Abstract
We explored interhemispheric facilitation (IHF) between (a) left and right primary motor cortex (M1) and (b) left dorsal premotor (dPM) and right M1 in 20 right-handed healthy human subjects using a paired pulse transcranial magnetic stimulation (TMS) protocol. A conditioning TMS pulse (CP) applied to left M1 or dPM with an intensity of 80% and 60% active motor threshold (CP80%AMT and CP60%AMT, respectively) was followed by a test pulse (TP) over right M1 induced by anterior–posterior- or posterior–anterior- (TPAP, TPPA) directed currents in the brain at interstimulus intervals (ISIs) of 3–8 and 10 ms. EMG was recorded from left first dorsal interosseous muscle. In the main experimental condition IHF was evoked by CP80%AMT over left M1 and TPAP at ISIs of 6 and 8 ms. The same CP80%AMT produced IHF at an ISI of 8 ms when applied over left dPM but only with TPPA. In addition, when CP60%AMT was given to M1, IHF was present at an ISI of 6 ms (but not 8 ms) when followed by TPPA, indicating that IHF elicited over dPM was not caused by current spread of the conditioning pulse to M1. We conclude that IHF can be induced differentially by conditioning M1 and dPM using subthreshold CP. These facilitatory interactions depended on the intensity and ISI of the CP as well as the current flow direction of the TP. We suggest that not only do the CPs activate separate anatomical pathways but also that these pathways project to different populations of interneurons in the receiving M1. These may correspond to elements involved in the generation of I3 and I1 waves, respectively.
Interhemispheric facilitation (IHF) has been investigated in a number of animal studies in the last century. For instance, IHF mediated through the corpus callosum was found in cats between homologous visual, acoustic and motor areas (for review see Bremer, 1958). Using electrical conditioning in cats, Asanuma & Okuda (1962) produced IHF between primary motor areas only in discrete areas whereas interhemispheric inhibition (IHI) could be elicited across an extended area in the conditioned hemisphere implying that IHF is focal and probably weak in contrast to IHI that appears to be a rather robust and widespread phenomenon.
In fact, in studies of interhemispheric interactions between motor areas in humans using transcranial magnetic stimulation (TMS), e.g. investigations of the ipsilateral silent period (Meyer et al. 1995), modulation of motor-evoked potential (MEP) amplitudes after conditioning the contralateral motor areas in a paired pulse protocol (Ferbert et al. 1992; Netz et al. 1995; Di Lazzaro et al. 1999; Daskalakis et al. 2002; Chen et al. 2003; De Gennaro et al. 2003, 2004; Mochizuki et al. 2004) or combining conditioning repetitive TMS (rTMS) and single-photon emission computed tomography (Okabe et al. 2003) IHI could clearly and readily be demonstrated. In good accordance with the study of Asanuma & Okuda (1962) IHF could be induced only under certain stimulation conditions in humans (Ugawa et al. 1993; Hanajima et al. 2001a). IHF between primary motor areas could be induced in a small window of slightly suprathreshold conditioning intensities and only in the pre-activated target muscle with an anterior–posterior- (AP) directed current flow over the test pulse (TP) hemisphere (Hanajima et al. 2001a). Ferbert et al. (1992) described IHF at short intervals in some subjects, but pointed out that this phenomenon was highly variable both within and between subjects.
Most studies in humans have used conditioning TMS pulses (CPs) over the hand area of the primary motor cortex (M1). However, anatomical data from monkeys showed that apart from direct M1 to M1 connections (Curtis, 1940; Jenny, 1979; Killackey et al. 1983; Gould et al. 1986) and connections between the dorsal premotor cortex (dPM) and contralateral homologous areas (Marconi et al. 2003) there are also pathways directly linking the dPM and contralateral M1 (Marconi et al. 2003). This raises the question of whether interhemispheric phenomena including IHF are also present and perhaps more robust in humans when dPM rather than M1 is conditioned. In fact, this issue was recently addressed by Mochizuki et al. (2004) who reported lower thresholds for induction of IHI when CPs were applied over dPM as compared to CPs given to M1. On the other hand, Hanajima et al. (2001a) could not produce IHF when conditioning 2 cm anteriorly to M1 (i.e. over dPM) using the same stimulation conditions that were effective over M1. Given the anatomical data in animals (see above) and studies in humans underscoring the role of dPM during bimanual coordination (Sadato et al. 1997; Schluter et al. 1998) it seems likely that facilitatory connections between dPM and contralateral M1 also exist in humans.
Recently, several studies have explored facilitatory and inhibitory interaction between ipsilateral dPM and M1 using single pulse (Civardi et al. 2001) or repetitive TMS (rTMS) conditioning over dPM (Gerschlager et al. 2001; Münchau et al. 2002; Bäumer et al. 2003; Rizzo et al. 2004). These studies showed that the effects of conditioning dPM crucially depend on the intensity of TMS pulses with effects being more specific at low intensities of stimulation.
The aim of the present study was to explore IHF further in healthy humans by comparing the effects of low intensity TMS conditioning of M1 and dPM. We hypothesized that (i) a low intensity CP over left M1 followed by a TP inducing an AP-directed current in contralateral M1 would produce IHF at certain interstimulus intervals (ISIs), and (ii) IHF could also be produced by conditioning left dPM but with different stimulation parameters.
Methods
Study design
The aims of the present study were threefold: (i) based on previous studies (see Introduction), to characterize focal short latency (3–8 ms and 10 ms) IHF of right M1 induced by a low-intensity CP given over left M1; (ii) to examine whether such IHF depends on the intensity of the CP and/or direction of the current flow-inducing TP; and (iii) to discriminate between IHF effects induced by CPs applied to left M1 and those following conditioning of left dPM. CPs were applied over the left hemisphere in right-handers because there is some evidence that interhemispheric interactions, at least with regard to inhibitory phenomena, are more homogeneous after conditioning the left hemisphere in right-handers (Kobayashi et al. 2003).
In a control experiment, conditions where most effective IHF could be elicited at rest were also tested during tonic pre-activation of the target muscle.
Subjects
All subjects were consistent right-handers according to the Edinburgh handedness inventory (EDI). We studied 7 healthy females (mean ± s.d.: age, 24 ± 1.7 years; EDI score, 90 ± 13.8) and 13 healthy males (age, 26.3 ± 5.3 years; EDI score, 90 ± 10.6). All participants gave their written informed consent prior to participation. The experiments conformed to the standards set by the Declaration of Helsinki and were carried out with the approval of the local Ethics Committee.
