERK inactivation. Together with previous findings, our data suggest that NMDARs, containing the NR2B subunit, exert a dual role in the regulation of ERK based on their localization; synaptic receptors activate ERK whereas the extrasynaptic ones control ERK inactivation.
Methods
Primary cultures of rat hippocampal neurons
Neurons from 18 day rat embryos were dissociated using trypsin and plated on coverslips coated with poly l-lysine at a density of 70 000 cells cm−2 in minimal essential medium (MEM) supplemented with 10% NU serum (BD Biosciences, Le Pont de Claix, France), 0.8% glucose, 1 mm sodium pyruvate, 2 mm glutamine, and 10 IU ml−1 penicillin–streptomycin as previously described (Krapivinsky et al. 2003). On days 7, 10 and 13 of culture incubation, one-half of the medium was changed to MEM with 2% B27 supplement (Invitrogen).
ERK activation and immunocytochemistry of cultured hippocampal neurons
Phospho-ERK immunocytochemistry
Twenty-four hours before stimulation (13 days in vitro (DIV)) one-half of the culture medium was changed to MEM with 2% B27 supplement. Three hours before stimulation, TTX (1 μm), CNQX (40 μm), D-AP5 (100 μm) and nifedipine (5 μm) were added to neurons. We gave special attention to the standardizing of the neuronal densities and maintenance of culturing conditions since any change of any of the parameters affected NMDAR-dependent ERK activation. For example, an increase of neuronal density led to a significant decrease of ERK responses induced by bath application of NMDA for more than 5 min (see Supplementary Fig. 1A). Omission of the 3 h preincubation step resulted in a high variability of basal ERK activity in control conditions and a decrease of the number of neurons showing ERK activation in response to applications of bicuculline or NMDA (see Supplementary Fig. 1B and C).
In experiments with no or weak effects of agonists or antagonists of NMDAR on ERK phosphorylation we routinely double-checked the effectiveness of the used solutions using whole-cell patch clamp recordings.
To activate the synaptic NMDAR (‘synaptic protocol’), neurons were first briefly plunged into the culture medium containing 10 μM bicuculline, 10 μM glycine, 5 μM nifedipine in order to remove traces of TTX, D-AP5 and CNQX, then transferred into wells containing the same solution and were incubated at 37°C for the desired time.
To activate the extrasynaptic NMDAR (‘extrasynaptic protocol’), we rinsed the neurons with the culture medium containing 50 μm MK-801, 5 μm nifedipine and then transferred them for 2 min into the medium containing 50 μm MK-801, 10 μm bicuculline, 10 μm glycine and 5 μm nifedipine in order to block the synaptic NMDAR. Thereafter, we briefly rinsed the coverslips with the culture medium containing 1 μm TTX, 40 μm CNQX and 5 μm nifedipine, and transferred them to the medium containing 10 μm NMDA, 10 μm glycine, 1 μm TTX, 40 μm CNQX and 5 μm nifedipine for the desired time.
To activate the entire population of NMDAR (‘entire NMDAR protocol’), we rinsed the neurons with the culture medium containing 1 μm TTX, 40 μm CNQX and 5 μm nifedipine, and transferred them to the medium containing the agonist of the NMDAR (NMDA or glutamate), 10 μm glycine, 1 μm TTX, 40 μm CNQX and 5 μm nifedipine. To activate the entire population of NMDARs in the absence of Mg2+ we rinsed and incubated neurons in the presence of the same cocktails of agonists and antagonists that were dissolved in the extracellular solution containing 140 mm NaCl, 2.5 mm KCl, 10 mm HEPES, 10 mmd-glucose, 2.0 mm CaCl2. MgCl2 (2 mm) was applied as mentioned.
