Abstract
Suppressor of cytokine signalling-3 (SOCS-3) has been implicated in the onset of insulin resistance in non-muscle tissue. Thus, we examined the effects of exercise training on SOCS-3 expression and the potential role of SOCS-3 in muscle. Female Sprague-Dawley rats (5–8 months) were treadmill trained for 12 weeks and the muscles were removed 24 h after the last bout of exercise. Exercise training increased SOCS-3 mRNA expression by 80% and 154% in the plantaris and soleus muscle, respectively. To mimic the effects of increased SOCS-3 expression, SOCS-3 cDNA was cotransfected with a NF-kappa B (NF-κB) luciferase construct into cultured C2C12 myotubes. SOCS-3 overexpression increased NF-κB transcriptional activity by 27-fold. The proximal region of the IL-6 gene promoter contains a NF-κB consensus site, which contributes to increased IL-6 expression in various tissues. SOCS-3 cDNA was cotransfected into cultured C2C12 myotubes with either the IL-6 luciferase construct or a mutated NF-κB IL-6 luciferase construct. SOCS-3 overexpression increased IL-6 transcriptional activity by 15-fold, however, when the NF-κB site was mutated SOCS-3 failed to increase IL-6 transcriptional activity. We subsequently found that IL-6 mRNA expression was elevated in the plantaris and soleus muscles of the trained animals compared to the sedentary animals. Finally, exercise induced a significant reduction in IκBα and increased phosphorylation of Iκκ suggesting that NF-κB activation was elevated after exercise training. These data suggest that training-induced elevations in SOCS-3 expression in skeletal muscle may contribute to the exercise-induced increase in IL-6 expression through alterations in the mechanisms that mediate NF-κB activity.
Increased physical activity results in phenotypic changes in skeletal muscle that alter both physiological and metabolic function (Spangenburg & Booth, 2003). Physical activity also results in improved insulin sensitivity of skeletal muscle (Holloszy, 2005). Numerous studies have reported that habitual exercise can prevent or attenuate the onset of various chronic conditions such as obesity or type 2 diabetes (Booth et al. 2002). Unfortunately, at this time the molecular and cellular mechanisms underlying the exercise-induced enhancement of skeletal muscle function remain elusive.
In 1982, it was first demonstrated that habitual exercise improved insulin sensitivity of skeletal muscle (Richter et al. 1982). The mechanism that mediates the enhancement of insulin sensitivity in response to exercise has remained elusive. For example, the enhanced insulin sensitivity after exercise is not due to an increase in activation of the insulin-signalling pathway (Treadway et al. 1989; Wojtaszewski et al. 1997, 2000). Thus, it appears that the effect on insulin sensitivity induced by exercise occurs independently of insulin's action on muscle (Holloszy, 2005). Another possibility to consider is that exercise may reduce the expression of genes that modulate the insulin-signalling pathway in muscle.
Recent investigations have suggested that members of the suppressor of cytokine signalling gene family (SOCS) contribute to the development of insulin resistance in peripheral tissue (Rui et al. 2002; Shi et al. 2004; Ueki et al. 2004). The SOCS gene family consists of eight family members (cis and SOCS-1–7), with each family member having a conserved SOCS-box motif in the C-terminal region of the protein (Alexander & Hilton, 2004). In addition, all of the family members contain a SH2-domain which allows the SOCS proteins to interact with key phosphotyrosine regions of various signalling proteins or membrane-bound receptors (Alexander & Hilton, 2004). SOCS-1 and SOCS-3 also contain a kinase inhibitory region in the N-terminal domain that can reduce or attenuate kinase activity of various signalling proteins. Expression of the SOCS gene family is induced by numerous 4cytokines (Emanuelli et al. 2001), growth factors (Spangenburg, 2005), and hormones (Emanuelli et al. 2000; Steppan et al. 2005). Recent data have suggested, that the onset of insulin resistance in peripheral tissue is induced by inflammatory cytokines released by macrophages present in adipose tissue (Fain et al. 2004). SOCS-1 and SOCS-3 expression can be induced by a number of cytokines including tumour necrosis factor (TNF)-α (Fasshauer et al. 2004). It is this described function that has made the SOCS family an attractive target for explaining the onset of insulin resistance in various tissues. Indeed, a recent investigation found that overexpression of SOCS-3 induced insulin resistance in liver cells by interacting with the IRS-1 and IRS-2 (Ueki et al. 2004). Similar findings have also been found for SOCS-3 in cultured COS-7 cells (Emanuelli et al. 2001) and adipocytes (Shi et al. 2004). These findings have led to the suggestion that increased SOCS-3 expression can result in insulin resistance and thus potentially lead to the onset of the metabolic syndrome.