Recording system
Subjects were seated in a comfortable armchair that was positioned in a subject and coil holder frame. Subjects' heads were fixed by a chin rest and a neck holder, which was adjusted individually to allow for a comfortable position. Both arms were supported by a pillow to ensure that arm muscles were completely relaxed. Subjects were instructed to relax but to keep their eyes open and fixate a visual target directly in front.
EMG was recorded with silver disc surface electrodes placed in differential pairs over the first dorsal interosseous (FDI) muscles bilaterally, using a belly tendon montage. The left FDI muscle was our target muscle for recording MEPs while the right FDI muscle was recorded to ensure complete relaxation. The earth electrode was placed at the wrist. EMG signals were amplified and filtered (20 Hz to 1 kHz) with a D360 amplifier (Digitimer Limited, Welwyn Garden City, UK). The signals were sampled at 5000 Hz, digitized using a laboratory interface (Micro1401; Cambridge Electronics Design (CED), Cambridge, UK) and stored on a personal computer for display and later off-line data analysis. To capture baseline EMG activity during the measurements EMG signals were continuously monitored acoustically with loudspeakers and visually by means of an oscilloscope.
TMS measurements
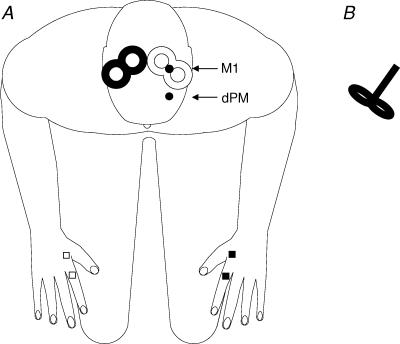
Measurements were performed with two Magstim 200 magnetic stimulators, each connected to a figure-of-eight-shaped coil with an outer winding diameter of approximately 70 mm (‘baby coil’; Magstim Company, Whitland, Dyfed, UK) with handles perpendicular to the coil windings (‘branding iron style’; Fig. 1B) both for CPs and TPs. The small diameter and the type of handle of these coils allowed the placement of each coil over the optimal target site without interfering with the coil positioning on the contralateral hemisphere (Fig. 1A). The magnetic stimulus had a nearly monophasic pulse configuration with a rise time of about 100 µs, decaying back to zero over about 0.8 ms.
Figure 1.
A, experimental set-up and position of custom-made 70 mm figure-of-eight TMS coils on subject's head. TMS test pulses were applied over right M1 (filled coil) inducing AP- or PA-directed current flows. Conditioning pulses were given over left primary motor cortex (M1) or dorsal premotor cortex (dPM) (open coil). MEPs were recorded from left first dorsal interosseus muscle (FDI). EMG was also recorded from right FDI to control for complete relaxation in the main experiment. B, custom-made ‘baby coils’ with handles perpendicular to the coil windings (‘branding iron style’).
Over the left hemisphere where CPs were applied the coil was always placed tangentially to the scalp at a 45 deg angle away from the midline, approximately perpendicular to the line of the central sulcus inducing a PA current in the brain. Over the right hemisphere where TPs were given, the same (mirrored) coil positions was used to induce a PA current flow. In addition, we also tested the effect of CPs given over the left side on TPs produced by an AP current flow in the right hemisphere by rotating the TP coil by 180 deg such that it was positioned 45 deg rotated towards the midline. We determined the optimal position for activation of the FDI muscles by moving the coil in 0.5 cm steps around the presumed motor hand area of the motor cortex of both hemispheres. The sites where stimuli of slightly suprathreshold intensity consistently produced the largest MEPs with the steepest negative slope in corresponding FDI muscle (referred to as the ‘motor hot spot’; M1) were marked with a red wax pen by drawing a semilunar line following the anterior bifurcation of the coil and a straight line indicating the orientation of the coil. The coil position for left dPM TMS was defined relative to the position of M1. A positron emission tomography (PET) study showed that the dPM is located approximately 2 cm anterior to M1 (Fink et al. 1997). By analogy to previous studies where we used conditioning dPM rTMS (Münchau et al. 2002; Bäumer et al. 2003) we calculated for each subject 8% of the distance between nasion and inion (typically about 3 cm) and defined the dPM area as this distance anterior to M1 (Fig. 1A) to minimize M1 activation when applying TMS pulses to dPM. For left dPM stimulation the coil had the same orientation as for left M1 stimulation, i.e. it induced a PA current flow in the brain. TMS coils were fixed to the frame using coil holders and placed at the marked stimulation sites.
Resting motor threshold (RMT) was defined as the minimum stimulus intensity that produced an MEP of more than 50 µV in 5 out of 10 consecutive trials. Active motor threshold (AMT) was defined as the lowest stimulus intensity at which MEPs were elicited in the tonically contracting FDI muscle of about 10% of maximum voluntary contraction. Motor thresholds were expressed as a percentage of maximum stimulator output (MSO).
RMT and AMT for FDI were determined bilaterally over M1 for both PA (left and right hemisphere) and AP (right hemisphere only) current direction and over left dPM which we referred to as resting and active premotor threshold (RMTdPM, AMTdPM).
Interhemispheric interaction was probed using a conditioning–test protocol. CPs were applied to left M1 or dPM as defined above and TPs given to right M1 inducing a PA- and AP-directed current flow in the brain. The intensity of the TP was set such that, when given alone, it would evoke an EMG response of approximately 1 mV peak-to-peak size in the left FDI muscle.
In the main experiment, subjects were studied at rest and left-to-right conditioning effects were tested at ISIs of 3, 4, 5, 6, 7, 8 and 10 ms. These eight conditions (TPs given alone and seven CPs at different ISIs) were applied in a block of 100 trials. In each block the control condition (TPs given alone) was tested 30 times and each of the CP–TP stimuli 10 times. For the CP, we chose a stimulus intensity of 80% or 60% of AMT (i.e. CP80%AMT and CP60%AMT). The rationale behind using very low intensities for the CP was threefold. (i) Studies in cats suggest that IHF is a very focal phenomenon that might be masked by extensive IHI (Asanuma & Okuda, 1962). By using stimulus intensities that were well below the threshold for inducing IHI, we were confident that any facilitatory effect on the opposite hemisphere would not be obscured by the presence of IHI. (ii) Intensities of the CP were also subthreshold for evoking descending corticospinal volleys (Di Lazzaro et al. 1998). Thus, spinal facilitatory effects cannot account for possible facilitation between hemispheres. (iii) The very low intensities also minimized current spread of TMS from M1 to dPM and vice versa.
