Abstract
The role of intramuscular, low pH saline injections during the neonatal period in the development and maintenance of visceral hyperalgesia has not been systematically studied. We aimed to investigate alterations in visceral sensation and neural circuitry that result from noxious stimuli in early life. Neonatal male Sprague–Dawley rats received sterile saline injections of pH 4.0 or 7.4 in the gastrocnemius muscle starting at postnatal day 8. Injections were given unilaterally every other day for 12 days ending on postnatal day 20. A third group received needle prick only on the same shedule as the second group, while a fourth group was left naïve. At 2 months of age, rats underwent assessment of cutaneous and deep somatic sensitivity using von Frey filaments and gastrocnemius muscle pinch, respectively. A visceromotor response (VMR) to graded colorectal distension (CRD; 10–80 mmHg for 30 s with 180 s interstimulus intervals) was recorded. Extracellular single-unit recordings from the thoracolumbar spinal neurons (T13–L1) were performed in adult pH 4.0 injected and naïve controls. There was no difference in the threshold for response to mechanical stimulation of the paw in rats injected with pH 4.0 saline compared to all other groups. Conversely, rats treated with pH 4.0 saline showed a significant bilateral reduction in withdrawal threshold to muscle pinch as adults (P < 0.05). At colorectal distensions ≥ 20 mmHg, an increase in the VMR was observed in the pH 4.0 injected group compared to all other groups (P < 0.05). Spinal neurons were classified as short latency abrupt (SL-A) or short latency sustained (SL-S). Spontaneous firing of SL-S (20.6 ± 2.2 impulses s−1), but not SL-A neurons (5.3 ± 0.9 impulses s−1) in the pH 4.0 treated rats was significantly higher than in control rats (SL-S, 2.6 ± 0.8 impulses s−1; SL-A, 3.1 ± 0.7 impulses s−1). The response of SL-S neurons to CRD in the pH 4.0 group was significantly higher at distension pressures ≥ 20 mmHg. Nociceptive somatic stimulation in neonatal rats results in chronic deep somatic and visceral hyperalgesia in adulthood. Colorectal distension-sensitive SL-S neurons are primarily sensitized to neonatal somatic stimulation.
The early neonatal period is a time during which the nociceptive neuronal circuits are formed, usually in the absence of painful stimuli. These circuits normally require use-dependent activity for appropriate development; however, noxious stimuli during this critical period may alter their development and subsequently result in decreased pain thresholds later in life (Anand, 1998). Animal models have demonstrated a critical time during development in which the spinal cord is vulnerable to permanent structural and functional alterations in pain pathways (Anand et al. 1999; Virgo et al. 2000; Lidow et al. 2001; Ren et al. 2004).
Alterations in visceral and somatic pain sensitivity resulting from noxious insults during the neonatal period have demonstrated conflicting data and have generated important controversy. For example, short-term unilateral inflammation of the hind paw results in visceral and somatic hypoalgesia (Wang et al. 2004). Others have noted somatic hypoalgesia in adult rats after neonatal inflammation with carrageenan, with subsequent hyperalgesia following re-inflammation (Lidow et al. 2001). Neonatal rat pups exposed to paw needle prick show decreased withdrawal latency in response to intense heat and increased latency in exploratory and defensive withdrawal behaviour as adults (Anand et al. 1999). Similarly, colonic irritation in neonatal rats increases colorectal sensitivity to mechanical distension in adults (Al-Chaer et al. 2000). It is now clear that the duration, location and intensity of the neonatal injury plays an important role in determining the long-term effects in sensory processing.
Perhaps the most striking results from these studies relates to the bilateral alterations in sensitivity following a unilateral insult. This observation suggests a central mechanism and reaffirms that the long-term effects are not somatotopically restricted (Ruda et al. 2000; Wang et al. 2004). Sensitization of spinal sensory neurons can result in enhanced neurotransmission, increased neuronal spontaneous activity and decreased firing thresholds (Ren & Dubner, 1996; Ji et al. 2003). Most spinal neurons that receive input from the visceral afferents in the lumbar spinal cord also receive convergent synaptic input from afferents of the deep somatic domain (Janig, 1996). Thereby, any acute alteration in synaptic input to central neurons during a period of great plasticity can potentially result in permanent plastic changes that are manifested in adulthood. The role of somatic stimuli in the development and maintenance of visceral hyperalgesia has not been clearly defined.
The relative role of viscerosomatic convergence in the pathophysiology of functional abdominal pain is not well understood owing to the lack of valid animal models. Alterations in pain responses can occur in human neonates as a result of mechanical stimulation or surgery (Fitzgerald et al. 1989; Andrews & Fitzgerald, 2002). We have previously reported the development of visceral hyperalgesia induced by non-inflammatory, noxious somatic stimulation. Two saline injections (pH 4.0) in the gastrocnemius muscle of adult rats result in visceral hyperalgesia that lasts up to 2 weeks (Miranda et al. 2004). Acute sensitization of colorectal distension-sensitive spinal neurons develops following low pH saline injections in the gastrocnemius muscle of adult rats (Peles et al. 2004). Since the structural and functional connectivity is quite different in the immature adult, increased somatic afferent activity early in the postnatal period may alter spinal neurons in areas of viscerosomatic convergence. We therefore aimed to investigate alterations in sensory processing of somatic and visceral stimuli in adults that result from a noxious somatic insult in the neonatal period.
Methods
Animals
Experiments were performed on 76 adult Male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) weighing 250–350 g. Neonatal rats were born in our facility and were not separated from their mother until 30 days post delivery. After weaning and prior to surgery, rats were housed in groups of two or three in plastic transparent cages containing woodchip bedding and maintained under controlled conditions with 12 h–12 h light–dark cycle. After completion of the experiments, rats were killed by lethal injection of Beuthanasia-D (390 mg pentobarbitone, 50 mg phenytoin sodium, 2% benzyl alcohol; Schering-Plough Animal Health) at a dose of 2 ml kg−1. All protocols were approved by the Animal Care and Use Committee at the Medical College of Wisconsin and are in accordance with the International Association of Pain (IASP) policies on use of laboratory animals.
Neonatal noxious somatic stimulation
Neonatal rats were separated into four experimental groups. The first group received unilateral injections of pH 4.0 saline (0.1 ml) to the gastrocnemius (GN) muscle starting on postnatal day 8 (P8) and contining every other day for 12 days (total of 6 injections; n = 25 rats). The second group received buffered pH 7.4 saline injections on the same schedule, while a third group received needle prick only without saline injections (n = 23 in each group). A fourth group (naïve control) did not receive any injections but was handled in a similar manner to the previous three groups. In general, neonatal rats appeared to experienced pain during the injection with saline in the GN muscle. This is based on the struggling behaviour observed during the injection and occasional vocalization. Vocalization with needle prick only was rare and less severe. No shaking or licking of the paws was noted in any of the groups following injections. Neonates were allowed to grow to 2 months of age without external interference and then underwent surgery for behavioural testing.