After stimulation, the neurons were fixed with 4% formaldehyde, permeabilized with 0.3% Triton X-100, blocked by 10% goat serum and labelled with rabbit antiphospho-p44/42 ERK (P-ERK) antibody (Cell Signalling) and with mouse anti-MAP2 (Sigma). Cy3-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, USA) and Alexa 488-conjugated goat anti-mouse IgG (Invitrogen) were used as secondary antibodies. Neuron permeabilization, block, overnight staining (4°C) with primary antibodies and staining with secondary antibodies were performed in a phosphate-free solution containing 140 mm NaCl, 5 mm KCl and 10 mm Hepes-Na, pH 7.4.
To confirm that the results obtained in the present study are comparable with results reported by others, we also checked the effects of the activation of synaptic/extrasynaptic NMDARs on properties of miniature postsynaptic currents of AMPA receptors (mEPSCs), cAMP response element binding protein (CREB) phosphorylation and neuronal death. In agreement with previous reports (Lu et al. 2001; Hardingham et al. 2002) activation of the synaptic NMDARs induced a sustained increase of mEPSC frequency (see Supplementary Fig. 2A) and phosphorylation of CREB (not shown). The activation of the extrasynaptic NMDARs resulted in a depression of mEPSCs frequency (see Supplementary Fig. 2B), a decrease of CREB phosphorylation and an induction of neuronal death (not shown).
NMDA and all blockers were from Tocris Neuramin. MEM and all supplements were from Invitrogen.
Image acquisition and analysis
Images were acquired with an Olympus Fluorview-500 confocal microscope (40 ×; 1.0 NA). For this, we first focused on neurons visualized with the MAP2 antibody (Alexa 488 fluorescence). Fluorescent images of MAP2 and P-ERK were then acquired. P-ERK fluorescence was analysed with the MetaMorph Imaging System (Universal Imaging, Westchester, PA, USA). Ten randomly chosen optical fields were analysed from each experiment (15–20 neurons per field). For analysis of the intensity of P-ERK staining in neuronal cells we first created a binary mask from MAP2-positive cells and then analysed P-ERK intensity in regions overlapping with the binary mask and normalized to the surface occupied by MAP2-positive neurons. This procedure allowed avoiding detection of P-ERK in non-neuronal cells. Some of the optical fields, however, included intensive P-ERK staining of non-neuronal (glial) cells that overlapped with neuronal P-ERK (see Supplementary Fig. 3). Such fields were omitted from analysis. All acquisitions and analysis were done blind. Acquisition parameters were same for every set of experiments. After analysis, data were normalized to the mean value of P-ERK in neurons activated with bicuculline for 5 min.
Electrophysiological recordings
Electrophysiological recordings from neurons were performed the same day as the analysis of ERK activity (14 DIV). Neurons were continuously perfused with an extracellular solution containing (mm): 140 NaCl, 2.5 KCl, 10 Hepes, 10 d-glucose and 2.0 CaCl2, pH 7.4. Recording electrodes (4–6 MΩ) were filled with a solution containing (mm): 100 caesium gluconate, 20 CsCl, 10 Hepes, 4 Mg-ATP (adenosine triphosphate), 0.4 Na-GTP (guanosine triphosphate), 10 mm sodium phosphocreatine and 10 mm EGTA (pH 7.2). To record whole cell responses of NMDAR, 10 μm of NMDA were applied by bath with an external solution containing 10 μm glycine, 1 μm tetrodotoxin and 1 μm strychnine. When needed, different concentrations of d-AP5 were co-applied with NMDA as indicated. Spontaneous excitatory postsynaptic currents were induced by bath application of 10 μm glycine, 10 μm bicuculline, 1 μm strychnine and 5 μm nifedipine in external solution containing 2 mm MgCl2. Recordings were made using an Axopatch-200A amplifier and pCLAMP acquisition software (Axon Instruments). Series resistance was < 20 MΩ. Data were low-pass filtered at 2 kHz and acquired at 10 kHz, and analysed using Clampfit software (Axon Instruments). All electrophysiology experiments were performed at 22–24°C.