The purpose of this investigation was to determine the effect of regular physical activity on the expression of suppressor of cytokine signalling-3 in skeletal muscle and examine a potential molecular mechanism for SOCS-3 expression. We hypothesized that SOCS-3 expression would decrease in skeletal muscle after regular physical activity. This hypothesis was generated based on the fact that regular physical activity improves insulin sensitivity in skeletal muscle (Holloszy, 2005), and overexpression of SOCS-3 results in insulin resistance in various cell culture models (Emanuelli et al. 2001; Shi et al. 2004).
Methods
Animal model and treadmill training
Female Sprague-Dawley rats (age 2–3 months) were randomly assigned to a chronic exercise training (trained) group (n = 9) or a sedentary group (n = 10). All animals were housed in the same facility with a 12: 12 h light–dark cycle, with food and water provided ad libitum. The first two weeks consisted of a familiarization period where animals ran 20 m min−1, with running duration gradually increased from 5 to 30 min over this time (Brown et al. 2003). Treadmill speed and running duration were increased over the next 6 weeks to 20 m min−1 (10 min), 28 m min−1 (30 min), and 35 m min−1 (20 min), and this protocol was maintained until 12 weeks (Brown et al. 2003). Twenty-four hours after the last exercise bout the animals were anaesthetized with sodium pentobarbital (35 mg kg−1; i.p. injection). The soleus and plantaris muscles were carefully dissected and frozen in liquid nitrogen. The muscles were then stored at −80°C until needed. The study was conducted under the guidelines accepted by the American Physiological Society and received prior approval from the Institutional Animal Care and Use Committee at the University of Colorado at Boulder.
Citrate synthase activity
Citrate synthase activity was measured according to previously described methods (Srere, 1969) and normalized to the protein content of the sample.
Reverse transcription of total RNA
Total RNA was isolated according to previously described methodology (Spangenburg et al. 2003). One microgram of total RNA was reversed transcribed. Briefly, the RNA was incubated with Super Script II reverse transcriptase (Invitrogen, San Diego, CA, USA), mixed oligo (dT), random decamers (Ambion, Austin, TX, USA) in a 25 μl reaction at 42°C for 50 min. The reaction was inactivated by incubation at 70°C for 15 min. The samples were subsequently stored at 4°C for later use.
PCR
All methods have been previously described (Spangenburg et al. 2003; Spangenburg, 2005). Briefly, a semiquantitative form of PCR was employed using 18S as a standard (Ambion) for each reaction. The following primer sequences were utilized (5′→ 3′): SOCS-3 sense (ATGGTCACCCACAGCAAGTTCCCG), SOCS-3 antisense (TTAAAGTGGAGCATCATACTGATCC). All primers were purchased from Invitrogen. The SOCS-3 primers produced a product size of 550 bp and were designed based on GenBank accession number AFO 75383.
Two microlitres of each reverse transcription reaction were mixed with 12.5 μl of AccuPrime Super Mix II (Invitrogen), 0.5 μm 18S primer/competimer mix, and 0.2 μm target primer mix in final 25 μl volume. SOCS-3 amplifications were performed in an Eppendorf Mastercycler with an initial denaturing step of 94°C for 2 min, followed by 30 cycles of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C as previously described (Huang et al. 2003). The final cycle ended with 10 min at 72°C. The signal determined for each target was subsequently normalized to the signal for the 18S target as previously described (Spangenburg et al. 2003; Spangenburg, 2005).
Skeletal muscle protein isolation
The muscle tissue was homogenized on ice in buffer containing 50 mm Hepes (pH 7.4), 0.1% Triton X-100, 4 mm EGTA, 10 mm EDTA, 15 mm Na4P2O7H2O, 100 mmβ-glycerophosphate, 25 mm NaF, 50 μg ml−1 leupeptin, 50 μg ml−1 pepstatin, 40 μg ml−1 aprotinin, 5 mm Na3VO4, and 1 mm PMSF. After homogenization, the samples were stored at −80°C. The protein concentration of the samples was determined in triplicate via the Bradford procedure (Bio-Rad Protein Assay, Hercules, CA, USA).
IκBα and Iκκ phosphorylation immunoblotting
Equal amounts of total protein (75 μg for plantaris muscle and 50 μg for the soleus muscle) were resolved on 8% SDS-PAGE gels and transferred to PVDF membranes. All blots were then incubated with Ponceau S (Sigma) to ensure equal loading in all lanes (data not shown). The membranes were then blocked with 5% non-fat dry milk in Tris-buffered saline with 0.1% Tween (TBS-T) and probed with either an antibody specific for total IκB or the phosphorylated form of Iκκ (Ser176/180) (Cell Signalling, Beverley MA (1: 1000)). The membranes were washed in TBS-T and then incubated for 1 h with horseradish peroxidase (HRP)-conjugated rabbit secondary antibody (1: 2000; Cell Signalling, Beverley MA, USA). Finally, the HRP activity was detected using enhanced chemiluminescence reagent (Pierce Rockford, IL, USA.). The membrane was exposed to autoradiographic film (Kodak-XAR5) with the exposure time adjusted to keep the integrated optical densities (IOD) within a linear and non-saturated range.
