Abstract
The oxygenation of endogenous cannabinoids (eCBs) 2-arachidonoyl glycerol (2-AG) and arachidonoyl ethanolamide by cyclooxygenase-2 (COX-2) produces novel types of prostanoids: prostaglandin glycerol esters (PG-Gs) and prostaglandin ethanolamides (PG-EAs). However, the physiological function of COX-2 oxidative metabolites of eCBs is still unclear. Here we demonstrate that PGE2-G, a COX-2 oxidative metabolite of 2-AG, induced a concentration-dependent increase in the frequency of miniature inhibitory postsynaptic currents (mIPSCs) in primary cultured hippocampal neurons, an effect opposite to that of 2-AG. This increase was not inhibited by SR141716, a CB1 receptor antagonist, but was attenuated by an IP3 or MAPK inhibitor. In addition, we also examined the effects of other prostanoids derived from COX-2 oxygenation of eCBs on mIPSCs. PGD2-G, PGF2α-G and PGD2-EA, but not PGE2-EA or PGF2α-EA, also increased the frequency of mIPSCs. The eCB-derived prostanoid-induced responses appeared to be different from those of corresponding arachidonic acid-derived prostanoids, implying that these effects are not mediated via known prostanoid receptors. We further discovered that the inhibition of COX-2 activity reduced inhibitory synaptic activity and augmented depolarization-induced suppression of inhibition (DSI), whereas the enhancement of COX-2 augmented the synaptic transmission and abolished DSI. Our results, which show that COX-2 oxidative metabolites of eCBs exert opposite effects to their parent molecules on inhibitory synaptic transmission, suggest that alterations in COX-2 activity will have significant impact on endocannabinoid signalling in hippocampal synaptic activity.
Cyclooxygenases (COXs) are key enzymes that convert arachidonic acid (AA) to prostaglandins (PGs). Of the three isozymes of COX that have been identified (Vane et al. 1998; Bazan & Flower, 2002; Chandrasekharan et al. 2002), COX-2 is the focus of growing attention not only because it is inducible in inflammation, but also because it is involved in synaptic transmission and plasticity (Yamagata et al. 1993; Kaufmann et al. 1996; Chen et al. 2002; Chen & Bazan, 2005a, b; Sang et al. 2005). Recent evidence indicates that COX-2 is also capable of oxygenating endogenous cannabinoids (eCBs) 2-arachidonoyl glycerol (2-AG) and arachidonoyl ethanolamide (AEA) to generate new types of prostanoids: prostaglandin glycerol esters (PG-Gs) and prostaglandin ethanolamides (PG-EAs) (Yu et al. 1997; Kozak et al. 2002, 2004; Chen & Bazan, 2005b). In particular, 2-AG has been shown to be a natural substrate for COX-2 and its oxygenation is as effective as that of AA (Kozak et al. 2000). These pioneer studies provide important information regarding COX-2 oxygenation as an important pathway for degrading or inactivating eCBs; however, its physiological significance is still not clear.
Endocannabinoids are involved in a variety of physiological and pathological processes in mammalian tissue via CB1 and CB2 receptors (Hajos & Freund, 2002a; Freund et al. 2003; Piomelli, 2003; Alger, 2004; Howlett et al. 2004; Di Marzo et al. 2004; Patrignani et al. 2005). In the brain, the main focus for eCBs in synaptic signalling is on the presynaptic CB1 receptor-mediated regulation of inhibitory synaptic transmission. However, the role of eCB-derived, COX-2-oxygenated prostanoids in synaptic signalling is unknown. We observed previously that PGE2, a major COX-2 oxidative metabolite of AA, modulates hippocampal synaptic transmission and plasticity (Chen et al. 2002; Chen & Bazan, 2005a; Sang et al. 2005). Here we report that COX-2 oxidative metabolites of 2-AG and AEA induced opposite effects to their precursors on inhibitory synaptic transmission via CB1- and prostanoid receptor-independent mechanisms. Our data suggest that COX-2-generated novel prostanoids, PG-Gs or PG-EAs, represent unique signalling mediators with potent activities distinct from their parent molecules, and exert biological responses extending the range of actions of their related PGs, implying that alterations in COX-2 activity will significantly influence endocannabinoid signalling in hippocampal synaptic function.
Methods
Primary hippocampal neuron culture
All experiments were carried out according to the guidelines approved by the Institutional Animal Care and Use Committee of Louisiana State University Health Sciences Center.
Primary hippocampal neurons were cultured as described before with some modifications (Chen & Bazan, 1999; Zhu et al. 2005; Sang et al. 2005). Briefly, mouse (FVB) pups (postnatal days 0–1) were rapidly decapitated, and hippocampi were immediately dissected out from the brain. The tissue was incubated in oxygenated trypsin for 10 min at 37°C and then mechanically triturated. Cells were spun down and resuspended in Neurobasal–B27 medium (Invitrogen) supplemented with 0.5 mml-glutamine, penicillin–streptomycin and 25 μm glutamate. Cells (1 × 106) were loaded into poly d-lysine-coated 35 mm culture dishes. One-third to one-half of the culture medium without glutamate was changed every 2–3 days. In some experiments where COX-2 activity was inhibited or enhanced, neurons were treated with interleukin-1β (IL-1β; Sigma, St Louis, MO, USA) or NS398 (Sigma) for 12 h. Cultures were used between 14 and 21 days in vitro.
Electrophysiological recordings
Miniature inhibitory postsynaptic currents (mIPSCs) were recorded in hippocampal neurons in culture under voltage clamp using an Axopatch-200B amplifier. Recording pipettes (4–5 MΩ) were pulled from borosilicate glass with a micropipette puller (Sutter Instrument Co.). The internal pipette solution contained (mm): 115.0 caesium gluconate, 15.0 CsCl, 4.0 NaCl, 10.0 Hepes, 0.5 EGTA, 4.0 Mg2ATP, and 0.5 Na2GTP (pH 7.25 with CsOH). The membrane potential was held at −70 mV. The external solution contained (mm): 130.0 NaCl, 2.5 KCl, 1.0 MgCl2, 10.0 Hepes, 1.25 NaH2PO4, 2.0 CaCl2, 25.0 glucose (pH 7.4 with NaOH). To isolate mIPSCs, TTX (0.5–1 μm), a voltage-gated Na+ channel blocker, 6,7-dinitro-quinoxaline-2,3-dione (DNQX; 10 μm), an AMPA receptor blocker, and d-aminophosphonovalerate (APV; 50 μm), an NMDA receptor blocker, were included in the external solution. All experiments were performed at room temperature (22–24°C). The frequency, amplitude and kinetics were analysed using the MiniAnalysis program.
