Abstract
Ubiquitously expressed volume-regulated anion channels (VRACs) are chloride channels which are permeable to a variety of small organic anions, including the excitatory amino acids (EAAs) glutamate and aspartate. Broad spectrum anion channel blockers strongly reduce EAA release in cerebral ischaemia and other pathological states associated with prominent astrocytic swelling. However, it is uncertain whether VRAC serves as a major pathway for EAA release from swollen cells. In the present study, we measured swelling-activated release of EAAs as d-[3H]aspartate efflux, and VRAC-mediated Cl− currents by whole-cell patch clamp in cultured rat astrocytes. We compared the pharmacological profiles of the swelling-activated EAA release pathway and Cl− currents. The expression of candidate Cl− channels was confirmed by RT-PCR. The maxi Cl− channel (p-VDAC) blocker Gd3+, the ClC-2 inhibitor Cd2+, and the MDR-1 blocker verapamil did not affect EAA release or VRAC currents. An antagonist of calcium-sensitive Cl− channels (CaCC), niflumic acid, had little effect on EAA release and only partially inhibited swelling-activated Cl− currents. The phorbol ester PDBu, which blocks ClC-3-mediated Cl− currents, had no effect on VRAC currents and up-regulated EAA release. In contrast, DCPIB, which selectively inhibits VRACs, potently suppressed both EAA release and VRAC currents. Two other relatively selective VRAC inhibitors, tamoxifen and phloretin, also blocked the VRAC currents and strongly reduced EAA release. Taken together, our data suggest that (i) astrocytic volume-dependent EAA release is largely mediated by the VRAC, and (ii) the ClC-2, ClC-3, ClC-4, ClC-5, VDAC, CaCC, MDR-1 and CFTR gene products do not contribute to EAA permeability.
Swelling-activated permeability pathways for organic osmolytes are present in the vast majority of eukaryotic cell types studied so far (Chamberlin & Strange, 1989; Kirk & Strange, 1998; Junankar & Kirk, 2000). These pathways potentially contribute to many cell functions, including cell volume regulation, apoptosis, transport of organic solutes and intercellular communication via release of bioactive organic substances (Strange et al. 1996; Lang et al. 1998; Kirk & Strange, 1998; Pasantes-Morales et al. 2000). In brain, in the supraoptic nucleus of the hypothalamus, volume-sensitive release of the non-essential sulphur-containing amino acid taurine from astrocytes regulates electrical activity of magnocellular neurons (Hussy et al. 1997; Deleuze et al. 1998; Hussy et al. 2000). Several brain pathologies, such as ischaemia, hyponatraemia and brain trauma, are associated with pronounced cell swelling, which is predominantly seen in astrocytes (Kimelberg, 1995; Pasantes-Morales et al. 2002; Mongin & Kimelberg, 2005a). Cell swelling was shown to activate the release of excitatory amino acids, glutamate and aspartate, in astrocyte cultures (Kimelberg et al. 1990). In the brain, excitatory amino acids can promote neuronal cell damage via over-activation of glutamate receptors (Dirnagl et al. 1999). Glutamate and aspartate release in the ischaemic cerebral cortex is sensitive to the broad spectrum chloride channel blockers, tamoxifen, 4-acetamido-4′-isothiocyanostilbene-2,2′-disulfonic acid (SITS), 4,4′-dinitrostilbene-2,2′-disulfonic acid (DNDS), 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB) and dipyridamole (Phillis et al. 1997; Phillis et al. 1998; Seki et al. 1999; Feustel et al. 2004).
One hypothetical route for swelling-activated release of organic osmolytes is the ubiquitously expressed volume-regulated anion channel(s) (VRAC), which is activated by cell swelling and is permeable to a variety of inorganic and small organic anions, including the amino acids taurine, glutamate and aspartate (Strange et al. 1996; Okada, 1997; Nilius & Droogmans, 2003). In electrophysiological studies VRAC currents are identified based on a combination of biophysical properties, such as moderate outward rectification, time-dependent inactivation at positive potentials, and Eisenman's type I anion selectivity sequence (SCN− > I−> Br− > Cl− > F− > gluconate) (Okada, 1997; Nilius et al. 1997). However, in spite of an extensive experimental search, the molecular nature of VRAC has not yet been identified.
Although the amino acid permeability of the VRAC has been demonstrated in a number of electrophysiological studies (Banderali & Roy, 1992; Jackson & Strange, 1993; Jackson et al. 1994; Roy, 1995; Boese et al. 1996), it is still uncertain whether the VRAC serves as the only, or even a major, pathway for cell swelling-activated amino acid release (Junankar & Kirk, 2000). Several groups have reported that, at least in some cell types, swelling-activated Cl− and organic osmolyte fluxes are mediated by separate transport mechanisms (Lambert & Hoffmann, 1994; Tomassen et al. 2004). Furthermore, other indirect evidence suggests that volume-sensitive organic osmolyte release may involve more than one permeability pathway (Ruhfus et al. 1996; Mongin et al. 1999).
Among Cl− channels that have been cloned to date, ClC-2, plasmalemmal VDAC (p-VDAC or VDACL), and ClC-3 exhibit volume sensitivity, but their other characteristics differ from VRAC properties (Grunder et al. 1992; Duan et al. 1997; Sabirov et al. 2001). At least two of these channels, VDAC and ClC-3, show a measurable permeability to excitatory amino acids (Duan et al. 1997; Sabirov et al. 2001). The objective of the present study was to identify Cl− channels that contribute entirely or partially to swelling-activated release of excitatory amino acids from swollen rat astrocytes. To achieve this aim we used a number of pharmacological agents, which discriminate between particular Cl− channels. Some of the data included in this manuscript have been presented in a preliminary form (Mongin et al. 2005).
Methods
Cell cultures
Confluent primary astrocyte cultures were prepared from the cerebral cortex of newborn Sprague-Dawley rats as previously described (Frangakis & Kimelberg, 1984), with minor modifications as listed below. All animal procedures were performed according to the NIH Guide for Animal Care and approved by the institutional animal care and use committee. Briefly, neonatal rats were killed by rapid decapitation, the cerebral cortices were removed and separated from meninges and basal ganglia, and tissue was dissociated with the neutral protease dispase. Dissociated cells were seeded on poly d-lysine-coated 18-mm glass coverslips (Carolina Biological Supply, Burlington, NC, USA) and grown for 3–4 weeks in minimal essential medium (MEM) supplemented with 10% heat-inactivated horse serum (HIHS), 50 U ml−1 penicillin, and 50 μg ml−1 streptomycin at 37°C in a humidified 5% CO2−95% air atmosphere. Culture medium was replaced twice a week. After 2 weeks of cultivation, penicillin and streptomycin were removed from the culture medium. Immunocytochemistry showed that ∼98% of the cells stained positively for the astrocytic marker glial fibrillary acid protein (GFAP).
Excitatory amino acid efflux measurements
Excitatory amino acid efflux measurements were performed as previously described (Mongin et al. 1999; Mongin & Kimelberg, 2002). Briefly, astrocytes grown on glass coverslips were loaded overnight with d-[3H]aspartate (4 μCi ml−1) in 2.5 ml of MEM containing 10% HIHS in an incubator set for 5% CO2−95% air at 37°C. Before the start of the efflux measurements, the cells were washed free of extracellular isotope and serum-containing medium in Hepes-buffered solution. The isoosmotic Hepes-buffered medium contained (mm) 135 NaCl, 3.8 KCl, 1.2 MgSO4, 1.3 CaCl2, 1.2 KH2PO4, 10 d-glucose and 10 Hepes, pH adjusted to 7.4 with NaOH. In those experiments where CdCl2 was tested, phosphate salts were removed from solutions. The coverslips were inserted into a Lucite perfusion chamber that had a depression precisely cut in the bottom to accommodate the coverslip and a Teflon screw top, leaving a space above the cells of ∼100–150 μm in height. The cells were superfused at a constant flow rate of 1.2 ml min−1 in an incubator set at 37°C with Hepes-buffered medium. In hypoosmotic medium NaCl concentration was reduced to 85 mm. The osmolarities of all buffers were checked with a freezing point osmometer (μOsmette, model 5004, Precision Systems, Natick, MA, USA) and were 287 ± 2 and 198 ± 2 for isoosmotic and hypoosmotic media, respectively. Superfusate fractions were collected at 1-min intervals. At the end of each experiment, the isotope remaining in the cells was extracted with a solution containing 1% sodium dodecylsulphate plus 4 mm EDTA. Four milliliters of Ecoscint scintillation cocktail (National Diagnostics, Atlanta, GA, USA) was added, and each fraction was counted for 3H in a Packard Tri-Carb 1900TR liquid scintillation analyser (PerkinElmer, Downers Grove, IL, USA). The percentage fractional isotope release for each time point was calculated by dividing radioactivity released in each 1-min interval by the radioactivity left in the cells (the sum of all the radioactive counts in the remaining fractions up to the beginning of the fraction being measured plus the radioactivity left in the cell digest) with a custom computer program.