C2C12 cell culture conditions
All cell culture experiments were performed using C2C12 mouse myoblasts (ATCC; Rockville, MD, USA), which were maintained at a subconfluent density at 37°C in 10% CO2 in Dulbecco's modified Eagle's medium (DMEM, Life Technologies, Rockville, MD, USA) supplemented with 20% fetal bovine serum (Life Technologies) and 1% penicillin–streptomycin antibiotic (Life Technologies). Differentiation was induced by transferring the myoblasts to DMEM media containing 2% horse serum (HS) and 1% penicillin–streptomycin antibiotic.
Transient transfection of myotubes
Transient transfections were performed with Lipofectamine PLUS reagent (Invitrogen, CA, USA) according to manufacture's directions and previously described methods (Spangenburg et al. 2004). The 2.1 Kb IL-6 promoter and the mutated IL-6 promoter (Xiao et al. 2004), pEF-driven SOCS-3 cDNA expression construct (Starr et al. 1997), and the NF-κB sensor construct (Stratagene, La Jolla, CA, USA) were utilized. The NF-κB sensor construct contains four multimerized consensus cis-binding elements for the NF-κB transcription factors and was utilized to quantify NF-κB activity in a similar manner as previously described (Spangenburg et al. 2004). The mutated IL-6 promoter contained a non-functional NF-κB binding site within the context of the 2.1 Kb promoter as previously described (Xiao et al. 2004). Briefly, all transfections were carried out on 80% confluent myoblasts in 24-well tissue culture plates with a total of 1.0 μg of DNA per transfection in serum-free DMEM. All assays were performed using equimolar ratios of DNA, with the total amount of DNA per transfection adjusted using the parent vector of SOCS-3 in order to ensure that each well received 1.0 μg of cDNA as previously described (Vyas et al. 2002; Spangenburg et al. 2004). After completion of the transfection, the culture media was changed to differentiation media for 96 h after completion of the transfection. After 96 h, the experiments were terminated by the lysis of the myotubes. Each experiment was conducted three different times (i.e. different sets on different days) with each set having n = 4 different cultures per experiment. All of the data were then pooled, resulting in 12 different measurements made for each group.
Cell lysis and luciferase measurements
Cell lysis and dual-luciferase measurements were performed utilizing the dual-luciferase assay kit (Promega, Madison, WI, USA), as previously described (Vyas et al. 2002). At the prescribed time point, the cells were gently washed twice with PBS, cell lysis buffer was then added, and the plates were gently rocked for 15 min at 4°C. The homogenates were then transferred to a microcentrifuge tube and centrifuged at 13 000 r.p.m. for 1 min. The supernatant was retained and stored at −80°C. All luciferase measurements were normalized to the thymidine kinase-driven Renilla plasmid as internal control as previously described (Vyas et al. 2002). For all conditions, at least three independent experiments were performed, each consisting of 3–4 separate measurements.
Statisitics
All data are expressed as mean ± s.e.m. Statistical significance was determined using a one-way analysis of variance for multiple comparisons followed by a Tukey's post hoc test. A P value of <0.05 was considered significant.
Results
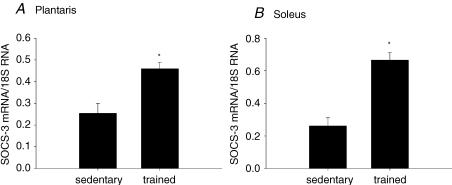
Rats were treadmill trained for 12 weeks, which resulted in a 62% statistically significant increase in citrate synthase activity in the plantaris muscle compared to the plantaris muscles of the sedentary animals (data not shown). The exercise training increased SOCS-3 mRNA expression by 80% and 154% in the plantaris and soleus muscles of the trained animals when compared to the muscles from the sedentary animals, respectively (Fig. 1A and B). There was no difference in 18S RNA expression between the sedentary and exercise-trained groups (data not shown). These data directly contradicted our initial hypothesis, in which we predicted a decline in SOCS-3 expression with exercise training.
Figure 1. Exercise training increases SOCS-3 mRNA expression in skeletal muscle.
SOCS-3 mRNA is increased in the plantaris (A) and soleus muscle (B) after 12 weeks of treadmill training compared to the sedentary animals. *Significantly different from the sedentary group (P < 0.05). n = 10 per group.
To understand the role of enhanced SOCS-3 expression in skeletal muscle, we utilized C2C12 myotubes (Fig. 2A) which allowed us to over-express SOCS-3 cDNA mimicking the SOCS-3 expression trends of our trained animals. Previously it has been suggested that a link may exist between NF-κB and SOCS-3 expression (Cai et al. 2005). Thus, SOCS-3 cDNA was cotransfected with a NF-κB sensor plasmid (i.e. contains four consensus-binding elements that drive the luciferase reporter construct) in cultured myotubes. Overexpression of SOCS-3 (pEF-SOCS-3) increased NF-κB transcriptional activity by 27-fold compared to the myotubes that were transfected with the empty plasmid (pEF) (Fig. 2B).