Chemicals and drugs
2-AG, AEA, PG-Gs (PGE2-G, PGD2-G and PGF2α-G) and PG-EAs (PGE2-EA, PGD2-EA and PGF2α-EA) were purchased from Cayman Chemical (Ann Arbor, MI, USA). These chemicals were dissolved in ethanol to make stock solutions at concentrations of 20 mm and distributed in small vials. To prevent the oxidation of lipids, the air in vials was expelled with nitrogen gas before being stored in a −80°C freezer. To ensure that these bioactive lipids were effective, the lipids were usually used within a very short period of time as soon as the vials were opened. In addition, we always used the lipids from different batches to ensure that the results could be duplicated. The stock solutions in the vials were diluted with the external solution to desired concentrations just before recordings. The drug solutions were applied by lowering the pipette (∼50 μm of tip size) to within 30–50 μm of the recorded cell, and the application was terminated by removal of the pipette from the bathing medium. 2-Aminoethoxydiphenyl borane (2-APB), PD98059 (PD), chelerythrine and KT5720 (Tocris, Ellisville, MO, USA), NS398 and SR141716 (provided by Chemical Synthesis and Drug Supply Program, the National Institute of Mental Health) were dissolved in DMSO to make up stock solutions at concentrations of 50–100 mm, and applied through bath perfusion. All other drugs and chemicals were obtained from Sigma unless stated otherwise. To rule out potentially non-specific effects of the solvents, the same amount of ethanol or DMSO was included in the control external solution.
Data analysis
Data are presented as means ± s.e.m. Unless stated otherwise, Student's t test was used for comparisons of before and after drug applications, analysis of variance (ANOVA) was used for between-group comparisons, and the Kolmogorov-Smirnov test was used for comparisons of mIPSC distribution. Differences were considered significant when P < 0.05.
Results
PGE2-G enhances mIPSCs
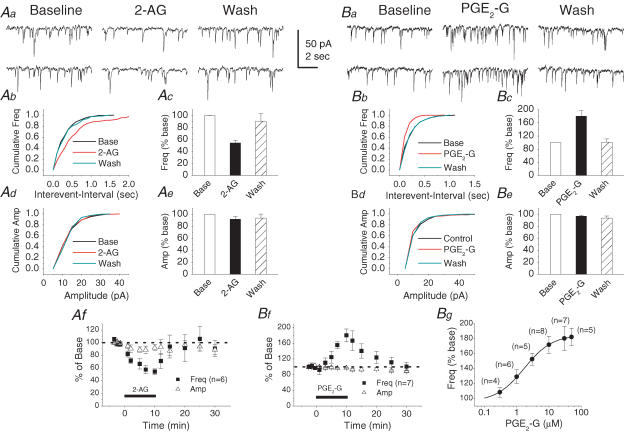
To address whether COX-2 oxidative metabolites of eCBs participate in synaptic signalling, we recorded mIPSCs in primary cultured hippocampal neurons. As shown in Fig. 1A, 2-AG (1 μm) significantly reduced the frequency of mIPSCs to 54.3 ± 4.4% of baseline (n = 6, P < 0.01) at 10 min following the application of 2-AG, but not the amplitude (92.1 ± 4.8% of baseline, n = 6, P > 0.05). The effect was reversible following 20 min of wash (90.7 ± 12.6% of baseline, n = 6, P > 0.05). In contrast to the effect of 2-AG, PGE2-G, a major COX-2 oxidative metabolite of 2-AG, at a concentration of 30 μm significantly increased the frequency of mIPSCs to 180.4 ± 16.3% of baseline (n = 7, P < 0.01), while it did not induce a significant change in the amplitude of mIPSCs (97.1 ± 1.7% of baseline, n = 7, P > 0.05). The PGE2-G-induced enhancement of the mIPSC frequency exhibited dose dependence (Fig. 1B). The EC50 for the PGE2-G-induced effect was 1.71 ± 0.05 μm. Similar to that of 2-AG, the PGE2-G-induced augmentation of mIPSC frequency was also reversible after 20 min of wash (100.7 ± 10.4% of baseline, n = 7, P > 0.05). These results indicate that PGE2-G, the COX-2 oxidative metabolite of 2-AG, augmented hippocampal inhibitory synaptic transmission, an effect opposite to its precursor.
Figure 1. 2-Arachidonoyl glycerol (2-AG) reduces and PGE2 glycerol ester (PGE2-G) enhances the frequency of mIPSCs in primary cultured hippocampal neurons.
Aa, representative sweeps of mIPSCs in the absence or presence of 2-AG (1 μm) and after washout. Miniature IPSCs were recorded in primary hippocampal neurons in culture from 14 to 21 days in vitro. The membrane potential was held at −70 mV. DNQX (10 μm), APV (50 μm) and TTX (0.5 μm) were included in the external solution. The synaptic events were analysed using the MiniAnalysis program. Ab, cumulative probability of mIPSC frequency in the absence or presence of 2-AG and after washout. Ac, mean percentage changes (normalized to the baseline) in the frequency of mIPSCs at 10 min after the application of 2-AG. 2-AG significantly reduces the frequency of mIPSCs (n = 6, P < 0.01). Ad, cumulative probability of mIPSC amplitude in the absence or presence of 2-AG and after washout. Ae, mean percentage changes in the amplitude of mIPSCs in the presence of 2-AG and after washout. Af, time course of the 2-AG-induced changes in frequency and amplitude of mIPSCs. Ba, representative sweeps of mIPSCs in the absence or presence of PGE2-G (30 μm) and after washout. Bb, cumulative probability of mIPSC frequency in the absence or presence of PGE2-G and after washout. Bc, mean percentage changes in the frequency of mIPSCs at 10 min after the application of PGE2-G. PGE2-G significantly enhances the frequency of mIPSCs (n = 7, P < 0.01). Bd, cumulative probability of mIPSC amplitude in the absence or presence of PGE2-G and after washout. Be, mean percentage changes in the amplitude of mIPSCs in the presence of PGE2-G and washout. Bf, time course of the PGE2-G-induced changes in frequency and amplitude of mIPSCs. Bg, PGE2-G induces a concentration-dependent increase in the frequency of mIPSCs. The curve is a non-linear least-squares fit to a logistic equation. EC50= 1.71 ± 0.05 μm.