Electrophysiological experiments
For electrophysiological measurements, primary astrocytes grown in confluent cultures were detached using recombinant protease TrypLE (Invitrogen, Carlsbad, CA, USA) and re-plated on poly d-lysine-treated glass coverslips at low density in MEM supplemented with 10% HIHS. The following day, serum-containing medium was replaced with serum-free Opti-MEM (Invitrogen) with addition of 300 μm dibutyryl-cyclicAMP (dbcAMP), and single cells were patch-clamped within 24–72 h. Whole-cell recordings were performed at room temperature as previously described (Kubo & Okada, 1992; Liu et al. 1998). Patch electrodes were fabricated from borosilicate glass capillaries using a micropipette puller (P-87, Sutter Instruments, Novato, CA, USA), and had a resistance of 3–3.5 MΩ when filled with pipette solution. Series resistance was ≤ 15 MΩ. Currents were recorded using an Axopatch 200B amplifier (Axon Instruments, Union City, CA, USA). pCLAMP software (version 9.2, Axon Instruments) was used for command pulse control, data acquisition and analysis. Current signals were filtered at 2 kHz using a four-pole Bessel filter and digitized at 4 kHz. The time course of current development was monitored by applying alternating 2-s step pulses every 15 s from a holding potential of 0 to ± 40 mV. After attaining significant activation of Cl− currents, we tested their biophysical properties by applying 2-s step pulses from 0 mV to test potentials of −100 to +100 mV in 20-mV increments. The isoosmotic external solution contained (mm): 110 CsCl, 2 CaCl2, 1 MgSO4, 5 glucose, 10 Hepes, and 60 mannitol (pH 7.4, 290 mosmol l−1). The hypoosmotic solution was made by omitting the mannitol from the isotonic solution and had an osmolarity of 230 mosmol. The pipette solution contained (mm): 110 CsCl, 1 MgSO4, 1 Na2-ATP, 0.3 Na2-GTP, 15 Na-Hepes, 10 Hepes, 1 EGTA and 10 mannitol (pH 7.3, 255 mosmol l−1). The osmolarity of the pipette solution was set lower than that of the isotonic bath solution in order to prevent spontaneous cell swelling after attaining the whole-cell mode (Worrell et al. 1989).
Total RNA isolation and RT-PCR
Total RNA was isolated from cultured astrocytes grown to confluency in 60-mm Petri dishes. TRIzol reagent (Invitrogen) was used according to the manufacturer's instructions as an improvement to the single-step RNA isolation method developed by Chomczynski & Sacchi (1986). RNA samples were incubated for 15 min at 29°C with DNase I mix in the presence of RNase inhibitor to digest any contaminating genomic DNA. The RNA samples were then transferred onto ice and 25 mm EDTA (pH 8.0) was added to each tube. After 5-min incubation at 75°C, the PCR tubes were immediately placed on ice again. A concentrated reagent mix, containing 10× PCR buffer, 10 mm dNTPs, 0.1 m DTT, RNAse inhibitor and 5 ng μl−1 random hexamers, was added to the reaction tubes. The reaction mixture was then heated for 3 min at 42°C. One-hundred units of Moloney murine leukaemia virus reverse transcriptase (Invitrogen) was then added, and the incubation continued for another 60 min. The enzyme was then inactivated by heating the reaction mixture for 10 min at 65°C. The RT reaction products were stored at −20°C until used in PCR.
We used the previously published sequences of oligonucleotide primers for ClC-1, ClC-2, ClC-3, ClC-4, ClC-5, CFTR, MDR-1 and VDAC-1 (see Table 1 for primer sequences and references). Ten-microlitre aliquots containing 10 pmol of each 5′- and 3′-primer were added to 10 μl of the RT reaction mixture and overlaid with 30 μl of mineral oil. The reaction tubes were placed in a thermocycler block (iCycler, Bio-Rad, Hercules, CA, USA), and heated for 2 min at 94°C. Five microlitres of the mixture containing dNTPs and 1.5 U of Taq polymerase (Invitrogen) was then added. The final concentrations of all components were as follows: 1 × PCR buffer without Mg2+ (Roche), 200 μm of each dNTP (Invitrogen), 2.0 mm MgCl2, 1 μm of each primer, and 30 mU μl−1 of Taq polymerase. Reactions were set for 38 cycles. Denaturation temperature was set at 94°C, elongation temperature at 72°C, and annealing temperature at 60°C.
Table 1. List of sequence-specific primers used for RT-PCR.
| Gene product | Forward primer | Reverse primer | Expected length | Refs |
|---|---|---|---|---|
| ClC-1 | TGTGGAACGCTCAGAACTGCAGTC | TCTAGTGCCAAGACACCTCTGAGC | 656 | Kulka et al. (2002) |
| ClC-2 | CAAGTTCCTCTCCCTCTTTG | GAACTGTCCAAAGCCAGGG | 499 | Enz et al. (1999) |
| ClC-3 | CCTCTTTCCAAAGTATAGCAC | TTACTGGCATTCATGTCATTTC | 552 | Enz et al. (1999) |
| ClC-4 | GGTACATGGCTGAACTCTTC | GAGTCATGTTGGGGTCATTG | 297 | Enz et al. (1999) |
| ClC-5 | TGCTGACTGTCCTTACTCAG | CAGGATGTTCCGAAGCTTCA | 269 | Kulka et al. (2002) |
| CFTR | CGCAGGTTCTCAGTGGACGATGCC | CCTCAACCAGAAAAACCAGCACGCA | 607 | Huber et al. (1998) |
| MDR1 | CCCATCATTGCAATAGCAGG | TGTTCAAACTTCTGCTCCTGA | 158 | Albermann et al. (2005) |
| VDAC-1 | GGACCGAGTATGGGCTGACG | GCTGCTATTCCAAAGCGAGTGTTAC | 478 | Bres et al. (2000) |
The PCR products (9 μl each) were separated on a 1.5% agarose gel preloaded with 1 μg ml−1 ethidium bromide, and visualized under ultraviolet light. The molecular weights of the PCR products were compared to a 100-bp DNA ladder (Invitrogen). Identities of the PCR products were confirmed by sequencing. Controls for the DNA contamination were as described above but excluding the reverse transcriptase from the reaction mixture.
Materials
d-[3H]Aspartate (specific activity ∼18 Ci mm−1) was obtained from PerkinElmer Life Sciences (Boston, MA, USA) or Amersham Biosciences (Piscataway, NJ, USA). Dispase (neutral protease dispase grade II) was purchased from Roche (Baton Rouge, LA, USA). All cell culture reagents were from Invitrogen (Carlsbad, CA, USA). 4-[(Butyl-6,7-dichloro-2-cyclopentyl-2,3-dihydro-1-oxo-1H-inden-5-yl)oxy]butanoic acid (DCPIB) and verapamil were obtained from Tocris (Ellisville, MO, USA). Phorbol 12,13-dibutyrate (PDBu) was from Calbiochem (La Jolla, CA, USA). Phloretin and all other reagents and salts were purchased from Sigma (St Louis, MO, USA) and were of highest grade available.
Data analysis
Amino acid release during the hypoosmotic medium exposure was analysed using repeated measures ANOVA to test for effects of drug and the effect of time and their interaction (P-values for the effects of drug are included in figure legends). Analysis also included planned comparison of mean peak release values in the presence or absence of the tested drug, using Fisher's LSD test (these P-values are presented in the text of the Results section). Peak release values were observed between the 2nd and 3rd minutes of exposure to hypoosmotic medium and represent the maximal activity of the amino acid permeability pathway. In electrophysiological experiments, effects of inhibitors were normalized to control Cl− currents in the same experiment. In these experiments significance of drug effects was tested using Student's t test comparing normalized currents in the presence of drug to 100% (P-values are presented in the text of the Results section). Statistical analysis was performed using Statistica 6.1 (StatSoft, Tulsa, OK, USA) or Origin 7.5 (OriginLab, Northampton, MA, USA).