Figure 2. Overexpression of SOCS-3 enhances NF-κB transcriptional activity in cultured myotubes.
A, a representative picture of the C2C12 myotubes after 96 h of differentiation. Scale bar, 100 μm. B, SOCS-3 cDNA (pEF-SOCS-3, 0.5 μg) or the empty vector (pEF, 0.5 μg), a NF-κB sensor luciferase construct, and a TK-renilla construct were transiently cotransfected into C2C12 myoblasts. The myoblasts were induced to differentiate for 96 h until myotubes readily covered the entire plate. These results are from three separate independent experiments. *Significantly different from the pEF group (P < 0.05).
Skeletal muscle has been shown to enhance production of interleukin-6 (IL-6) after bouts of exercise (Steensberg et al. 2001). This increase in IL-6 expression appears to be regulated at the transcriptional level (Steensberg et al. 2001). Interestingly, in the proximal region of the IL-6 promoter is a NF-κB-binding element, which appears to play an important regulatory role in IL-6 gene transcription (Xiao et al. 2004) (Fig. 3A). Thus, we cotransfected the C2C12 myotubes with the SOCS-3 cDNA and the 2.1 Kb IL-6 promoter reporter construct (IL-6-luc). We found that overexpression of SOCS-3 resulted in a significant 15-fold increase in IL-6 promoter activity compared to the myotubes transfected with the empty plasmid (Fig. 3B). Subsequently, we cotransfected the myotubes with the SOCS-3 cDNA and either the IL-6 promoter construct or an IL-6 promoter construct in which the NF-κB-binding element has been mutated (IL-6-mut-luc) rendering it non-functional. Interestingly, we found two effects. First, mutation of the NF-κB-binding element resulted in enhanced basal IL-6 promoter activity over the control IL-6 promoter construct (Fig. 3B). However, when the mutated IL-6 promoters were cotransfected with the SOCS-3 cDNA there was no significant difference in IL-6 promoter activity (Fig. 3B). This suggests that SOCS-3 is increasing IL-6 promoter activity by some mechanism involving NF-κB-driven transcriptional activity.
Figure 3. Overexpression of SOCS-3 enhances IL-6 transcriptional activity through the proximal NF-κB-binding element in cultured myotubes.
A, the proximal region of the IL-6 promoter contains a NF-κB-binding element (noted in bold letters and underlined). Please note that the IL-6 promoter that was transfected was ∼2.1 Kb in length and that only a portion of the promoter is shown here. B, SOCS-3 cDNA (pEF-SOCS-3, 0.5 μg) or the empty vector (pEF, 0.5 μg), IL-6 luciferase construct (IL-6-luc) or the IL-6 promoter construct with the mutated proximal NF-κB consensus site (IL-6 mut-luc) (Xiao et al. 2004), and a TK-Renilla construct were transiently cotransfected into C2C12 myoblasts. The horizontal bars indicate the quantitative difference when the cells either overexpress or do not overexpress SOCS-3, with pEF-SOCS-3 statistically increasing IL-6 luciferase activity by 15-fold and pEF-SOCS-3 inducing a non-statistical (NS) increase in the mut-IL-6 promoter activity of 2-fold. The myoblasts were induced to differentiate for 96 h until myotubes readily covered the entire plate. These results are from three independent experiments. *Significantly different from the pEF-IL-6 luc group (P < 0.05) and #signicantly different from the pEF-SOCS-3-IL-6 luc group (P < 0.05).
Based on our results in our SOCS-3 overexpression studies, we sought to determine whether IL-6 expression was elevated after exercise training. Indeed, we found that exercise training increased basal IL-6 mRNA expression by 20% and 27% in the plantaris and soleus muscles compared to the muscles taken from the sedentary animals, respectively (Fig. 4A and B).
Figure 4. IL-6 mRNA is increased in muscle after exercise training.
IL-6 mRNA is increased in the plantaris (A) and soleus muscle (B) after 12 weeks of treadmill training compared to the sedentary animals. *Significantly different from the sedentary group (P < 0.05). n = 10 per group.
NF-κB's ability to increase transcription is based on the localization of the transcription factor in either the cytoplasm or the nucleus (Delhalle et al. 2004). More specifically, the location of the transcription factor is determined by the binding of NF-κB to Iκα (Delhalle et al. 2004). In order to induce translocation of NF-κB, IκBα is ubiquinated and degraded, which results in the release of NF-κB allowing it to translocate to the nucleus (Delhalle et al. 2004). The activation of the sequence that results in the ubiquination of IκBα is preceded by the phosphorylation of Iκκ, which subsequently targets Iκα for ubiquination (Delhalle et al. 2004). Thus, as previously described (Ji et al. 2004; Ho et al. 2005) we measured the total expression of IκBα and the phosphorylation levels of Iκκ as an index of NF-κB activation. We found that the total expression of IκBα was significantly decreased by 16% and 26% in the plantaris and soleus muscles compared to the sedentary animals, respectively (Fig. 5A and B). The phosphorylation status of Iκκ was significantly increased by 44% in the soleus muscle from the trained group compared to the sedentary group (Fig. 6A and B). However, no differences were detected in the phosphorylation status of Iκκ between the plantaris muscles taken from the sedentary and trained groups.