Six subjects participated in a control experiment that was designed to test IHF with CP80%AMT given to left M1 and left dPM at ISIs of 6 and 8 ms during 10% maximum voluntary contraction of the target muscle. CPs over M1 were followed by AP-directed TPs and CPs over dPM by PA-directed TPs. TP intensity was set to produce MEP amplitudes of about 0.3–0.5 mV.
Data analysis
At each ISI the mean peak-to-peak amplitude of the conditioned MEP was expressed as a percentage of the mean peak-to-peak size of the unconditioned MEP. Measurements were made on each individual trial.
It has previously been shown that an AP-directed current flow in M1 preferentially induces corticospinal I3 waves, and a PA-directed current flow I1 waves, with latencies of corticospinal volleys induced by a PA current flow being approximately 3 ms shorter than those induced by an AP current flow, at least under pre-activation (Sakai et al. 1997; Hanajima et al. 2001a). To verify whether this is also true in the relaxed muscle we determined latencies of all unconditioned test MEPs using a customized Matlab script (Matlab 6.51) as follows. Each trial was integrated and rectified. MEP onset was defined by the first of five consecutive data points exceeding a threshold of 10 µV over and above the baseline within a time frame of 15–90 ms after the TP. To validate these automated measurements we also determined MEP latencies manually in three subjects and calculated the correlation between both measurements.
In the control experiment MEP latencies elicited in the pre-activated FDI were determined manually trial by trial.
Statistical analyses
Unconditioned test MEP amplitudes were compared between the different stimulation conditions using repeated measures analysis of variance (ANOVA).
Mean test MEP latencies in the PA stimulation block were compared with those induced by an AP current flow using paired sample t tests. To correlate automated and manual MEP latency measurements at rest the Pearson correlation coefficient was used.
To compare the conditioning effects of all experimental conditions, mean conditioned MEP amplitudes across all ISIs were entered into a three-factor repeated measures ANOVA with the factors ‘site’ (2 levels; M1 and dPM), ‘intensity’ (2 levels; 60 and 80% AMT) and ‘direction’ of the TP current flow (2 levels; PA and AP). One condition turned out to differ significantly from all other conditions (CP80%AMT given to left M1 followed by TPAP to right M1 referred to as ‘condition A’). In condition A, we compared absolute MEP amplitudes of conditioned MEPs at each ISI with unconditioned test MEPs using paired sample t tests to test if and at which ISI IHF occurred. The Bonferroni method was used to correct for multiple non-independent comparisons.
To test for differential effects at these ISIs across experimental conditions, we carried out repeated measures ANOVA using mean (relative) MEP amplitudes at each ISI as dependent variables. Conditioned MEP amplitudes at each ISI were expressed as a percentage of the unconditioned test MEP amplitude. This ANOVA model had four factors: ‘site’ of CP (left M1 versus left dPM), ‘intensity’ of CP (CP80%AMTversus CP60%AMT), current ‘direction’ of the TP (AP versus PA orientation) and ISI between CP and TP (6 and 8 ms). To analyse the effect of tonic pre-activation on IHF we compared conditioned MEP amplitudes using a three-factor repeated measures ANOVA model with the factors ‘activation state’ (2 levels; active and rest), ‘site’ (2 levels; M1 and dPM) and ISI (2 levels; 6 and 8 ms).
For all statistical analysis the Greenhouse-Geisser correction was used to correct for non-sphericity. Conditional on a significant F value in ANOVA, post hoc tests were performed (Fisher test). A P value of < 0.05 was considered significant. Data are given as mean ± 1 s.d.
Results
None of the subjects reported any side-effects during or after the experiments. RMT and AMT were significantly higher in the non-dominant right hemisphere. Motor thresholds over left dPM were significantly higher than those over left M1 (Table 1). Intensities of the TP were significantly higher for AP- (65.2 ± 12.2) than for PA- (50.2 ± 8.7) directed current flows (T = −10.4; P < 0.0001).
Table 1. Active and resting motor thresholds (AMT and RMT) of right and left motor cortex (M1) and resting and active thresholds of left dorsal premotor cortex (RMTdPM and AMTdPM, respectively).
| Site | Threshold | Right hemisphere (% of MSO) | Left hemisphere (% of MSO) | Significance (2-sided) |
|---|---|---|---|---|
| M1 | RMT | 40.4 ± 6.0 | 36.1 ± 6.4 | T = 3.9; P = 0.001 |
| AMT | 30.0 ± 4.9 | 25.6 ± 5.9 | T = 4.7; P < 0.001 | |
| dPM | RMTdPM | — | 49.5 ± 10.9 | — |
| AMTdPM | — | 37.7 ± 10.7 | — | |
| Significance | RMT | — | T =−6.9; P < 0.001 | — |
| (2-sided) | AMT | — | T =−6.5; P < 0.001 | — |
Values are mean ± s.d. RMT and AMT were higher in the non-dominant right hemisphere and also higher over left dorsal premotor cortex (dPM) as compared to left M1. Thresholds are expressed as percentage of maximum stimulator output (MSO).
MEP amplitudes and latencies
A three-factorial repeated measures ANOVA of unconditioned test MEP amplitudes revealed no significant main effect or interaction of the factors ‘site’, ‘intensity’ and ‘direction’, indicating that baseline excitability did not differ between experimental conditions (Table 2).
Table 2. Test MEP amplitudes of the eight experimental conditions.
| Condition | Test MEP amplitude (mV) |
|---|---|
| TPPA (M1–CP60%AMT) | 0.92 ± 0.09 |
| TPPA (M1–CP80%AMT) | 0.93 ± 0.08 |
| TPAP (M1–CP60%AMT) | 0.91 ± 0.08 |
| TPAP (M1–CP80%AMT) | 0.92 ± 0.10 |
| TPPA (dPM–CP60%AMT) | 1.03 ± 0.08 |
| TPPA (dPM–CP80%AMT) | 0.89 ± 0.08 |
| TPAP (dPM–CP60%AMT) | 0.85 ± 0.08 |
| TPAP (dPM–CP80%AMT) | 0.82 ± 0.07 |
Values are mean ± s.e.m. TP, test pulse; PA, anterior-directed current; AP, posterior-directed current; M1–CP, conditioning pulse applied to M1; dPM–CP, conditioning pulse applied to dPM; AMT, active motor threshold.