Results
Swelling-activated d-[3H]aspartate release and Cl− currents in cultured rat astrocytes are sensitive to a broad spectrum Cl− channel blocker NPPB
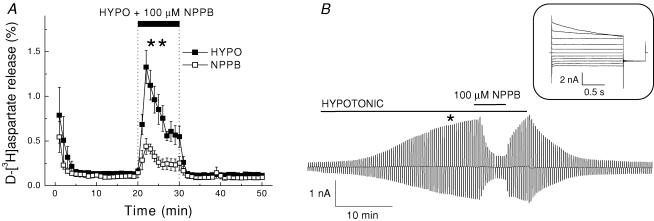
In cultured rat astrocytes, 30% reduction in medium osmolarity induced transient efflux of d-[3H]aspartate with the maximal release rate of ∼1.0–1.5%, which was 10–20 times higher that that seen in non-stimulated cells under isoosmotic conditions (Fig. 1A). The broad spectrum Cl− channel blocker NPPB, at 100 μm, blocked the peak release by 67 ± 7% (significantly different from control release, P = 0.003, Fig. 1A). In parallel experiments, we measured whole-cell Cl− currents in response to 20% reduction in medium osmolarity. The extremely flat morphology of cultured astrocytes made it difficult to patch cells and maintain the seal during exposure to hypoosmotic medium. Therefore, in electrophysiological experiments we pretreated cells with dibutyryl-cAMP to make the cell bodies rounder. Such treatment improved experimental success rate, while not causing any substantial changes in either current densities or the biophysical properties of swelling-activated Cl− currents (data not shown). As seen in Fig. 1B, hypotonic stress activated large whole-cell Cl− currents. Average steady-state current density increased from 3.1 ± 0.5 pA pF−1 under isoosmotic conditions to 19.7 ± 3.3 pA pF−1 at +40 mV (n = 5, data not shown). These currents exhibited moderate outward rectification and time-dependent inactivation at large positive potentials (Fig. 1B, inset). As shown in Fig. 1B, 100 μm NPPB strongly and reversibly inhibited swelling-activated Cl− currents, reducing them by 95.2 ± 3.4% (n = 5, P < 0.001) of control. Because of the slower activation of Cl− currents in electrophysiological experiments (Fig. 1B), compared to activation of d-[3H]aspartate release in efflux experiments (Fig. 1A), we additionally performed simultaneous measurements of d-[3H]aspartate and 36Cl− fluxes in hypoosmotically swollen cells. In intact cells, activation of d-[3H]aspartate and 36Cl− releases occurred at the same time (online Supplemental material, Supplemental Fig. 1).
Figure 1. The broad spectrum anion channel blocker NPPB potently inhibits swelling-activated excitatory amino acid release and Cl− currents in cultured rat astrocytes.
A, effect of NPPB on swelling-activated d-[3H]aspartate release. Cells were exposed to hypoosmotic medium for 10 min, as indicated, in the presence (□) or absence (▪) of 100 μm NPPB. Data are means ± s.e.m. of 5 experiments. **P = 0.007, effect NPPB versus control, repeated measures ANOVA. B, representative of 5 electrophysiological recordings of Cl− currents in single cultured astrocytes exposed to hypoosmotic medium. Cells were held at 0 mV and alternative pulses to ± 40 mV were applied to measure activation of Cl− currents. NPPB (100 μm) was applied after Cl− currents approached steady-state level. Inset: Cl− current responses to step pulses from −100 to +100 mV in 20 mV increments at the time point indicated by asterisk.
Expression of Cl− channels in cultured astrocytes
To determine which candidate Cl− channels are expressed in our preparation of cultured astrocytes, we assayed the mRNA in these cells using an RT-PCR method with previously published sequence-specific primers (Huber et al. 1998; Enz et al. 1999; Bres et al. 2000; Kulka et al. 2002; Albermann et al. 2005; for primer sequences see Table 1). As seen in Fig. 2, all the candidate Cl− channels were present in our preparation at the mRNA level. The resulting PCR products corresponded to their predicted molecular weights, and their molecular identity was confirmed by sequencing. The protein expression for Cl− channels of interest in cultured astrocytes, with the exception of ClC-4, as well as similar mRNA expression has been shown previously by Parkerson & Sontheimer (2004). We used two negative controls for the RT-PCR reactions. To check for genomic DNA contamination we omitted reverse transcriptase from the reaction mixtures. This resulted in disappearance of the PCR signal. Additionally, we used the sequence-specific primers for the ClC-1 gene product, which is not expressed in the brain or in cultured astrocytes. As expected, there was no signal corresponding to ClC-1 (Fig. 2).
Figure 2. mRNA transcript expression for the different Cl− channels in primary astrocyte cultures.
Ethidium bromide staining of the PCR products for transcripts of the various Cl− channel candidates studied. PCR was performed using sequence-specific primers after reverse transcriptase processing of mRNA samples treated with DNase to remove any genomic DNA contamination. For primer sequences and other details see Methods. Visible bands correspond to the expected products for ClC-1 (lanes 2, 3), ClC-2 (4, 5), ClC-3 (6, 7), ClC-4 (8, 9), ClC-5 (10, 11), CFTR (12, 13), MDR-1 (14, 15), and p-VDAC (16, 17). ClC-1 product was not detected. When indicated, reverse transcriptase (RT) was omitted from the reaction mixture as a negative control. Lanes 1 and 18 contained a 100-bp MW ladder.
Effect of the plasmalemmal VDAC inhibitor Gd3+ on swelling-activated d-[3H]aspartate release and Cl− currents
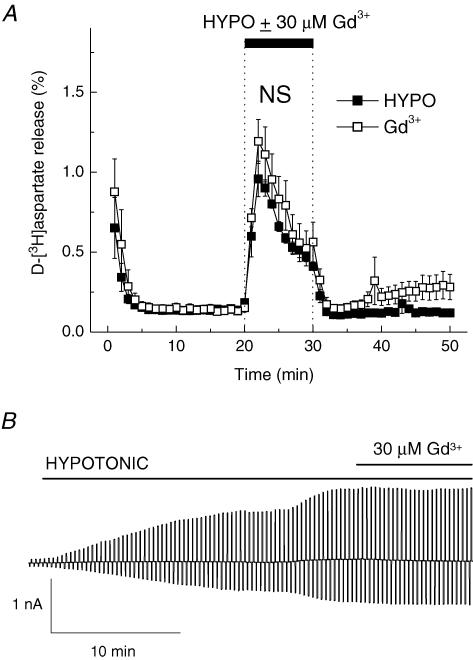
Maxi chloride channels (p-VDAC, or VDACL) were initially cloned from the bovine brain (Dermietzel et al. 1994) and are functionally expressed in cultured rat cortical astrocytes (Jalonen, 1993; Parkerson & Sontheimer, 2004). p-VDACs are volume sensitive and have a large enough pore to conduct excitatory amino acids (Sabirov et al. 2001; Sabirov & Okada, 2004). To test for p-VDAC contribution to excitatory amino acid release, we used 30 μm Gd3+, which at this concentration discriminates between p-VDAC and VRAC (Sabirov et al. 2001). As seen in Fig. 3, Gd3+ had a trend to slightly stimulate both swelling-activated d-[3H]aspartate release (P = 0.219) and Cl− currents (P = 0.050).
Figure 3. The p-VDAC blocker Gd3+ does not affect swelling-activated d-[3H]aspartate release and Cl− currents.
A, effect of Gd3+ on swelling-activated d-[3H]aspartate release. Cells were exposed to hypoosmotic medium for 10 min, as indicated, in the presence (□) or absence (▪) of 30 μm Gd3+. Data are means ± s.e.m. of 5 experiments. NS, not significant (P = 0.181), Gd3+versus control, repeated measures ANOVA. B, effect of Gd3+ on swelling-activated Cl− currents. Representative of 6 electrophysiological recordings. Gd3+ (30 μm) was applied after Cl− currents approached steady-state level.
