Abstract
Potassium channels that are inhibited by intracellular ATP (ATPi) were first identified in ventricular myocytes, and are referred to as ATP-sensitive K+ channels (i.e. KATP channels). Subsequently, K+ channels with similar characteristics have been demonstrated in many other tissues (pancreatic β-cells, skeletal muscle, central neurones, smooth muscle). Approximately one decade ago, KATP channels were cloned and were found to be composed of at least two subunits: an inwardly rectifying K+ channel six family (Kir6.x) that forms the ion conducting pore and a modulatory sulphonylurea receptor (SUR) that accounts for several pharmacological properties. Various types of native KATP channels have been identified in a number of visceral and vascular smooth muscles in single-channel recordings. However, little attention has been paid to the molecular properties of the subunits in KATP channels and it is important to determine the relative expression of KATP channel components which give rise to native KATP channels in smooth muscle. The aim of this review is to briefly discuss the current knowledge available for KATP channels with the main interest in the molecular basis of native KATP channels, and to discuss their possible linkage with physiological functions in smooth muscle.
It is generally accepted that K+ channel activation prevents the development of action potentials in smooth muscles, so allowing graded changes in membrane potential to regulate their tone. Several K+ channels, with different molecular bases, contribute to the regulatory basal K+ conductance in smooth muscle cells: (i) voltage-gated K+ channels (Kv); (ii) large conductance Ca2+-activated K+ channels (BKCa); (iii) small conductance Ca2+-activated K+ channels (SKCa); (iv) inward rectifier K+ channels (Kir); (v) two-pore domain K+ channels (TASK); (vi) stretch-dependent K+ channels and (vii) KATP channels (Thorneloe & Nelson, 2005).
KATP channels were first identified in cardiac myocytes (Noma, 1983). Being selective for K+ and activated by a fall in the internal concentration of ATP, KATP channel activity may confer a voltage-independent ‘brake’ which limits myogenic depolarization and controls myogenic reactivity. Several endogenous agonists (such as calcitonin gene-related peptide (CGRP), adenosine, etc.) activate KATP channels leading to hyperpolarization and relaxation, a response that is mimicked by treatment with KATP channel openers. In contrast, various neurotransmitters (noradrenaline (norepinephrin), 5-hydroxytryptamine (5-HT), neuropeptide Y, etc.) and vasoconstrictors (angiotensin II, endothelin-1, etc.) inhibit KATP channels leading to depolarization and contraction (Quayle et al. 1997). Thus, modulation of KATP channels allows the contribution of native KATP channels to be finely tuned, so regulating the contractility of smooth muscle.
KATP channels are octameric complexes of pore-forming and modulatory subunits
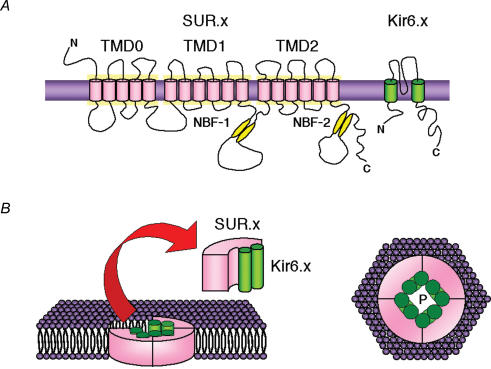
Recent progress in defining the molecular basis of KATP channels in different tissues indicates that there is functional diversity which results from cell-specific expression of different subunit proteins (Aguilar-Bryan & Bryan, 1999). When KATP channels were cloned (Inagaki et al. 1995), they were found to contain four pore-forming, inwardly rectifying channel subunits (Kir6.x) and four modulatory sulphonylurea receptor subunits (SUR.x) that are members of the ATP-binding cassette (ABC) super-family of proteins (Fig. 1). To date, two Kir6 isoforms, Kir6.1 and Kir6.2, and two SUR isoforms, SUR1 and SUR2, have been identified (Aguilar-Bryan & Bryan, 1999). Kir6.x subunits have two transmembrane domains, M1 and M2, cytoplasmic N- and C-termini and a pore-forming loop typical of inward rectifier K+ channels (Aschroft & Gribble, 1998; Aguilar-Bryan & Bryan, 1999; Seino, 1999). Channels containing Kir6.1 have a unitary conductance of ∼35 pS, whereas for Kir6.2 channels this is ∼70 pS in symmetrical 140 mm K+ conditions. Alternative splicing of exon 38 results in two species of SUR2, i.e. SUR2A (Inagaki et al. 1996) and SUR2B (Isomoto et al. 1996). The 42 amino acid residues located in the carboxyl-terminal end of SUR2B is divergent from that of SUR2A but highly homologous to that of SUR1 (Isomoto et al. 1996). SURs possess large cytoplasmic domains containing two conserved nucleotide binding folds (NBFs), NBF1 and NBF2, with Walker A and B motifs (Fig. 1). The endoplasmic reticulum (ER) retention motifs are present in the cytoplasmic domains of the Kir6 and SURs; they preclude cell surface expression unless both subunits are present (Zerangue et al. 1999).