Muscle histology
To evaluate possible inflammatory alterations in the GN muscle as a result of pH 4.0 saline injections in the neonatal period, histological analysis of the muscle tissue was performed in adult rats (n = 4). The rats were killed by lethal injection of Beuthanasia-D and the ipsilateral GN muscle was removed and sectioned at 100 μm intervals and stained with Haematoxylin and Eosin. The proximal, middle and distal areas of the muscle were examined by light microscopy in the pH 4.0 injected rats and compared to non-injected controls.
Mechanical withdrawal threshold
Von Frey filaments of various bending forces (100–400 mN) were used to assess any alterations in hind leg sensitivity prior to any surgical procedures in all four groups (pH 4.0, pH 7.4, needle prick and naive). The experimenter was blinded to the neonatal groups. The rats were placed on a screen platform and allowed to acclimate to the environment for 15–20 min before testing. Progressive, increasing forces of filaments were applied to the planter surface of the hind paw bilaterally until the paw was withdrawn. Two trials were recorded in each leg with 5 min intervals between trials. The lowest bending force required to stimulate a withdrawal reflex was recorded as the mechanical withdrawal threshold.
Assessment of deep somatic tissue sensitivity
Compression thresholds of the GN muscle in adult rats were used to assess alteration in deep somatic tissue sensitivity in all four groups. This method has been validated previously in rats using a strain gauge attached to the inner arm of a pair of forceps (Yu et al. 2002; Skyba et al. 2005). The devise was assembled in-house by using strain gauges attached to the tip of a pair of large blunt forceps (contact area 4 × 4 mm). The tip of the forceps during compression of the GN muscle produces an output that is proportional to the applied force. Rats were trained to stay in a gardening glove for 1 h a day for 3 days prior to measurements. Animals were further restrained during testing by placing a hand on their back over the glove while the experimenter extended the hind limb to expose the GN muscle for compression using the devise. The output voltage, after calibration using a known weight suspended by a string, was recorded (grams of force) at the time of either forceful limb withdrawal or vocalization. The experimenter was blinded to the neonatal groups. Three measurements were recorded in each leg at 5 min intervals. The measurements were averaged and taken as the final value for each limb.
Surgical preparation for behavioural study
All surgical procedures were performed after deep anaesthesia with sodium pentobarbitone (50 mg kg−1i.p.). Penicillin G (30 000 U, i.m.) was given in the contralateral thigh preoperatively in all animals to prevent infection. For electromyography (EMG), a pair of Teflon-coated electrodes (Cooner wire, part no. A5631) was implanted in the external oblique muscle. The pair of electrodes was externalized subcutaneously and protected using a siliconized tube sutured to the dorsal aspect of the neck. Postoperatively, animals were given subcutaneous buprenorphine hydrochloride (0.1 mg kg−1) to relieve pain. Rats were housed separately following surgery and allowed to recuperate for at least 7 days before testing.
Visceromotor response (VMR)
The visceromotor response (VMR) to colorectal distension (CRD) was used as an objective measure of visceral sensation in all groups, given its reliability and reproducibility (Ness & Gebhart, 1988b; Ness et al. 1990, 1991). Electromyogram recordings from the external oblique muscles quantified contraction of the abdominal wall musculature in response to graded CRD. Training of individual rats involved a 1 h session in a Bollman cage for three consecutive days. On the recording day a distensible latex balloon (5 cm in length) was inserted into the descending colon and rectum before placing the rats in the Bollman cage. The balloon was attached to a distension device by polyethylene tubing and was kept in place by taping the catheter to the tail. A pressure transducer monitored the intralumen pressure during the stimulus–response function (SRF). Distension pressures (5, 10, 20, 30, 40, 50, 60 and 80 mmHg) were held constant during the 30 s stimulus period, with a 180 s interstimulus interval. The EMG signal from the external oblique muscle was amplified through a low-noise AC differential amplifier (model 1700, A-M Systems, Inc.) and recorded on-line using the Spike 2/CED 1401 data acquisition program (CED 1401; Cambridge Electronic Design, Cambridge, UK).
Adult behavioural study
To confirm that the observed changes in adult rats were a result of changes in the nociceptive neuronal circuitry and not due to a persistent injury lasting longer than 5 weeks, similar studies were carried out in 8 week old adult animals. Adult male Sprague–Dawley rats (350–400 g) received pH 4.0 injections every other day for 12 days (total of 6 injections) 5 days following EMG electrode implantation as described in the Surgical preparation section (n = 8). Similar to the neonatally sensitized adult rats, all animals underwent von Frey and muscle pinch testing as well as assessment of the VMR in response to CRD. Rats were tested before the series of injections and 5 weeks following the six low pH injections.
Electrophysiology
Surgical preparation
Rats were deprived of food, but not water, for 16–18 h before the experiment. Rats were anaesthetized with an initial dose of sodium pentobarbitone (50 mg kg−1i.p.) and maintained with a constant intravenous infusion of 5–10 mg kg−1 h−1 through the right femoral vein. To monitor blood pressure, the left carotid artery was also cannulated. Following tracheal intubation, the rats were paralysed with an initial dose of gallamine triethiodide (10 mg kg−1i.v., Flaxedil, Sigma, St. Louis, MO, USA) and mechanically ventilated with room air (∼60 cycles min−1). Subsequent doses of gallamine triethiodide (5 mg kg−1 h−1) were given as needed to maintain paralysis. The body temperature was kept within the physiological range (36–37°C) with an overhead lamp. The rats were placed in a stereotaxic head holder, and the thoracolumbar (T13–L1) spinal cord was exposed by laminectomy. After removal of the dura membrane, a 1–2 cm saline-soaked gelatin sponge (Gelfoam, Pharmacia Upjohn Company, Michigan, MI, USA) was used to cover the exposed spinal cord segment. The skin was reflected laterally to make a pool for agar solution (Sigma) that was allowed to cool to 38°C prior to pouring. The agar was allowed to harden, and the dorsal surface of the spinal cord was exposed by removing a cubical slice of agar with a scalpel blade. The exposed surface of the spinal cord was covered with warm mineral oil (37°C).