Figure 5. Exercise training decreases IκBα expression in skeletal muscle.
IκBα total protein expression decreases in the plantaris (A) and soleus muscle (B) after 12 weeks of treadmill training compared to the sedentary animals. *Significantly different from the sedentary group (P < 0.05). n = 6 per group.
Figure 6. Iκκ phosphorylation levels after exercise training in skeletal muscle.
Iκκ phosphorylation levels was not enhanced in the plantaris (A), but was increased in the soleus muscle (B) after 12 weeks of treadmill training compared to the sedentary animals. *Significantly different from the sedentary group (P < 0.05). n = 6 per group.
Discussion
There are several novel aspects of this study. For the first time we present data indicating that exercise training results in enhanced SOCS-3 mRNA expression in skeletal muscles that contain both a fast and slow phenotype. Our data also demonstrate for the first time in cultured myotubes that increased SOCS-3 expression results in enhanced NF-κB transcriptional activity. The increase in NF-κB transcriptional activity appears to mediate increases in IL-6 promoter activity. Subsequently, we found that exercise training also resulted in increased basal levels of IL-6 mRNA expression, which was associated with decreased expression of IκBα and enhanced phosphorylation of Iκκ both of which are necessary for NF-κB activation. These data suggest for the first time a potential molecular mechanism mediated by exercise-induced SOCS-3 expression which results in increased NF-κB activation (Fig. 7).
Figure 7. A hypothetical model which depicts habitual exercise training increasing SOCS-3 mRNA (and potentially SOCS-3 protein) expression in skeletal muscle, which may activate mechanisms responsible for increased IL-6 production.
The increase in SOCS-3 expression appears to have the ability to increase NF-κB transcriptional activity by potentially increasing the degradation of IκBα. The increase in SOCS-3 expression may enhance the transcriptional activation of the IL-6 promoter through the increased activity of NF-κB, resulting increased IL-6 expression. It should be noted that portions of this figure (depicted in underlined font) are based on the following previously published literature: the exercise-induced transcriptional activation of NF-κB (Ji et al. 2004; Ho et al. 2005), the increased secretion of IL-6 protein from the muscle cell (Steensberg et al. 2002; Febbraio & Pedersen, 2005), and the increased degradation of IκBα/β (Ji et al. 2004).
Exercise training has been know for numerous years to increase insulin sensitivity of skeletal muscle (Richter et al. 1982; Heath et al. 1983; Holloszy, 2005). This is a critical finding since quantitatively, skeletal muscle is the dominant insulin-sensitive tissue in the body (Holloszy, 2005). This would lead one to predict that any gene product that potentially may induce insulin resistance would exhibit reduced expression in skeletal muscle with exercise training. Previously, a number of investigations in non-muscle tissues have found that increased expression of SOCS-3 results in decreased insulin sensitivity, and reduced SOCS-3 expression results in increased insulin sensitivity (Emanuelli et al. 2000, 2001; Shi et al. 2004). These published data led us to hypothesize that in skeletal muscle regular exercise training would reduce SOCS-3 expression. The observations that exercise training elicited an increase in SOCS-3 expression in both the soleus and plantaris muscles were unanticipated. Consequently, we have confirmed these findings in another set of exercised animals (data not shown). Thus, these data indicate that since exercise does not induce insulin resistance in skeletal muscle (Richter et al. 1982; Heath et al. 1983; Holloszy, 2005), it is unlikely that increased expression of SOCS-3 induces insulin resistance in skeletal muscle under the conditions of this experiment. These findings concurs with prior conclusions that found that SOCS-3 expression was not related to insulin resistance in human skeletal muscle taken from obese non-diabetic individuals (Rieusset et al. 2004). This suggests that the role of SOCS-3 in skeletal muscle may differ from what was previously defined in other tissues, and warrants further potential investigation. This possibility is not unreasonable based on the findings that overexpression of SOCS-6 in skeletal muscle improved insulin sensitivity (Li et al. 2004).