Figure 1. Schematic illustration of the predicted topologies of Kir6.x and SUR.x subunits and the assembly of these subunits into a KATP channel.
The transmembrane domain model proposed by Tusnady et al. (1997) is given. NBF-1 and -2 represent the two nucleotide-binding folds with Walker A and B consensus motifs. N and C indicate the N and C termini of the proteins (adapted from Cole & Clément-Chomienne, 2003). P, channel pore; Kir6.x, inwardly rectifying K+ channels; SUR.x, sulphonylurea receptors; TMD, transmembrane domain.
Kir6.x–SUR.x combination
Different combinations of Kir6.x and SUR.x isoforms/variants yield tissue-specific KATP channel subtypes with different features and distinct functional properties. In functional expression studies, it is accepted that SUR1–Kir6.2 forms the pancreatic β-cell KATP channel and that SUR2A–Kir6.2 forms the cardiac KATP channel (Aschroft & Gribble, 1998; Aguilar-Bryan & Bryan, 1999). However, the molecular identity of smooth muscle-type KATP channels has not been established with the same certainty. Two types of smooth muscle-type KATP channels have been cloned and identified (Table 1), namely Kir6.2–SUR2B channels (Isomoto et al. 1996) and Kir6.1–SUR2B channels (Yamada et al. 1997).
Table 1. Smooth muscle-type recombinant KATP channels.
| Subunit composition | Conductance (140 mm K+) | Native channel | Reference |
|---|---|---|---|
| Kir6.2–SUR2B | 80 pS | KATP channel | Isomoto et al. 1996 |
| Kir6.1–SUR2B | 33 pS | KNDP channel | Yamada et al. 1997 |
Isomoto et al. (1996) have succeeded in isolating a cDNA that encodes a novel variant of SUR, termed SUR2B. Coexpression of SUR2B with Kir6.2 produces activity which is enhanced by KATP channel openers. Moreover, RT-PCR analysis indicates that transcripts for SUR2B are widely distributed in various smooth muscles (Isomoto et al. 1996). These results suggest that Kir6.1–SUR2B is a native smooth muscle-type KATP channel. However, the electrophysiological properties of Kir6.1–SUR2B channels are not consistent with those of all KATP channels detected in several smooth muscles (Quayle et al. 1997).
Yamada et al. (1997) reported that reconstituted Kir6.1–SUR2B channels resemble nucleotide diphosphate (NDP)-sensitive KNDP channels in some vascular smooth muscle (Beech et al. 1993; Zhang & Bolton, 1996; Cole et al. 2000). Characteristically they demonstrate: (1) a unitary conductance of 33 pS with no voltage dependency; (2) channel activity is enhanced by KATP channel openers with burst-like openings, which are inhibited by glibenclamide; (3) no channel opening is observed in the absence of KATP channel openers; (4) internal application of NDPs can restore activity even after run-down is complete; (5) ATPi appears to be required to maintain activity in the presence of Mg2+, even though channel current–voltage relationships are linear and show no inward rectification in the presence of Mg2+. Furthermore, Kir6.1–SUR2B channels are not suppressed by physiological concentrations of ATPi. Thus, it is somewhat uncertain whether or not Kir6.1–SUR2B channels may be classified into a category of ‘KATP channels’, although they do resemble KNDP channels.