Spinal neuron recordings
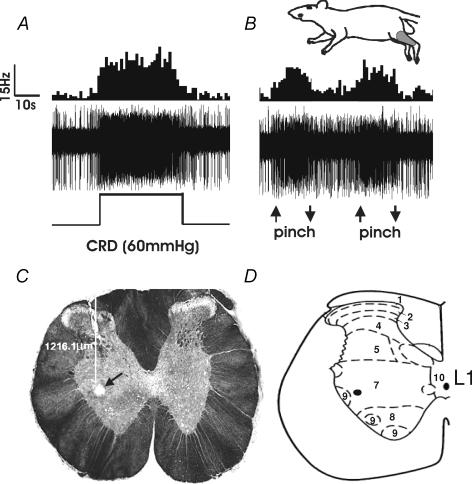
Stainless-steel microelectrodes (3–4 MΩ, FHC, Bowdoinham, ME, USA) were used for extracellular single-unit recording from the thoracolumbar (T13–L1) spinal segments. The placement of the electrode was 0.1–1.0 mm lateral to the spinal mid-line and 0.6–1.8 mm ventral to the dorsal surface. The action potentials were amplified through a low-noise AC differential amplifier (model 3000; A-M Systems) and continuously monitored and displayed on an oscilloscope. A dual window discriminator (model DDIS-1; BAK Electronics) was used to discriminate the action potentials and to convert the signal to a rectangular TTL pulse. The frequency of TTL pulses was counted on-line by using the Spike 2/CED 1401 data acquisition system (Cambridge Electronic Design). Action potentials, intracolonic pressure and blood pressure were recorded on-line. After the experiments, data were analysed using the Wave-Mark analysis method of the Spike 4 software (Cambridge Electronic Design) to distinguish individual action potentials. In order to document the recording site of a CRD-sensitive neuron having a receptive field in the GN muscle, an electrolytic lesion (200 μA, 20 s DC anodal) was made in the spinal cord of a naïve rat. After the electrolytic lesion, the animal was immediately perfused transcardially with ice-cold phosphate buffer solution (pH 7.4) followed by 4% paraformaldehyde. The exposed segments of the spinal cord were then removed and stored overnight in paraformaldehyde. The fixed tissue was then transferred to 30% sucrose buffer for cryoprotection. Serial sections of 30 μm were cut and viewed under a light microscope to locate the lesion.
In order to study the possible alterations in spinal neurons that can account for the visceral and somatic hyperalgesia observed in the behavioural studies, only adult rats in the pH 4.0 group and naïve controls underwent electrophysiological testing. Somatic and visceral sensitivity did not differ in the pH 7.4 and needle prick groups when compared to control animals and so they were not studied. Recordings from the thoracolumbar (T13–L1) spinal cord examined populations of neurons in the two groups. A distensible latex balloon (5 cm) was inserted through the anus into the colon and rectum. The balloon was attached to polyethylene tubing and held in place by taping the tubing to the tail. Colorectal distension-sensitive neurons were identified using brief CRD as the search stimulus (60 mmHg). The neurons were classified as either short latency abrupt (SL-A) or short latency sustained (SL-S) based on their response characteristics to CRD (Ness & Gebhart, 1987, 1991a,b). Short latency abrupt neurons demonstrate increased firing during CRD with an abrupt cessation of response after termination of the distending stimulus, while the SL-S neurons maintain a sustained response (> 4 s) following the distending stimulus. The spontaneous neuronal activity was recorded for each neuron, followed by recording of responses to graded CRD (10, 20, 30, 40, 60 and 80 mmHg of 30 s duration and with 3 min interstimulus time intervals).
Neurons with cutaneous receptive fields were tested to noxious pinch using a pair of non-serrated forceps. Neurons that responded to CRD and tail rotation were considered proprioceptive and were not included in the study.
Data analysis
Statistical analysis was performed using SigmaStat (V2.03, SPSS Inc., Chicago, IL, USA). Analysis of the mechanical withdrawal threshold and GN muscle compression thresholds were conducted by one-way analysis of variance (ANOVA). The contralateral and ipsilateral sides were compared independently, and the data are expressed as the means ± s.e.m. The area under the curve of the EMG recordings during the 30 s stimulus was measured. Data were analysed with two-way repeated measures (ANOVA) and by Student–Newman–Keuls test for multiple comparisons. The baseline spontaneous firing of spinal neurons was calculated by measuring the action potential count over a period of 30 s prior to any distension (data expressed as means ± s.e.m.). In order to obtain changes in response of the neurons to CRD, the spontaneous firing was subtracted from the total action potential counts for each distension pressure during the stimulus–response function. Responses of neurons to CRD were measured as action potential counts over 30 s distension, and were analysed at each distension pressure using one-way repeated measures ANOVA with Bonferoni correction. P < 0.05 was taken as significant.
Results
Mechanical withdrawal thresholds
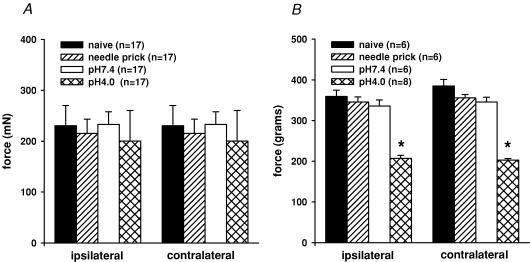
Although no formal testing was performed, gross observation did not demonstrate any alterations in gait or abnormal behaviour in the adult rats. Alterations in hind paw cutaneous sensitivity was quantified by measuring the mechanical withdrawal threshold (MWT) to pricking the plantar surface of the paw with von Frey filaments. There was no difference in the threshold for response to mechanical stimulation of the paw by the various bending forces in rats injected with pH 4.0 saline (200 ± 60 mN) when compared to pH 7.4 saline (230 ± 40 mN), needle prick (215 ± 28 mN), or naïve controls (233 ± 25 mN; Fig. 1A). This suggests the absence of cutaneous hypersensitivity.
Figure 1. Bar graphs representing the bending force of von Frey filaments required to induce paw withdrawal.
Four groups of neonates were tested as adults. A, the naïve controls, needle prick, pH 7.4 and pH 4.0 treated rats did not show any significant alterations in the threshold for response to mechanical stimulation of the paw, suggesting the absence of cutaneous hyperalgesia. B, bar graphs of GN muscle pinch force necessary to initiate leg withdrawal. Only pH 4.0 treated rats showed significant reduction in withdraw threshold, suggesting deep-tissue somatic hyperalgesia (P < 0.05 compared to control rats).