In the relaxed FDI muscle, mean latency of unconditioned test MEPs following TPPA (24.6 ± 1.4 ms) was shorter than that after TPAP (25.7 ± 1.1 ms; T = 4.3; P < 0.001). In addition, latencies of unconditioned and conditioned MEPs (at 6 and 8 ms ISI) in experimental conditions producing significant IHF (TPPA with CP80%AMT to dPM and CP60%AMT to M1 versus TPAP with CP80%AMT to M1) were also shorter in TPPA as compared to TPAP conditions (for all: T > 3; P < 0.005). There were no latency differences between unconditioned and conditioned MEPs in TPPA and TPAP conditions, respectively (Table 3).
Table 3. Latencies of experimental conditions producing significant facilitation.
| Conditioned MEP latency (ms) | |||
|---|---|---|---|
| Condition | Unconditioned MEP latency (ms) | ISI of 6 ms | ISI of 8 ms |
| TPAP (M1–CP80%AMT) | 25.3 ± 0.27 | 25.3 ± 0.31 | 25.2 ± 0.30 |
| TPPA (dPM–CP80%AMT) | 24.4 ± 0.33 | 24.3 ± 0.32 | 24.1 ± 0.33 |
| TPPA (M1–CP60%AMT) | 24.3 ± 0.30 | 24.1 ± 0.28 | 24.2 ± 0.28 |
Values are mean ± s.e.m. TP, test pulse; PA, anterior-directed current; AP, posterior-directed current; M1–CP, conditioning pulse applied to M1; dPM–CP, conditioning pulse applied to dPM; AMT, active motor threshold; ISI, interstimulus interval.
Comparison of the script based and the manually determined MEP latencies in three subjects revealed a high correlation of both methods (r = 0.93; P < 0.001) with script-based latencies being some 0.5 ms longer.
In the activated FDI mean MEP latency following TPPA (20.8 ± 1.7 ms) was shorter than that after TPAP (23.5 ± 1 ms; T = 4.1; P = 0.001).
Conditions producing IHF
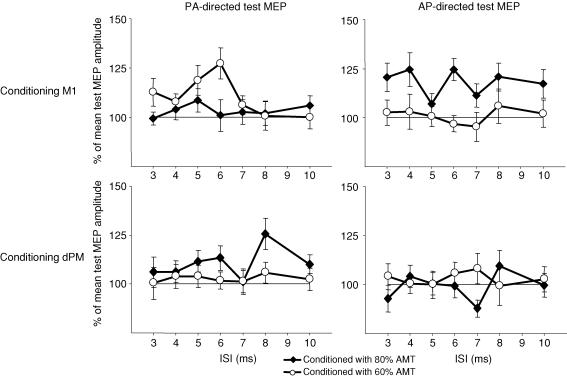
Figure 2 shows conditioned MEP amplitudes at different ISIs for the eight experimental conditions. There is IHF at several ISIs in some but not in other conditions. To identify conditions where differential IHF occurred as compared to other conditions without IHF (thereby avoiding a type 1 error of multiple comparisons for multiple conditions) we first carried out a three-factorial ANOVA with the factors ‘site’ (2 levels; M1 and dPM), ‘intensity’ (2 levels; 60 and 80% AMT) and ‘direction’ (2 levels; AP and PA) where conditioned MEP amplitudes at the different ISIs were collapsed. There was an effect of ‘site’ (F1;19 = 4.9; P < 0.05) and a significant interaction of the factors ‘site’בintensity’בdirection’ (F1; 19 = 4.2; P < 0.05) showing that there were significant conditioning effects in some but not other conditions. Post hoc tests, comparing all conditions with each other showed that there was only one condition that significantly differed from other conditions (Table 4). In this condition (referred to as ‘condition A’) TPAP to right M1 was conditioned by CP80%AMT to left M1. No other condition differed from any other.
Figure 2.
Mean relative MEP amplitudes of conditioned MEPs at interstimulus intervals (ISIs) of 3–10 ms in the different experimental conditions in the main experiment. Mean values (± s.e.m.) are shown.
Table 4. P values of post hoc tests following a significant three-factor ANOVA testing IHF in all experimental conditions (comparisons with condition A).
| M1 | dPM | |||
|---|---|---|---|---|
| AP | PA | AP | PA | |
| 60% AMT | 0.03 | 0.28 | 0.03 | 0.07 |
| 80% AMT | — | 0.09 | 0.03 | 0.34 |
P < 0.05 (in italics)
We then identified those ISIs in condition A at which IHF had occurred. Comparing absolute MEP amplitudes of conditioned MEPs with unconditioned TPs, CP80%AMT induced some facilitation of the MEPs at ISIs of 3, 4, 6, 8 and 10 ms (Fig. 2). However, significant IHF was only found at ISIs of 6 and 8 ms (P < 0.05, with Bonferroni correction).
To address the question of whether IHF at 6 and 8 ms was a specific phenomenon depending on the stimulation parameters, we carried out a four-factorial repeated measures ANOVA at these ISIs where effects of ‘site’, ‘intensity’ and ‘direction’ of the current flow inducing TP on relative MEP amplitudes were tested. There was a significant main effect of ‘intensity’ (F1; 19 = 4.5; P = 0.05) and a significant interaction of ‘site’בdirection’בintensity’בISI’ (F1; 19 = 9.5; P < 0.01) indicating that IHF at these ISIs is a current flow-, intensity- and site-specific phenomenon.
On the basis of this analysis we explored the experimental conditions in which significant IHF at ISIs of 6 and 8 ms occurred by comparing conditioned MEP amplitudes at these ISIs (absolute values) with test MEP amplitudes in each condition. In addition to condition A, paired sample t tests (with Bonferroni correction) also revealed significant IHF in the following experimental conditions: (i) ‘condition B’; CP60%AMT over left M1 and TPPA at an ISI of 6 ms (P < 0.05, corrected); and (ii) ‘condition C’; CP80%AMT over left dPM and TPPA at an ISI of 8 ms (P < 0.05, corrected).
In addition, to exclude a type 2 error we also compared conditioned MEPs at ISIs of 3, 4, 5, 7 and 10 ms in separate ANOVAs between all experimental conditions. There was no significant effect on conditioned MEP size at any of these ISIs. Therefore, we restricted further analyses to conditioned MEPs at ISIs of 6 and 8 ms.