Statistical analysis
All population data were expressed as mean ± s.e.m., unless otherwise indicated. Student's t test was employed to examine the statistical significance of the differences between groups of data. Significantly different values (P < 0.05) are indicated in figures with asterisks.
Results
To activate the synaptic pool of NMDAR (‘synaptic protocol’) we stimulated a synaptic release of glutamate in cultured 14 DIV hippocampal neurons by application of bicuculline, a blocker of GABAA receptors that removes inhibitory action of GABA and thus induces an increase of the glutamate-driven activity of the neuronal network (Hardingham et al. 2002). Figure 1A illustrates a bicuculline-induced increase of the frequency of spontaneous excitatory postsynaptic currents (EPSCs) reflecting a neuronal activity.
Figure 1. Activation of ERK by different subpopulations of NMDARs.
A, example of voltage clamp whole cell recording of spontaneous excitatory postsynaptic currents (EPSCs) that reflect activity of the neuronal network during bath application of bicuculline and co-application of MK-801 1 min later. Vh=−60 mV. B, neuron treatment with MK-801 in presence of bicuculline effectively inhibits synaptic NMDAR component of EPSCs. Superimposed traces illustrate spontaneous EPSCs recorded in the presence of bicuculline before (black) and after (grey) neuron incubation with MK-801. Plot shows MK-801-induced decrease of the amplitude of slow (NMDAR) component of EPSCs measured 100 ms after peak value. Mean data from 4 experiments, 5–7 events in each experiment. C, images of phosphorylated ERK1, 2 (P-ERK) and MAP2, in neurons activated via synaptic and extrasynaptic NMDARs. D, time course of ERK phosphorylation after activation of different populations of NMDARs. Abscissa indicates the time of treatment of cultures using mentioned protocols. Mean ± s.e.m. from 4 experiments. Bicuculline induced significantly different phosphorylation of ERK (P < 0.05) at all studied time points as compared with other experimental conditions.
Such bicuculline-dependent activation of excitatory synapses resulted in a relatively rapid and strong increase of ERK activity in neuronal cells that was evaluated using immunostaining with an antibody recognizing the active (phosphorylated) form of ERK (Payne et al. 1991) (Fig. 1C and D). The peak of ERK phosphorylation was observed in neurons incubated with bicuculline for 3–5 min. An increase of the time of neuron exposure to bicuculline resulted in the progressive decline of the level of ERK phosphorylation. The bicuculline-induced ERK phosphorylation was NMDA dependent since it was effectively inhibited by MK-801, a specific blocker of the open NMDA channel (Fig. 1D). The effect of MK-801 was not related to decreased neuronal activity since the application of MK-801 that inhibited the slow (NMDAR) component of the EPSCs (Fig. 1A and B) had no significant effect on the EPSCs interevents interval (6.9 ± 1.3 and 9.2 ± 3.7 s before and after MK-801 application, respectively, n = 7, P = 0.57). The slight residual activity of the ERK observed after co-application of MK-801 and bicuculline (Fig. 1D) could be the result of a brief NMDA-channel activation occurring prior to their blockage by MK-801 or activation of the AMPA receptors. To avoid possible ‘contamination’ of the NMDAR-dependent ERK phosphorylation by Ca2+ influx through L-type voltage-gated calcium channels (Dolmetsch et al. 2001; Zhao et al. 2005) all external solutions contained nifedipine, a specific blocker of these channels (see Methods).