Molecular basis of native KATP channels in smooth muscle
A point-for-point quantitative comparison between native and recombinant KATP channels is required to determine the pattern of KATP channel subunit expression in smooth muscle. In visceral and vascular smooth muscle, the molecular properties of native KATP channels have been investigated in RT-PCR analysis in addition to electrophysiological observations. Table 2 summarizes the published molecular and electrical properties of native KATP channels. Various combinations of Kir6.x and SUR.x convey the heterogeneity.
Table 2. Native KATP channels in visceral and vascular smooth muscles (RT-PCR analysis).
| Transcript | |||||||
|---|---|---|---|---|---|---|---|
| Smooth muscle | Kir6.1 | Kir6.2 | SUR1 | SUR2B | Subunit composition | Conductance (140 mm K+) | Reference |
| Murine colon | − | + | − | + | Kir6.2–SUR2B | 27 pS | Koh et al. 1998 |
| Guinea-pig stomach | + | + | − | + | Kir6.1–SUR2B | 37 pS | Sim et al. 2002 |
| Pig urethra | + | + | + | + | Kir6.1–6.2–SUR1 | 43 pS | Teramoto et al. 1997b |
| Kir6.1–6.2–SUR2B | Teramoto et al. 2000 | ||||||
| Yunoki et al. 2002 | |||||||
| Cultured human | + | − | − | + | Kir6.1–SUR2B | 28 pS | Cui et al. 2002 |
In murine colon, mRNAs of Kir6.2 and SUR2B are detected and the unitary channel conductance is 27 pS, suggesting that Kir6.1–SUR2B forms the KATP channels in this tissue (Koh et al. 1998). In guinea-pig stomach, the transcripts of Kir6.1, Kir6.2 and SUR2B but not SUR1 are present and channel conductance is 37 pS, suggesting that KATP channels are composed of Kir6.1 and SUR2B (Sim et al. 2002). In pig urethra, RT-PCR analysis demonstrates the presence of SUR1 and SUR2B transcripts and both SUR1 and SUR2B coexist as functional SUR subunits (Yunoki et al. 2002, 2003). The transcripts of Kir6.1 and Kir6.2 have been detected in pig urethra (Teramoto et al. 2000, 2003) and the conductance of urethral KATP channels is 43 pS (an intermediate conductance between that of Kir6.1 and Kir6.2; Teramoto et al. 1997b), suggesting that the nature of the pore region of urethral KATP channels differs from that of vascular KATP channels (Tomoda et al. 2005) and that urethral KATP channels may possess a heteromeric channel structure with the channel pore composed of Kir6.1 and Kir6.2 subunits (Teramoto et al. 2003). In smooth muscle-type KATP channels, it still remains elusive as to which molecular factor(s) may modify the expression of KATP channel components (such as Kir6.x and SUR.x) in cell plasma membrane. On the other hand, in Kir6.2–SUR1 (i.e. pancreatic β cell-type KATP channels), trafficking of KATP channel complexes out of the ER is controlled by a tri-peptide Arg-Lys-Arg (RKR) retention/retrieval signal present in each of the Kir6.2 and SUR1 subunits (Zerangue et al. 1999). Upon successful assembly of SUR and Kir into an octameric complex, the -RKR- motifs are concealed to allow the channel to translocate from the ER to the Golgi, where the sugar moiety on SUR1 is modified before further translocation to the plasma membrane (Raab-Graham et al. 1999; Zerangue et al. 1999; Conti et al. 2002). Thus, in Kir6.2–SUR1, the -RKR- trafficking signal provides a quality control mechanism to prevent individual subunits as well as incompletely assembled channel complexes from trafficking to the cell surface.
