Abstract
The carbonic anhydrase inhibitor acetazolamide may have both inhibitory and stimulatory effects on breathing. In this placebo-controlled double-blind study we measured the effect of an intravenous dose (4 mg kg−1) of this agent on the acute isocapnic hypoxic ventilatory response in 16 healthy volunteers (haemoglobin oxygen saturation 83–85%) and examined whether its inhibitory effects on this response could be reversed by antioxidants (1 g ascorbic acid i.v. and 200 mg α-tocopherol p.o.). The subjects were randomly divided into an antioxidant (Aox) and placebo group. In the Aox group, acetazolamide reduced the mean normocapnic and hypercapnic hypoxic responses by 37% (P < 0.01) and 55% (P < 0.01), respectively, and abolished the O2–CO2 interaction, i.e. the increase in O2 sensitivity with rising PCO2. Antioxidants completely reversed this inhibiting effect on the normocapnic hypoxic response, while in hypercapnia the reversal was partial. In the placebo group, acetazolamide reduced the normo- and hypercapnic hypoxic responses by 33 and 47%, respectively (P < 0.01 versus control in both cases), and also abolished the O2–CO2 interaction. Placebo failed to reverse these inhibitory effects of acetazolamide in this group. We hypothesize that either an isoform of carbonic anhydrase may be involved in the regulation of the redox state in the carotid bodies or that acetazolamide and antioxidants exert independent effects on oxygen-sensing cells, in which both carbonic anhydrase and potassium channels may be involved. The novel findings of this study may have clinical implications, for example with regard to a combined use of acetazolamide and antioxidants at high altitude.
A major defence of the mammalian body to acute hypoxia is a rapid increase in pulmonary ventilation called the acute hypoxic response (AHR). This vital chemoreflex is primarily mediated by the carotid bodies located at the bifurcations of the common carotid arteries (Gonzalez et al. 1994). A decrease in oxygen tension in the blood leads to a complex cascade of events in carotid body type I cells, in which closure of one or more classes of potassium channels results in membrane depolarization (Lopez-Barneo et al. 1999; Lahiri, 2000; Prabhakar, 2000). The mechanistic link between hypoxia and potassium channel closure is unknown; however, potassium channels are sensitive to oxidizing and reducing agents, and reactive oxygen species (ROS; Kouri, 1998; Liu & Gutterman, 2002). Whether ROS indeed play a mediating role in oxygen sensing is controversial (Gonzalez et al. 2002). Recently, Hildebrandt et al. (2002) have shown that after a 5-day oral application, N-acetylcysteine increased the hypoxic response in young adults indicating a possible specific involvement of gluthathione in oxygen sensing. In older women with an age-related reduction of hypoxic sensitivity, ascorbic acid supplementation appeared to increase their hypoxic response (Pokorski & Marczak, 2003). Recently, we showed that an antioxidant mixture, consisting of oral α-tocopherol and intravenous ascorbic acid was able to reverse the reduction of the hypoxic response in humans by subanaesthetic concentrations of inhalational anaesthetics (Teppema et al. 2002, 2005). These studies indicate that antioxidants may be able to increase carotid body sensitivity in a situation wherein their output is reduced. If this is a general property of antioxidants remains to be seen, and in this context it would be of interest to investigate whether they would also be able to reverse carotid body inhibition that is induced by other means than anaesthetics or age-related phenomena. One other, pharmacological, means to reduce carotid body sensitivity is acetazolamide, a well-known carbonic anhydrase inhibitor. The mechanism by which acetazolamide reduces the hypoxic response in man (Swenson & Hughes, 1993) and animals (Teppema et al. 1992; Teppema & Dahan, 2004) is unclear, but could involve opening of potassium channels (Tricarico et al. 2004) and/or inhibition of one or more carbonic anhydrase iso-enzymes that have been shown to be present in the carotid bodies (Yamamoto et al. 2003) and at least one of which appears to act as a scavenger of reactive oxygen species in cultured cells (Raisanen et al. 1999). Previous studies have shown that the effect of acetazolamide on the hypoxic response strongly depends on the dose and route of administration (Teppema et al. 1992; Swenson & Hughes, 1993; Teppema & Dahan, 2004). The aims of the present study in healthy volunteers were twofold. First, we wished to examine if an intravenous acetazolamide dose as low as 4 mg kg−1 would reduce the carotid body-mediated acute hypoxic response in man. Second, we wished to examine if a reduction of the acute hypoxic response by acetazolamide could be reversed by antioxidants (1 g ascorbic acid i.v. and 200 mg α-tocopherol), suggesting a modulating role of the redox status of blood in oxygen sensing or a potential role of a carbonic anhydrase isoenzyme via an influence of the (extra) cellular redox state in the carotid bodies.
Methods
Subjects and apparatus
Sixteen volunteers (8 men, 8 women, equally distributed over two subject groups, age 19–24 years; all of them gave informed consent) participated in the study after approval from the local human ethics committee. All experiments conformed to the Declaration of Helsinki. All subjects were healthy, did not smoke or use any illicit drugs. They underwent a series of test experiments to familiarize them with the apparatus and experimental procedures. The subjects were instructed not to eat or drink for at least 8 h prior to the study. After arrival in the laboratory, a catheter was inserted into the right antecubital vein for drug infusion and into the left radial artery for arterial blood gas analysis (ABL 700 Radiometer, Copenhagen, Denmark).