It has been demonstrated that mIPSCs consist of both ‘basal mIPSCs’ and ‘Ca2+-enhanced mIPSCs’ in the bath solution containing 2 mm Ca2+ (Yamasaki et al. 2006). To examine whether the PGE2-G-induced enhancement of mIPSCs is associated with Ca2+ influx from the external solution, we have recorded mIPSCs in cultured hippocampal neurons in external solution containing Cd2+ (200 μm). We observed that both frequency and amplitude of mIPSCs were not affected (frequency: 106.1 ± 18.9% of control, n = 3; amplitude: 101.9 ± 6.8% of control, n = 3). Then we examined the effect of PGE2-G on mIPSCs in the presence of Cd2+. It appeared that PGE2-G (10 μm) still increased the frequency of mIPSCs (173.0 ± 24.0% of baseline, n = 4, P < 0.01), similar to that in the control solution. These results indicate that blockade of the voltage-dependent Ca2+ channels had no significant effects on either inhibitory synaptic transmission, or the PGE2-G-induced increase in the frequency of mIPSCs in cultured hippocampal neurons.
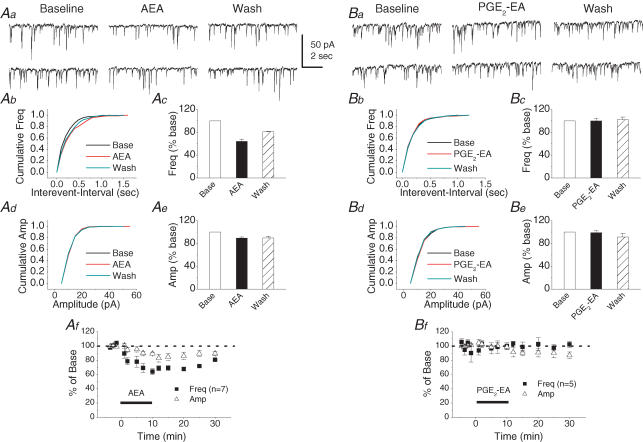
PGE2-EA does not induce an effect on mIPSCs
To determine whether COX-2 oxidative metabolites of AEA are involved in hippocampal synaptic signalling, we recorded synaptic responses in the presence of PGE2-EA, a major COX-2 oxidative metabolite of AEA (Ross et al. 2002). While AEA (5 μm) significantly induced a reversible reduction in the frequency of mIPSCs at 10 min following the application of AEA (64.2 ± 4.0% of baseline n = 7, P < 0.01), PGE2-EA (30 μm) did not elicit a detectable response in either the frequency (100.1 ± 4.3% of baseline, n = 5, P > 0.05) or amplitude (98.6 ± 3.7% of baseline, n = 5, P > 0.05) of mIPSCs (Fig. 2). These data show that PGE2-EA does not have an effect on GABAergic synaptic transmission in primary cultured hippocampal neurons.
Figure 2. PGE2 ethanolamide (PGE2-EA) does not affect mIPSCs in primary cultured hippocampal neurons.
Aa, representative sweeps of mIPSCs in the absence or presence of arachidonoyl ethanolamide (AEA; 5 μm) and after washout. Ab, cumulative probability of mIPSC frequency in the absence or presence of AEA and after washout. Ac, AEA significantly reduces the frequency of mIPSCs (n = 7, P < 0.01). Ad, cumulative probability of mIPSC amplitude in the absence or presence of AEA and after washout. Ae, mean percentage changes in the amplitude of mIPSCs in the presence of AEA and after washout. Af, time course of the AEA-induced changes in frequency and amplitude of mIPSCs. Ba, representative sweeps of mIPSCs in the absence or presence of PGE2-EA (30 μm) and after washout. Bb, cumulative probability of mIPSC frequency in the absence or presence of PGE2-EA and after washout. Bc, mean percentage changes in the frequency of mIPSCs at 10 min after application of PGE2-EA. Bd, cumulative probability of mIPSC amplitude in the absence or presence of PGE2-EA and after washout. Be, mean percentage changes in the amplitude of mIPSCs in the presence of PGE2-EA and after washout. Bf, time course of the PGE2-EA-induced changes in frequency and amplitude of mIPSCs.
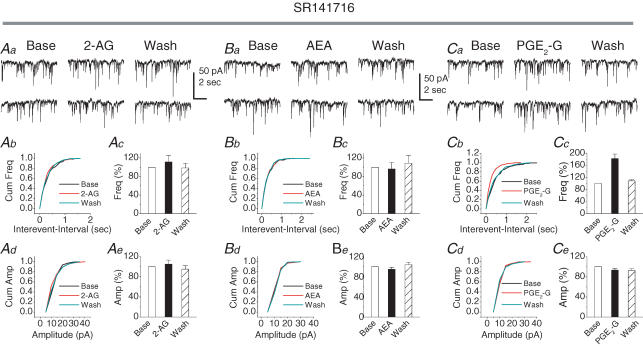
The PGE2-G-induced increase in the frequency of mIPSCs is not mediated by the CB1 receptor
In the brain, the eCB-induced effect is mainly mediated by the CB1 receptor (CB1R; Felder, 1998; Sullivan, 2000; Wilson & Nicoll, 2001, 2002; Hajos & Freund, 2002a, b; McAllister & Glass, 2002; Freund et al. 2003; Piomelli, 2003; Hajos et al. 2004; Isokawa & Alger, 2005). To determine whether the PGE2-G-induced enhancement of mIPSCs is mediated by the CB1R, we examined the effects of 2-AG, AEA and PGE2-G on mIPSCs in the presence of SR141716 (SR), a CB1R antagonist (provided by the National Institute of Mental Health Chemical Synthesis and Drug Supply Program). As indicated in Fig. 3A and B, bath application of SR (1 μm) for 30 min blocked 2-AG and AEA-induced reduction in the frequency of mIPSCs (111.4 ± 14.1% of baseline, n = 3, P > 0.05; and 92.3 ± 13.9% of baseline, n = 4, P > 0.05, respectively). However, PGE2-G still augmented the frequency of mIPSCs in neurons pre-treated with SR (1 μm) for 30 min (182.5 ± 15.1% of baseline, n = 7, P < 0.01) (Fig. 3C). These results indicate that the PGE2-G-induced effect on synaptic transmission is not mediated via the CB1 receptor, and provide further evidence that the PGE2-G-induced response is different from its parent molecule.