Skeletal muscle has been shown to produce and release IL-6, which results in significant increases of IL-6 in the serum (Carey & Febbraio, 2004; Febbraio & Pedersen, 2005). Further, evidence has shown that acute exercise results in increased levels of IL-6 mRNA in skeletal muscle, although the mechanisms for this induction remain undefined (Steensberg et al. 2001). Febbraio & Pedersen, (2005) dubbed IL-6 as a ‘myokine’ and suggest that IL-6 may act to alter or enhance mobilization of substrates that could be used as energy sources by skeletal muscle. In addition, recent data have suggested that IL-6 can improve insulin sensitivity in skeletal muscle (Weigert et al. 2005). Although, exercise clearly results in the production of IL-6 by skeletal muscle, the mechanism that induces IL-6 mRNA upregulation is unclear at the moment. Here our data suggest that exercise-induced increases in SOCS-3 expression have the ability to enhance activation of NF-κB in skeletal muscle. Interestingly, the IL-6 promoter contains a consensus NF-κB-binding site (Fig. 3A) in the proximal region of the promoter (Xiao et al. 2004), which appears to be critical for the induction of increased IL-6 promoter activity by SOCS-3. This finding is in agreement, with previously published data that overexpression of SOCS-3 in cultured macrophages results in enhanced transcriptional activation of NF-κB (Park et al. 2003). It also should be noted that in cultured myotubes, IL-6 promoter activity is inhibited by NF-κB since the mutation of the NF-κB-binding site resulted in increased activity, however, when SOCS-3 was co-expressed with the mutated IL-6 promoter there was failure to significantly increase activity of the IL-6 promoter. This mechanistic dichotomy of NF-κB may occur due to the fact that the transcription factor usually exists as a dimer, which can be made up of any combination of p50(p105), p52 (p100), p65, Rel-B, and c-Rel (Delhalle et al. 2004). Thus, it is possible that depending upon which complex of proteins is recruited to the promoter, there will be differing activation of the IL-6 promoter. There is precedence for this in muscle, as it has been found that during muscle atrophy NF-κB-driven gene transcription was increased; however, the NF-κB complex did not consist of the prototypical p50/p65 heterodimer, instead they found that Bcl-3-p50 heterodimer was contributing to the activation of the NF-κB-driven transcription (Hunter et al. 2002). This contrasts the other recent data which found that acute exercise induced translocation of p50 to the nucleus in muscle (Ji et al. 2004). Thus, it is possible that activation levels of the promoter for that target gene depend on which NF-κB heterodimer is present on the cis-element. Our culture data were in agreement with our animal data in that basal levels of IL-6 mRNA were in fact elevated in the trained soleus and plantaris muscles. Further, decreased expression of IκBα and enhanced phosphorylation of Iκκ, both of which are necessary for activation of NF-κB were found in the trained muscles versus the muscles taken from the sedentary animals. These data agree with very recent suggestions that NF-κB is activated in skeletal muscle by exercise (Ji et al. 2004; Ho et al. 2005). Thus, our data coupled with previously published data suggest that exercise increases SOCS-3 expression in muscle, and the increase in SOCS-3 potentially contributes to increases in IL-6 transcription through an unknown interaction with the NF-κB signalling pathway (Fig. 7).
These findings are interesting in that SOCS-3, IL-6, and NF-κβ have all been suggested to contribute to peripheral insulin resistance (Perseghin et al. 2003; Ueki et al. 2004) and thus contribute to the onset type 2 diabetes. However, in our hands and in the hands of others all of these gene products (SOCS-3 or IL-6) or activation levels (NF-κB) have been shown to increase in skeletal muscle with exercise (Steensberg et al. 2001; Ji et al. 2004; Ho et al. 2005). The generation of transgenic mice that specifically overexpress IL-6 (Lieskovska et al. 2003) and activated Iκκ (Cai et al. 2004) (leads to hyperactivation of NF-κB) have been shown to develop smaller skeletal muscles. Interestingly, hyperactivation of NF-κB in skeletal muscle did not result in insulin resistance of the muscle, however, NF-κB activation in the liver did result in overall systemic insulin resistance (Cai et al. 2004, 2005). Further, infusion of recombinant IL-6 onto the tibalis anterior muscle resulted in muscle atrophy, which correlated with increase SOCS-3 mRNA expression (Haddad et al. 2005). Finally, increased SOCS-3 expression in skeletal muscle has been implicated in the muscle atrophy process as well (Lieskovska et al. 2003; Haddad et al. 2005). Thus, at this moment it is unclear how three distinct genes appear to have some sort of complex interplay and yet are all found to be active in very contradicting processes (e.g. exercise, insulin resistance, and muscle atrophy). One possible explanation is that NF-κB actually exists as a complex of proteins (Delhalle et al. 2004). More specifically, NF-κB can be a homodimer of p50 or p65 or a hetrodimer of p50 and p65 (Delhalle et al. 2004). It has been suggested that the form of NF-κB that is activated may dictate what ultimate physiological result is achieved in skeletal muscle. It is possible that a similar finding may apply for SOCS-3 and IL-6, in the context that elevation of expression of these genes becomes critical for the determination of the functional outcome in the muscle. What is clear is that these findings indicate that it is necessary to conduct more detailed experiments concerning the role of SOCS-3 in skeletal muscle.