The subjects breathed through a face mask, which was connected to a gas mixing system which received oxygen, carbon dioxide and nitrogen from three mass flow controllers. Gas flow was measured with a pneumotachograph (Fleisch, Lausanne, Switzerland) connected to a pressure transducer and electronically integrated to yield a volume signal. This signal was calibrated with a motor-driven piston pump (stroke volume 1 l at a frequency of 20 strokes min−1). Corrections were made for the changes in gas viscosity due to changes in oxygen concentration of the inhaled gas mixtures. The pneumotachograph was connected to a T-piece. One arm of the T-piece received a gas mixture (with a flow of 50 l min−1) from a gas mixing system consisting of three mass-flow controllers (Bronkhorst High-Tec, Veenendaal, the Netherlands). A personal computer provided control signals to the mass-flow controllers so that the composition of the inspired gas mixtures could be adjusted to force end-tidal oxygen concentration (PET,O2) to follow a specified pattern in time while the end-tidal carbon dioxide concentration (PET,CO2) is kept constant (Dahan et al. 1994; van den Elsen et al. 1995). The oxygen and carbon dioxide concentrations of inspired and expired gases were measured with a gas monitor (Multicap, Datex-Engstrom, Helsinki, Finland) by paramagnetic and infrared analysis. The gas monitor was calibrated with gas mixtures of known concentration delivered by a gas-mixing pump (Wösthoff, Bochum, Germany). The arterial haemoglobin-O2 saturation obtained via a finger probe (Sa,O2) was measured by pulse oximetry (Sattelite Plus, Datex-Engstrom).
Study design
Subjects were randomly divided into two groups and exposed to three treatments: control followed by acetazolamide (Actz) and antioxidants for the antioxidants (Aox) group and control followed by acetazolamide (Actz) and placebo for the placebo group. In both groups, two hypoxic exposures were applied during each of the three treatments, one at a constant normocapnic end-tidal PCO2 (PET,CO2, ‘normocapnic experiments’ in Table 2, resulting in normocapnic clamped levels of ventilation), and one at a constant hypercapnic PET,CO2 (‘hypercapnic experiments’ in Table 2, resulting in hypercapnic clamped levels of ventilation). The order of these CO2 levels was random. Hypoxia was induced with a ‘dynamic end-tidal forcing’ system by which steps from normoxia (PET,O2 15 kPa) into hypoxia (PET,O2 6.2 kPa obtained within four to six breaths) were applied (Dahan et al. 1994; van den Elsen et al. 1995; Teppema et al. 2002; Teppema et al. 2005). Since peak hypoxic ventilatory responses occur within 3 min (van den Elsen et al. 1995), hypoxia was maintained for 3 min, after which hyperoxia was introduced for 5 min (FIO2 > 0.5). Subsequently, the PET,O2 sequence was repeated at the alternate end-tidal PCO2 (PET,CO2) level.
Table 2.
Effects of acetazolamide, antioxidants and placebo on normocapnic and hypercapnic hypoxic responses
| Aox group | Placebo group | |||||
|---|---|---|---|---|---|---|
| Control | Actz | Actz + Aox | Control | Actz | Actx + Aox | |
| Resting ventilation (l min−1) | 8.9 ± 0.4 | 9.3 ± 0.5 | 9.4 ± 0.4 | 9.1 ± 0.8 | 9.4 ± 0.5 | 9.2 ± 0.3 |
| Resting PET,CO2 (kPa) | 5.6 ± 0.1 | 5.5 ± 0.1 | 5.3 ± 0.2 | 5.4 ± 0.2 | 5.3 ± 0.2 | 5.2 ± 0.2 |
| Normocapnic experiments | ||||||
| Clamped PET,CO2 (kPa) | 5.9 ± 0.1 | 5.9 ± 0.1 | 5.9 ± 0.1 | 5.7 ± 0.2 | 5.7 ± 0.2 | 5.7 ± 0.2 |
| Normoxic ventilation (l min−1) | 11.1 ± 0.5 | 12.1 ± 0.8 | 14.0 ± 0.7c | 10.6 ± 0.8 | 11.7 ± 0.9 | 13.7 ± 0.6e |
| Hypoxic ventilation (l min−1) | 20.4 ± 0.7 | 17.0 ± 1.0 | 22.8 ± 1.0 | 23.7 ± 0.9 | 16.8 ± 0.9 | 19.5 ± 0.9 |
| Hypoxic response (l min−1%−1) | 0.65 ± 0.1 | 0.41 ± 0.0* | 0.67 ± 0.1 | 0.61 ± 0.1 | 0.41 ± 0.1* | 0.46 ± 0.1* |
| Hypoxic Sa,O2 (%) | 85.0 ± 0.8 | 84.0 ± 0.7 | 84.8 ± 0.6 | 85.1 ± 0.8 | 83.1 ± 0.6 | 83.0 ± 1.0 |
| Hypercapnic experiments | ||||||
| Clamped PET,CO2 (kPa) | 6.4 ± 0.1 | 6.4 ± 0.1 | 6.4 ± 0.1 | 6.2 ± 0.2 | 6.2 ± 0.2 | 6.2 ± 0.2 |
| Normoxic ventilation (l min−1) | 16.0 ± 0.8 | 18.1 ± 1.0 | 20.0 ± 0.8d | 15.9 ± 1.0 | 17.3 ± 1.5 | 18.2 ± 0.7f |
| Hypoxic ventilation (l min−1) | 27.3 ± 0.8 | 23.1 ± 1.2 | 29.8 ± 1.1 | 27.6 ± 1.1 | 23.8 ± 1.6 | 24.3 ± 0.8 |
| Hypoxic response (l min−1%−1) | 0.86 ± 0.1† | 0.39 ± 0.0*‡ | 0.74 ± 0.1§ | 0.82 ± 0.1† | 0.44 ± 0.1*,a | 0.53 ± 0.1*,b |
| Hypoxic Sa,O2 (%) | 85.1 ± 0.8 | 85.1 ± 0.6 | 84.8 ± 1.0 | 84.6 ± 0.5 | 82.9 ± 0.8 | 82.9 ± 0.8 |
Effect of acetazolamide (Actz) and placebo on resting ventilatory parameters and the acute normocapnic and hypercapnic hypoxic responses. Data are means ± s.e.m. Note that in the Aox group Actz reduces the hypoxic response both in normocapnia and hypercapnia and that these effects are reversed by Aox. In the placebo group, no reversal is seen.