Figure 3. The PGE2-G-induced increase in frequency of mIPSCs is not mediated via the CB1 receptor in primary cultured hippocampal neurons.
Aa, representative sweeps of mIPSCs in the absence or presence of 2-AG (1 μm) and after washout in neurons pre-treated with SR141716 (SR; 1 μm) for 30 min. Ab, cumulative probability of mIPSC frequency. Ac, mean percentage changes (normalized to the baseline) in the frequency of mIPSCs at 10 min after application of 2-AG in the presence of SR. Bath application of SR blocked 2-AG-induced reduction in the frequency of mIPSCs (111.4 ± 14.1% of baseline, n = 3, P > 0.05). Ad, cumulative probability of mIPSC amplitude. Ae, mean percentage changes in the amplitude of mIPSCs. Ba, representative sweeps of mIPSCs in the absence or presence of AEA (5 μm) and after washout in neurons pre-treated with SR (1 μm) for 30 min. Bb, cumulative probability of mIPSC frequency. Bc, mean percentage changes in the frequency of mIPSCs at 10 min after application of AEA in the presence of SR. Bath application of SR for 30 min blocked AEA-induced reduction in the frequency of mIPSCs (92.3 ± 13.9% of baseline, n = 4, P > 0.05). Bd, cumulative probability of mIPSC amplitude. Be, mean percentage changes in the amplitude of mIPSCs. Ca, representative sweeps of mIPSCs in the absence or presence of PGE2-G (30 μm) and after washout in neurons pre-treated with SR (1 μm) for 30 min. Cb, cumulative probability of mIPSC frequency. Cc, mean percentage changes in the frequency of mIPSCs at 10 min after application of PGE2-G (30 μm) in the presence of SR. PGE2-G still augmented the frequency of mIPSCs in neurons pre-treated with SR for 30 min (182.5 ± 15.1% of baseline, n = 7, P < 0.01). Cd, cumulative probability of mIPSC amplitude. Ce, mean percentage changes in the amplitude of mIPSCs.
The PG-G- and PG-EA-induced effects on mIPSCs are not mediated via prostanoid receptors
Since PGE2-G and PGE2-EA, major COX-2 oxidative metabolites of 2-AG and AEA, produced distinct effects on mIPSCs in cultured hippocampal neurons, we then decided to examine the effects of other COX-2 oxidative metabolites of 2-AG and AEA on inhibitory synaptic transmission. We individually applied PGD2-G, PGF2α-G, PGD2-EA and PGF2α-EA in cultured hippocampal neurons. As shown in Table 1, PGD2-G (30 μm), PGF2α-G (30 μm) and PGD2-EA (30 μm) significantly augmented the frequency of mIPSCs (158.7 ± 10.5% of baseline, n = 6, P < 0.01; 140.9 ± 7.2% of baseline, n = 10, P < 0.01; 134.6 ± 7.0% of baseline, n = 6, P < 0.01, respectively), but not the amplitude. Similar to that of PGE2-EA, PGF2α-EA (30 μm) did not induce significant effects on the frequency and amplitude of mIPSCs. These results reveal that these novel prostanoids may be involved in hippocampal synaptic signalling. However, it was not clear which receptor(s) mediate(s) these novel prostanoid-induced synaptic responses. Indeed, recent evidence indicates that eCB metabolites resulting from the COX-2 oxygenation may be novel signal mediators (Kozak et al. 2004), and may have biological efficacy through prostanoid receptor-dependent and -independent mechanisms (Ross et al. 2002; Matias et al. 2004; Nirodi et al. 2004). Because there are very few prostanoid receptor antagonists commercially available, at least for PGE2 receptors, we decided to directly apply AA-derived PGs (PGE2, PGD2 and PGF2α) in hippocampal neurons to determine their effects on mIPSCs, and compared the effects with those of eCB-derived prostanoids. Interestingly, application of PGE2 (5 μm) and PGD2 (5 μm) significantly decreased the frequency of mIPSCs (81.9 ± 7.1% of baseline, n = 9, P < 0.01; 58.3 ± 4.4% of baseline, n = 7, P < 0.01, respectively), an effect opposite to that of PGE2-G and PGD2-G, eCB-derived prostanoids. Meanwhile, we observed that PGF2α (5 μm) did not induce significant changes in the frequency and amplitude of mIPSCs. These results demonstrate that the eCB-derived prostanoid-induced effect on mIPSCs is different from that of AA-derived prostanoids, implying that there may be undefined receptors rather than prostanoid receptors mediating the responses.
Table 1. Effects of prostaglandin glycerol esters (PG-Gs), prostaglandin ethanolamides (PG-EAs) and prostaglandins (PGs) on mIPSCs in primary cultured hippocampal neurons.
| Frequency (% of baseline) | Amplitude (% of baseline) | |
|---|---|---|
| PGD2-G (30 μm, n = 6) | 158.7 ± 10.5** | 100.6 ± 3.8 |
| PGF2α-G (30 μm, n = 10) | 140.9 ± 7.2** | 103.3 ± 5.6 |
| PGD2-EA (30 μm, n = 6) | 134.6 ± 7.0** | 97.1 ± 2.1 |
| PGF2α-EA (30 μm, n = 5) | 97.3 ± 9.9 | 100.2 ± 4.8 |
| PGE2 (5 μm, n = 9) | 81.9 ± 7.1** | 89.2 ± 2.7 |
| PGD2 (5 μm, n = 7) | 58.3 ± 4.4** | 90.7 ± 2.8 |
| PGF2α (5 μm, n = 10) | 88.7 ± 9.0 | 95.9 ± 2.2 |
Values are means ± s.e.m.