Assessment of deep somatic tissue sensitivity
Long-term changes in somatic sensory perception in all four groups of rats were assessed using deep muscle pinch in the GN muscle. The GN muscle pinch force necessary to initiate leg withdrawal did not differ in the pH 7.4 (ipsilateral, 335 ± 14.9 g and contralateral, 345 ± 11.6 g) and needle-prick groups (ipsilateral, 345 ± 12.5 g and contralateral, 355 ± 8.6 g) when compared to naïve controls (ipsilateral, 359 ± 15.2 g and contralateral, 385 ± 15.7 g, n.s.). Only pH 4.0 treated rats showed a significant bilateral reduction in withdraw threshold suggesting deep-tissue somatic hyperalgesia (ipsilateral, 207 ± 7.2 g and contralateral, 202 ± 3.8 g, P < 0.01, Fig. 1B).
Muscle histology
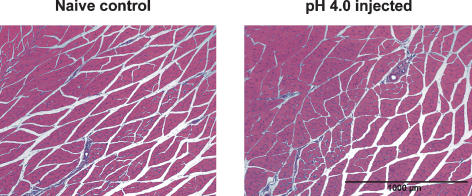
Analysis of tissue histology showed no evidence of tissue necrosis, oedema or lymphocytic infiltration as a result of the low pH injections (n = 4, Fig. 2). Although it is possible that muscle repair could have taken place following the last neonatal injection, these findings are in accordance with previous studies that failed to show significant alterations in muscle histology at different time intervals following pH 4.0 injections in adult rats (Sluka et al. 2001). Figure 2 shows an example of GN muscle histology.
Figure 2. Photomicrographs of muscle histology.
Frozen sections of adult rat GN muscle injected neonatally with pH 4.0 saline was stained with Haematoxylin and Eosin and compared to naïve control muscle. There was no visible damage, inflammation or necrosis of the ipsilateral muscle in the control or pH 4.0 injected rats.
Behavioural response to colorectal distension
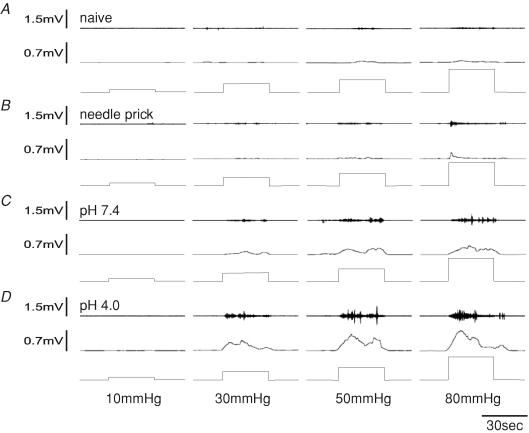
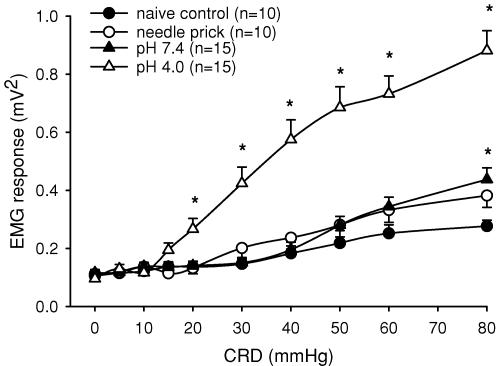
The VMR to CRD was assessed in all groups (pH 4.0 injected, pH 7.2 injected, needle prick and naïve controls) at 2 months of age. All rats exhibited an increase in EMG activity to graded intensities of distension (≥ 30 mmHg). A significant increase in the VMR was observed in the pH 4.0 injected group compared to control rats at distension pressures ≥ 20 mmHg. Since 20 mmHg is not considered noxious in rats and humans (Ness et al. 1991), the altered visceral sensation observed has the characteristics of both allodynia and hyperalgesia (Fig. 3). The naïve control group, which received no injection but similar handling to other groups, exhibited a response greater than baseline at distension pressures ≥ 30 mmHg that peaked at 80 mmHg (0.28 ± 0.3 mV2, Fig. 3). Similarly, the pH 7.4 neonatally treated rats exhibited a progressive increase in response to distension pressures ≥ 30 mmHg that was not different from control rats except at 80 mmHg (0.62 ± 0.3 mV2, n.s. versus controls). Overall, the visceromotor response of needle-prick controls and pH 7.2 injected rats did not differ from that of control rats, suggesting that the low pH injections are necessary to produce visceral allodynia and hyperalgesia at noxious distensions below 80 mmHg. Figure 4 shows the summary data for the VMR responses of the four groups.
Figure 3. Visceromotor responses represented as EMG activity to graded CRD (10, 30, 50 and 80 mmHg, 30 s duration) from a naïve control (A), needle prick (B), a pH 7.4 (C) and a pH 4.0 injected rat (D).
The top trace represents EMG recordings, the middle trace represents rectified EMG recordings (2.5 s intervals) and the bottom trace represents distension pressures. All rats showed a response at distension pressures ≥ 30 mmHg. Responses of the rat injected with pH 4.0 saline (D) were significantly greater than those of the naïve control rat (A) and rat subjected to needle prick (B) at distensions ≥ 20 mmHg.
Figure 4. Summary data of mean VMR at 2 months of age to graded CRD (5–80 mmHg) in naïve control, needle prick, pH 7.4 and pH 4.0 neonatally treated rats.
The pH 4.0 group exhibited a significantly greater response than the control group. Significance was seen at distension pressures of ≥ 20 mmHg, representing the development of visceral hyperalgesia and allodynia as a result of neonatal somatic stimulation with pH 4.0 saline injections (*P < 0.05 versus control group). The pH 7.4 response was also greater than that of the control rats but was only significant at 80 mmHg CRD distension (*P < 0.05 versus control group).