P < 0.010 versus control
P = 0.0002 versus normocapnia
P = 0.52 versus normocapnia
P = 0.02 versus normocapnia
P = 0.24 versus normocapnia
P = 0.19 versus normocapnia
P = 0.004 versus control
P = 0.015 versus control
P = 0.016 versus control
P = 0.015 versus control.
In all subjects, control hypoxic studies at both CO2 levels (no drugs given) were followed by the intravenous administration of 4 mg kg−1 acetazolamide (AHP Pharma SA, France). Twenty minutes after the Actz infusion, a second set of hypoxic studies was performed at both CO2 levels. Next, eight subjects received the antioxidant cocktail (Aox group), eight others placebo (placebo group). The antioxidant mixture consisted of 200 mg α-tocopherol (dl-α-tocoferolacetaat, OPG Farma, the Netherlands) ingested with a cup of yoghurt and 1 g ascorbic acid (Ascorbinezuur CF, Centrafarm, the Netherlands), infused intravenously 35 min after α-tocopherol application. The placebo mixture (manufactured by the local pharmacy) consisted of cellulose tablets (ingested with yoghurt), and saline, infused 35 min after the ingestion of cellulose. The final sets of hypoxic studies were performed 10 min after the infusions of Aox and saline in the Aox and placebo groups, respectively. With respect to the administration of the antioxidants and placebo, the study had a double-blind, randomized design.
The breath-to-breath data of the last 10 breaths of normoxia (prior to hypoxia) and of hypoxia (prior to hyperoxia) were averaged. Hypoxic ventilatory sensitivity (or acute hypoxic ventilatory response; units l min−1%−1) was calculated as (Dahan et al. 1994):
The statistical analysis was performed using SPSS v11.0 for Windows (SPSS Inc. Chicago, IL, USA). To detect a significance of difference among the three treatment levels of each session, a two-way repeated measures ANOVA with post hoc Bonferroni correction was performed to detect significant differences among the three treatment levels within the Aox group (control, Actz, Aox) and the placebo group (control, Actz, placebo). P values < 0.05 were considered significant. Unless otherwise indicated, reported values are means ± s.e.m.
Results
All subjects completed the sessions without side-effects, except one subject belonging to the placebo group who withdrew after one session for unknown reasons.
The effects of acetazolamide on resting arterial acid–base and [K+] are shown in Table 1. In both groups of volunteers, the agent induced small reductions in mean potassium and bicarbonate concentrations and base excess. No significant differences in mean resting minute ventilation and end-tidal PCO2 were observed between control and Actz or Actz + Aox in the Aox group and control and Actz or Actz + placebo in the placebo group (Table 2). After acetazolamide administration, there was no detectable increase in the arterial-to-end-tidal PCO2 gradient indicating incomplete erythrocytic carbonic anhydrase inhibition. We observed no effect of low intravenous dose of acetazolamide on arterial blood pressure (data not shown).
Table 1.
Effects of acetazolamide on blood acid–base status
| Control | Acetazolamide | |
|---|---|---|
| Aox group (n = 8) | ||
| Pa,CO2 (kPa) | 5.6 ± 0.3 | 5.4 ± 0.4 |
| Pa,CO2−PET,CO2 (kPa) | −0.00 ± 0.15 | −0.08 ± 0.24 |
| pHa | 7.39 ± 0.01 | 7.37 ± 0.01† |
| Arterial [bicarbonate] (mm) | 24.8 ± 1.4 | 22.7 ± 1.6† |
| Base excess (mm) | −0.3 ± 1.1 | −2.5 ± 1.2† |
| Arterial [K+] (mm) | 4.1 ± 0.3 | 3.9 ± 0.3§ |
| Sa,O2 (%) | 97.6 ± 0.4 | 96.8 ± 1.1* |
| Placebo group (n = 7) | ||
| Pa,CO2 (kPa) | 5.4 ± 0.5 | 5.2 ± 0.4 |
| Pa,CO2−PET,CO2 (kPa) | 0.04 ± 0.26 | −0.09 ± 0.33 |
| pHa | 7.38 ± 0.03 | 7.37 ± 0.02* |
| Arterial [bicarbonate] (mmol l−1) | 24.1 ± 1.8 | 21.9 ± 1.6‡ |
| Base excess (mm) | −0.9 ± 1.6 | −3.0 ± 1.2† |
| Arterial [K+] (mm) | 4.1 ± 0.2 | 3.7 ± 0.3† |
| Sa,O2 (%) | 97.6 ± 0.2 | 97.7 ± 0.4 |
Effect of acetazolamide on resting blood (gas) and electrolyte parameters in eupnoeic resting conditions. Data are means ± s.d.