Habitual exercise training induces many phenotypic changes in skeletal muscle. At this time investigators have only begun to recently unravel the molecular and cellular mechanisms that contribute to these training-induced changes. Here, our data indicate that chronic exercise induced increases in SOCS-3 gene expression in both the soleus and plantaris muscles. We have suggested that this increase in SOCS-3 expression may result in increased basal levels of IL-6 mRNA in the muscle due to increased transcriptional activation of the IL-6 gene promoter. All of our measures that were taken from the animal were made 24 h after the last bout of exercise. We expect that this was sufficient time to ensure that any changes measured are due to an effect of the chronic training and not of the last bout of exercise completed by the animal. However, it is possible and should not be ruled out that the increased SOCS-3 and IL-6 mRNA levels could in fact be due to changes induced by the last bout of exercise completed by the animal. This point must be considered since there is precedence for elevations in gene expression to be totally returned to baseline 24 h after the last bout of exercise; however, the same investigation also found that transcriptional activation of other genes had not returned to baseline 24 h after the last bout of exercise (Hildebrandt et al. 2003). Clearly, more studies will need to be conducted to clarify this point.
In conclusion, these data demonstrate for the first time that exercise training increases SOCS-3 expression in both fast and slow fibre-dominated skeletal muscle. In addition, the data suggest that exercise-induced increases in IL-6 production may be mediated by SOCS-3's ability to affect NF-κB-induced transcription of the IL-6 promoter. Clearly, the role of SOCS-3 in skeletal muscle is complex and will require more experiments to elucidate the role it plays in muscle function.
Acknowledgments
The authors wish to thank Emily Pettycrew and Brittany Kearney for their expert technical assistance. The authors also wish to thank Dr William Farrar for his kind contribution of the IL-6 and mutated IL-6 luciferase plasmids. These experiments were supported in part by National Institutes of Health grants HL 40306-15 (RLM) and HL 72790-02 (RLM).
References
- Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size after ischemia-reperfusion in rat heart. J Appl Physiol. 2003;95:2510–2518. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- Cai D, Frantz JD, Tawa NE, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey AL, Febbraio MA. Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia. 2004;47:1135–1142. doi: 10.1007/s00125-004-1447-y. [DOI] [PubMed] [Google Scholar]
- Delhalle S, Blasius R, Dicato M, Diederich M. A beginner's guide to NF-kappaB signaling pathways. Ann N Y Acad Sci. 2004;1030:1–13. doi: 10.1196/annals.1329.002. [DOI] [PubMed] [Google Scholar]
- Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944–47949. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- Emanuelli B, Peraldi P, Filloux C, Sawka-Verhelle D, Hilton D, Van Obberghen E. SOCS-3 is an insulin-induced negative regulator of insulin signaling. J Biol Chem. 2000;275:15985–15991. doi: 10.1074/jbc.275.21.15985. [DOI] [PubMed] [Google Scholar]
- Fain JN, Bahouth SW, Madan AK. TNFalpha release by the nonfat cells of human adipose tissue. Int J Obes Relat Metab Disord. 2004;28:616–622. doi: 10.1038/sj.ijo.0802594. [DOI] [PubMed] [Google Scholar]
- Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Insulin resistance-inducing cytokines differentially regulate SOCS mRNA expression via growth factor- and Jak/Stat-signaling pathways in 3T3-L1 adipocytes. J Endocrinol. 2004;181:129–138. doi: 10.1677/joe.0.1810129. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev. 2005;33:114–119. doi: 10.1097/00003677-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Heath GW, Gavin JR, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol. 1983;55:512–517. doi: 10.1152/jappl.1983.55.2.512. [DOI] [PubMed] [Google Scholar]
- Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- Hildebrandt AL, Pilegaard H, Neufer PD. Differential transcriptional activation of select metabolic genes in response to variations in exercise intensity and duration. Am J Physiol Endocrinol Metab. 2003;285:E1021–E1027. doi: 10.1152/ajpendo.00234.2003. [DOI] [PubMed] [Google Scholar]
- Ho RC, Hirshman MF, Li Y, Cai D, Farmer JR, Aschenbach WG, Witczak CA, Shoelson SE, Goodyear LJ. Regulation of IkappaB kinase and NF-kappaB in contracting adult rat skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C794–C801. doi: 10.1152/ajpcell.00632.2004. [DOI] [PubMed] [Google Scholar]
- Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. 2005;99:338–343. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- Huang KC, Chen CW, Chen JC, Lin WW. Statins induce suppressor of cytokine signaling-3 in macrophages. FEBS Lett. 2003;555:385–389. doi: 10.1016/s0014-5793(03)01297-3. [DOI] [PubMed] [Google Scholar]