P < 0.01 versus baseline.
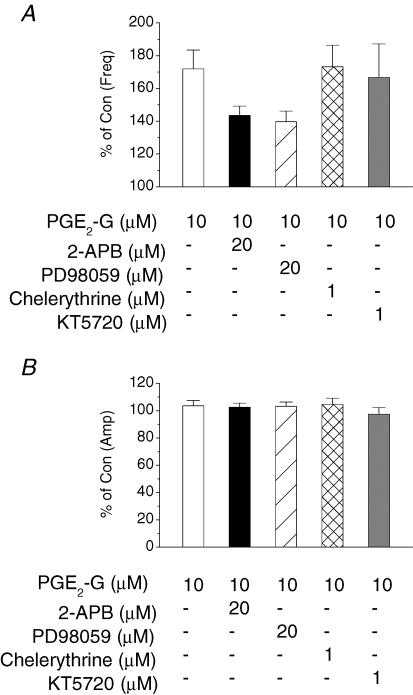
The PGE2-G-induced enhancement of synaptic transmission is mediated via the inositol 1,4,5-trisphosphate or mitogen-activated protein kinase pathway
A recent report has shown that PGE2-G mobilizes intracellular calcium, triggers inositol 1,4,5-trisphosphate (IP3) synthesis, and activates protein kinase C (PKC) in RAW264.7 macrophage cells (Nirodi et al. 2004). To probe the possible signal transduction pathway for the PGE2-G-induced response in hippocampal synaptic signalling, we applied 2-APB, an IP3 inhibitor, PD98059 (PD), a mitogen-activated protein kinase (MAPK) inhibitor, chelerythrine, a PKC inhibitor, and KT5720 (KT), a PKA inhibitor. As illustrated in Fig. 4, bath application of 2-APB (20 μm) for 30 min significantly attenuated the PGE2-G (10 μm)-induced increase in mIPSC frequency (from 171.9 ± 11.5% to 143.5 ± 5.7% of baseline, n = 8, P < 0.05). Similarly, PD (20 μm) reduced the enhancement to 139.7 ± 6.5% (n = 7, P < 0.05). However, chelerythrine (1 μm) or KT (1 μm) did not affect the PGE2-G (10 μm)-induced increase in mIPSC frequency. These data provided evidence that the PGE2-G-induced enhancement of synaptic transmission may be mediated via IP3 and MAPK pathways.
Figure 4. The PGE2-G-induced enhancement of synaptic transmission is mediated via IP3 and MAPK pathways in primary cultured hippocampal neurons.
A, mean percentage changes in the frequency of mIPSCs at 10 min after the application of PGE2-G (10 μm) in the presence of 2-aminoethoxydiphenyl borane (2-APB; 20 μm, n = 8), PD98059 (20 μm, n = 7), chelerythrine (1 μm, n = 5), or KT5720 (1 μm, n = 5). Kinase inhibitors were bath applied for 30 min before administration of PGE2-G. B, mean percentage changes in the amplitude of mIPSCs at 10 min after application of PGE2-G in the presence of these inhibitors.
Effects of COX-2 activity on inhibitory synaptic transmission
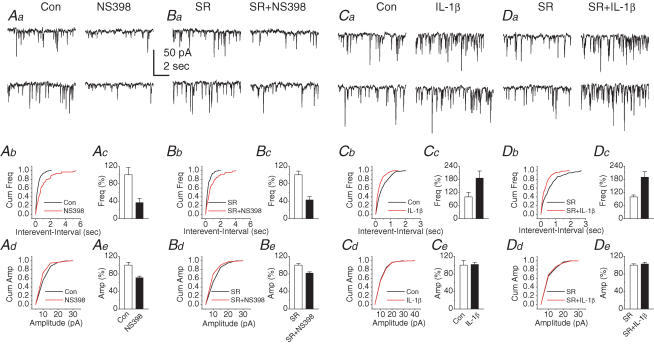
If COX-2 plays an important role in the metabolism of eCBs, and its oxidative metabolites of eCBs participate in synaptic signalling, then, up- or down-regulation of COX-2 expression or activity should alter eCB signalling in synaptic transmission. To test this hypothesis, neurons were treated with NS398, a selective COX-2 inhibitor (Chen et al. 2002; Chen & Bazan, 2005a; Sang et al. 2005), and IL-1β, a known COX-2 inducer. As indicated in Fig. 5A, the basal inhibitory synaptic transmission was significantly reduced in neurons treated with NS398 (20 μm) for 12 h (frequency: 36.7 ± 9.4% of control, n = 7, P < 0.05; amplitude: 71.1 ± 3.6% of control, n = 7, P < 0.05). The NS398-induced reduction of mIPSCs was not blocked by 1 μm SR (frequency: 42.6 ± 7.7% of control, n = 7, P < 0.01; amplitude: 81.7 ± 2.9%, n = 7, P < 0.05). There were no significant differences in the reductions of the frequency and amplitude of mIPSCs between the absence and presence of SR in NS398-treated neurons. This phenomenon is in harmony with results reported by others (Kim & Alger, 2004). To test whether the COX-2 inhibition-induced reduction of mIPSCs resulted from accumulated AA, we examined the effect of AA on mIPSCs. AA (10 μm) significantly reduced the frequency of mIPSCs to 57.5 ± 8.0% (P < 0.01, n = 6) of the baseline, but not the amplitude (97.3 ± 3.2% of the baseline, P > 0.05, n = 6). The AA-induced decrease in the frequency of mIPSCs was not inhibited by SR (60.3 ± 5.4%, P < 0.01; n = 5). In contrast, the frequency of mIPSCs was significantly elevated in neurons treated with IL-1β (20 ng ml−1) for 12 h (184.8 ± 32.7% of control, n = 9, P < 0.05), but not the amplitude (101.4 ± 4.3% of control, n = 9, P > 0.05). Application of SR failed to block the IL-1β-induced potentiation of the frequency (189.4 ± 25.1% of control, n = 6, P < 0.05). These results indicate that alterations in basal mIPSCs in neurons treated with NS398 or IL-1β may not be mediated by CB1R. To confirm that the IL-1β-induced enhancement of mIPSCs may be due to COX-2 oxidative metabolites of eCBs, we applied 2-APB and PD to hippocampal neurons treated with IL-1β (20 ng ml−1) for 12 h. We found that both IP3 and MAPK inhibitors significantly attenuated the IL-1β-induced enhancement of mIPSC frequency by 58.8 ± 5.7% (n = 8, P < 0.05) and 52.0 ± 6.3% (P < 0.01, n = 10), respectively. These data indicate that the COX-2 up-regulation-induced enhancement of inhibitory synaptic activity is probably mediated by oxidative metabolites of 2-AG.