P < 0.05
P < 0.01
P < 0.02
P < 0.03.
Blood gas analysis was performed to check for possible arterial-to-end-tidal PCO2 differences following acetazolamide infusion.
Normocapnic clamped PET,CO2 values were 5.9 ± 0.1 and 5.7 ± 0.1 kPa in the Aox and placebo group, respectively. In both subject groups, the clamped PET,O2 was increased by 0.5 kPa to perform a hypercapnic acute hypoxic response (AHR). When going from the normocapnic to the hypercapnic PET,O2, both subject groups showed an about equal increase in AHR (O2–CO2 interaction, Table 2, also see Fig. 1).
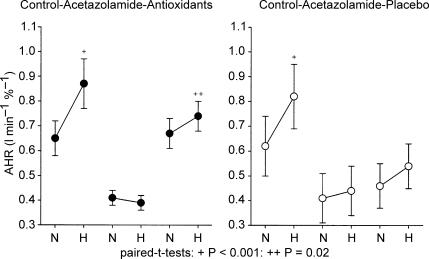
Figure 1. Effects of antioxidants (left panel) and placebo (right panel) on acetazolamide-induced inhibition of normocapnic (N) and hypercapnic (H) hypoxic responses.
Control data are shown at left in both panels. Note the increase in hypoxic sensitivity when going from normocapnia to hypercapnia in control that is abolished after acetazolamide (shown in the middle of both panels). The left panel shows that after antioxidant administration, the normocapnic hypoxic response in fully restored, while an O2–CO2 interaction reappears. After placebo (right panel), the normocapnic and hypercapnic hypoxic responses clearly remain below control and no O2–CO2 interaction reappears. Data are means ± s.e.m.; paired t tests; +P < 0.001; ++P = 0.02.
The effects of Actz, Aox and placebo on the mean AHR in both subject groups are summarized in Table 2 and graphically presented in Fig. 1. The mean normocapnic AHRs were reduced by 37 and 33% in the Aox and placebo groups (N.S.), respectively (Table 2). This inhibiting effect on the hypercapnic AHR was even larger (52 and 47% reduction in the Aox and placebo groups, respectively, not significantly different between the two groups): in fact, Actz abolished any O2–CO2 interaction (Fig. 1, Table 2). In the subjects of the Aox group that showed a reduced normocapnic and hypercapnic AHR upon acetazolamide, this effect was reversed by Aox. In normocapnia, this reversal was total, while in hypercapnia a complete or almost complete reversal occurred in all individuals except one in which it was partial (for mean data see Table 2). After Aox, all these individuals showed a larger hypercapnic than normocapnic AHR indicating a reversal of the O2–CO2 interaction. Note that both the normocapnic and hypercapnic AHRs after Actz + Aox did not significantly differ from those during control.
In contrast to the subjects receiving Aox, those receiving placebo did not show reversal of the acetazolamide-induced inhibition of the normocapnic and hypercapnic AHR (Fig. 2). At both CO2 levels, the AHR after Actz + placebo was not different from that after Actz alone. After Actz + placebo, some subjects in this group showed a somewhat larger, and others an equal or somewhat smaller hypercapnic than normocapnic AHR so that in contrast to those treated with Aox, the subjects of this group showed on average no signs of a re-appearing O2–CO2 interaction.
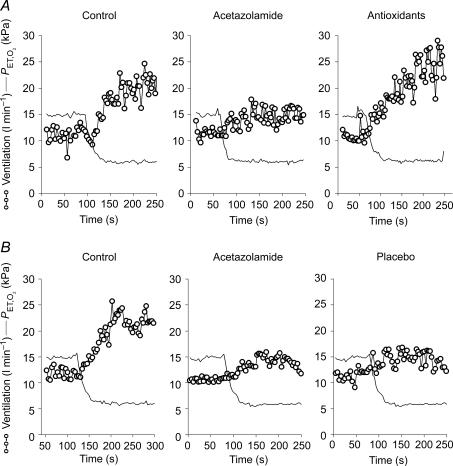
Figure 2. Effects of antioxidants (A) and placebo (B) on the acetazolamide-induced depression of the normoxic acute hypoxic ventilatory response in one individual.
Aox reverses the depression but placebo fails to do so. Ventilatory data are shown breath-by-breath.