In cultured human pulmonary arterial smooth muscle cells, the expression of Kir6.1 and SUR2B mRNAs has been reported (Cui et al. 2002). Since the unitary conductance is 28 pS, Kir6.1–SUR2B is likely to be the predominant isoform of the native KATP channel, possessing ATPi sensitivity (Cui et al. 2002). In contrast, Kir6.1–SUR2B channels expressed in HEK-293 cells are entirely insensitive to ATPi (≤ 5 mm). It is not certain whether or not this discrepancy may be due to cultured smooth muscle cells or different recording conditions. Cui et al. (2002) suggest that the expression of the recombinant KATP channels in HEK-293 cells may alter ATPi sensitivity, changing basal phosphorylation states since the activity of native KATP channels in smooth muscle cells is readily modulated by several kinases (PKA, Wellman et al. 1998; PKC, Bonev & Nelson, 1993; and tyrosine kinase, Hatakeyama et al. 1995). Alternatively, this discrepancy is related to other factors governing the regulation of KATP channels in the native environment.
Recently, a gene-targeting strategy to generate mice with disrupted muscle-specific KATP channel regulatory subunits has been carried out to improve the understanding of the role of KATP channels (reviewed by Seino & Miki, 2004). Since Kir6.1-containing KATP channels are involved in regulation of vascular tonus (Li et al. 2003), it would be of interest to investigate the functional properties of vascular smooth muscle in the Kir6.1 null mouse. Furthermore, it has been also reported that the Kir6.1 null mouse is a model of variant angina pectoris (i.e. Prinzmetal's angina or spontaneous angina pectoris) in human by disruption of the gene encoding Kir6.1 (Miki et al. 2002). Miki et al. (2002) suggest that smooth muscle-type KATP channels are likely to be defective in Kir6.1 null mice, concluding that Kir6.1 is a constituent of smooth muscle-type KATP channels on the plasma membrane of vascular smooth muscle.
In summary, native KATP channels in smooth muscle show considerable heterogeneity in several notable respects. Significantly, the functional expression studies also show that heteromultimerization readily occurs between Kir6.1 and Kir6.2, producing functional recombinant KATP channels that possess distinct unitary conductance values which are intermediate between the levels observed for the homomeric channels (Cui et al. 2001). These results suggest that multiple types of native KATP channels exist in different species and types of smooth muscle and that mixed populations of Kir6.x and SURs subunits form hybrid KATP channels.
Physiological roles of the native KATP channels
KATP channels are characteristically activated by declining concentrations of ATPi or elevated concentrations of NDPs, followed by changing the ratio of ADP/ATP. Thus, it is thought that KATP channels provide a link between cell metabolism and membrane excitability. Furthermore, KATP channels appear to be the target of a number of neuropeptides and neurotransmitters. In in vivo experiments, the existence of active native KATP channels in smooth muscle has been inferred through the ability of glibenclamide to produce excitation (reviewed by Quayle et al. 1997).
Resting membrane potential and basal tone
A number of in vitro studies have reported that glibenclamide (≤ 1 μm), which blocks KATP channels, increases muscle tone and causes depolarization in vascular smooth muscle (rabbit mesenteric artery, Nelson et al. 1990; canine saphenous vein, Nakashima & Vanhoutte, 1995) and non-vascular smooth muscle (guinea-pig trachea, Murray et al. 1989; dog bronchial smooth muscle, Kamei et al. 1994; pig urethra, Teramoto et al. 1997a). Furthermore, in vivo studies also show that glibenclamide significantly increases vascular resistance and decreases arterial diameter (Quayle et al. 1997). Although the interpretation of theses studies solely depends on the sensitivity of glibenclamide, direct measurements of channel activity show that brief openings of native KATP channels occasionally occurred in the absence of KATP channel openers (Teramoto et al. 1997a). Presumably this is related to a low density or a low open probability of native KATP channels. These results suggest that KATP channels play important roles in regulating the resting membrane potential of several smooth muscles with a small amount of channel activity.
Interaction between cytoskeletal networks and the regulating mechanisms of KATP channels
The integrity of the microenviroment, in particular the actin filament network, surrounding KATP channel proteins may play an important role in modulating the channel activity of KATP channels (Van Wagoner & Lamorgese, 1994). DNase I, one of the actin microfilament disrupters, has been shown to stimulate the activity of KATP channels in cardiomyocytes (Terzic & Kurachi, 1996). Similarly, cytochalasin B enhanced the activity of KATP channels in native KATP channels of smooth muscle (Teramoto et al. 2002). The actin filament network and its related proteins might be involved in signal transaction between the inhibitory regulatory proteins and KATP channels.