To activate the extrasynaptic NMDARs (‘extrasynaptic protocol’) we used an approach previously described by Tovar & Westbrook (1999) and Hardingham et al. (2002). For this we first irreversibly blocked the synaptic NMDAR by a co-application of bicuculline and MK-801 to neuronal cultures for 2 min. An electrophysiological recording of the kinetic of EPSCs revealed that in our experimental conditions even 1 min of incubation with bicuculline and MK-801 is sufficient to inhibit the synaptic NMDAR in all EPSCs recorded after wash-out of the blockers (not shown). After wash-out of MK-801 we stimulated the remaining NMDAR extrasynaptic receptors by bath application of 10 μm NMDA. Since the synaptic NMDARs were already blocked by MK-801, NMDA application presumably only activated the extrasynaptic pool of NMDARs. Surprisingly, such activation of the extrasynaptic NMDARs did not cause ERK phosphorylation (Fig. 1C and D). The absence of ERK stimulation was not caused by a lack of NMDAR activation since the same protocol induced a prominent NMDA current (Fig. 2A). This current comprised approximately 50% of the current induced by NMDA bath application without preliminary treatment with bicuculline and MK-801 (‘entire NMDAR protocol’). To rule out the possibility that the NMDA current induced by extrasynaptic protocol is too small to activate ERK we suppressed 50% of current stimulated by the ‘entire NMDAR protocol’ using competitive NMDA antagonist D-AP5 (Fig. 2B). We assumed that this competitive inhibitor equally inhibited synaptic and extrasynaptic NMDARs. Figure 2C shows that in the presence of D-AP5 there was still strong ERK activation. Therefore, the similar amplitude NMDA current is sufficient to activate ERK if it is carried via both synaptic and extrasynaptic NMDARs but does not activate ERK when it is generated by only extrasynaptic NMDARs. Thus, stimulation of extrasynaptic NMDARs does not result in ERK activation.
Figure 2. Differential sensitivity of P-ERK to reduction of NMDA current through extrasynaptic and entire pool of NMDARs.
A, currents induced by bath activation of 10 μm NMDA before and after block of the synaptic NMDARs with MK-801 (entire NMDA and extrasynaptic NMDA, respectively). B, dose dependence of the inhibition of NMDA (10 μm)-induced currents by d-AP5. n = 7. Example shows current recorded during application of 10 μm NMDA and NMDA with 10 μm D-AP5. C, inhibition of the NMDAR with 20 μmd-AP5 does not affect the NMDA-induced ERK phosphorylation. Neurons were incubated with indicated agonists and blockers for 5 min. Mean ± s.e.m. from 5 experiments.
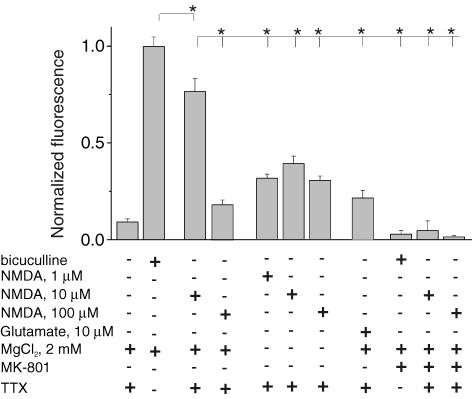
If extrasynaptic NMDARs do not activate ERK, then bath application of NMDA (entire NMDAR protocol) should induce ERK phosphorylation similar to those induced by bicuculline (synaptic protocol). However, we noticed that in most experiments the entire NMDAR protocol was less effective than the synaptic protocol in activating ERK (Figs 2C and 3). One of the possible explanations of this difference could be less effective NMDAR opening during bath application of agonist compared with bicuculline stimulation, due to Mg2+ block of the receptor at negative potentials (Nowak et al. 1984), difference in local agonist concentration, etc. However, the removal of the extracellular Mg2+ as well as an increase of NMDA concentration did not facilitate ERK activation (Fig. 3); on the contrary, stronger activation of NMDARs resulted in even weaker ERK phosphorylation. Bath application of glutamate (10 μm) also caused much weaker ERK activation than bicuculline (Fig. 3).
Figure 3. ERK phosphorylation at different strengths of NMDAR activation.
Phosphorylation of the ERK after NMDAR activation during 5 min with bicuculline, glutamate, different concentrations of NMDA and under different experimental conditions as indicated by (+) and (−). The basal external solution was as described in Methods. n = 4.