Discussion
In this study in healthy volunteers, acetazolamide (4 mg kg−1, i.v.) reduced the normocapnic and hypercapnic hypoxic response by about 35 and 50%, respectively, and prevented the increase in hypoxic response that normally occurs with a rise in arterial PCO2 (O2–CO2 interaction; note also that the inhibiting effect of acetazolamide occurred despite a small significant decrease in arterial pH that normally would tend to increase the AHR). A mixture of antioxidants (α-tocopherol, 200 mg p.o. and ascorbic acid, 1 g i.v.) reversed these inhibiting effects of acetazolamide on the hypoxic response.
Methodology and study design: general considerations
Two issues that should be discussed concern the acetazolamide dose and the timing of the ventilatory measurements. The acetazolamide dose that is needed to obtain an acute maximal physiological effect from carotid body carbonic anhydrase (CA) inhibition has not been systematically explored. For most organs it is 5–10 mg kg−1, but for kidneys, containing an enzyme concentration similar to carotid bodies, it is smaller (Maren, 1967, 1977). From available human data (Lehmann et al. 1969) we estimate a free unbound (diffusible) drug concentration in our subjects of about 8 μm at the beginning of the elimination phase which is insufficient for impairment of red cell function (Maren, 1967, 1977) but well within a concentration range necessary for lowering intraocular pressure (EC50≈ 7 μm (Yano et al. 1998). The concentration needed for maximal inhibition of carotid body function probably lies between 8 and 15 μm, because already at 12–15 μm the hypoxic response is abolished (Swenson & Hughes, 1993). In order to reach this concentration of ∼15 μm, we considered initially to administer 500 mg to our subjects, because this could have told us whether the hypoxic response after Aox would reappear after its abolishment by acetazolamide. However, this could have the disadvantage of studying an effect that could be confounded by sufficient erythrocytic CA inhibition to result in tissue acidosis (Maren, 1977) and the inability to perform real isocapnic measurements (Teppema et al. 2001; note the absence of arterial-to-end-tidal PCO2 after acetazolamide infusion –Table 1). Nevertheless, together with those of Swenson & Hughes (1993) our data indicate a strong dose-dependent effect of acetazolamide on carotid body function in a narrow concentration range.
The time course of the effect of intravenous acetazolamide on the control of breathing is not well described. In the anaesthetized cat, an intravenous dose of 4 mg kg−1 abolishes the O2–CO2 interaction and reduces hypoxic sensitivity by half, effects that are still demonstrable about 100 min after infusion (Teppema et al. 2001; Teppema & Dahan, 2004). Similar to Swenson & Hughes (1993), we performed the ventilatory measurements within one elimination half-time of acetazolamide which after an intravenous bolus is ∼1.7 h (Maren & Robinson, 1960; Lehmann et al. 1969). The estimated prevailing plasma concentration (∼5 μm) at the time of the last ventilatory measurements (hypercapnic AHR after Actz + Aox/placebo) is well within the range reported to cause physiological effects in various peripheral tissues except erythrocytes (Maren, 1967, 1977; Lehmann et al. 1969; Yano et al. 1998). A long-lasting inhibition of carotid body function by acetazolamide was confirmed in our subjects receiving Actz plus placebo because on average they still showed a hypoxic response that was significantly lower than control.
Reduction of the AHR by acetazolamide; reversal by Aox
Our observations are from intact organisms and this compels us to be cautious with extrapolating them back to processes occurring at the cellular level. However, available data obtained from reduced preparations may help us to place our observations into a proper context. For the sake of brevity, we will only discuss two scenarios that may be helpful to explain our findings.
Scenario 1
Inhibition of the AHR by acetazolamide is due to inhibition of a CA isoform. It is assumed that the antioxidants that we administered did not influence acetazolamide's binding to the isoenzyme but rather exerted an independent reducing effect, for example by scavenging reactive oxygen species (ROS; Burton et al. 1983; Frei et al. 1989). As Aox reversed an inhibitory effect of a carbonic anhydrase inhibitor, we consider a possible role of some CA isoform as a ROS scavenger. Two candidates for such an antioxidant function could be CA III and IV, the latter because it contains cysteine groups that may participate in redox reactions (Hurt et al. 1998), and the former because it behaves as an important ROS scavenger (Raisanen et al. 1999) and is present in type I carotid body cells from rat (Yamamoto et al. 2003). CA III is a cytoplasmic enzyme with a relatively low affinity for sulphonamide inhibitors (Kararli & Silverman, 1985), while at the same time acetazolamide is only moderately permeable (Holder & Hayes, 1965). These two factors, limited permeability of acetazolamide and its low affinity for CA III could then explain why a relatively high dose is needed to completely abolish the hypoxic ventilatory response (Teppema et al. 1992). With regard to CA IV, we are not aware of data showing a role of this protein in regulating the (extracellular) redox state, for example by S-glutathiolation of one or more of its cysteine residues (Hurt et al. 1998). It should also be mentioned that as yet the presence of CA IV in the carotid bodies has not been demonstrated.
Since Aox reversed the acetazolamide-induced depression of the AHR, scenario 1 could involve a role for CA as ROS scavenger in hypoxia which then could contribute to (i.e. increase) the magnitude of the ventilatory response. However, this remains a matter of speculation since experimental evidence is lacking. Also, note that it is unsettled if hypoxia is associated with either an increase or decrease in [ROS] and that ROS do not seem to be directly involved in oxygen sensing (Gonzalez et al. 2002), and our results do not shed more light on this. It is interesting to note that Aoxs are also able to reverse cutaneous photosensitivity by acetazolamide and other sulphonamide-derived diuretics and antidiabetics (Selvaag, 1996; Moore, 2002), and that Aoxs such as vitamin E may affect CA activity (Ciftci et al. 2005).