Effect of Cd2+ on swelling-activated d-[3H]aspartate release and Cl− currents
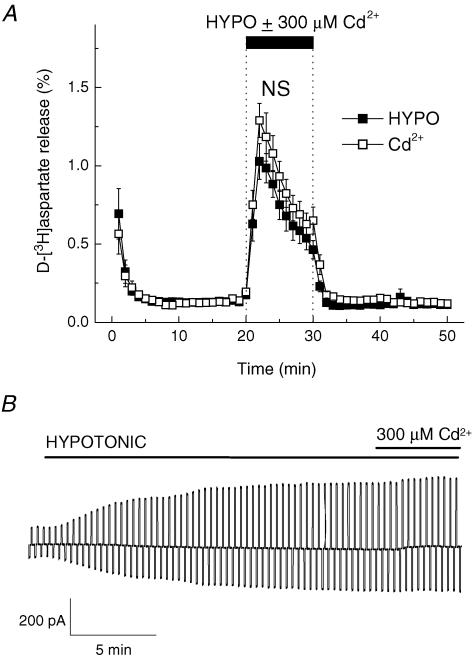
We have used 300 μm CdCl2 to probe for the potential involvement of the ClC-2 channel. ClC-2 is a broadly expressed Cl− channel, which is also regulated by cell swelling (Grunder et al. 1992), and is expressed in cultured astrocytes (Ferroni et al. 1997; Parkerson & Sontheimer, 2004). Cd2+ (300 μm) inhibits ClC-2 currents by ∼70% (Clark et al. 1998). However, in our experiments Cd2+ failed to inhibit either swelling-activated d-[3H]aspartate release (Fig. 4A, P = 0.134) or Cl− currents (Fig. 4B, P = 0.689).
Figure 4. The ClC-2 inhibitor Cd2+does not affect swelling-activated d-[3H]aspartate release and Cl− currents.
A, effect of Cd2+ on swelling-activated d-[3H]aspartate release. Cells were exposed to hypoosmotic medium for 10 min, as indicated, in the presence (□) or absence (▪) of 300 μm Cd2+. Data are means ± s.e.m. of 5–6 experiments. NS, not significant (P = 0.294), Cd2+versus control, repeated measures ANOVA. B, effect of 300 μm Cd2+ on swelling-activated Cl− currents. Representative of 6 electrophysiological recordings.
Effect of the phorbol ester PDBu on d-[3H]aspartate release and Cl− currents
The ClC-3 channel has been cloned from rat brain, and is potently inhibited by activation of PKC (Kawasaki et al. 1994). The PKC activator PDBu strongly down-regulates swelling-activated Cl− currents in cardiomyocytes and pulmonary artery smooth muscle cells (Duan et al. 1999; Zhong et al. 2002). ClC-3 is expressed in cultured astrocytes (Parkerson & Sontheimer, 2004). In our hands, 500 nm PDBu had no effect on swelling activated Cl− currents (Fig. 5B, P = 0.727), and strongly up-regulated d-[3H]aspartate release (Fig. 5A, peak release 170.4 ± 11.6% of control, P < 0.001).
Figure 5. PDBu, which down-regulates ClC-3 activity via PKC-dependent mechanism, does not inhibit swelling-activated d-[3H]aspartate release and Cl− currents.
A, effect of 500 nm PDBu on swelling-activated d-[3H]aspartate release. Cells were exposed to hypoosmotic medium for 10 min, as indicated, in the presence (□) or absence (▪) of PDBu. PDBu was applied 10 min before and during exposure to hypoosmotic medium. Data are means ± s.e.m. of 5 experiments. **P = 0.002, PDBu versus control, repeated measures ANOVA. B, effect of 500 nm PDBu on swelling-activated Cl− currents. Representative of 6 electrophysiological recordings.
Effect of the calcium-activated Cl− channel blocker, niflumic acid, on swelling-induced d-[3H]aspartate release and Cl− currents
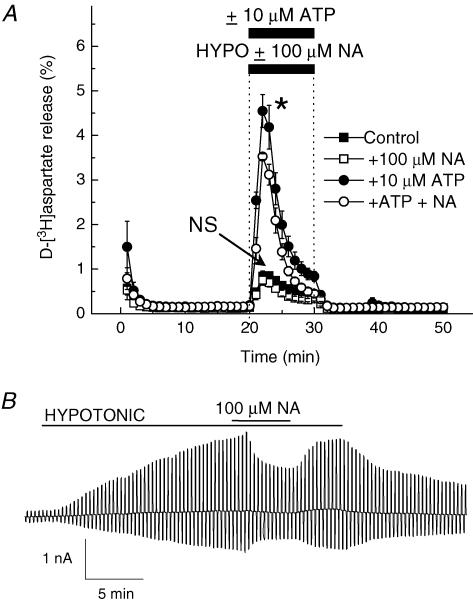
Hypoosmotic media are known to elevate intracellular Ca2+ concentration in many cells (McCarty & O'Neil, 1992), which may trigger activation of calcium-sensitive Cl− channels. ATP and other Ca2+-mobilizing agents strongly potentiate swelling-induced release of organic osmolytes in astrocytes and several other cell types (Mongin & Kimelberg, 2002; Loveday et al. 2003; Franco et al. 2004), implying a potential contribution of calcium-sensitive Cl− channels. These channels are completely blocked by 100 μm niflumic acid (Large & Wang, 1996; Pedersen et al. 1998). In our experiments, 100 μm niflumic acid was ineffective against swelling-activated excitatory amino acid release (Fig. 6A, P = 0.585). When ATP was coapplied with hypoosmotic medium, it strongly potentiated d-[3H]aspartate release, which now was weakly sensitive to 100 μm niflumic acid (Fig. 6A, 22.5 ± 1.4% inhibition, P = 0.047). Swelling-activated Cl− currents were more sensitive to niflumic acid (Fig. 6B, 41.1 ± 2.5% inhibition, P < 0.001).
Figure 6. A blocker of Ca2+-activated Cl− channels, niflumic acid, weakly affects swelling-activated d-[3H]aspartate release and Cl− currents.
A, effect of 100 μm niflumic acid (NA) on swelling-activated d-[3H]aspartate release in the presence or absence of 10 μm ATP. Cells were exposed to hypoosmotic medium for 10 min, as indicated, in the presence (□, •) or absence (▪, •) of 100 μm niflumic acid. ATP was present in hypoosmotic medium where indicated (○, •). Data are means ± s.e.m. of 5 experiments. NS, not significant (P = 0.074), niflumic acid versus control; *P = 0.04, ATP versus ATP plus niflumic acid, repeated measures ANOVA. B, effect of 100 μm niflumic acid (NA) on swelling-activated Cl− currents. Representative of 6 electrophysiological recordings.
Effect of verapamil on swelling-induced d-[3H]aspartate release and Cl− currents
The product of the multidrug resistance-1 gene, P-glycoprotein or MDR-1, was formerly proposed to be VRAC, but it is now thought to function only as a VRAC modulator (Valverde et al. 1992; Wine & Luckie, 1996). This protein is expressed in cultured astrocytes (Ronaldson et al. 2004). We used 100 μm verapamil, which at this concentration completely inhibits MDR-1 function (Luckie et al. 1994), to test for direct or indirect MDR-1 involvement in excitatory amino acid release. In our experiments, verapamil did not affect peak d-[3H]aspartate release (P = 0.402), but strongly inhibited the time-dependent inactivation of the release during exposure to hypoosmotic medium (Fig. 7A). In the corresponding electrophysiological study, 100 μm verapamil failed to affect swelling-activated Cl− currents (Fig. 7B, P = 0.323).
Figure 7. The MDR-1 inhibitor verapamil does not block swelling-activated d-[3H]aspartate release and Cl− currents.
A, effect of 100 μm verapamil (VRP) on swelling-activated d-[3H]aspartate release. Cells were exposed to hypoosmotic medium for 10 min, as indicated, in the presence (□) or absence ▪) of verapamil. Data are means ± s.e.m. of 5 experiments. *P = 0.015, verapamil versus control (main effect); ***P < 0.001, verapamil versus control (group and time interaction effect), repeated measures ANOVA. This significant interaction effect reflects the markedly affected time course of release in the verapamil group. B, effect of 100 μm verapamil on swelling-activated Cl− currents. Representative of 6 electrophysiological recordings.