We suggested that stimulation of extrasynaptic NMDARs by agonist bath application results in the blocking of ERK activation or ERK inactivation. To verify this hypothesis we activated ERK by bicuculline application for 5 min, irreversibly blocked activated (synaptic) receptors by incubation with MK-801 for 2 min, washed the blocker out and then stimulated extrasynaptic receptors by bath application of NMDA for 10 min (for scheme of experiment see Fig. 4A). Figure 4B shows that 85 ± 4% of ERK activated with bicuculline remained phosphorylated 10 min after inhibition of the synaptic activity. An activation of the extrasynaptic pool of NMDA, subsequent to the neurons' treatment with bicuculline, resulted in a significant decrease of ERK phosphorylation to 48 ± 5%. Thus, the activation of the extrasynaptic NMDARs increases the rate of ERK dephosphorylation.
Figure 4. Activation of the extrasynaptic NMDAR induces dephosphorylation of P-ERK.
A, scheme of the experiment. Horizontal bars indicate the duration of incubation of neurons with indicated drugs. B, mean data from 3 experiments showing that activation of the extrasynaptic pool of NMDAR induces dephosphorylation of P-ERK. C, ERK phosphorylation in neuronal cultures incubated during indicated times with bicuculline, bicuculline + DL-TBOA (50 μm), and NMDA (10 μm). Mean data from 4 experiments. Asterisks indicate values of ERK phosphorylation significantly different from others measured at the same time points (P < 0.01).
To verify if extrasynaptic NMDA-dependent dephosphorylation of ERK might be activated by endogenous glutamate, we studied the bicuculline-dependent ERK phosphorylation in the presence of dl-TBOA (threo-β-benzyloxyaspartate, 50 μm), a blocker of glutamate uptake that facilitates spillover of glutamate from the synaptic cleft and favours activation of the extrasynaptic pool of NMDARs. Consistent with our hypothesis, the co-application of DL-TBOA with bicuculline resulted in a significant decrease of the level of ERK phosphorylation as compared with the synaptic protocol and was similar to those induced by bath application of NMDA (Fig. 4C).
Discussion
The main finding of the present study in cultured rat hippocampal neurons at 14 DIV is that the extrasynaptic pool of NMDARs does not participate in the activation of the ERK signalling pathway; on the contrary, it favours the inactivation of active ERK. Our work also shows that two opposed NMDAR-dependent regulatory pathways of ERK activity are spatially separated; this provides a unique opportunity for future studies of these pathways.
Previously it has been shown that ERK could be activated by synaptic NMDARs (Hardingham et al. 2001; Krapivinsky et al. 2003). The present study extends this finding and shows that only this pool of NMDARs activates ERK. Previous work has suggested also that NMDARs can exert bidirectional control of ERK activity (Chandler et al. 2001; Kim et al. 2005). Chandler et al. (2001) described a bell-shaped dose–response curve of ERK activation in cortical neuronal cultures and postulated that the activation of ERK is related to a weak calcium rise induced by low (10 or 25 μm) concentrations of NMDA, whereas the inactivation process was related to a strong increase of intracellular calcium induced by a high (100 μm) concentration of NMDA. Kim et al. (2005) showed that a high concentration of NMDA (70 μm) inhibited ERK activity in a time- and development-dependent manner and attributed the activatory effect of NMDA to the NR2A subunit, whereas the inhibitory effect was attributed to the NR2B subunit without discriminating between synaptic and extrasynaptic pools of the NMDARs containing this subunit. Curiously, in the same work the authors showed that NR2A knockdown did not modify the ability of the NMDARs to activate ERK and concluded that ‘NR2A is not essential for ERK activation’.