Cellular pathways for KATP channel modulation
The activity of native KATP channels is increased by several vasodilators (e.g. adenosine, CGRP, prostacyclin, β agonists) which activate PKA through the formation of cyclic AMP, whereas contractile agonists (e.g. angiotensin II, endothelin-1, serotonin, noradrenaline) or α agonists (Figs 2 and 3; author's unpublished data), vasopressin, neuropeptide (Y), which activate PKC pathways, decrease the activity of native KATP channels, causing depolarization and contraction (Quayle et al. 1997; Cole et al. 2000). Although channel phosphorylation is essential for the regulation of native KATP channels in smooth muscle, the molecular basis and mechanisms by which native KATP channels are affected by PKC- and PKA-mediated phosphorylation remains unknown.
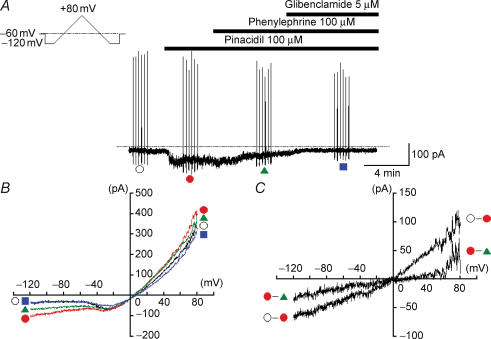
Figure 2. Effects of phenylephrine on the pinacidil-induced KATP current in smooth muscle cells dispersed from rat tail artery.
Whole-cell recording, bath solution 140 mm K+ solution, pipette solution 140 mm K+ containing 5 mm EGTA. A, current trace. The vertical lines are responses to triangular ramp potential pulses of 200 mV s−1 from −120 mV to +80 mV, applied after an initial 300 ms conditioning pulse to −120 mV (see inset). Pinacidil (100 μm) caused an inward membrane current which was sustained. The current was inhibited by application of 100 μm phenylephrine. Additional application of glibenclamide suppressed the pinacidil-induced KATP current in rat tail artery. The dashed line indicates zero current. B, current–voltage relationships measured from the negative-going limb (the falling phase) of the ramp pulse. Each symbol is the same as in the current trace (A). The lines are mean membrane currents from the six ramps in each condition. C, net membrane currents. The phenylephrine-sensitive membrane current was obtained by subtraction of the membrane currents in the absence and presence of 100 μm phenylephrine when 100 μm pinacidil was present in the bath solution.
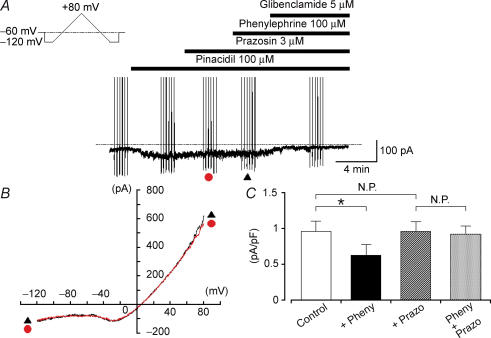
Figure 3. The phenylephrine-induced inhibition of KATP current was blocked by prazosin in rat tail artery.
Whole-cell recording, bath solution 140 mm K+ solution, pipette solution 140 mm K+ containing 5 mm EGTA. A, current trace. The vertical lines are responses to triangular ramp potential pulses of 200 mV s−1 from −120 mV to +80 mV, applied after an initial 300 ms conditioning pulse to −120 mV (see inset). Pinacidil caused an inward membrane current which was sustained. Prazosin caused no effect on the pinacidil-induced KATP current. Similarly, additional application of phenylephrine had no effect on the pinacidil-induced KATP current in the presence of prazosin. Subsequently, application of glibenclamide suppressed the pinacidil-induced KATP current. The dashed line indicates zero current. B, current–voltage relationships measured from the negative-going limb (the falling phase) of the ramp pulse. Each symbol is the same as in the current trace (A). The lines are mean membrane currents from the six ramps in each condition. C, summary of the data. The open column indicates the current density (pA pF−1) of the pinacidil-induced KATP currents (i.e. Control). The black column shows the current density of the pinacidil-induced KATP currents in the presence of 100 μm phenylephrine (+ Pheny). The hatched column represents the current density of the pinacidil-induced KATP currents in the presence of 3 μm prazosin (+ Prazo). The grey column indicates the current density of the pinacidil-induced KATP currents in the presence of both prazosin and phenylephrine (Pheny + Prazo). *Statistically significant difference, demonstrated using a paired t test (P < 0.01). Each column represents the relative mean value with s.d. (n = 5).