Electrophysiology
Characteristics of CRD-sensitive neurons
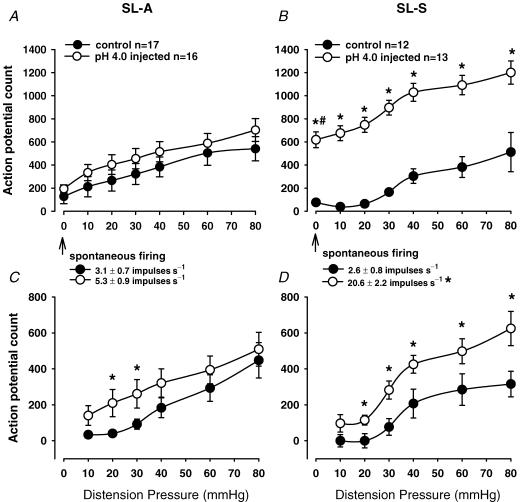
A total of 29 spinal neurons from the thoracolumbar (T13–L1) spinal cord were identified as responsive to CRD from the control group of rats and 29 neurons from the neonatally pH 4.0 injected group. The neurons were classified as SL-A or SL-S in each of the two groups. Figure 5A and B shows an example of a neuron responsive to CRD with the corresponding somatic receptive field in the GN muscle. An example of the recording site of this neuron in the L1 segment is also shown in Fig. 5C and D. Of the 29 neurons identified in the control group, 17 were SL-A and 12 were SL-S. In the pH 4.0 group, 16 were SL-A and 13 were SL-S. The mean spontaneous discharge rate for the SL-A and SL-S neurons in the pH 4.0 group was 5.3 ± 0.9 and 20.6 ± 2.2 impulses s−1, respectively. The baseline spontaneous firing of the SL-A neurons was not different from the control group (3.1 ± 0.7 impulses s−1, n.s.). In contrast, the mean spontaneous activity of SL-S neurons from the pH 4.0 injected group was significantly greater than that of the SL-S control neurons (2.6 ± 0.8 impulses s−1, P < 0.01, Student's paired t test). Figure 6A and B shows the action potential count of the SL-A and SL-S neurons, respectively, compared to control rats. At distension pressure zero, the action potential count represents the spontaneous firing of the neurons over 30 s.
Figure 5. Example of a CRD-sensitive neuron in the L1 spinal segment of a naïve rat with a somatic receptive field in the distal hind limb.
A illustrates the response on the neuron to CRD. The top trace shows the increased response to CRD (60 mmHg, 30 s) represented as a frequency histogram (1 s bin width), the middle trace is the neuron action potential and the bottom trace is the distension pressure. B illustrates the response of the same neuron to pinch of the ipsilateral gastrocnemius muscle. The onset and termination of pinch is indicated by up and down arrows, respectively. The convergent somatic receptive field to pinch in the gastrocnemius muscle is represented in the shaded area of the cartoon. C, the photomicrograph shows the electrolytic lesion (200 μA, 20 s DC anodal) of the recording site for this neuron in the L1 spinal segment (arrow). The depth of recording was 1216.1 μm from spinal cord dorsum. D, schematic representation of the L1 spinal cord segment with corresponding laminae. The black dot represents the recording site.
Figure 6. Summary data of CRD-sensitive spinal neurons in naïve and neonatally injected rats.
Summary data of the mean spontaneous firing rate of CRD-sensitive SL-A (A) and SL-S spinal neurons (B) from the naïve control and neonatally pH 4.0 saline injected rats (total firing). C and D represent the response to distension of SL-A and SL-S neurons, respectively (total minus spontaneous firing). The spontaneous firing of the SL-A neurons in the pH 4.0 group (A) was not different from controls. The response of SL-A neurons to CRD in the pH 4.0 saline injected group was higher at 20–30 mmHg (C, P < 0.05). In contrast, the spontaneous firing of the SL-S neurons in the pH 4.0 group (B) was significantly higher than that of the control group (*P < 0.01) and SL-A pH 4.0 group (#P < 0.01). Similarly, the responses of SL-S neurons to CRD in the pH 4.0 group were significantly higher at distension pressures > 10 mmHg (D, P < 0.05 versus control group).
All neurons studied had convergent cutaneous receptive fields excited by noxious pinch. The skin receptive fields were located in the base of the tail, ipsilateral GN muscle, thigh, gluteal area, and contralateral thigh and gluteal areas. Expansion of the somatic receptive field in the pH 4.0 group was evident in a small percentage of SL-A and SL-S. Approximately 13% of SL-A and 23% of SL-S neurons responded to pinch of the contralateral thigh, while none of the neurons in either the naïve control group had expansion of somatic receptive field that included the contralateral thigh. The characteristics of the neurons studied are given in Table 1.
Table 1. Characteristics of CRD-sensitive thoracolumbar spinal neurons in adult rats.
| Control subgroups | pH 4.0 subgroups | |||
|---|---|---|---|---|
| SL-A | SL-S | SL-A | SL-S | |
| Spontaneous activity (impulses s−1) | 3.1 ± 0.7 | 2.6 ± 0.8 | 5.3 ± 0.9 | 20.6 ± 2.2 |
| Convergent inputs | ||||
| (1) Tail base | 11/17 (65%) | 6/12 (50%) | 10/16 (62%) | 8/13 (62%) |
| (2) Ipsilateral GN muscle | 15/17 (88%) | 10/12 (83%) | 8/16 (50%) | 9/13 (69%) |
| (3) Ipsilateral thigh | 5/17 (30%) | 3/12 (25%) | 9/16 (56%) | 8/13 (62%) |
| (4) Ipsilateral gluteal region | 8/17 (47%) | 7/12 (58%) | 8/16 (50%) | 7/13 (54%) |
| (5) Contralateral thigh | 0/17 (0%) | 0/12 (0%) | 2/16 (13%) | 3/13 (23%) |
The pH 4.0 group received saline injections (pH 4.0) in the GN muscle during the neonatal period and underwent testing at age 2 months. The control group was handled similarly but did not undergo injections. The neurons that responded to CRD were classified in each group as SL-A or SL-S, as described in the Methods. The spontaneous activity of the SL-S neurons in the pH 4.0 group was significantly higher than controls (*P < 0.01), while the SL-A naurons demonstrated no significant difference. Data are means ± s.e.m. Convergent input indicates the number of units with convergent excitatory cutaneous receptive fields exited by noxious stimulus.
Responses of spinal neurons to graded CRD
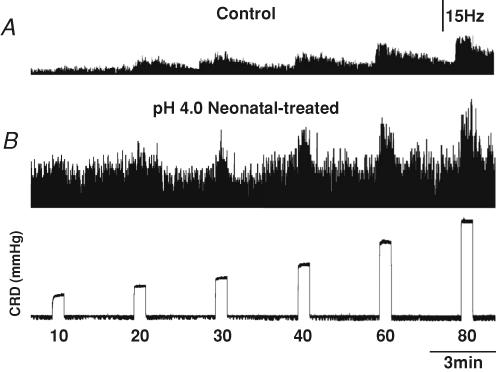
Neuronal responses to graded CRD were recorded in both groups. Figure 7 shows the response of two SL-S neurons from a control (Fig. 7A) and a neonatally saline pH 4.0 injected rat (Fig. 7B) to graded CRD. The spontaneous firing of each individual neuron was subtracted from the total response to CRD for each distension pressure and compared to control values. This gave a true representation of the actual change in neuronal response to colorectal distension, since there was a significant difference in the baseline, spontaneous firing between the SL-A and SL-S neurons in the pH 4.0 group. Comparison of the average responses of SL-A neurons isolated from the pH 4.0 injected group shows that the average response to CRD was greater than in control rats at distension pressures of 20 and 30 mmHg but did not differ from control rats at higher intensities (40, 60 and 80 mmHg). Although the average response of SL-A neurons at these greater intensities of CRD was higher in the pH 4.0 injected group, the possibility of random sampling variability could not be excluded. Figure 6A and C shows the action potential count summary data of the neuronal responses of SL-A in the control and pH 4.0 injected groups. The response to CRD of SL-S neurons in the pH 4.0 injected group was significantly higher than the response in the control rats at intensities ≥ 20 mmHg (Fig. 6B and D). This observation, in addition to the significant increase in spontaneous firing, suggests that SL-S neurons may play a more important role than SL-A neurons in the spinal sensitization resulting from noxious somatic stimulation early in development.