- Hunter RB, Stevenson E, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. Faseb J. 2002;16:529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. Faseb J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- Li L, Gronning LM, Anderson PO, Li S, Edvardsen K, Johnston J, Kioussis D, Shepherd PR, Wang P. Insulin induces SOCS-6 expression and its binding to the p85 monomer of phosphoinositide 3-kinase, resulting in improvement in glucose metabolism. J Biol Chem. 2004;279:34107–34114. doi: 10.1074/jbc.M312672200. [DOI] [PubMed] [Google Scholar]
- Lieskovska J, Guo D, Derman E. Growth impairment in IL-6-overexpressing transgenic mice is associated with induction of SOCS3 mRNA. Growth Horm IGF Res. 2003;13:26–35. doi: 10.1016/s1096-6374(02)00135-1. [DOI] [PubMed] [Google Scholar]
- Park SH, Kim KE, Hwang HY, Kim TY. Regulatory effect of SOCS on NF-kappaB activity in murine monocytes/macrophages. DNA Cell Biol. 2003;22:131–139. doi: 10.1089/104454903321515931. [DOI] [PubMed] [Google Scholar]
- Perseghin G, Petersen K, Shulman GI. Cellular mechanism of insulin resistance: potential links with inflammation. Int J Obes Relat Metab Disord. 2003;27(Suppl. 3):S6–S11. doi: 10.1038/sj.ijo.0802491. [DOI] [PubMed] [Google Scholar]
- Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982;69:785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieusset J, Bouzakri K, Chevillotte E, Ricard N, Jacquet D, Bastard JP, Laville M, Vidal H. Suppressor of cytokine signaling 3 expression and insulin resistance in skeletal muscle of obese and type 2 diabetic patients. Diabetes. 2004;53:2232–2241. doi: 10.2337/diabetes.53.9.2232. [DOI] [PubMed] [Google Scholar]
- Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- Shi H, Tzameli I, Bjorbaek C, Flier JS. Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J Biol Chem. 2004;279:34733–34740. doi: 10.1074/jbc.M403886200. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE. SOCS-3 induces myoblast differentiation. J Biol Chem. 2005;280:10749–10758. doi: 10.1074/jbc.M410604200. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Abraha T, Childs TE, Pattison JS, Booth FW. Skeletal muscle IGF-binding protein-3 and -5 expressions are age, muscle, and load dependent. Am J Physiol Endocrinol Metab. 2003;284:E340–E350. doi: 10.1152/ajpendo.00253.2002. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Booth FW. Molecular regulation of individual skeletal muscle fibre types. Acta Physiol Scand. 2003;178:413–424. doi: 10.1046/j.1365-201X.2003.01158.x. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Bowles DK, Booth FW. Insulin-like growth factor-induced transcriptional activity of the skeletal alpha-actin gene is regulated by signaling mechanisms linked to voltage-gated calcium channels during myoblast differentiation. Endocrinology. 2004;145:2054–2063. doi: 10.1210/en.2003-1476. [DOI] [PubMed] [Google Scholar]
- Srere P. Citrate synthase. Meth Enzymol. 1969;13:3–5. [Google Scholar]
- Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Febbraio MA, Osada T, Schjerling P, Van Hall G, Saltin B, Pedersen BK. Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol. 2001;537:633–639. doi: 10.1111/j.1469-7793.2001.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, Keller C, Starkie RL, Osada T, Febbraio MA, Pedersen BK. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E1272–E1278. doi: 10.1152/ajpendo.00255.2002. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Wang J, Whiteman EL, Birnbaum MJ, Lazar MA. Activation of SOCS-3 by resistin. Mol Cell Biol. 2005;25:1569–1575. doi: 10.1128/MCB.25.4.1569-1575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway JL, James DE, Burcel E, Ruderman NB. Effect of exercise on insulin receptor binding and kinase activity in skeletal muscle. Am J Physiol. 1989;256:E138–E144. doi: 10.1152/ajpendo.1989.256.1.E138. [DOI] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas DR, Spangenburg EE, Abraha TW, Childs TE, Booth FW. GSK-3beta negatively regulates skeletal myotube hypertrophy. Am J Physiol Cell Physiol. 2002;283:C545–C551. doi: 10.1152/ajpcell.00049.2002. [DOI] [PubMed] [Google Scholar]
- Weigert C, Hennige AM, Brodbeck K, Haring HU, Schleicher ED. Interleukin-6 acts as insulin sensitizer on glycogen synthesis in human skeletal muscle cells by phosphorylation of Ser473 of Akt. Am J Physiol Endocrinol Metab. 2005;289:E251–E257. doi: 10.1152/ajpendo.00448.2004. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Hansen BF, Gade Kiens B, Markuns JF, Goodyear LJ, Richter EA. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. doi: 10.2337/diabetes.49.3.325. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Hansen BF, Kiens B, Richter EA. Insulin signaling in human skeletal muscle: time course and effect of exercise. Diabetes. 1997;46:1775–1781. doi: 10.2337/diab.46.11.1775. [DOI] [PubMed] [Google Scholar]
- Xiao W, Hodge DR, Wang L, Yang X, Zhang X, Farrar WL. NF-kappaB activates IL-6 expression through cooperation with c-Jun and IL6-AP1 site, but is independent of its IL6-NFkappaB regulatory site in autocrine human multiple myeloma cells. Cancer Biol Ther. 2004;3:1007–1017. doi: 10.4161/cbt.3.10.1141. [DOI] [PubMed] [Google Scholar]