Effect of VRAC blockers, DCPIB, tamoxifen and phloretin, on the swelling-induced d-[3H]aspartate release and Cl− currents
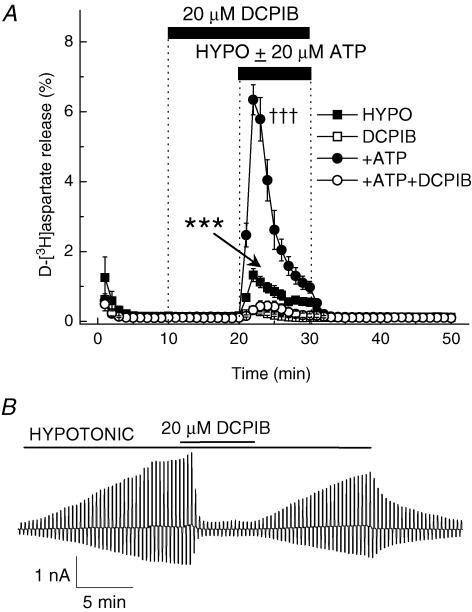
As seen in Fig. 1B, application of hypotonic solution induced whole-cell Cl− currents with moderate outward rectification and inactivation at large positive potentials, which are typical for VRAC. To explore the contribution of VRAC activity to excitatory amino acid release, we tested a novel selective VRAC blocker, DCPIB. DCPIB inhibits VRAC currents in calf pulmonary artery endothelial cells (CPAE), guinea-pig atrial cardiomyocytes and Xenopus oocytes, but does not affect either endogenous or heterologously expressed ClC-1, ClC-2, ClC-4, ClC-5, CFTR, and calcium-activated Cl− channels (Decher et al. 2001). The IC50 for DCPIB inhibition of VRAC currents is 4.1 μm (Decher et al. 2001). In our experiments, 20 μm DCPIB blocked swelling-activated d-[3H]aspartate release by 76.0 ± 4.5% (Fig. 8A, P < 0.001). DCPIB was even more effective when hypoosmotic medium was coapplied with 20 μm ATP (Fig. 8A, 94.9 ± 1.7% inhibition, P < 0.001). Consistent with effects reported in the literature, 20 μm DCPIB nearly completely inhibited swelling-activated Cl− currents (Fig. 8B, 93.5 ± 1.7% inhibition, P < 0.001).
Figure 8. The selective VRAC blocker DCPIB potently inhibits swelling-activated d-[3H]aspartate release and Cl− currents.
A, effect of 20 μm DCPIB on swelling-activated d-[3H]aspartate release in the presence or absence of 20 μm ATP. Cells were exposed to hypoosmotic medium for 10 min, as indicated, in the presence (□, ○) or absence (▪, •) of DCPIB, which was given 10 min before and during application of hypoosmotic medium. ATP was present in hypoosmotic medium only (○, •). Data are means ± s.e.m. of 5 experiments. ***P < 0.001, DCPIB versus control; †††P < 0.001; ATP versus ATP plus DCPIB, repeated measures ANOVA. B, effect of 20 μm DCPIB on swelling-activated Cl− currents. Representative of 6 electrophysiological recordings.
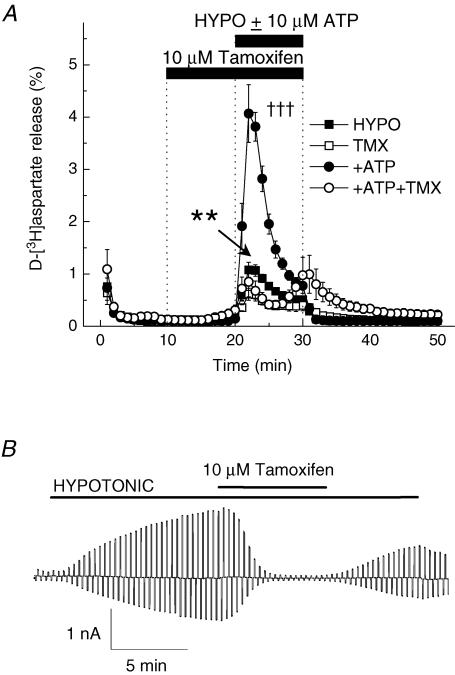
Another well known inhibitor of swelling-activated Cl− channels, tamoxifen, completely inhibited the whole-cell VRAC currents (Fig. 9B, 102.6 ± 6.3% inhibition, P < 0.001). Surprisingly, acute application of tamoxifen with hypoosmotic medium produced only weak inhibition of the swelling-activated d-[3H]aspartate release (data not shown). When we exposed cells to tamoxifen for 10 min before and then during application of hypoosmotic medium, inhibition was substantially stronger (Fig. 9A, 43.2 ± 10.7% inhibition, P = 0.040). In the cells exposed to hypoosmotic medium and 10 μm ATP, tamoxifen potency was further increased (Fig. 9A, 79.1 ± 7.1% inhibition, P < 0.001).
Figure 9. The VRAC blocker tamoxifen moderately inhibits swelling-activated d-[3H]aspartate release and completely suppresses volume-sensitive Cl− currents.
A, effect of 10 μm tamoxifen on swelling-activated d-[3H]aspartate release in the presence or absence of 10 μm ATP. Cells were exposed to hypoosmotic medium for 10 min, as indicated, in the presence (□, ○) or absence (▪, •) of 10 μm tamoxifen, which was applied 10 min before and during exposure to hypoosmotic medium. ATP was present in hypoosmotic medium only (○, •). Data are means ± s.e.m. of 5–7 experiments. **P = 0.002, tamoxifen versus control; †††P < 0.001, ATP versus ATP plus tamoxifen, repeated measures ANOVA. B, effect of 10 μm tamoxifen on swelling-activated Cl− currents. Representative of 6 electrophysiological recordings.
As reported previously (Mongin & Kimelberg, 2002; Haskew-Layton et al. 2005) another relatively selective VRAC blocker, phloretin, strongly inhibits the volume-sensitive d-[3H]aspartate release. Here, we compared the effects of phloretin on VRAC currents and excitatory amino acid release in the same cell preparation. Phloretin (100 μm) partially inhibited the excitatory amino acid release (Fig. 10 and Supplemental Fig. 2, 49.9 ± 5.0% inhibition, P < 0.001). VRAC current inhibition was somewhat stronger (Fig. 10 and Supplemental material, 82.3 ± 5.3% inhibition, P < 0.001). The expected VRAC inhibition at this phloretin concentration was ∼80%, as originally shown by Fan et al. (2001) in T84, C127/CFTR and intestinal 407 cells.
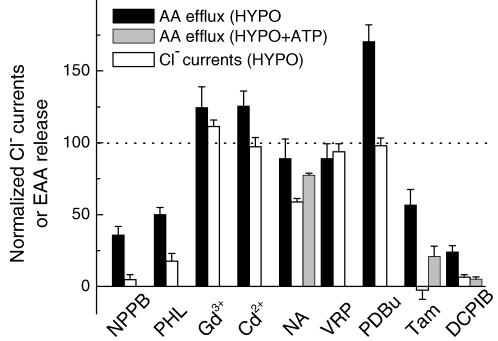
Figure 10. Summary of effects of different inhibitors on swelling activated excitatory amino acid release and Cl− currents in rat cultured astrocytes.
Normalized (relative to hypoosmotic controls) release values in the presence of all tested inhibitors are plotted for swelling-activated excitatory amino acid release next to normalized VRAC currents. When available, normalized excitatory amino acid release in the presence of ATP is also shown. PHL, phloretin; NA, niflumic acid; VRP, verapamil; Tam, tamoxifen.
Discussion
In the vast majority of cell types studied so far, cell swelling activates release of organic osmolytes, and this release is sensitive to broad spectrum Cl− channel blockers (Strange et al. 1996; Kirk & Strange, 1998; Lang et al. 1998; Junankar & Kirk, 2000). In cultured glial cells, swelling-induced excitatory amino acid release is potently suppressed by NPPB, DIDS, SITS, DNDS, dideoxyforskolin and phloretin, all agents known to inhibit VRAC currents (Jackson & Strange, 1993; Sanchez-Olea et al. 1993; Okada, 1997; Bres et al. 2000; Mongin & Kimelberg, 2002; Haskew-Layton et al. 2005). However, because of the low selectivity of these compounds, it remains uncertain whether VRAC serves as a major pathway for the volume-dependent excitatory amino acid fluxes. In the present study we took advantage of several commercially available compounds which discriminate among a number of cloned and characterized Cl− channels. Our pharmacological data strongly suggest that ClC-2, ClC-3, p-VDAC, CaCC, CFTR and MDR-1 do not mediate any substantial part of astrocytic excitatory amino acid release. On the other hand, all the VRAC blockers tested in this study strongly inhibited d-[3H]aspartate release from swollen cells, indicating that the putative VRAC is a major contributor to volume-sensitive excitatory amino acid fluxes.