Figure 7. Responses of CRD-sensitive spinal neurons from a control (A) and a pH 4.0 neonatally treated rat (B) to graded CRD.
The top two traces are the activity of the neuron represented as a frequency histogram (1 s bin width), and the bottom trace is the intraluminal pressure of the colon. The neuron from the control rat (depth, 1154.7 μm) exhibited a markedly lower baseline firing rate than the neuron from pH 4.0 neonatally treated rat (1276.0 μm). The neuron from the pH 4.0 neonatally treated rat exhibited a greater response to CRD than the neuron from the control rat.
Adult behavioural study
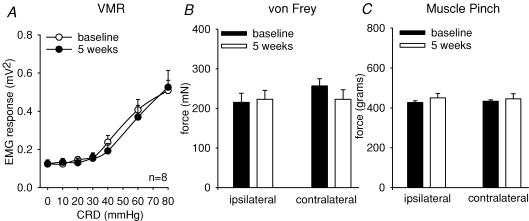
Adult rats that received six pH 4.0 injections showed no change from baseline in the threshold for response to mechanical stimulation (ipsilateral, 215.0 ± 22 mN and contralateral, 256.76 ± 18 mN) when compared at 5 weeks post injection (ipsilateral, 223.2 ± 24 mN and contralateral, 225.6 ± 16 mN; Fig. 8B). Similarly, the GN muscle pinch force at baseline necessary to initiate leg withdrawal did not differ from baseline (ipsilateral, 432.8 ± 7.6 g and contralateral, 427.1 ± 8.2 g) when tested at 5 weeks post injection (ipsilateral, 459.4 ± 6.4 g and contralateral, 450.1 ± 5.5 g; Fig. 8C). The VMR to CRD was also assessed prior to low pH injections and compared at 5 weeks post injection. There was no change in the visceromotor response as a result of the low pH injections in the adult rats (Fig. 8A).
Figure 8. Summary data of adult rats before and 5 weeks following six low pH injections in the GN muscle.
A represents the mean VMR to graded CRD (5–80 mmHg). Rats did not demonstrate any alteration in the VMR as a result of the noxious somatic stimulus (n.s.). B represents the bending force of von Frey filaments required to induce paw withdrawal, and C represents the GN muscle pinch force necessary to initiate leg withdrawal. Rats did not show any alterations in the threshold for response to mechanical stimulation of the paw or muscle pinch, suggesting the absence of cutaneous and deep somatic hyperalgesia.
Discussion
The present study demonstrated that nociceptive somatic stimulation in the form of low pH saline injections applied early in the rat neonatal period results in chronic somatic and visceral hyperalgesia. Two groups of rats treated with unilateral GN muscle saline injections (pH 4.0 or 7.4) on alternating days spanning P8 through P21 were tested as adults. A third group received only needle pricks on the same schedule, while the fourth group was left naïve. Only those that received the noxious pH 4.0 injections demonstrated an increase in the magnitude of the visceromotor response and a lower threshold for response to deep somatic pinch as adults. The present data demonstrate that the likely site of sensitization occurs in spinal areas of viscerosomatic convergence mainly through SL-S neurons. Since no alterations in visceral or somatic sensitivity were observed in adult rats with a similar somatic insult, the present findings validate the existence of a critical, vulnerable window early in the development of the nervous system, which can be associated with long-term neural plasticity and central sensitization.
Transient alterations in visceral sensation resulting from noxious, low pH injections in the GN muscle of adult rats occur through spinal segments of viscerosomatic convergence (Miranda et al. 2004; Peles et al. 2004). The thoracolumbar and lumbosacral segments of the spinal cord receive input from visceral afferents originating from the distal colon and rectum. In adult rats the thoracolumbar (T13–L1) spinal neurons that are excited by visceral afferents have convergent input from afferents of the superficial skin and deep somatic areas (Cervero & Tattersall, 1986). Chronic neonatal hind paw inflammation with Freund's adjuvant (CFA) has been shown to result in spinal segmental alterations (Ruda et al. 2000). In the present study, only spinal segments with known convergence were examined, thus further studies that involve neuronal recording from spinal areas distant from segments of convergence are required in order to explore possible segmental expansion.
The relative contribution of primary sensory afferents in the development and maintenance of visceral and somatic hyperalgesia in the present study remains uncertain. Previous studies have demonstrated sensitization of visceral primary sensory afferents in the thoracolumbar and lumbosacral spinal segments in addition to spinal dorsal horn neurons as a result of neonatal colonic pain and irritation (Al-Chaer et al. 2000; Lin & Al-Chaer, 2003). It is possible that nociceptive nerve terminals from the GN muscle may undergo chronic peripheral sensitization as a result of the low pH injections. This is further suggested by the observed somatic hyperalgesia. However, the bilateral alterations in sensitivity that result from a unilateral stimulus suggest a central mechanism. Nevertheless, an initial barrage of peripheral afferent input is probably necessary to at least initiate and possibly maintain the observed excitability of spinal neurons in the present study. Although an increased density of primary afferents has been shown in previous studies of neonatal injury with CFA (Ruda et al. 2000; Walker et al. 2003), the type and duration of the somatic stimulus used is significantly different from that in the present study and thus warrants further investigation in this model.