Effects of current flow on IHF
Next, we examined the impact of the current flow direction of the TP (TPPAversus TPAP) and the site of the CP (left M1 versus left dPM) on IHF using post hoc tests (relative amplitudes).
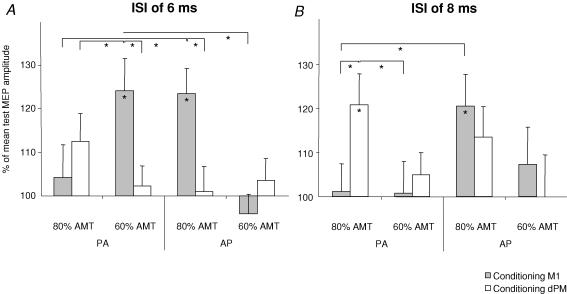
At a given ISI and a given intensity and site of the CP the amount of IHF was dependent on the current flow of the TP (Fig. 3). Thus, IHF following CP60%AMT given over left M1 was significantly larger with TPPA (condition B) than with TPAP at an ISI of 6 ms (P < 0.001) (Fig. 3A). In contrast IHF was significantly larger when CP80%AMT over M1 was followed by TPAP (condition A) than by TPPA at this ISI (P = 0.001) (Fig. 3A) and also at an ISI of 8 ms (P = 0.001) (Fig. 3B). A dependence of IHF on the current flow direction of the TP was not found for dPM–M1 IHF. The magnitude of IHF induced by CP80%AMT given to dPM was not significantly different when probed with TPPA or TPAP.
Figure 3.
Conditioned relative MEP amplitudes (mean values ± s.e.m.) at ISIs of 6 ms (A) and 8 ms (B) in all stimulation conditions. Conditions producing IHF are marked with an asterisk. Brackets signify comparisons between experimental conditions. *P < 0.05. AMT, active motor threshold. AP, anterior–posterior current flow of the TMS test pulse; PA, posterior–anterior current flow of the TMS test pulse.
Differential effects of M1 and dPM conditioning
At a given intensity of the CP, ISI and current flow of the TP IHF was site specific.
Following CP60%AMT coupled with TPPA (condition B) IHF was significantly more pronounced when given over left M1 as compared to left dPM at an ISI of 6 ms (P < 0.001) (Fig. 3A). In condition A, CP80%AMT followed by a TPAP was significantly more effective in inducing IHF when applied to left M1 as compared to left dPM at an ISI of 6 ms (P < 0.001) (Fig. 3A). On the other hand, CP80%AMT to left dPM followed by TPPA was significantly more effective in producing IHF than conditioning left M1 with the same intensity at an ISI of 8 ms (P = 0.001) (Fig. 3B).
It is possible that some of the effects observed following TMS conditioning of left dPM might be explained by current spread to left M1. Given that motor thresholds with the stimulation coil positioned over dPM were some 35% (resting condition) to 50% (active condition) higher than thresholds over M1 (Table 1) CP80%AMT applied over dPM would lead to effective stimulation of M1 in the order of 40% to about 52% AMT which is less than the lowest stimulation intensity that we used for conditioning M1 (60% AMT). This notwithstanding, we also compared conditions where IHF was induced by CP80%AMT given to dPM with those where CP60%AMT was applied to M1 at the same ISI.
At an ISI of 6 ms (using TPPA) there was IHF following CP80%AMT given to dPM and CP60%AMT given to M1 which was significant only after M1 stimulation (condition B). Moreover, IHF was significantly larger after conditioning M1 (P < 0.05) (Fig. 3A). Conceivably, at this ISI dPM stimulation (with 80% AMT) was indeed acting on the same neural elements that were also stimulated, though more effectively, by CP over M1.
However, CP80%AMT over dPM at an ISI of 8 ms followed by TPPA was significantly more effective in producing IHF than CP60%AMT applied to M1 (P < 0.05) (Fig. 3B). In fact, with these simulation parameters conditioning left M1 had no facilitatory effect at all.
Finally, given that the latency difference of 2 ms between effective left M1 (CP60%AMT; ISI 6 ms) and dPM (CP80%AMT; ISI 8 ms) conditioning on TP induced by PA current flow could represent a delay caused by one synapse in the same pathway (left dPM to left M1 to right M1) we have also compared effective IHF between these conditions. There was no difference (P < 0.05). It is thus possible that these effects are mediated by the same pathway probably running from the left dPM through left M1 to right M1.
Taken together, these results can be taken as evidence that apart from effective stimulation of M1 through current spread away from dPM it is also possible to produce IHF through direct stimulation of dPM under certain experimental conditions.
Effects of pre-activation
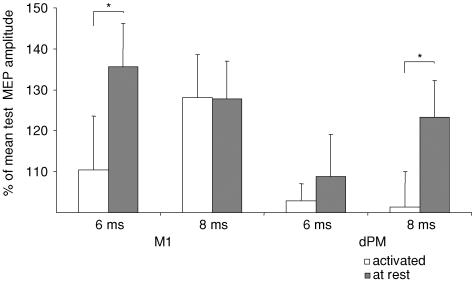
Differential effects on IHF induced by conditioning left dPM or M1 were also investigated in the pre-activated FDI with TP MEP amplitudes of 0.3–0.5 mV. Comparing the resting and pre-activated states there was a significant interaction of the factors ‘activation state’בsite’בISI’ (F1; 5 = 6.2; P < 0.05). Post hoc tests showed that IHF following M1 conditioning at an ISI of 6 ms and following dPM conditioning at an ISI of 8 ms was significantly stronger at rest as compared to the pre-activated state (P < 0.05). In contrast, IHF induced by conditioning M1 at an ISI of 8 ms was not different between the active and resting conditions.
Comparing conditioned with unconditioned mean MEP amplitudes there was significant IHF at ISIs of 6 and 8 ms when M1 was conditioned at rest, and at 8 ms when dPM was conditioned in this same subgroup at rest (Fig. 4). Under pre-activation IHF was present only at an ISI of 8 ms when M1 was conditioned (P < 0.05).
Figure 4.
Interhemispheric facilitation induced by conditioning M1 or dPM at rest or under pre-activation of the target muscle (mean values ± s.e.m.). Brackets signify comparisons between experimental conditions. *P < 0.05.