In recombinant KATP channel studies, the activation of Kir6.2–SUR1 and/or Kir6.2–SUR2A channels by PKC and PKA has been studied and potential phosphorylation sites have been identified by mutational analysis (Beguin et al. 1999; Lin et al. 2000; Manning Fox et al. 2004), but such studies have not been carried out on SUR2B-containing recombinant channels. PKA-mediated phosphorylation of Kir6.2–SUR1 channels was initially studied in vitro, and residues (i.e. Kir6.2-S372 and SUR1-S1571) were identified as potential phosphorylation sites (Beguin et al. 1999). Subsequent analysis suggested that these sites may not be relevant for control of gating as the SUR site is not conserved in other species, and substitution of Kir6.2-T224, but not S372, prevented PKA-mediated stimulation of gating (Lin et al. 2000). However, in both studies, the effects of PKA were studied in non-physiological conditions: the effect of PKA on the inhibition by ATP was assessed, but no attempt was made to evaluate the actions of PKA in the presence of MgADP. Consideration of the effects of kinase-mediated regulation of KATP channels in the presence of MgADP is essential because the relative changes in ATP sensitivity identified in these studies would be irrelevant within the normal physiological range of ATPi of 1–5 mm. The importance of this point was illustrated in a recent study by Manning Fox et al. (2004) which reveals a more complex modulation involving additional sites in the presence of MgADP.
Conclusions
Several observations suggest that the Kir6.1–SUR2B channel is likely to be the predominant isoform of native KATP channel in some vascular smooth muscles. Conversely, many studies have identified the molecular properties of native KATP channels and have suggested that more than one type of KATP channel is expressed in smooth muscle. Clearly many queries remain about the nature of smooth muscle-type KATP channels: (1) Are native KATP channels composed of additional and different regulatory subunits? (2) Do any endogenous ligands regulate KATP channels? (3) What are the physiological roles of membrane phospholipids (e.g. phosphatidylinositol 4,5-bisphosphates (PIP2)) in the control of native KATP channels? (4) What is the localized distribution of the subunits of Kir6.x and SUR.x in a range of smooth muscles? (5) What are the components of native KATP channels, and how does the stoichiometry of Kir6.x and SUR.x proteins vary between differing smooth muscles? Further studies are required to understand the full complexity of native KATP channels in smooth muscle.
Acknowledgments
The author wishes to thank Professors Yushi Ito, William C. Cole (University of Calgary, Calgary, Canada) and G. David S. Hirst (Australia National University, Canberra, Australia) for helpful discussion and critical reading of the manuscript. This work was supported by both a Grant-in-Aid for Scientific Research (B)-(2) (Noriyoshi Teramoto, Grant No. 16390067) from the Japanese Society for the Promotion of Science and a Grant-in-Aid for Exploratory Research (Grant No. 17659075) from the Ministry of Education, Culture, Sports, Science and Technology, Japan as well as by Grants-in-aid from the Clinical Research Foundation (Fukuoka, Japan) in 2005.