Anatomical differences in the primary afferent input or in the local circuitry, such as projection neurons and interneurons, could account for the observed differential effects on the neuronal subtypes observed in this study. Interestingly, the descending inhibitory controls on spinal neurons are immature at birth and do not become fully developed until 2–3 weeks postnatally, the period during which the pH 4.0 injections were given in the present study (Boucher et al. 1998; Fitzgerald & Koltzenburg, 1998; Jennings & Fitzgerald, 1998). Thus, persistent sensory inputs in the immature spinal cord may not be properly modulated, ultimately making inhibitory processing less pronounced in the adult spinal cord. Differences in connectivity, neuronal size, or expression of glutamate receptors early in development may also contribute to altered development of neuronal processing. The precise mechanisms, however, remain poorly defined. Previous studies have noted an overexpression of glutamate receptors in the spinal cord during the perinatal period (Kalb et al. 1992). Expression of NMDA subunits in the rat spinal cord has been shown to peak between P1 and P7 with a decline to adult levels by P35 (Brown et al. 2002). The noxious somatic stimulus in the present study was not given during the peak expression of these receptors, but is likely to have covered a window of amplified expression in the rat spinal cord (P8–P20). Thus, a possible mechanism underlying the present findings of increased neuronal excitability may relate to a decrease in inhibitory interneurons. Overexpression of ionotropic glutamate (NMDA) receptors early may lead to increased calcium fluxes and result in excitotoxicity of inhibitory interneurons in the dorsal horn, resulting in disinhibition and hyperexcitabily of second-order sustained neurons (Mayer & Gebhart, 1994; Fukuoka et al. 1998).
Although it is becoming clear that long-lasting effects develop from perturbation of the immature neuronal circuitry, the exact ‘window of vulnerability’ still remains unclear. Models of neonatal injury vary in duration, intensity and location of injury based on types of inflammatory agents used and the postnatal period at which the stimulus is administered. Certain studies have failed to demonstrate long-term alterations in mechanical or thermal thresholds after neonatal inflammation (Walker et al. 2003; Alvares et al. 2000). Others have shown that inflammation with carrageenan results in long-term abnormal withdrawal responses to noxious stimulation only if the inflammation is induced early (P0). These changes are not evident if the inflammatory insult is given at a later time (P14; Lidow et al. 2001). The central nervous system of rat pups during the first week of development has been postulated to correspond to that of premature human infants (Marsh et al. 1997). Thus, while the present results demonstrate the vulnerability of the immature central nervous system to early life events, it does not theoretically correspond to the premature period in human babies, adding further uncertainty to the ‘window’ of vulnerability. Clinically the development of visceral hyperalgesia is likely to be multifactorial, with psychosocial influences playing a major role. One can also speculate that these long-term alterations in sensory processing are ultimately influenced by neonatal stress. In neonatal pups, handling and maternal separation have been shown to result in visceral and somatic hyperalgesia (Pieretti et al. 1991; Coutinho et al. 2002). The present findings suggest alterations in the spinal components of the nociceptive circuitry as a result of the noxious low pH stimulus rather than neonatal stress, since the other groups remained largely unaffected by handling and needle prick.
A notable finding of the present study was the differential effect of the neonatal somatic stimulus on the activity of the two subtypes of neurons that encode for CRD. Both abrupt and sustained spinal neurons have long axonal projections to the brain and are involved in visceral nociception (Ness & Gebhart, 1987, 1988a). Abrupt neurons in adult rats can be consistently inhibited by noxious stimulation in non-segmental somatic receptive fields and thus have central inhibitory mechanisms that are not present in sustained neurons (Ness & Gebhart, 1991a, 1991b, 2001). Multiple studies have demonstrated that abrupt and sustained spinal neurons can be differentially modulated pharmacologically. The response of CRD-sensitive sustained neurons is dose-dependently inhibited by the κ-opioid receptor agonist fedotozine or the non-NMDA receptor antagonist CNQX, and has minimal effects on abrupt neurons (Ness, 1999; Ji & Traub, 2002). The present study, along with previous reports of a selective increase in the activity of sustained neurons following colonic inflammation in adult rats (Ness et al. 2000) suggests that SL-S neurons may play a more important role in central sensitization and visceral hyperalgesia than SL-A.
There was no alteration in the thresholds for mechanical withdrawal of the paw in response to cutaneous stimulation using von Frey filaments. Furthermore, the excitatory cutaneous receptive field was very similar in the pH 4.0 injected rats and naïve controls. It is known that noxious visceral stimulation in adult animals induces expansion of the somatic receptive field and results in sensitization of responses to mechanical stimuli (Cervero et al. 1992; Euchner-Wamser et al. 1993; Gebhart et al. 1993). A notable difference in the present study was the expansion of the cutaneous receptive field to noxious pinch that included the contralateral thigh only during spinal recording of the pH 4.0 groups. This difference, however, was not enough to exclude the possibility of random sampling variability.
In human neonates, hypersensitivity can occur as a result of surgery, heel lances or gastric suctioning (Andrews & Fitzgerald, 2002; Fitzgerald et al. 1989; Anand et al. 2004). Longitudinal studies are very difficult to perform. Somatic pain experienced early in childhood, such as trauma, surgery or even painful vaccinations, may influence the response and behaviour of central spinal neurons that may ultimately lead to central hyperexcitability. The exact mechanisms leading to chronic, functional abdominal pain in children remain poorly understood and are likely to be multifactorial. However, based on the results of the present study we can postulate that persistent, poorly controlled somatic pain early in development in areas of viscerosomatic convergence may be of great importance in the development and maintenance of central sensitization and consequently altered visceral sensation in children.
Acknowledgments
The work was supported by Children's Research Institute grant awarded to Dr Adrian Miranda and NIH RO1 (DK062312-01A2) awarded to Dr Jyoti N. Sengupta.