An important finding of this study is the nearly complete inhibition of the swelling-activated organic osmolyte release by the selective VRAC blocker DCPIB. In a recent study by Decher et al. (2001), DCPIB inhibited swelling-activated Cl− currents, i.e. VRACs, in several cell types, while not affecting Cl− currents carried by either endogenous or heterologously expressed ClC-1, ClC-2, ClC-4, ClC-5, CFTR and calcium-activated Cl− channels. Thus, VRAC is likely to be the predominant pathway responsible for the volume-dependent release of organic osmolytes in cultured astrocytes. Consistent with such a conclusion, 10 mm extracellular ATP, which at high concentrations acts as a VRAC open channel blocker (Tsumura et al. 1996), potently suppresses swelling-activated excitatory amino acid release in cultured astrocytes (Mongin & Kimelberg, 2002; Haskew-Layton et al. 2005). Furthermore, 100 μm phloretin, which distinguishes VRAC from CFTR and Ca2+-activated Cl− channels (Fan et al. 2001), also strongly inhibits astrocytic amino acid release (Mongin & Kimelberg, 2002; Haskew-Layton et al. 2005; and Fig. 10 in the present study).
Although the data of Decher and colleagues on the specific inhibition of VRAC by DCPIB are very convincing, the selectivity of this compound has not yet been verified by other laboratories. Therefore, the information obtained with other Cl− channel blockers is useful in order to corroborate the DCPIB data and test for the possible contribution of VRAC-independent permeability pathways. In our study Gd3+ and Cd2+ did not affect swelling-activated excitatory amino acid release. Since Gd3+ and Cd2+ potently block p-VDAC and ClC-2, respectively (Sabirov et al. 2001; Clark et al. 1998), these results rule out involvement of these two volume-sensitive pathways. Contribution of MDR-1 to organic osmolyte release was excluded based on the absence of inhibiton by verapamil, which potently blocks MDR-1 activity (Luckie et al. 1994). Interestingly, verapamil markedly inhibited time-dependent inactivation of d-aspartate release in hypoosmotically swollen cells (see Fig. 6A). Such inactivation is thought to be due to a regulatory volume decrease (RVD). Verapamil blocks RVD in several cell types and may act via inhibition of Ca2+ influx or K+ conductance (McCarty & O'Neil, 1990; De Smet et al. 1998).
Unlike all the candidate channels mentioned above, ClC-3 does not have a selective inhibitor. The sensitivity of ClC-3 to DCPIB has not been tested. However, a unique feature of this channel is a strong inhibition by PKC. Phorbol esters, such as phorbol 12-myristate 13-acetate (PMA) and PDBu, eliminate ClC-3-mediated Cl− currents in osmotically swollen cells (Duan et al. 1997; Zhong et al. 2002). On the contrary, PDBu and PMA stimulate VRAC-mediated Cl− currents and swelling-activated organic osmolyte release at least in some cell types (Jackson & Strange, 1993; Loveday et al. 2003; Gong et al. 2004). In our experiments, 500 nm PDBu strongly potentiated swelling-activated organic osmolyte fluxes, thus making ClC-3 involvement unlikely. PDBu did not stimulate Cl− currents in our electrophysiological experiments. One possible explanation is that PDBu acts via one of the Ca2+-dependent PKC isoforms, which is not activated in the absence of Ca2+ in our pipette solution.
We next tested for the involvement of Ca2+-activated Cl− channels (CaCCs). Although CaCCs are not volume sensitive, we included them in a list of candidates because cell swelling increases intracellular Ca2+ and therefore may indirectly activate Ca2+-sensitive transport pathways. It has been also found that ATP and other Ca2+ mobilizing agonists strongly potentiate volume-sensitive efflux of 125I− and organic osmolytes in swollen cells (Tilly et al. 1994; Loveday et al. 2003; Franco et al. 2004). In cultured astrocytes, ATP potentiates hypoosmotic medium-induced d-[3H]aspartate release by 2- to 3-fold and this effect is completely dependent on intracellular Ca2+ and calmodulin (Mongin & Kimelberg, 2005b). It is possible that ATP activates a separate Ca2+-sensitive osmolyte release route in swollen cells, additional to VRAC. Lee et al. (2004) have recently reported in a preliminary form that ATP, bradykinin and other Ca2+-releasing agonists induce astrocytic glutamate release via CaCCs. In their work, CaCCs were completely blocked by 100 μm niflumic acid, as in a number of other studies (Large & Wang, 1996; Pedersen et al. 1998; Lee et al. 2004). In our hands, 100 μm niflumic acid produced negligible inhibition of swelling-activated excitatory amino acid release both in the absence and in the presence of extracellular ATP. These results seem to rule out the involvement of CaCCs.
Perhaps the most perplexing result of our study is the relatively weak inhibition of swelling-activated amino acid release by tamoxifen. Tamoxifen is a potent VRAC blocker, which is commonly used in electrophysiological studies. However, the sensitivity of VRAC to this compound varies among cell types, from high sensitivity in HEK293 and I-407 cells to partial inhibition in human cancer KB3 and rat RBL-2H3 cells, to complete insensitivity in cultured cortical neurons (Nilius et al. 1994; Inoue et al. 2005). In our experiments, 10 μm tamoxifen completely blocked swelling-activated Cl− currents but, surprisingly, was only partially effective (∼40% inhibition) against the volume-dependent d-[3H]aspartate release. Moreover, its inhibitory effect on organic osmolyte release required preincubation. In the cells, where swelling-activated excitatory amino acid release was potentiated by application of ATP, tamoxifen suppressed the release by ∼80%, approaching the efficacy of the selective VRAC blocker, DCPIB. Such differential sensitivity to tamoxifen may be explained by the indirect, calmodulin-dependent effects of this compound on VRAC, as originally suggested by Kirk & Kirk (1994). Consistent with such an idea, other calmodulin antagonists more potently block swelling-activated amino acid release in cells stimulated by ATP, where increases in intracellular Ca2+ up-regulate calmodulin-dependent processes (Mongin & Kimelberg, 2005b).
As seen in the summary Fig. 10, in cultured rat astrocytes swelling-activated Cl− currents and excitatory amino acid release share a similar pharmacological profile. In particular, the recently characterized selective VRAC blocker DCPIB nearly completely inhibited volume-dependent organic osmolyte release, as well as VRAC currents. The data obtained with other Cl− channel inhibitors are consistent with the idea of DCPIB selectivity, and rule out contributions of ClC-2, ClC-3, p-VDAC, CaCC, CFTR and MDR-1 to organic osmolyte release from swollen cells. Therefore we now propose that VRAC is a principal candidate pathway for mediating organic osmolyte release. Furthermore, DCPIB is a valuable tool to probe for VRAC contribution to physiological and pathological release of organic osmolytes in vivo and in vitro.