Our work shows that NMDAR-dependent activating/inactivating pathways are spatially separated and only extrasynaptic NMDAR mediates ERK inactivation. The slight inactivation of ERK occurring during a prolonged activation of the synaptic NMDARs with bicuculline might be the result of the activation of the perisynaptic NMDARs occurring during elevated neuronal activity (Scimemi et al. 2004; Lozovaya et al. 2004) or spontaneous (background) dephosphorylation of P-ERK.
Synaptic NMDARs are multimers including the NR1, NR2A and NR2B subunits, whereas extrasynaptic NMDARs include mostly NR1 and NR2B subunits (Cull-Candy et al. 2001). Recent analysis of spatial distribution of NMDAR subunits extends this conclusion and suggests that both NR2A- and NR2B-containing NMDARs can be located in either synaptic or extrasynaptic compartments (Thomas et al. 2006). This finding also supports the idea that spatial distribution rather then subunit composition of NMDARs determines the variety of the NMDA-dependent signalling. We have shown previously that the ERK signalling cascade is brought to the NMDAR by a physical interaction between the NR2B subunit of the NMDAR with RasGRF1, and ERK activation occurs predominantly via NMDAR containing the NR2B subunit (Krapivinsky et al. 2003). Based on these results and results of the present study we suggest that the NR2B subunit exerts a dual role in the regulation of the ERK signalling cascade: the synaptic NR2B is responsible for ERK phosphorylation and activation, while stimulation of the extrasynaptic NR2B contributes to ERK dephosphorylation and inactivation. This hypothesis is in agreement with recent findings showing the crucial role of the synaptic NR2B, but not the NR2A subunit in neuronal plasticity (Berberich et al. 2005; Weitlauf et al. 2005; Barria & Malinow, 2005).
The signalling pathways that mediate a positive link between NMDARs and ERK are most probably converged on RasGRF1 and RasGRF2, calcium–calmodulin-dependent Ras-specific GDP/GTP exchange factors. The double knock-out of these factors results in a complete loss of NMDAR-dependent ERK activation (Tian et al. 2004). The pathway conducting the inhibitory signal from extrasynaptic NMDARs to ERK remains to be identified. It is probably linked to some ERK phosphatases, since stimulation of the extrasynaptic NMDAR accelerates dephosphorylation of active ERK. Several phosphatases including dual-specificity phosphatases (Camps et al. 2000), serine/threonine phosphatases (Alessi et al. 1995), and tyrosine phosphatases (Pulido et al. 1998) effectively dephosphorylate ERK. At least one of them, a striatal-enriched tyrosine phosphatase (STEP), is involved in NMDAR-dependent regulation of ERK activity (Paul et al. 2003). Identification of the functional links between STEP or other phosphatases and extrasynaptic NMDARs provide an attractive direction in study of the novel signalling cascades.
Several works have previously shown the opposing effects of synaptic and extrasynaptic pools of NMDARs on different physiological responses. Thus, the activation of the synaptic pool of NMDAR is implicated in LTP induction (Lu et al. 2001), CREB activation (Hardingham et al. 2002) and revealed a neuroprotective effect (Hardingham et al. 2002) whereas activation of the extrasynaptic NMDAR supported LTD (Lu et al. 2001; Massey et al. 2004), shut off the CREB pathway (Hardingham et al. 2002) and induced neuronal death (Hardingham et al. 2002). We propose that the bidirectional control of the ERK signalling cascade by different pools of NMDARs is an earlier signalling event that allows discrimination of large diversities of physiological and/or pathological processes. Numerous works have shown that NMDAR-dependent ERK activation is crucial for synaptic plasticity including long-term memory, LTP, spine formation (for review see Adams & Sweatt, 2002; Thomas & Huganir, 2004). On the contrary, the functional significance of NMDAR-dependent ERK inactivation has not been studied yet. The activation of the extrasynaptic NMDA is attributed mainly to global release of glutamate occurring at pathological conditions including hypoxic/ischaemic insults, traumas, and epileptic brain damage (Lipton, 1999; Arundine & Tymianski, 2004). Therefore, the negative control of the ERK activity by extrasynaptic NMDARs might be one of the first signalling events detecting pathology. It remains unclear whether such ERK inactivation initiates neuronal death or, on the contrary, launches a neuroprotective program. Therefore an understanding of the functional role of ERK inactivation is an important subject for future studies and is a putative target of new pharmacological applications.