Scenario 2
Acetazolamide causes intracellular alkalosis in type I carotid body cells, which results in a decrease in carotid body output and sensitivity. Aoxs reverse this by an independent effect on potassium channels. Acetazolamide causes alkalosis in type I carotid body cells from neonatal rat (Buckler et al. 1991) and in guinea pig mesenteric smooth muscle cells, it induces alkalosis followed by hyperpolarization, opening of maxi-K (BK) channels and a decreased Ca2+ influx (Pickkers et al. 1999). Apart from TASK-1 channels (Buckler et al. 2000), maxi-K channels have been shown to play a role in oxygen sensing type I cells from rat carotid body (Wyatt & Peers, 1995; Williams et al. 2004; note that in rabbit and mouse, Kv channels have been shown to be involved –Riesco-Fagundo et al. 2001; Perez-Garcia et al. 2004). A reversal by Aoxs of the inhibitory effects of acetazolamide on the hypoxic response could be explained by the known redox sensitivity of potassium channels (Kourie, 1998; Liu & Gutterman, 2002). In rabbit type I glomus cells, reducing agents decrease the open probability of potassium channels (Lopez-Barneo et al. 1999).
In rat muscle cells, acetazolamide has a BK channel-opening effect which may be mediated by a direct action and is not shared by the more lipophilic carbonic anhydrase inhibitor methazolamide (Tricarico et al. 2004). In cats, 4 mg kg−1 acetazolamide has a similar inhibitory effect on the hypoxic response as we report here in humans (Teppema et al. 2004). Recently, we have compared the effect of low-dose acetazolamide and methazolamide on the hypoxic response in this species and the surprising finding was that even high-dose methazolamide did not affect it (authors' unpublished observations). This suggests that at least in the cat, but possibly also in humans, the inhibition of the hypoxic response by acetazolamide is not related to inhibition of carbonic anhydrase (thereby making the above scenario 1 less likely) but rather to some other pharmacological effect of the agent. It remains a matter of speculation whether the inhibiting effect of acetazolamide on the hypoxic response may be mediated via a specific effect on one or more potassium channels, or, alternatively, to a possible action on various types of L-type Ca2+ and/or sodium channels (Bendahhou et al. 2001; MacNaughton et al. 2004).
Clinical implications?
As at this stage the possible clinical implications of our observations are a matter of speculation we only briefly address two issues, the first of which concerns a possible effect of Aox on the hypoxic response per se. Since in previous studies (Teppema et al. 2002, 2005) we were unable to demonstrate an effect of Aox on normoxic ventilation and the AHR, we decided not to include a separate protocol with Aox alone. Putting our present and previous findings together, it seems that in resting conditions, in the absence of inhibiting factors, Aox may not alter carotid body output but may increase it when such factors are present. This could be important in elderly people (> 70 years): they have been reported to possess a considerably lower O2 sensitivity than healthy young adults (Kronenberg & Drage, 1973; Peterson et al. 1981), while in women 60–80 years of age ascorbic acid supplementation appeared to increase their hypoxic response (Pokorski & Marczak, 2003). The ability of antioxidants to alter the hypoxic response may vary between different reducing agents. For example, Hildebrandt et al. (2002) showed that after a 5-day oral application, N-acetylcysteine increased the hypoxic response in young adults indicating a possible specific involvement of glutathione in oxygen sensing.
A second issue concerns the possibility that Aox may also alter physiological effects of acetazolamide other than the depression of the hypoxic response. For example, the agent has well-known beneficial effects in the prevention and treatment of acute mountain sickness (Bartsch et al. 2004). Ascent to high altitude is associated with increased production of ROS that may have both advantageous and adverse roles in the adaptation to low environmental PO2 (Bailey, 2003). Acetazolamide may prevent or ameliorate symptoms of AMS by an acidosis-induced rise in ventilation and/or by reducing pulmonary vasoconstriction (Hohne et al. 2004), an oxygen-sensitive process that, similar to O2 sensing in the carotid bodies, involves potassium channels. It remains to be seen whether supplemental antioxidants at altitude might interfere with the beneficial effect of acetazolamide or influence adaptive processes in a hypobaric environment.