Acknowledgments
We thank Artur Chernoguz for his experimental contribution, Dr Paul J. Feustel for critical reading of the manuscript and help with statistical analysis, and Dr Min Zhou for helpful discussion of the electrophysiological experiments. This study was supported in part by NINDS grant NS-35205 to H.K.K.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2005.103820
http://jp.physoc.org/cgi/content/full/jphysiol.2005.103820/DC1 and contains supplemental material consisting of two figures:
Simultaneous measurements of d-[3H]aspartate and 36Cl− release from cultured astrocytes exposed to hypoosmotic medium
VRAC blocker phloretin strongly inhibits swelling-activated d-[3H]aspartate release and Cl−currents
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Albermann N, Schmitz-Winnenthal FH, Z'graggen K, Volk C, Hoffmann MM, Haefeli WE, Weiss J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem Pharmacol. 2005;70:949–958. doi: 10.1016/j.bcp.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Banderali U, Roy G. Anion channels for amino-acids in Mdck cells. Am J Physiol. 1992;263:C1200–C1207. doi: 10.1152/ajpcell.1992.263.6.C1200. [DOI] [PubMed] [Google Scholar]
- Boese SH, Wehner F, Kinne RK. Taurine permeation through swelling-activated anion conductance in rat IMCD cells in primary culture. Am J Physiol. 1996;271:F498–F507. doi: 10.1152/ajprenal.1996.271.3.F498. [DOI] [PubMed] [Google Scholar]
- Bres V, Hurbin A, Duvoid A, Orcel H, Moos FC, Rabie A, Hussy N. Pharmacological characterization of volume-sensitive, taurine permeable anion channels in rat supraoptic glial cells. Br J Pharmacol. 2000;130:1976–1982. doi: 10.1038/sj.bjp.0703492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin ME, Strange K. Anisosmotic cell volume regulation: a comparative view. Am J Physiol. 1989;257:C159–C173. doi: 10.1152/ajpcell.1989.257.2.C159. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1986;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark S, Jordt SE, Jentsch TJ, Mathie A. Characterization of the hyperpolarization-activated chloride current in dissociated rat sympathetic neurons. J Physiol. 1998;506:665–678. doi: 10.1111/j.1469-7793.1998.665bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet P, Li J, Van Driessche W. Hypotonicity activates a lanthanide-sensitive pathway for K+ release in A6 epithelia. Am J Physiol. 1998;275:C189–C199. doi: 10.1152/ajpcell.1998.275.1.C189. [DOI] [PubMed] [Google Scholar]
- Decher N, Lang HJ, Nilius B, Bruggemann A, Busch AE, Steinmeyer K. DCPIB is a novel selective blocker of ICl,swell and prevents swelling-induced shortening of guinea-pig atrial action potential duration. Br J Pharmacol. 2001;134:1467–1479. doi: 10.1038/sj.bjp.0704413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleuze C, Duvoid A, Hussy N. Properties and glial origin of osmotic-dependent release of taurine from the rat supraoptic nucleus. J Physiol. 1998;507:463–471. doi: 10.1111/j.1469-7793.1998.463bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Hwang TK, Buettner R, Hofer A, Dotzler E, Kremer M, Deutzmann R, Thinnes FP, Fishman GI, Spray DC. Cloning and in situ localization of a brain-derived porin that constitutes a large-conductance anion channel in astrocytic plasma membranes. Proc Natl Acad Sci U S A. 1994;91:499–503. doi: 10.1073/pnas.91.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Duan D, Cowley S, Horowitz B, Hume JR. A serine residue in ClC-3 links phosphorylation-dephosphorylation to chloride channel regulation by cell volume. J General Physiol. 1999;113:57–70. doi: 10.1085/jgp.113.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- Enz R, Ross BJ, Cutting GR. Expression of the voltage-gated chloride channel ClC-2 in rod bipolar cells of the rat retina. J Neurosci. 1999;19:9841–9847. doi: 10.1523/JNEUROSCI.19-22-09841.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HT, Morishima S, Kida H, Okada Y. Phloretin differentially inhibits volume-sensitive and cyclic AMP-activated, but not Ca-activated, Cl− channels. Br J Pharmacol. 2001;133:1096–1106. doi: 10.1038/sj.bjp.0704159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferroni S, Marchini C, Nobile M, Rapisarda C. Characterization of an inwardly rectifying chloride conductance expressed by cultured rat cortical astrocytes. Glia. 1997;21:217–227. doi: 10.1002/(sici)1098-1136(199710)21:2<217::aid-glia5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Feustel PJ, Jin Y, Kimelberg HK. Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke. 2004;35:1164–1168. doi: 10.1161/01.STR.0000124127.57946.a1. [DOI] [PubMed] [Google Scholar]
- Franco R, Rodriguez R, Pasantes-Morales H. Mechanisms of the ATP potentiation of hyposmotic taurine release in Swiss 3T3 fibroblasts. Pflugers Arch. 2004;449:159–169. doi: 10.1007/s00424-004-1322-1. [DOI] [PubMed] [Google Scholar]
- Frangakis MV, Kimelberg HK. Dissociation of neonatal rat brain by dispase for preparation of primary astrocyte cultures. Neurochem Res. 1984;9:1689–1698. doi: 10.1007/BF00968079. [DOI] [PubMed] [Google Scholar]
- Gong W, Xu H, Shimizu T, Morishima S, Tanabe S, Tachibe T, Uchida S, Sasaki S, Okada Y. ClC-3-independent, PKC-dependent activity of volume-sensitive Cl channel in mouse ventricular cardiomyocytes. Cell Physiol Biochem. 2004;14:213–224. doi: 10.1159/000080330. [DOI] [PubMed] [Google Scholar]
- Grunder S, Thiemann A, Pusch M, Jentsch TJ. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature. 1992;360:759–762. doi: 10.1038/360759a0. [DOI] [PubMed] [Google Scholar]
- Haskew-Layton RE, Mongin AA, Kimelberg HK. Hydrogen peroxide potentiates volume-sensitive excitatory amino acid release via a mechanism involving Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:3548–3554. doi: 10.1074/jbc.M409803200. [DOI] [PubMed] [Google Scholar]
- Huber S, Braun G, Burger-Kentischer A, Reinhart B, Luckow B, Horster M. CFTR mRNA and its truncated splice variant (TRN-CFTR) are differentially expressed during collecting duct ontogeny. FEBS Lett. 1998;423:362–366. doi: 10.1016/s0014-5793(98)00112-4. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Desarmenien MG, Moos FC. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 2000;62:113–134. doi: 10.1016/s0301-0082(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Pantaloni A, Desarmenien MG, Moos F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol. 1997;502:609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Mori S, Morishima S, Okada Y. Volume-sensitive chloride channels in mouse cortical neurons: characterization and role in volume regulation. Eur J Neurosci. 2005;21:1648–1658. doi: 10.1111/j.1460-9568.2005.04006.x. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am J Physiol. 1994;267:C1203–C1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol. 1993;265:C1489–C1500. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- Jalonen T. Single-channel characteristics of the large-conductance anion channel in rat cortical astrocytes in primary culture. Glia. 1993;9:227–237. doi: 10.1002/glia.440090308. [DOI] [PubMed] [Google Scholar]
- Junankar PR, Kirk K. Organic osmolyte channels: a comparative view. Cell Physiol Biochem. 2000;10:355–360. doi: 10.1159/000016368. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Uchida S, Monkawa T, Miyawaki A, Mikoshiba K, Marumo F, Sasaki S. Cloning and expression of a protein kinase C-regulated chloride channel abundantly expressed in rat brain neuronal cells. Neuron. 1994;12:597–604. doi: 10.1016/0896-6273(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J Neurosurg. 1995;83:1051–1059. doi: 10.3171/jns.1995.83.6.1051. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk J, Kirk K. Inhibition of volume-activated I− and taurine efflux from HeLa cells by P-glycoprotein blockers correlates with calmodulin inhibition. J Biol Chem. 1994;269:29389–29394. [PubMed] [Google Scholar]
- Kirk K, Strange K. Functional properties and physiological roles of organic solute channels. Annu Rev Physiol. 1998;60:719–739. doi: 10.1146/annurev.physiol.60.1.719. [DOI] [PubMed] [Google Scholar]
- Kubo M, Okada Y. Volume-regulatory Cl− channel currents in cultured human epithelial cells. J Physiol. 1992;456:351–371. doi: 10.1113/jphysiol.1992.sp019340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka M, Schwingshackl A, Befus AD. Mast cells express chloride channels of the ClC family. Inflamm Res. 2002;51:451–456. doi: 10.1007/pl00012411. [DOI] [PubMed] [Google Scholar]