Discussion
The aim of this study was to characterize IHF of right M1 by TMS conditioning of left M1 and dPM, respectively, in healthy human subjects. The main novel finding was that IHF could be induced by sub-motor-threshold TMS conditioning (60 and 80% AMT) over left M1 or dPM at short ISIs (6 and 8 ms) in a differential manner. The ISI, the intensity of the CP, the current direction of the TP and tonic contraction all had an impact on the magnitude of IHF. The influence of these factors on IHF differed depending on the site of conditioning, indicating that dPM-to-M1 and M1-to-M1 IHF is mediated by different neuronal circuits in humans.
Interhemispheric inhibition may mask interhemispheric facilitation
In the seminal paper by Ferbert et al. (1992) on IHI of M1 produced by TMS conditioning of the opposite M1 in healthy humans there is also a mention of IHF at short ISIs (3 and 5 ms). However, the latter was referred to as being ‘capricious and would often disappear if the block of trials was repeated, only to reappear again in the same subject on another same day.’ A more recent study by Hanajima et al. (2001a) could produce IHF at ISIs of 4–5 ms between both M1 using slightly suprathreshold CPs of 105% AMT but not at 5% higher or lower intensities. In addition, M1-to-M1 IHF was only present in the pre-activated target muscle with TPAP (Hanajima et al. 2001a, b). However, under the same experimental conditions there were no effects following TMS conditioning of the dPM. In one study IHF was reported to occur after conditioning M1 with intensities of 150% of RMT at short latencies (1–5 ms) (Salerno & Georgesco, 1996), but other investigators always produced robust IHI at such high conditioning intensities (Ferbert et al. 1992; Daskalakis et al. 2002; Chen et al. 2003).
Taken together, these studies suggest that in healthy humans IHF is subtle and highly variable, whereas IHI is a consistent phenomena that can readily be produced at intensities above individual RMT both at rest and during contraction (Ferbert et al. 1992; Netz et al. 1995; Di Lazzaro et al. 1999; Daskalakis et al. 2002; Chen et al. 2003; De Gennaro et al. 2003, 2004). The TMS data obtained in humans are in good agreement with a study on interhemispheric interactions between motor cortical areas in cats. Asanuma & Okuda (1962) could demonstrate that interhemispheric inhibitory neurons are abundant but facilitatory neurons are scarce. There were only a few areas in the motor cortex where IHF could be elicited. If such an area was found only small changes in the position of the electrode would cause IHF to disappear and be replaced by IHI in a wide area of surrounding cortex. These studies imply that IHF is indeed subtle with a narrow window of effective stimulation. Outside this window inhibition prevails probably cancelling out facilitatory effects.
The present study lends support to the notion that IHF is a focal phenomenon best studied with low intensity conditioning stimulation. One of the main findings was that IHF could be produced with CP80%AMT and even CP60%AMT. Using such low intensity pulses, both M1 and dPM conditioning lead to IHF. Importantly, apart from the stimulation intensity, these effects crucially depended on the ISI between CP and TP and the current flow direction of the TP.
Intensity of the conditioning stimulus
Two other studies have previously investigated the effects of sub-motor-threshold CPs on interhemispheric interaction. De Gennaro et al. (2004) could not produce interhemispheric effects using a conditioning intensity of 80% RMT over M1. Mochizuki et al. (2004) reported IHI but no IHF following conditioning right M1 and dPM at 90% RMT. In addition, Hanajima et al. (2001)a found IHF at intensities of 105% AMT after conditioning M1 only during slight activation of the target muscle (see above). Also, only medially directed CP currents were effective in producing IHF.
The apparent discrepancy between the present study and previous work might be explained by differences in (i) study design and (ii) stimulation intensities.
First, in some previous studies where IHF was addressed CPs were applied to the right and TPs to the left hemisphere (Hanajima et al. 2001a) whereas we opted for the reverse set-up because of some evidence that interhemispheric interactions are more homogeneous after conditioning the left hemisphere in right-handers (Kobayashi et al. 2003).
Second, we used very low intensities for CPs. CPs of 80% or even 60% of AMT were sufficient to elicit IHF. Such low intensities have not previously been explored. Given that IHF is probably very focal and readily cancelled out by stronger IHI, it is likely that weak IHF was missed in previous studies where higher CP intensities were used. In our study, the intensity of the CP was well below the threshold for inducing IHI so that IHF was not masked by IHI.
Several recent TMS studies have shown that TMS conditioning with intensities of 80% and 90% AMT can lead to effective activation of neuronal populations that mediate dPM-to-M1 interactions in the left hemisphere using single or repetitive TMS pulses (Civardi et al. 2001; Gerschlager et al. 2001; Münchau et al. 2002; Bäumer et al. 2003; Rizzo et al. 2004). For example, a single CP with an intensity of 90% AMT applied to the left dPM leads to inhibition of a TP given to the left M1 at an ISI of 6 ms (Civardi et al. 2001) while a CP at 120% RMT leads to facilitation of a TP at the same ISI. Following a 20 min train of 1 Hz rTMS given to dPM at an intensity of 80% AMT intracortical excitability was increased in ipsilateral M1 as studied with the Kujirai et al. (1993) paired pulse protocol (Münchau et al. 2002). Importantly, these effects were intensity specific. Slightly higher intensities of 90% AMT rTMS applied to the dPM did not alter intracortical excitability (Münchau et al. 2002). Instead, this intensity caused a reduction of net corticospinal excitability as indexed by the MEP size (Gerschlager et al. 2001).
These patterns of dependence on stimulation intensity of ipsilateral dPM-to-M1 interactions, IHI and IHF may indicate that the underlying inhibitory and facilitatory neuronal circuits involved are distinct. Taken together, these experiments further support the concept that TMS conditioning of motor areas at very low intensities provides an interesting means of producing subtle but specific effects on cortico-cortical connectivity among frontal motor areas.
Latency of interhemispheric facilitation
As regards effective ISIs between CP and TP in this study 6 and 8 ms compare well to previous experiments of IHI. The onset of significant inhibition of MEPs by a contralateral CP is typically 6 ms (Ferbert et al. 1992). Because TMS pulses may take 1–2 ms to excite corticospinal neurons and can produce repetitive activity in pyramidal neurons lasting 3–5 ms (Day et al. 1989) some 7 ms or so may elapse until the last corticospinal volley induced by a single TP leaves the cortex. All volleys contribute to the peak-to-peak size of the MEP so that even late arrival of transcallosal inputs would affect the size of the MEP. The time taken for conditioning pulses to produce facilitation of the output of descending corticospinal volleys may thus have been up to 13 ms (6 plus 7).