References
- Bailey DM. Radical dioxygen: from gas to (unpaired!) electrons. Adv Exp Med Biol. 2003;543:201–221. [PubMed] [Google Scholar]
- Bartsch P, Bailey DM, Berger MM, Knauth M, Baumgartner RW. Acute mountain sickness: controversies and advances. High Alt Med Biol. 2004;5:110–124. doi: 10.1089/1527029041352108. [DOI] [PubMed] [Google Scholar]
- Bendahhou S, Cunmmins TR, Griggs RC, Fu YH, Ptacek LJ. Sodium channel inactivation defects are associated with acetazolamide-exacerbated hypokalemic periodic paralysis. Ann Neurol. 2001;50:417–420. doi: 10.1002/ana.1144. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD, Peers C, Nye PC. Intracellular pH and its regulation in isolated type I carotid body cells of the neonatal rat. J Physiol. 1991;436:107–129. doi: 10.1113/jphysiol.1991.sp018542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GW, Joyce A, Ingold KU. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983;221:281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Ciftci M, Bülbül M, Gül M, Gümüstekin K, Dane S, Süleyman H. Effects of nicotine and vitamin E on carbonic anhydrase activity in some rat tissues in vivo and in vitro. J Enzyme Inhib Med Chem. 2005;20:103–108. doi: 10.1080/14756360400002098. [DOI] [PubMed] [Google Scholar]
- Dahan A, van den Elsen MJ, Berkenbosch A, DeGoede J, Olievier IC, van Kleef JW, Bovill JG. Effects of subanesthetic halothane on the ventilatory responses to hypercapnia and acute hypoxia in healthy volunteers. Anesthesiology. 1994;80:727–738. doi: 10.1097/00000542-199404000-00004. [DOI] [PubMed] [Google Scholar]
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Sanz-Alfayate G, Agapito MT, Gomez-Nino A, Rocher A, Obeso A. Significance of ROS in oxygen sensing in cell systems with sensitivity to physiological hypoxia. Respir Physiol Neurobiol. 2002;132:17–41. doi: 10.1016/s1569-9048(02)00047-2. [DOI] [PubMed] [Google Scholar]
- Hildebrandt W, Alexander S, Bartsch P, Droge W. Effect of N-acetyl-cysteine on the hypoxic ventilatory response and erythropoietin production. linkage between plasma thiol redox state and O2 chemosensitivity. Blood. 2002;99:1552–1555. doi: 10.1182/blood.v99.5.1552. [DOI] [PubMed] [Google Scholar]
- Hohne C, Krebs MO, Seiferheld M, Boemke W, Kaczmarczyk G, Swenson ER. Acetazolamide prevents hypoxic pulmonary vasoconstriction in conscious dogs. J Appl Physiol. 2004;97:515–521. doi: 10.1152/japplphysiol.01217.2003. [DOI] [PubMed] [Google Scholar]
- Holder LB, Hayes SL. Diffusion of sulfonamides in aqueous buffers and into red cells. Mol Pharmacol. 1965;1:266–279. [PubMed] [Google Scholar]
- Hurt JD, Tu C, Laipis PJ. Isolation and expression of murine carbonic anhydrase IV. Protein Expr Purif. 1998;12:7–16. doi: 10.1006/prep.1997.0801. [DOI] [PubMed] [Google Scholar]
- Kararli T, Silverman DN. Inhibition of the hydration of CO2 catalyzed by carbonic anhydrase III from cat muscle. J Biol Chem. 1985;260:3484–3489. [PubMed] [Google Scholar]
- Kourie JI. Interaction of reactive oxygen species with ion transport mechanisms. Am J Physiol. 1998;275:C1–C24. doi: 10.1152/ajpcell.1998.275.1.C1. [DOI] [PubMed] [Google Scholar]
- Kronenberg RS, Drage CW. Attenuation of the ventilatory and heart rate responses to hypoxia and hypercapnia with aging in normal men. J Clin Invest. 1973;52:1812–1819. doi: 10.1172/JCI107363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri S. Historical perspectives of cellular oxygen sensing and responses to hypoxia. J Appl Physiol. 2000;88:1467–1473. doi: 10.1152/jappl.2000.88.4.1467. [DOI] [PubMed] [Google Scholar]
- Lehmann B, Linner E, Wistrand PJ. The pharmacokinetics of acetazolamide in relation to its use in the treatment of glaucoma and to its effect as an inhibitor of carbonic anhydrases. Adv Biosci. 1969;5:197–217. [Google Scholar]
- Liu Y, Gutterman DD. Oxidative stress and potassium channel function. Clin Exp Pharmacol Physiol. 2002;29:305–311. doi: 10.1046/j.1440-1681.2002.03649.x. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia MT, Colinas O, Miguel-Velado E, Moreno Dominguez A, Lopez-Lopez JR. Characterisation of the Kv channels of mouse carotid body chemoreceptors cells and their role in oxygen sensing. J Physiol. 2004;557:457–471. doi: 10.1113/jphysiol.2004.062281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Montoro RJ, Smani T, Garcia-Hirschfeld J, Urena J. K+ and Ca2+ channel activity and cytosolic [Ca2+] in oxygen-sensing tissues. Respir Physiol. 1999;115:215–227. doi: 10.1016/s0034-5687(99)00016-x. [DOI] [PubMed] [Google Scholar]
- MacNaughton NCL, Davies CH, Randall A. Inhibition of alpha (1E) Ca2+ channels by carbonic anhydrase inhibitors. J Pharmacol Sci. 2004;95:240–247. doi: 10.1254/jphs.fp0040032. [DOI] [PubMed] [Google Scholar]