Acknowledgments
We are grateful to Dr Grigory Krapivinsky for critical reading and comments on the manuscript and Galina Medyna for help in preparing the manuscript.
Supplementary material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.105510
http://jp.physoc.org/cgi/content/full/jphysiol.2006.105510/DC1 and contains three supplemental figures.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol. 1995;5:283–295. doi: 10.1016/s0960-9822(95)00059-5. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bading H, Greenberg ME. Stimulation of protein tyrosine phosphorylation by NMDA receptor activation. Science. 1991;253:912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. doi: 10.1016/j.neuron.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Berberich S, Punnakkal P, Jensen V, Pawlak V, Seeburg PH, Hvalby O, Kohr G. Lack of NMDA receptor subtype selectivity for hippocampal long-term potentiation. J Neurosci. 2005;25:6907–6910. doi: 10.1523/JNEUROSCI.1905-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Dorairaj NR, Norwood D. N-methyl D-aspartate receptor-mediated bidirectional control of extracellular signal-regulated kinase activity in cortical neuronal cultures. J Biol Chem. 2001;276:2627–2636. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- Goldin M, Segal M. Protein kinase C and ERK involvement in dendritic spine plasticity in cultured rodent hippocampal neurons. Eur J Neurosci. 2003;17:2529–2539. doi: 10.1046/j.1460-9568.2003.02694.x. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Kurino M, Fukunaga K, Ushio Y, Miyamoto E. Activation of mitogen-activated protein kinase in cultured rat hippocampal neurons by stimulation of glutamate receptors. J Neurochem. 1995;65:1282–1289. doi: 10.1046/j.1471-4159.1995.65031282.x. [DOI] [PubMed] [Google Scholar]
- Li JH, Wang YH, Wolfe BB, Krueger KE, Corsi L, Stocca G, Vicini S. Developmental changes in localization of NMDA receptor subunits in primary cultures of cortical neurons. Eur J Neurosci. 1998;10:1704–1715. doi: 10.1046/j.1460-9568.1998.00169.x. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Lozovaya NA, Grebenyuk SE, Tsintsadze TS, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape ‘superslow’ afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, Collingridge GL, Bashir ZI. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–7828. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Payne DM, Rossomando AJ, Martino P, Erickson AK, Her JH, Shabanowitz J, Hunt DF, Weber MJ, Sturgill TW. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase) EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J. 1998;17:7337–7350. doi: 10.1093/emboj/17.24.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A, Fine A, Kullmann DM, Rusakov DA. NR2B-containing receptors mediate cross talk among hippocampal synapses. J Neurosci. 2004;24:4767–4777. doi: 10.1523/JNEUROSCI.0364-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and Extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Tian X, Gotoh T, Tsuji K, Lo EH, Huang S, Feig LA. Developmentally regulated role for Ras-GRFs in coupling NMDA glutamate receptors to Ras, Erk and CREB. EMBO J. 2004;23:1567–1575. doi: 10.1038/sj.emboj.7600151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf C, Honse Y, Auberson YP, Mishina M, Lovinger DM, Winder DG. Activation of NR2A-containing NMDA receptors is not obligatory for NMDA receptor-dependent long-term potentiation. J Neurosci. 2005;25:8386–8390. doi: 10.1523/JNEUROSCI.2388-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Adams JP, Dudek SM. Pattern-dependent role of NMDA receptors in action potential generation: consequences on extracellular signal-regulated kinase activation. J Neurosci. 2005;25:7032–7039. doi: 10.1523/JNEUROSCI.1579-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.