References
- Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphospate-sensitive potassium channels. Endocrine Rev. 1999;20:101–135. doi: 10.1210/edrv.20.2.0361. [DOI] [PubMed] [Google Scholar]
- Aschroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Zhang H, Nakao K, Bolton TB. K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br J Pharmacol. 1993;110:573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin P, Nagashima K, Nishimura M, Gonoi T, Seino S. PKA-mediated phosphorylation of the human KATP channel: separate roles of Kir6.2 and SUR1 subunit phosphorylation. EMBO J. 1999;18:4722–4732. doi: 10.1093/emboj/18.17.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonev AD, Nelson MT. Muscarinic inhibition of ATP-sensitive K+ channels by protein kinase C in urinary bladder smooth muscle. Am J Physiol. 1993;265:C1723–C1728. doi: 10.1152/ajpcell.1993.265.6.C1723. [DOI] [PubMed] [Google Scholar]
- Cole WC, Clément-Chomienne O. ATP-sensitive K+ channels of vascular smooth muscle cells. J Cardiovasc Electrophysiol. 2003;14:94–103. doi: 10.1046/j.1540-8167.2003.02376.x. [DOI] [PubMed] [Google Scholar]
- Cole WC, Malcolm T, Walsh MP, Light PE. Inhibition by protein kinase C of the KNDP subtype of vascular smooth muscle ATP-sensitive potassium channel. Circ Res. 2000;87:112–117. doi: 10.1161/01.res.87.2.112. [DOI] [PubMed] [Google Scholar]
- Conti LR, Radeke CM, Vandenberg CA. Membrane targeting of ATP-sensitive potassium channel. J Biol Chem. 2002;277:25416–25422. doi: 10.1074/jbc.M203109200. [DOI] [PubMed] [Google Scholar]
- Cui Y, Giblin JP, Clapp LH, Tinker A. A mechanism for ATP-sensitive potassium channel diversity: Functional coassembly of two pore-forming subunits. Proc Natl Acad Sci U S A. 2001;98:729–734. doi: 10.1073/pnas.011370498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Tran S, Tinker A, Clapp LH. The molecular composition of KATP channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am J Respir Cell Mol Biol. 2002;26:135–143. doi: 10.1165/ajrcmb.26.1.4622. [DOI] [PubMed] [Google Scholar]
- Hatakeyama N, Wang Q, Goyal RK, Akbarali HI. Muscarinic suppression of ATP-sensitive K+ channel in rabbit esophageal smooth muscle. Am J Physiol. 1995;268:C877–C885. doi: 10.1152/ajpcell.1995.268.4.C877. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, 4th, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Kamei K, Yoshida S, Imagawa J, Nabata H, Kuriyama H. Regional and species differences in glyburide-sensitive K channels in airway smooth muscles as estimated from actions of KC128 and levcromakalim. Br J Pharmacol. 1994;113:889–897. doi: 10.1111/j.1476-5381.1994.tb17076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh SD, Bradley KK, Rae MG, Keef KD, Horowitz B, Sanders KM. Basal activation of ATP-sensitive potassium channels in murine colonic smooth muscle cell. Biophys J. 1998;75:1793–1800. doi: 10.1016/S0006-3495(98)77621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Wu J, Jiang C. Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. J Membr Biol. 2003;196:61–69. doi: 10.1007/s00232-003-0625-z. [DOI] [PubMed] [Google Scholar]
- Lin YF, Jan YN, Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J. 2000;19:942–955. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning Fox JE, Nichols CG, Light PE. Activation of adenosine triphosphate-sensitive potassium channels by acyl coenzyme A esters involves multiple phosphatidylinositol 4,5-bisphosphate-interacting residues. Mol Endocrinol. 2004;18:679–686. doi: 10.1210/me.2003-0431. [DOI] [PubMed] [Google Scholar]
- Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- Murray MA, Boyle JP, Small RC. Cromakalim-induced relaxation of guinea-pig isolated trachealis: antagonism by glibenclamide and by phentolamine. Br J Pharmacol. 1989;98:865–874. doi: 10.1111/j.1476-5381.1989.tb14615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Vanhoutte PM. Isoproterenol causes hyperpolarization through opening of ATP-sensitive potassium channels in vascular smooth muscle of the canine saphenous vein. J Pharmacol Exp Ther. 1995;272:379–384. [PubMed] [Google Scholar]
- Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344:770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Raab-Graham KF, Cirilo LJ, Boettcher AA, Radeke CM, Vandenberg CA. Membrane topology of the amino-terminal region of the sulfonylurea receptor. J Biol Chem. 1999;274:29122–29129. doi: 10.1074/jbc.274.41.29122. [DOI] [PubMed] [Google Scholar]
- Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Ann Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- Seino S, Miki T. Gene targeting approach to clarification of ion channel function: studies of Kir6.x null mice. J Physiol. 2004;554:295–300. doi: 10.1113/jphysiol.2003.047175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim JH, Yang DK, Kim YC, Park SJ, Kang TM, So I, Kim KW. ATP-sensitive K+ channels composed of Kir6.1 and SUR2B subunits in guinea pig gastric myocytes. Am J Physiol. 2002;282:G137–G144. doi: 10.1152/ajpgi.00057x.2002. [DOI] [PubMed] [Google Scholar]
- Teramoto N, Creed KE, Brading AF. Activity of glibenclamide-sensitive K+ channels under unstimulated conditions in smooth muscle cells of pig proximal urethra. Naunyn-Schmiedeberg's Arch Pharmacol. 1997a;356:418–424. doi: 10.1007/pl00005071. [DOI] [PubMed] [Google Scholar]
- Teramoto N, Doira N, Ito Y. Electrophysiological and molecular evidence of inwardly rectifying ATP-sensitive K+ channels expressed in pig urethral myocytes. J Pharmacol Sci. 2003;91(Suppl. I):246P. [Google Scholar]
- Teramoto N, McMurray G, Brading AF. Effects of levcromakalim and nucleoside diphosphates on glibenclamide-sensitive K+ channels in pig urethral myocytes. Br J Pharmacol. 1997b;120:1229–1240. doi: 10.1038/sj.bjp.0701033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto N, Tomoda T, Yunoki T, Brading AF, Ito Y. Modification of ATP-sensitive K+ channels by proteolysis in smooth muscle cells from pig urethra. Life Sci. 2002;72:475–485. doi: 10.1016/s0024-3205(02)02284-1. [DOI] [PubMed] [Google Scholar]
- Teramoto N, Yunoki T, Tanaka K, Takano M, Masaki I, Yonemitsu Y, Sueishi K, Ito Y. The effects of caffeine on ATP-sensitive K+ currents in smooth muscle cells from pig urethra. Br J Pharmacol. 2000;131:505–513. doi: 10.1038/sj.bjp.0703586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzic A, Kurachi Y. Actin microfilament disrupters enhance KATP channel opening in patches from guinea-pig cardiomyocytes. J Physiol. 1996;492:395–404. doi: 10.1113/jphysiol.1996.sp021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83:215–242. doi: 10.1139/y05-016. [DOI] [PubMed] [Google Scholar]
- Tomoda T, Yunoki T, Naito S, Ito Y, Teramoto N. Multiple actions of U-37883A, an ATP-sensitive K+ channel blocker, on membrane currents in pig urethra. Eur J Pharmacol. 2005;524:1–10. doi: 10.1016/j.ejphar.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Tusnady GE, Bakos E, Varadi A, Sarkadi B. Membrane topology distinguishes a subfamily of the ATP-binding cassette (ABC) transporters. FEBS Lett. 1997;402:1–3. doi: 10.1016/s0014-5793(96)01478-0. [DOI] [PubMed] [Google Scholar]
- Van Wagoner DR, Lamorgese M. Ischemia potentiates the mechnosensitive modulation of atrial ATP-sensitive potassium channels. Ann N Y Acad Sci. 1994;723:392–395. [PubMed] [Google Scholar]
- Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507:117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunoki T, Teramoto N, Ito Y. Functional involvement of sulphonylurea receptor (SUR) type 1 and 2B in the activity of pig urethral ATP-sensitive K+ channels. Br J Pharmacol. 2002;139:652–660. doi: 10.1038/sj.bjp.0705268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunoki T, Teramoto N, Takano N, Seki N, Creed KE, Naito S, Ito Y. The effects of MCC-134 on the ATP-sensitive K+ channels in pig urethra. Eur J Pharmacol. 2003;482:287–295. doi: 10.1016/j.ejphar.2003.09.068. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Zhang H-L, Bolton TB. Two types of ATP-sensitive potassium channels in rat portal vein smooth muscle cells. Br J Pharmacol. 1996;118:105–114. doi: 10.1111/j.1476-5381.1996.tb15372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]