It is interesting to note that IHF in previous studies was noted between 3 and 5 ms after the CP was given to the contralateral M1 (Ferbert et al. 1992; Hanajima et al. 2001a). There was also some IHF at an ISI of 4 ms (122%; P = 0.05; see Fig. 3) in the present study, but this was not significant after correction for multiple comparisons. However, significant IHF occurred at ISIs of 6 and 8 ms.
How can these differences in latency be explained?Hanajima et al. (2001a) studied IHF during tonic contraction of the target muscle, while we assessed IHF at rest. Moreover, previous studies used higher intensities to condition the M1. It is conceivable that ICF is mediated by different sets of neurons with some fast conducting elements being activated at higher intensities or by pre-activation (Ferbert et al. 1992; Hanajima et al. 2001a) and slightly slower conducting fibres being preferentially activated by low intensity stimulation (present study). The latter may not be activated by higher intensity stimulation or may simply be overwhelmed by accompanying stronger IHI.
Of note, effective IHF of TP induced by PA current flow could be produced both by conditioning left M1 (CP60%AMT) at an ISI of 6 ms and dPM (CP80%AMT) at an ISI of 8 ms. This latency difference of 2 ms could represent a delay caused by one synapse in the same pathway (left dPM to left M1 to right M1).
Sensitivity of interhemispheric facilitation to the current flow directions of the TMS pulse
If right M1 was conditioned with CP80%AMT, M1-to-M1 IHF was more effective with TPAP. On the other hand, dPM-to-M1 IHF at an ISI of 8 ms was more pronounced with TPPA.
This differential pattern of M1-to-M1 IHF and dPM-to-M1 IHF suggests that these phenomena are mediated by different interhemispheric circuits. We infer that interhemispheric volleys generated in left M1 and dPM, respectively, target different neuronal populations in the right hemisphere.
It was previously shown that AP-directed TMS pulses lead to activation of the corticospinal tract preferentially by inducing I3 waves whereas PA-directed currents did the same by inducing I1 waves (Sakai et al. 1997; Hanajima et al. 2001a). In accordance with this notion, unconditioned and conditioned MEP latencies to TPAP were longer than those to TPPA in the present study. Therefore, we conclude that CP80%AMT to left dPM preferentially lead to facilitation of I1, while CP80%AMT to left M1 caused preferential facilitation of I3 waves in right M1. The excitability pattern differed when an even lower intensity was used to provoke IHF. CP60%AMT to dPM no longer induced IHF, whereas CP60%AMT to left M1 did, provided it was coupled with TPPA. This indicates that conditioning of left M1 at 60% of AMT preferentially modulated circuits involved in the generation of the I1 volley, whereas conditioning at 80% of AMT facilitated circuits subserving the generation of the I3 volley.
Importantly, when IHF was probed with CP80%AMT given to left M1 (followed by AP-directed TP) and left dPM (followed by PA-directed TP) at ISIs of 6 and 8 ms during pre-activation of the target muscle then significant IHF was present only at an ISI of 8 ms following conditioning of M1 but could not be produced by premotor conditioning.
This supports the concept that IHF through motor and premotor pathways is mediated by different circuits. Moreover, it also suggests a functional segregation with circuits originating in M1 being more directly engaged in the control of contralateral corticospinal motor output. Interhemispheric premotor-to-motor interaction could be more relevant to other aspects of motor control such as visuo-motor integration (Wise et al. 1997) and might be suppressed during simple muscle contraction as tested here.
Site of stimulation and interhemispheric transfer
Because IHF could be induced by conditioning M1 and dPM the question arises of whether some of the effects following dPM conditioning might be explained by current spread to M1. In fact, at an ISI of 6 ms (using TPPA) IHF following conditioning M1 with CP60%AMT was significantly stronger than that after conditioning dPM with CP80%AMT. Thus, at this ISI IHF in the dPM stimulation condition can probably be explained by current spread from dPM to M1. However, at an ISI of 8 ms CP80%AMT (combined with TPPA) only produced effective IHF when dPM was conditioned which can be taken as evidence that under these experimental conditions we did indeed selectively stimulate the dPM.
The precise route of interhemispheric transfer of IHF cannot be clarified with our experiments. However, the short latency between CP and TP at which IHF occurred is in favour of a more or less direct route across the corpus callosum. In addition, the fact that subthreshold TMS pulses do not give rise to descending cortico-spinal volleys (Di Lazzaro et al. 1998) makes a subcortical route unlikely. Moreover, previous studies on IHI have demonstrated short latency changes in motor cortical excitability in the hemisphere contralateral to CP using both supra-motor- and sub-motor-threshold CPs (Di Lazzaro et al. 1999; Mochizuki et al. 2004) which argues against a subcortical route. This view is supported by reduced or absent IHI in patients with lesions in callosal pathways or in the corpus callosum (Boroojerdi et al. 1996, 1998).
Whether IHF was mediated through direct M1-to-M1 connections is unclear. Also, the route of CP following dPM stimulation remains to be determined. Both dPM to ipsilateral M1 to contralateral M1 or dPM to contralateral dPM to M1 pathways are possible. It is well established that there are direct homotopic callosal connections between both motor cortices in monkeys passing though the anterior half of the corpus callosum (Pandya & Seltzer, 1986). However, these connections are more abundant between cortical representation areas of proximal body parts and only modest between hand representation zones (Jenny, 1979). On the other hand, there is recent evidence of direct homotopic and heterotopic connections between dPM, at least in monkeys (Marconi et al. 2003).
In this respect it is interesting to note that, as pointed out above, similar IHF of TPPA could be induced by conditioning left dPM at an ISI of 8 ms (CP80%AMT) and also left M1 (CP60%AMT) at an ISI of 6 ms, i.e. 2 ms later as compared to the left dPM. We speculate that, at least under these conditions, a route from left dPM (ISI 8 ms) via left M1 (ISI 6 ms) to right M1 is most likely.
Future studies in patients with well-defined M1 or dPM lesions may shed further light on the precise anatomy of interhemispheric circuits involved in IHF in humans.