Figure 5. Alterations in COX-2 activity affect basal inhibitory synaptic transmission in primary cultured hippocampal neurons.
Aa, representative sweeps of mIPSCs recorded from control and NS398-treated neurons. The culture was treated with NS398 (20 μm) for 12 h. Ab, cumulative probability of mIPSC frequency recorded from control and NS398-treated neurons. Ac, the frequency of mIPSCs is significantly reduced in NS398-treated neurons (n = 7, P < 0.01). Ad, cumulative probability of mIPSC amplitude. Ae, the amplitude of mIPSCs is significantly reduced in neurons treated with NS398 (n = 7, P < 0.05). Ba, representative sweeps of mIPSCs recorded from neurons treated with and without NS398 in the presence of SR (1 μm). The recordings were made 30 min following application of SR. Bb, cumulative probability of mIPSC frequency recorded from the neurons treated with NS398 in the presence of SR. Bc, the frequency of mIPSCs is still reduced in the presence of SR in neurons treated with NS398 (n = 7, P < 0.01). Bd, cumulative probability of mIPSC amplitude. Be, the amplitude of mIPSCs is decreased in the presence of SR in neurons treated with NS398 (n = 7, P < 0.05). Ca, representative sweeps of mIPSCs recorded from control and IL-1β-treated neurons. The culture was treated with IL-1β (20 ng ml−1) for 12 h. Cb, cumulative probability of mIPSC frequency recorded from the control and IL-1β-treated neurons. Cc, the frequency of mIPSCs is enhanced in the neurons treated with IL-1β (n = 9, P < 0.01). Cd, cumulative probability of mIPSC amplitude. Ce, the amplitude of mIPSCs is not altered in IL-1β-treated neurons (n = 9, P > 0.05). Da, representative sweeps of mIPSCs recorded from neurons treated with and without IL-1β (20 ng ml−1) in the presence of SR. Db, cumulative probability of mIPSC frequency recorded from neurons treated with and without IL-1β in the presence of SR. Dc, the frequency of mIPSCs is enhanced in the neurons treated with IL-1β in the presence of SR (n = 6, P < 0.01). Dd, cumulative probability of mIPSC amplitude. De, the amplitude of mIPSCs is not changed in the presence of SR in IL-1β-treated neurons (n = 6, P > 0.05).
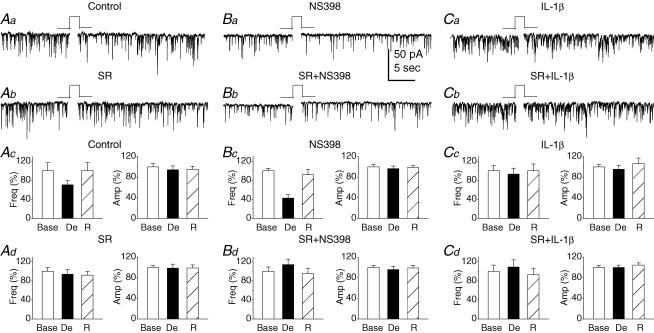
Several lines of evidence indicate that an increase or decrease the synthesis/degradation of eCBs alters depolarization-induced suppression of inhibition (DSI; Wilson & Nicoll, 2001, 2002; Freund et al. 2003). In particular, recent evidence shows that COX-2 inhibitors augment DSI (Kim & Alger, 2004), indicating that alterations in COX-2 activity affect endocannabinoid signalling. To test this idea, DSI was induced by a 5 s depolarizing step to 0 mV from a holding potential of −70 mV in neurons treated with the COX-2 inhibitor or inducer. As shown in Fig. 6, depolarization produced a significant reduction in the frequency of mIPSCs to 70.7 ± 8.4% of the baseline (n = 7, P < 0.01) during the first 10 s period just after depolarization under control conditions. However, in neurons treated with NS398 (20 μm) for 12 h, depolarization decreased the frequency of mIPSCs to 42.7 ± 7.1% of baseline (n = 6, P < 0.01). There is a significant enhancement in DSI in neurons treated with NS398 when compared to that of controls (P < 0.05). These results are consistent with the observations reported by others who demonstrate that the COX-2 inhibition augments DSI (Kim & Alger, 2004). To examine whether the increase in COX-2 expression or activity that oxidizes eCBs alters synaptic transmission, we treated neurons with IL-1β (20 ng ml−1) for 12 h. DSI was virtually absent in neurons treated with IL-1β (93.0 ± 12.4% of baseline, n = 9, P > 0.05). To determine whether DSI is mediated via the CB1 receptor, SR (1 μm) was bath applied for 30 min. In the presence of SR, DSI was inhibited in neurons treated either with NS398 (94.0 ± 9.8% of baseline, n = 7, P > 0.05) or vehicle (114.3 ± 11.0% of baseline, n = 7, P > 0.05). These observations demonstrate that COX-2 plays an important role in the regulation of eCB signalling by oxygenating eCBs and producing new signal mediators.
Figure 6. Alterations in COX-2 activity affect depolarization-induced suppression of inhibition (DSI) in primary cultured hippocampal neurons.