References
- Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Alvares D, Torsney C, Beland B, Reynolds M, Fitzgerald M. Modelling the prolonged effects of neonatal pain. Prog Brain Res. 2000;129:365–373. doi: 10.1016/S0079-6123(00)29028-6. [DOI] [PubMed] [Google Scholar]
- Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73:1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- Anand KJ, Coskun V, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term behavioral effects of repetitive pain in neonatal rat pups. Physiol Behav. 1999;66:627–637. doi: 10.1016/s0031-9384(98)00338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand KJ, Runeson B, Jacobson B. Gastric suction at birth associated with long-term risk for functional intestinal disorders in later life. J Pediatr. 2004;144:449–454. doi: 10.1016/j.jpeds.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Andrews K, Fitzgerald M. Wound sensitivity as a measure of analgesic effects following surgery in human neonates and infants. Pain. 2002;99:185–195. doi: 10.1016/s0304-3959(02)00100-8. [DOI] [PubMed] [Google Scholar]
- Boucher T, Jennings E, Fitzgerald M. The onset of diffuse noxious inhibitory controls in postnatal rat pups: a C-Fos study. Neurosci Lett. 1998;257:9–12. doi: 10.1016/s0304-3940(98)00779-4. [DOI] [PubMed] [Google Scholar]
- Brown KM, Wrathall JR, Yasuda RP, Wolfe BB. Quantitative measurement of glutamate receptor subunit protein expression in the postnatal rat spinal cord. Brain Res Dev Brain Res. 2002;137:127–133. doi: 10.1016/s0165-3806(02)00435-2. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM, Pozo MA. Selective changes of receptive field properties of spinal nociceptive neurones induced by noxious visceral stimulation in the cat. Pain. 1992;51:335–342. doi: 10.1016/0304-3959(92)90219-2. [DOI] [PubMed] [Google Scholar]
- Cervero F, Tattersall JE. Somatic and visceral sensory integration in the thoracic spinal cord. Prog Brain Res. 1986;67:189–205. doi: 10.1016/s0079-6123(08)62763-6. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Euchner-Wamser I, Sengupta JN, Gebhart GF, Meller ST. Characterization of responses of T2–T4 spinal cord neurons to esophageal distension in the rat. J Neurophysiol. 1993;69:868–883. doi: 10.1152/jn.1993.69.3.868. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Res. 1998;389:261–270. doi: 10.1016/0165-3806(86)90194-x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Millard C, MacIntosh N. Cutaneous hypersensitivity following peripheral tissue damage in newborn infants and its reversal with topical anesthesia. Pain. 1989;39:31–36. doi: 10.1016/0304-3959(89)90172-3. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABAA receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78:13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Gebhart GF, Meller ST, Euchner-Wamser I, Senguta JN. Modeling visceral pain. In: Vecchiet L, Albe-Fessard D, Lindblom U, editors. New Trends in Referred Pain and Hyperalgesia. Amsterdam: Elsevier; 1993. pp. 129–147. chap. 12. [Google Scholar]
- Janig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- Jennings E, Fitzgerald M. Postnatal changes in responses of rat dorsal horn cells to afferent stimulation: a fibre-induced sensitization. J Physiol. 1998;509:859–868. doi: 10.1111/j.1469-7793.1998.859bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Ji Y, Traub RJ. Differential effects of spinal CNQX on two populations of dorsal horn neurons responding to colorectal distension in the rat. Pain. 2002;99:217–222. doi: 10.1016/s0304-3959(02)00106-9. [DOI] [PubMed] [Google Scholar]
- Kalb RG, Lidow MS, Halsted MJ, Hockfield S. N-Methyl-d-aspartate receptors are transiently expressed in the developing spinal cord ventral horn. Proc Natl Acad Sci U S A. 1992;89:8502–8506. doi: 10.1073/pnas.89.18.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow MS, Song ZM, Ren K. Long-term effects of short-lasting early local inflammatory insult. Neuroreport. 2001;12:399–403. doi: 10.1097/00001756-200102120-00042. [DOI] [PubMed] [Google Scholar]
- Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- Marsh DF, Hatch DJ, Fitzgerald M. Opioid systems and the newborn. Br J Anaesth. 1997;79:787–795. doi: 10.1093/bja/79.6.787. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Ness TJ. Kappa opioid receptor agonists differentially inhibit two classes of rat spinal neurons excited by colorectal distention. Gastroenterology. 1999;117:388–394. doi: 10.1053/gast.1999.0029900388. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J Neurophysiol. 1987;57:1867–1892. doi: 10.1152/jn.1987.57.6.1867. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neurons responsive to noxious colorectal distension in the T13–L2 spinal cord of the rat. J Neurophysiol. 1988a;60:1419–1438. doi: 10.1152/jn.1988.60.4.1419. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988b;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. I. Noxious cutaneous stimuli inhibit visceral nociceptive neurons and reflexes. J Neurophysiol. 1991a;66:20–28. doi: 10.1152/jn.1991.66.1.20. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. II. Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol. 1991b;66:29–39. doi: 10.1152/jn.1991.66.1.29. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Acute inflammation differentially alters the activity of two classes of rat spinal visceral nociceptive neurons. Neurosci Lett 2000 Mar 10. 2000;281:131–134. doi: 10.1016/s0304-3940(00)00832-6. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Inflammation enhances reflex and spinal neuron responses to noxious visceral stimulation in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:649–657. doi: 10.1152/ajpgi.2001.280.4.G649. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Metcalf AM, Gebhart GF. A psychophysiological study in humans using phasic colonic distension as a noxious visceral stimulus. Pain. 1990;43:377–386. doi: 10.1016/0304-3959(90)90035-C. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Randich A, Gebhart GF. Further behavioral evidence that colorectal distension is a ‘noxious’ visceral stimulus in rats. Neurosci Lett. 1991;131:113–116. doi: 10.1016/0304-3940(91)90349-x. [DOI] [PubMed] [Google Scholar]
- Peles S, Miranda A, Shaker R, Sengupta JN. Acute nociceptive somatic stimulus sensitizes neurones in the spinal cord to colonic distension in the rat. J Physiol. 2004;560:291–302. doi: 10.1113/jphysiol.2004.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti S, d'Amore A, Loizzo A. Long-term changes induced by developmental handling on pain threshold: effects of morphine and naloxone. Behav Neurosci. 1991;105:215–218. doi: 10.1037//0735-7044.105.1.215. [DOI] [PubMed] [Google Scholar]
- Ren K, Anseloni V, Zou SP, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Enhanced descending modulation of nociception in rats with persistent hindpaw inflammation. J Neurophysiol. 1996;76:3025–3037. doi: 10.1152/jn.1996.76.5.3025. [DOI] [PubMed] [Google Scholar]
- Ruda MA, Ling QD, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–631. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. J Pain. 2005;6:41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Virgo L, Dekkers J, Mentis GZ, Navarrete R, de Belleroche J. Changes in expression of NMDA receptor subunits in the rat lumbar spinal cord following neonatal nerve injury. Neuropathol Appl Neurobiol. 2000;26:258–272. doi: 10.1046/j.1365-2990.2000.00244.x. [DOI] [PubMed] [Google Scholar]
- Walker SM, Meredith-Middleton J, Cooke-Yarborough C, Fitzgerald M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105:185–195. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- Wang G, Ji Y, Lidow MS, Traub RJ. Neonatal hind paw injury alters processing of visceral and somatic nociceptive stimuli in the adult rat. J Pain. 2004;5:440–449. doi: 10.1016/j.jpain.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Yu YC, Koo ST, Kim CH, Lyu Y, Grady JJ, Chung JM. Two variables that can be used as pain indices in experimental animal models of arthritis. J Neurosci Meth. 2002;115:107–113. doi: 10.1016/s0165-0270(02)00011-0. [DOI] [PubMed] [Google Scholar]