- Maren TH. Carbonic anhydrase: chemistry, physiology and inhibition. Physiol Rev. 1967;47:595–761. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren TH. Use of inhibitors in physiological studies of carbonic anhydrase. Am J Physiol. 1977;232:F291–F297. doi: 10.1152/ajprenal.1977.232.4.F291. [DOI] [PubMed] [Google Scholar]
- Maren TH, Robinson B. The pharmacology of acetazolamide as related to cerebrospinal fluid and the treatment of hydrocephalus. Bull Johns Hopkins Hosp. 1960;106:1–24. [PubMed] [Google Scholar]
- Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002;25:345–372. doi: 10.2165/00002018-200225050-00004. [DOI] [PubMed] [Google Scholar]
- Peterson DD, Pack AI, Silage DA, Fishman AP. Effects of aging on ventilatory and occlusion pressure responses to hypoxia and hypercapnia. Am Rev Respir Dis. 1981;124:387–391. doi: 10.1164/arrd.1981.124.4.387. [DOI] [PubMed] [Google Scholar]
- Pickkers P, Garcha RS, Schachter M, Smits P, Hughes AD. Inhibition of carbonic anhydrase accounts for the direct vascular effects of hydrochlorothiazide. Hypertension. 1999;33:1043–1048. doi: 10.1161/01.hyp.33.4.1043. [DOI] [PubMed] [Google Scholar]
- Pokorski M, Marczak M. Ascorbic acid enhances hypoxic ventilatory reactivity in elderly subjects. J Int Med Res. 2003;31:448–457. doi: 10.1177/147323000303100514. [DOI] [PubMed] [Google Scholar]
- Prabhakar N. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- Raisanen SR, Lehenkari P, Tasanen M, Rahkila P, Harkonen PL, Vaananen HK. Carbonic anhydrase III protects cells from hydrogen peroxide-induced apoptosis. FASEB J. 1999;13:513–522. doi: 10.1096/fasebj.13.3.513. [DOI] [PubMed] [Google Scholar]
- Riesco-Fagundo AM, Perez-Garcia MT, Gonzalez C, Lopez-Lopez JR. O2 modulates large-conductance Ca2+-dependent K+ channels of rat chemoreceptor cells by a membrane-restricted and CO-sensitive mechanism. Circ Res. 2001;89:430–436. doi: 10.1161/hh1701.095632. [DOI] [PubMed] [Google Scholar]
- Selvaag E. Inhibition of thiazide photohemolysis in vitro by antioxidants and a nitrogen atmosphere. Photodermatol Photoimmunol Photomed. 1996;12:211–215. doi: 10.1111/j.1600-0781.1996.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Swenson ER, Hughes JM. Effects of acute and chronic acetazolamide on resting ventilation and ventilatory responses in men. J Appl Physiol. 1993;74:230–237. doi: 10.1152/jappl.1993.74.1.230. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A. Low-dose acetazolamide reduces the hypoxic ventilatory response in the anesthetized cat. Respir Physiol Neurobiol. 2004;140:43–51. doi: 10.1016/j.resp.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Dahan A, Olievier CN. Low-dose acetazolamide reduces CO2–O2 stimulus interaction within the peripheral chemoreceptors in the anaesthetised cat. J Physiol. 2001;537:221–229. doi: 10.1111/j.1469-7793.2001.0221k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Nieuwenhuijs D, Sarton E, Romberg R, Olievier CN, Wards DS, Dahan A. Antioxidants prevent depression of the acute hypoxic ventilatory response by subanaesthetic halothane in men. J Physiol. 2002;544:931–938. doi: 10.1113/jphysiol.2002.025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ, Rochette F, Demedts M. Ventilatory effects of acetazolamide in cats during hypoxemia. J Appl Physiol. 1992;72:1717–1723. doi: 10.1152/jappl.1992.72.5.1717. [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Romberg RR, Dahan A. Antioxidants reverse reduction of the human hypoxic ventilatory response by subanesthetic isoflurane. Anesthesiology. 2005;102:47–53. doi: 10.1097/00000542-200504000-00009. [DOI] [PubMed] [Google Scholar]
- Tricarico D, Barbieri M, Mele A, Carbonara G, Camerino DC. Carbonic anhydrase inhibitors are specific openers of skeletal muscle BK channel of K+-deficient rats. FASEB J. 2004;18:760–761. doi: 10.1096/fj.03-0722fje. [DOI] [PubMed] [Google Scholar]
- van den Elsen MJLJ, Dahan A, DeGoede J, Berkenbosch A, van Kleef J. Influences of subanesthetic isoflurane on ventilatory control in humans. Anesthesiology. 1995;83:478–490. doi: 10.1097/00000542-199509000-00006. [DOI] [PubMed] [Google Scholar]
- Williams SE, Wootton P, Mason MS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- Wyatt CN, Peers C. Ca2+-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J Physiol. 1995;483:559–565. doi: 10.1113/jphysiol.1995.sp020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Fujimura M, Nishita T, Nishijima K, Atoji Y, Suzuki Y. Immunohistochemical localization of carbonic anhydrase isozymes in the rat carotid body. J Anat. 2003;202:573–577. doi: 10.1046/j.1469-7580.2003.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano I, Takayama A, Takano M, Inatami M, Tanihara H, Ogura I, Honda Y, Inui K. Pharmacokinetics and pharmacodynamics of acetazolamide in patients with transient intraocular pressure elevation. Eur J Clin Pharmacol. 1998;54:63–68. doi: 10.1007/s002280050422. [DOI] [PubMed] [Google Scholar]