Aa, representative sweeps of mIPSCs recorded before and after depolarization in control neurons. DSI was elicited by a 5 s depolarizing step to 0 mV from a holding potential of −70 mV. Ab, representative sweeps of mIPSCs recorded before and after depolarization in neurons treated with SR (1 μm) for 30 min before recordings. Ac, mean percentages of the depolarization (De) -induced changes in frequency and amplitude of mIPSCs calculated from the first 10 s period just after depolarization and normalized to the 10 s period before delivering a depolarization step in control neurons (n = 7, P < 0.01). Ad, mean percentages of the depolarization-induced changes in frequency and amplitude of mIPSCs in SR-treated neurons (n = 7, P > 0.05). Ba, representative sweeps of mIPSCs recorded before and after depolarization in neurons treated with NS398 (20 μm) for 12 h. Bb, representative sweeps of mIPSCs recorded before and after depolarization in the presence of SR in neurons treated with NS398. Bc, mean percentages of the depolarization-induced changes in the frequency and amplitude of mIPSCs in NS398-treated neurons (n = 6, P < 0.01). Bd, mean percentages of the depolarization-induced changes in frequency and amplitude in the presence of SR in NS398-treated neurons (n = 7, P > 0.05). Ca, representative sweeps of mIPSCs recorded before and after depolarization in neurons treated with IL-1β (20 ng ml−1) for 12 h. Cb, representative sweeps of mIPSCs before and after depolarization in the presence of SR in IL-1β-treated neurons. Cc, mean percentages of the depolarization-induced changes in frequency and amplitude in IL-1β-treated neurons (n = 9, P > 0.05). Cd, mean percentage of the depolarization-induced changes in frequency and amplitude in the presence of SR in IL-1β-treated neurons (n = 6, P > 0.05).
Discussion
In the present study, we discovered that COX-2 oxidative metabolites of eCBs modulate GABAergic receptor-mediated inhibitory synaptic transmission in primary cultured hippocampal neurons. The modulation of mIPSCs by these eCB-derived prostanoids appears to be opposite to that of their parent molecules, 2-AG and AEA, and different from that of AA-derived prostanoids. This is the first evidence that these prostanoids, derived from the COX-2 oxygenation of eCBs, may be a new type of signalling mediator participating in hippocampal synaptic transmission.
In addition to the well-studied hydrolytic modes of eCB metabolism, these bioactive lipids are also susceptible to oxidative metabolism by a number of fatty acid oxygenases, including cyclooxygenases, lipoxygenases, and cytochrome P450s known to be involved in eicosanoid production from AA (Kozak et al. 2004). It has recently been demonstrated that COX-2 oxygenates 2-AG and AEA (Yu et al. 1997; So et al. 1998; Kozak et al. 2000, 2001). However, the biological consequences of this connection have only begun to be investigated. The eCB-derived COX-2 oxygenated products might represent unique signal mediators with potent activities distinct from their precursors and corresponding AA-derived prostanoids (Kozak et al. 2004; Patrignani et al. 2005). We found in the present study that PGE2-G, a main COX-2 oxidative metabolite of 2-AG, significantly increased the frequency of mIPSCs, and the increase was not mediated via the CB1R. Meanwhile, PGD2-G, PGF2α-G and PGD2-EA also increased the frequency of mIPSCs, but not PGE2-EA and PGF2α-EA. Our findings suggest that some COX-2 oxidative metabolites of eCBs act as signalling molecules and modulate synaptic transmission, while others may not serve as signal modulators in hippocampal synaptic transmission, but may participate in some undefined functions.
At present, little is known about the mechanisms of PG-G- and PG-EA-induced functional roles in hippocampal synaptic transmission (Kozak et al. 2004; Chen & Bazan, 2005b). It has been proposed that these novel lipids may have biological efficacy through prostanoid receptor-dependent and -independent mechanisms (Ross et al. 2002; Kozak et al. 2004; Matias et al. 2004; Nirodi et al. 2004). For instance, a recent study shows that PGE2-EA has a similar profile of action to that of PGE2 in that it binds to PGE2 receptors (EP) (Ross et al. 2002). However, our results indicate that PG-G- and PG-EA-induced effects on mIPSCs are different from those induced by corresponding AA-derived PGs, suggesting that the eCB-derived prostanoid-induced effects are not mediated via known prostanoid receptors. Recent evidence also shows that the PGE2-G triggers intracellular Ca2+ mobilization via IP3 and PKC pathways in RAW264.7 macrophage cells (Nirodi et al. 2004). However, we observed that the PGE2-G-induced increase in the frequency of mIPSCs is attenuated by IP3 and MAPK inhibitors, but not by a PKC or protein kinase A (PKA) inhibitor in hippocampal neurons in culture. This discrepancy between our observations and others is probably due to the different preparations used (Nirodi et al. 2004).
Since COX-2 metabolizes 2-AG and AEA, and eCB-derived prostanoids exert opposite effects to that of 2-AG and AEA on inhibitory synaptic transmission, it is possible that alterations in COX-2 expression or activity would significantly influence the amount of eCBs available, resulting in changes in synaptic activity. This postulation has been confirmed by a recent study in which COX-2 inhibition augments DSI in the hippocampus (Kim & Alger, 2004). Our present study provides further evidence that the inhibition of COX-2 retards the inactivation of eCBs, thereby raising the eCB levels and promoting the eCB-mediated responses, whereas the enhancement of COX-2 accelerates the metabolism of eCBs, thereby lowering the eCB levels and reducing the eCB-mediated responses. Interestingly, we observed that NS398 significantly decreases the basal inhibitory synaptic activity in terms of frequency and amplitude of mIPSCs, and the decrease appears not to be mediated by the CB1R, similar to the findings of Kim & Alger (2004). While the mechanisms by which the COX-2 inhibition induces reduction in basal inhibitory synaptic transmission are still not completely understood (Kim & Alger, 2004), we observed in the present study that AA (10 μm) significantly inhibited the frequency of mIPSCs, and the inhibition was not blocked by SR. This information suggests that accumulated AA during the COX-2 inhibition may be, at least in part, responsible for the reduced synaptic activity in neurons treated with the COX-2 inhibitor. On the other hand, the IL-1β-induced increase in the basal inhibitory synaptic transmission probably results from the reduced eCBs, and elevated their COX-2 oxidative metabolites, PG-Gs and PG-EAs. This assumption was supported by the experiments where SR failed to block the IL-1β-induced enhancement of mIPSCs, and the IP3 and MAPK inhibitors significantly reduced the frequency of mIPSCs in neurons treated with IL-1β. These observations suggest that there was, at least, an increased amount of PGE2-G in the culture where COX-2 expression was enhanced by IL-1β.