- Lambert IH, Hoffmann EK. Cell swelling activates separate taurine and chloride channels in Ehrlich mouse ascites tumor cells. J Membr Biol. 1994;142:289–298. doi: 10.1007/BF00233436. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Lee CJ, Dravid SM, Traynelis SF. 2004 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience Program No. 405.25; 2004. Glutamate permeation through Ca2+ activated anion channels expressed in astrocytes. [Google Scholar]
- Liu Y, Oiki S, Tsumura T, Shimizu T, Okada Y. Glibenclamide blocks volume-sensitive Cl− channels by dual mechanisms. Am J Physiol. 1998;275:C343–C351. doi: 10.1152/ajpcell.1998.275.2.C343. [DOI] [PubMed] [Google Scholar]
- Loveday D, Heacock AM, Fisher SK. Activation of muscarinic cholinergic receptors enhances the volume-sensitive efflux of myo-inositol from SH-SY5Y neuroblastoma cells. J Neurochem. 2003;87:476–486. doi: 10.1046/j.1471-4159.2003.02021.x. [DOI] [PubMed] [Google Scholar]
- Luckie DB, Krouse ME, Harper KL, Law TC, Wine JJ. Selection for MDR1/P-glycoprotein enhances swelling-activated K+ and Cl− currents in NIH/3T3 cells. Am J Physiol. 1994;267:C650–C658. doi: 10.1152/ajpcell.1994.267.2.C650. [DOI] [PubMed] [Google Scholar]
- McCarty NA, O'Neil RG. Dihydropyridine-sensitive cell volume regulation in proximal tubule: the calcium window. Am J Physiol. 1990;259:F950–F960. doi: 10.1152/ajprenal.1990.259.6.F950. [DOI] [PubMed] [Google Scholar]
- McCarty NA, O'Neil RG. Calcium signaling in cell volume regulation. Physiol Rev. 1992;72:1037–1061. doi: 10.1152/physrev.1992.72.4.1037. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Abdullaev IF, Rudkouskaya A, Kimelberg HK. Comparison of pharmacological profiles of volume-regulated Cl− currents and excitatory amino acid release in cultured astrocytes (Abstract) J Neurochem. 2005;94(Suppl. 1):17. [Google Scholar]
- Mongin AA, Kimelberg HK. ATP potently modulates anion channel-mediated excitatory amino acid release from cultured astrocytes. Am J Physiol Cell Physiol. 2002;283:C569–C578. doi: 10.1152/ajpcell.00438.2001. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Kimelberg HK. Astrocytic swelling in neuropathology. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford/New York: Oxford University Press; 2005a. pp. 550–562. [Google Scholar]
- Mongin AA, Kimelberg HK. ATP regulates anion channel-mediated organic osmolyte release from cultured rat astrocytes via multiple Ca2+-sensitive mechanisms. Am J Physiol Cell Physiol. 2005b;288:C204–C213. doi: 10.1152/ajpcell.00330.2004. [DOI] [PubMed] [Google Scholar]
- Mongin AA, Reddi JM, Charniga C, Kimelberg HK. [3H]Taurine and D-[3H]aspartate release from astrocyte cultures are differently regulated by tyrosine kinases. Am J Physiol Cell Physiol. 1999;276:C1226–C1230. doi: 10.1152/ajpcell.1999.276.5.C1226. [DOI] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Buyse G, Manolopoulos V, Droogmans G. Properties of volume-regulated anion channels in mammalian cells. Prog Biophys Mol Biol. 1997;68:69–119. doi: 10.1016/s0079-6107(97)00021-7. [DOI] [PubMed] [Google Scholar]
- Nilius B, Sehrer J, Viana F, De Greef C, Raeymaekers L, Eggermont J, Droogmans G. Volume-activated Cl− currents in different mammalian non-excitable cell types. Pflugers Arch. 1994;428:364–371. doi: 10.1007/BF00724520. [DOI] [PubMed] [Google Scholar]
- Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- Parkerson KA, Sontheimer H. Biophysical and pharmacological characterization of hypotonically activated chloride currents in cortical astrocytes. Glia. 2004;46:419–436. doi: 10.1002/glia.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasantes-Morales H, Franco R, Ordaz B, Ochoa LD. Mechanisms counteracting swelling in brain cells during hyponatremia. Arch Med Res. 2002;33:237–244. doi: 10.1016/s0188-4409(02)00353-3. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Franco R, Torres-Marquez ME, Hernandez-Fonseca K, Ortega A. Amino acid osmolytes in regulatory volume decrease and isovolumetric regulation in brain cells: contribution and mechanisms. Cell Physiol Biochem. 2000;10:361–370. doi: 10.1159/000016369. [DOI] [PubMed] [Google Scholar]
- Pedersen SF, Prenen J, Droogmans G, Hoffmann EK, Nilius B. Separate swelling- and Ca2+-activated anion currents in Ehrlich ascites tumor cells. J Membr Biol. 1998;163:97–110. doi: 10.1007/s002329900374. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Song D, O'Regan MH. Inhibition by anion channel blockers of ischemia-evoked release of excitotoxic and other amino acids from rat cerebral cortex. Brain Res. 1997;758:9–16. doi: 10.1016/s0006-8993(97)00155-8. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Song D, O'Regan MH. Tamoxifen, a chloride channel blocker, reduces glutamate and aspartate release from the ischemic cerebral cortex. Brain Res. 1998;780:352–355. doi: 10.1016/s0006-8993(97)01352-8. [DOI] [PubMed] [Google Scholar]
- Ronaldson PT, Bendayan M, Gingras D, Piquette-Miller M, Bendayan R. Cellular localization and functional expression of P-glycoprotein in rat astrocyte cultures. J Neurochem. 2004;89:788–800. doi: 10.1111/j.1471-4159.2004.02417.x. [DOI] [PubMed] [Google Scholar]
- Roy G. Amino-acid current through anion channels in cultured human glial-cells. J Membr Biol. 1995;147:35–44. doi: 10.1007/BF00235396. [DOI] [PubMed] [Google Scholar]
- Ruhfus B, Tinel H, Kinne RK. Role of G-proteins in the regulation of organic osmolyte efflux from isolated rat renal inner medullary collecting duct cells. Pflugers Arch. 1996;433:35–41. doi: 10.1007/s004240050245. [DOI] [PubMed] [Google Scholar]
- Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J General Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabirov RZ, Okada Y. Wide nanoscopic pore of maxi-anion channel suits its function as an ATP-conductive pathway. Biophys J. 2004;87:1672–1685. doi: 10.1529/biophysj.104.043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Olea R, Pena C, Moran J, Pasantes-Morales H. Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl− transport in cultured astrocytes. Neurosci Lett. 1993;156:141–144. doi: 10.1016/0304-3940(93)90458-w. [DOI] [PubMed] [Google Scholar]
- Seki Y, Feustel PJ, Keller RW, Jr, Tranmer BI, Kimelberg HK. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke. 1999;30:433–440. doi: 10.1161/01.str.30.2.433. [DOI] [PubMed] [Google Scholar]
- Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- Tilly BC, Edixhoven MJ, van den Berghe BN, Bot AG, De Jonge HR. Ca2+-mobilizing hormones potentiate hypotonicity-induced activation of ionic conductances in Intestine 407 cells. Am J Physiol. 1994;267:C1271–C1278. doi: 10.1152/ajpcell.1994.267.5.C1271. [DOI] [PubMed] [Google Scholar]
- Tomassen SF, Fekkes D, De Jonge HR, Tilly BC. Osmotic swelling-provoked release of organic osmolytes in human intestinal epithelial cells. Am J Physiol Cell Physiol. 2004;286:C1417–C1422. doi: 10.1152/ajpcell.00468.2003. [DOI] [PubMed] [Google Scholar]
- Tsumura T, Oiki S, Ueda S, Okuma M, Okada Y. Sensitivity of volume-sensitive Cl− conductance in human epithelial cells to extracellular nucleotides. Am J Physiol. 1996;271:C1872–C1878. doi: 10.1152/ajpcell.1996.271.6.C1872. [DOI] [PubMed] [Google Scholar]
- Valverde MA, Diaz M, Sepulveda FV, Gill DR, Hyde SC, Higgins CF. Volume-regulated chloride channels associated with the human multidrug-resistance P-glycoprotein. Nature. 1992;355:830–833. doi: 10.1038/355830a0. [DOI] [PubMed] [Google Scholar]
- Wine JJ, Luckie DB. Cell-volume regulation: P-glycoprotein – a cautionary tale. Curr Biol. 1996;6:1410–1412. doi: 10.1016/s0960-9822(96)00744-0. [DOI] [PubMed] [Google Scholar]
- Worrell RT, Butt AG, Cliff WH, Frizzell RA. A volume-sensitive chloride conductance in human colonic cell line T84. Am J Physiol. 1989;256:C1111–C1119. doi: 10.1152/ajpcell.1989.256.6.C1111. [DOI] [PubMed] [Google Scholar]
- Zhong J, Wang GX, Hatton WJ, Yamboliev IA, Walsh MP, Hume JR. Regulation of volume-sensitive outwardly rectifying anion channels in pulmonary arterial smooth muscle cells by PKC. Am J Physiol Cell Physiol. 2002;283:C1627–C1636. doi: 10.1152/ajpcell.00152.2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Simultaneous measurements of d-[3H]aspartate and 36Cl− release from cultured astrocytes exposed to hypoosmotic medium
VRAC blocker phloretin strongly inhibits swelling-activated d-[3H]aspartate release and Cl−currents