Conclusions
This study shows that differential and specific IHF can be elicited by conditioning M1 and dPM at short latencies (6 and 8 ms) in healthy humans using low intensity TMS. The pattern of most effective IHF following M1 stimulation differed from that induced by dPM conditioning with regard to the intensity of the CP, the ISI between CP and TP, the current flow direction of the TP, and also the activation state of the target muscle. This implies that interhemispheric M1-to-M1 and dPM-to-M1 facilitatory interactions are mediated by different neuronal pathways. This IHF protocol might complement future studies of patients with abnormal activity in interhemispheric connections, e.g. patients with lesions after stroke.
Acknowledgments
A. Münchau (grant I/78 553) and H. R. Siebner (grant I/79 932) were supported by the Volkswagenstiftung.
References
- Asanuma H, Okuda O. Effects of transcallosal volleys on pyramidal tract cell activity of cat. J Neurophysiol. 1962;25:198–208. doi: 10.1152/jn.1962.25.2.198. [DOI] [PubMed] [Google Scholar]
- Bäumer T, Lange R, Liepert J, Weiller C, Siebner HR, Rothwell JC, Münchau A. Repeated premotor rTMS leads to cumulative plastic changes of motor cortex excitability in humans. Neuroimage. 2003;20:550–560. doi: 10.1016/s1053-8119(03)00310-0. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Hungs M, Mull M, Topper R, Noth J. Interhemispheric inhibition in patients with multiple sclerosis. Electroencephalogr Clin Neurophysiol. 1998;109:230–237. doi: 10.1016/s0924-980x(98)00013-7. [DOI] [PubMed] [Google Scholar]
- Bremer F. Physiology of the Corpus Callosum. Res Publ Assoc Res Nerv Ment Dis. 1958;36:424–428. [PubMed] [Google Scholar]
- Chen R, Yung D, Li JY. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Curtis H. Intercortical connections of corpus callosum as indicated by evoked potentials. J Neurophysiol. 1940;3:407–413. [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543:317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Rothwell JC, Thompson PD, Maertens de Noordhout A, Nakashima K, Shannon K, Marsden CD. Delay in the execution of voluntary movement by electrical or magnetic brain stimulation in intact man. Evidence for the storage of motor programs in the brain. Brain. 1989;112:649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Bertini M, Pauri F, Cristiani R, Curcio G, Ferrara M, Rossini PM. Callosal effects of transcranial magnetic stimulation (TMS): the influence of gender and stimulus parameters. Neurosci Res. 2004;48:129–137. doi: 10.1016/j.neures.2003.10.004. [DOI] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Bertini M, Pauri F, Cristiani R, Curcio G, Romei V, Fratello F, Rossini PM. Reproducibility of callosal effects of transcranial magnetic stimulation (TMS) with interhemispheric paired pulses. Neurosci Res. 2003;46:219–227. doi: 10.1016/s0168-0102(03)00060-9. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res. 1999;124:520–524. doi: 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57:449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Gould H, III, Cusick C, Pons T, Kaas J. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and frontal eye fields in owl monkeys. Comp Neurol. 1986;247:297–325. doi: 10.1002/cne.902470303. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I. Interhemispheric facilitation of the hand motor area in humans. J Physiol. 2001a;531:849–859. doi: 10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Okabe S, Yuasa K, Shiio Y, Iwata NK, Kanazawa I. Interhemispheric interaction between the hand motor areas in patients with cortical myoclonus. Clin Neurophysiol. 2001b;112:623–626. doi: 10.1016/s1388-2457(01)00477-1. [DOI] [PubMed] [Google Scholar]
- Jenny AB. Commissural projections of the cortical hand motor area in monkeys. J Comp Neurol. 1979;188:137–145. doi: 10.1002/cne.901880111. [DOI] [PubMed] [Google Scholar]
- Killackey H, Gould H, III, Cusick C, Pons T, Kaas J. The relation of corpus callosum connections to architectonic fields and body surface maps in sensorimotor cortex of new and old world monkeys. J Comp Neurol. 1983;219:384–419. doi: 10.1002/cne.902190403. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Schlaug G, Pascual-Leone A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. Neuroimage. 2003;20:2259–2270. doi: 10.1016/s1053-8119(03)00220-9. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi B, Genovesio A, Giannetti S, Molinari M, Caminiti R. Callosal connections of dorso-lateral premotor cortex. Eur J Neurosci. 2003;18:775–788. doi: 10.1046/j.1460-9568.2003.02807.x. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Röricht S, Gräfin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Huang YZ, Rothwell JC. Interhemispheric interaction between human dorsal premotor and contralateral primary motor cortex. J Physiol. 2004;561:331–338. doi: 10.1113/jphysiol.2004.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci. 2002;22:554–561. doi: 10.1523/JNEUROSCI.22-02-00554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz J, Ziemann U, Homberg V. Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res. 1995;104:527–533. doi: 10.1007/BF00231987. [DOI] [PubMed] [Google Scholar]
- Okabe S, Hanajima R, Ohnishi T, Nishikawa M, Imabayashi E, Takano H, Kawachi T, Matsuda H, Shiio Y, Iwata NK, Furubayashi T, Terao Y, Ugawa Y. Functional connectivity revealed by single-photon emission computed tomography (SPECT) during repetitive transcranial magnetic stimulation (rTMS) of the motor cortex. Clin Neurophysiol. 2003;114:450–457. doi: 10.1016/s1388-2457(02)00408-x. [DOI] [PubMed] [Google Scholar]
- Pandya D, Seltzer B. The topography of commissural fibers. In: Leporè F, Ptito M, Jasper H, editors. Two Hemispheres – One Brain: Functions of the Corpus Callosum. New York: Alan R. Liss; 1986. pp. 47–73. [Google Scholar]
- Rizzo V, Siebner HR, Modugno N, Pesenti A, Munchau A, Gerschlager W, Webb RM, Rothwell JC. Shaping the excitability of human motor cortex with premotor rTMS. J Physiol. 2004;554:483–495. doi: 10.1113/jphysiol.2003.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadato N, Yonekura Y, Waki A, Yamada H, Ishii Y. Role of the supplementary motor area and the right premotor cortex in the coordination of bimanual finger movements. J Neurosci. 1997;17:9667–9674. doi: 10.1523/JNEUROSCI.17-24-09667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furubayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp Brain Res. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Salerno A, Georgesco M. Interhemispheric facilitation and inhibition studied in man with double magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1996;101:395–403. [PubMed] [Google Scholar]
- Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Hanajima R, Kanazawa I. Interhemispheric facilitation of the hand area of the human motor cortex. Neurosci Lett. 1993;160:153–155. doi: 10.1016/0304-3940(93)90401-6. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]