Our results provide evidence that eCB-derived COX-2-oxygenated prostanoids are probably a new class of signalling mediator involved in hippocampal synaptic transmission. This means that COX-2 plays a central role in metabolizing AA and eCBs and generating AA- and eCB-derived prostanoids. Thus, the excessive expression of COX-2 or enhanced COX-2 activity resulting from inflammation, traumatic injury, epilepsy or degenerative processes will have significant impact on eCBs, AA- and eCB-derived prostanoid signalling in synaptic activity, indicating that unravelling this important signal transduction pathway will be of substantial significance in our understanding of the roles of COX-2-mediated events in physiological, pharmacological and pathological functions.
Acknowledgments
This work was supported by National Institutes of Health and the Alzheimer's Association grants (to C.C.).
References
- Alger BE. Endocannabinoids: getting the message across. Proc Natl Acad Sci U S A. 2004;101:8512–8513. doi: 10.1073/pnas.0402935101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan NG, Flower RJ. Lipid signals in pain control. Nature. 2002;420:135–138. doi: 10.1038/420135a. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan NV, Dai H, Roos KLT, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Bazan NG. Platelet-activating factor inhibits ionotropic GABA receptor activity in cultured hippocampal neurons. Neuroreport. 1999;10:3831–3835. doi: 10.1097/00001756-199912160-00020. [DOI] [PubMed] [Google Scholar]
- Chen C, Bazan NG. Endogenous PGE2 regulates membrane excitability and synaptic transmission in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2005a;93:929–941. doi: 10.1152/jn.00696.2004. [DOI] [PubMed] [Google Scholar]
- Chen C, Bazan NG. Lipid signaling: Sleep, synaptic plasticity, and neuroprotection. Prostaglandins Other Lipid Mediat. 2005b;77:65–76. doi: 10.1016/j.prostaglandins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002;87:2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Felder CC. Cannabinoid receptors and their endogenous agonists. Annu Rev Pharmacol. 1998;38:179–200. doi: 10.1146/annurev.pharmtox.38.1.179. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem Phys Lipids. 2002a;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- Hajos N, Freund TF. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacol. 2002b;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Hajos N, Kathuria S, Dinh T, Piomelli D, Freund TF. Endocannabinoid transport tightly controls 2-arachidonoyl glycerol actions in the hippocampus: effects of low temperature and the transport inhibitor AM404. Eur J Neurosci. 2004;19:2991–2996. doi: 10.1111/j.0953-816X.2004.03433.x. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacol. 2004;47(Suppl. 1):345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Alger BE. Retrograde endocannabinoid regulation of GABAergic inhibition in the rat dentate gyrus granule cell. J Physiol. 2005;567:1001–1010. doi: 10.1113/jphysiol.2005.094219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson P. COX-2, a synaptically induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2317–2321. doi: 10.1073/pnas.93.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoid, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J Biol Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Prusakiewicz JL, Marnett LJ. Oxidative metabolism of endocannabinoids by COX-2. Curr Pharm Des. 2004;10:659–667. doi: 10.2174/1381612043453081. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Prusakiewicz JL, Rowlinson SW, Schneider C, Marnett LJ. Metabolism of prostaglandin glycerol esters and prostaglandin ethanolamides in vitro and vivo. J Biol Chem. 2001;276:30072–30077. doi: 10.1074/jbc.M105854200. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glycerol prostaglandins by COX-2. J Biol Chem. 2000;27:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- McAllister SD, Glass M. CB1 and CB2 receptor-mediated signaling: a focus on endocannabinoids. Prostagland Leuk Essen Fatty Acid. 2002;66:161–171. doi: 10.1054/plef.2001.0344. [DOI] [PubMed] [Google Scholar]
- Matias I, Chen J, Petrocellis LD, Bisogno T, Ligresti A, Fezza F, Krauss AHP, Shi L, Protzman CE, Li C, Liang Y, Niexes L, Kedzie KM, Burk RM, Marzo VD, Woodward DF. Prostaglandin ethanolamides (prostamides): in vitro pharmacology and metabolism. J Pharmacol Exp Ther. 2004;309:745–757. doi: 10.1124/jpet.103.061705. [DOI] [PubMed] [Google Scholar]
- Nirodi CS, Crews BC, Kozak KR, Morrow JD, Marnett LJ. The glyceryl ester of prostaglandin E2 mobilizes calcium and activates signal transduction in RAW264.7 cells. Proc Natl Acad Sci U S A. 2004;101:1840–1845. doi: 10.1073/pnas.0303950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrignani P, Tacconelli S, Sciulli MG, Capone ML. New insight into COX-2 biology and inhibition. Brain Res Rev. 2005;48:352–359. doi: 10.1016/j.brainresrev.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signaling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Ross RA, Craib SJ, Stevenson LA, Pertwee RG, Henderson A, Toole J, Ellington HC. Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. J Pharmacol Exp Ther. 2002;301:900–907. doi: 10.1124/jpet.301.3.900. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So OY, Scarafia LE, Mak AY, Callan OH, Swinney DC. The dynamics of prostaglandin H synthases. Studies with prostaglandin h synthase 2 Y355F unmask mechanisms of time-dependent inhibition and allosteric activation. J Biol Chem. 1998;273:5801–5807. doi: 10.1074/jbc.273.10.5801. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn Mem. 2000;7:132–139. doi: 10.1101/lm.7.3.132. [DOI] [PubMed] [Google Scholar]
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenase 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapse. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoids signaling in the brain. Science. 2002;296:678–681. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Hashimoto K, Kano M. Miniature synaptic events elicited by presynaptic Ca2+ rise are selectively suppressed by cannabinoid receptor activation in cerebellar Purkinje cells. J Neurosci. 2006;26:86–95. doi: 10.1523/JNEUROSCI.2258-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- Zhu P, Genc A, Zhang X, Zhang J, Bazan NG, Chen C. Heterogeneous expression and regulation of PGE2 receptors in the hippocampus. J Neurosci Res. 2005;81:817–826. doi: 10.1002/jnr.20597. [DOI] [PubMed] [Google Scholar]