Abstract
The initial skin blood flow response to rapid local heating is an axon reflex, which may be mediated by calcitonin gene-related peptide and substance P released from C-fibres. We investigated the role of nitric oxide (NO) and noradrenaline on the temperature threshold for the axon reflex during gradual local heating. 36 subjects participated in two studies. Using microdialysis, we examined the following interventions: NO synthase inhibition (10 mmNG-nitro-l-arginine methyl ester, l-NAME); low-dose NO infusion (1.0 μm sodium nitroprusside, SNP); adrenergic blockade (10 mm bretylium tosylate); and low-dose (0.1 μm) noradrenaline infusion. Laser-Doppler flowmetry was used to measure red blood cell flux. Skin was heated at a rate of 0.1°C min−1 from 33°C to 40°C. Compared to control skin sites, the axon reflex response was shifted to a higher temperature in 4 subjects in the l-NAME sites (control, 37.0 ± 0.3°C, n = 16; l-NAME, 39.8 ± 0.1°C, n = 4; P < 0.001) and absent in 12 subjects. The response was also absent in l-NAME plus low-dose SNP sites and not altered by low-dose SNP infusion alone. Adrenergic blockade, with and without low-dose noradrenaline infusion, also abolished the axon reflex response in all subjects. Low-dose noradrenaline infusion alone shifted the axon reflex to a significantly lower temperature threshold compared to control sites (control, 38.2 ± 0.5°C; noradrenaline, 37.7 ± 0.4°C, P < 0.05, n = 5). These results suggest that endogenous NO and noradrenaline contribute to the temperature threshold of the axon reflex response during gradual local heating of the skin.
In humans, the skin blood flow response to a non-painful, rapid heat stimulus consists of two phases: an immediate increase in skin blood flow, known as an initial peak and nadir response, followed by a prolonged secondary plateau response. The initial phase is considered to be mediated by the axon reflex, whereas the secondary plateau phase is approximately 70% dependent on the production of nitric oxide (NO) (Kellogg et al. 1999; Minson et al. 2001, 2002); however, the specific independent and interdependent mechanisms of the axon reflex and secondary plateau responses are complex and not well understood. This thermal hyperaemia may involve numerous mechanisms, as these responses are altered (i) by the rate at which heat is applied (Magerl & Treede, 1996), (ii) in the presence of conscious pain (Kellogg et al. 1995) and (iii) in patients with diabetic dermopathy (Wigington et al. 2004), postural tachychardic syndrome (Medow et al. 2005) and secondary Raynaud's syndrome (Boignard et al. 2005).
It has been suggested that the axon reflex in human skin is a response neurally mediated by C-fibre nociceptors (Magerl & Treede, 1996; Schmelz et al. 2000), which have been shown to stimulate antidromic vasodilatation via the release of calcitonin gene-related peptide (CGRP) and substance P (Wallengren & Hakanson, 1987; Sann & Pierau, 1998). CGRP has been shown to induce a potent and prolonged vasodilatation in human skin (Brain et al. 1985; Weidner et al. 2000), whereas the response to microdialysis infusion of substance P is robust but short-lived (Weidner et al. 2000; Klede et al. 2003; Wong et al. 2005). Substance P has also been shown to limit the prolonged vasodilator action of CGRP to a transient increase in cutaneous blood flow (Brain & Williams, 1988; Wallengren & Wang, 1993), a response that is qualitatively similar to the observed pattern of skin blood flow of the axon reflex during rapid local heating.
Basal levels of NO contribute to resting vascular tone and may contribute to the axon reflex response. Previous work from our laboratory has shown that NO synthase inhibition decreased, rather than abolished, the axon reflex to rapid local heat, which indicated a possible interaction of NO with other mechanisms in this response (Minson et al. 2001, 2002). One possibility is that NO may influence the release or action of CGRP and substance P from C-fibre nociceptors in human skin. Prior investigations have shown that NO synthase inhibition significantly attenuated the flare response induced by capsaicin, but not CGRP (Hughes & Brain, 1994), and attenuated substance P-mediated dilatation (Klede et al. 2003; Wong et al. 2005). Taken together, these data suggest that the release of CGRP and the vasoactive properties of substance P may be partially NO-dependent.
Noradrenaline also contributes to resting vascular tone and has been shown to enhance C-fibre nociceptor sensitivity in animal models (Nam et al. 2000; Banik et al. 2001, 2004; Sato et al. 2005). Although the possible interaction between noradrenaline and C-fibres is less-well understood in humans, preliminary evidence suggests that noradrenaline may also contribute to the axon reflex response. Indeed, iontophoresis of noradrenaline has been shown to enhance the hyperalgesic (Drummond, 1995, 1998) and axon reflex (Drummond & Lipnicki, 1999) responses in human skin.
Given that C-fibre nociceptors may have a role in the axon reflex response and that NO and noradrenaline may influence antidromic vasodilatation, we sought to investigate the contribution of NO and noradrenaline to the onset of the axon reflex in human skin. To identify the temperature for the onset of the axon reflex response, we utilized a progressive local heating protocol by which the local heater temperature was raised from 33°C to 40°C at the rate of 0.1°C min−1. In previous studies we have used a more-rapid local heating protocol of 0.1°C s−1 (Minson et al. 2001, 2002; Wong et al. 2006). The slower rate of temperature rise employed in this study elicited a gradual increase in skin blood flow punctuated by a sharp rise to a peak at an average temperature of 37°C, which we attributed to being mediated by an axon reflex. This slower, progressive local heating protocol allowed us to better assess the temperature threshold for the axon reflex than with a more-rapid temperature increase.
With the slower, progressive local heating protocol, we tested three hypotheses. First, we hypothesized that NO synthase inhibition and adrenergic blockade would shift the onset of the first observed axon reflex response to a higher temperature threshold. Second, we hypothesized that giving a low dose of an exogenous form of NO during NO synthase inhibition, and noradrenaline during adrenergic blockade, would restore the axon reflex to the same temperature threshold as in control conditions. Third, we hypothesized that low-dose NO and noradrenaline alone would shift the onset of the first axon reflex to a lower temperature threshold in comparison to control conditions.
Methods
Subjects
17 male (25 ± 4 years of age) and 19 female (24 ± 3 years of age) subjects participated in two studies. Several subjects participated in multiple protocols. All subjects were healthy, non-smokers who were not taking prescription medication (with the exception of oral contraceptives) and had no history of cardiovascular disease. All protocols were approved by the Institutional Review Board at the University of Oregon and conformed to the guidelines contained within the Declaration of Helsinki. Verbal and written informed consent was obtained from each subject prior to the participation of each protocol.
Instrumentation
On the day of testing, each subject was equipped with a five-lead electrocardiogram, to continuously monitor heart rate, and a blood pressure cuff on the dominant arm to monitor blood pressure every 5 min by automatic brachial auscultation (Cardiocap, Datex Ohmeda Tewksbury, MA, USA). A water-perfused suit covered the body with the exception of the face, hands and the area of skin to be studied. The water-perfused suit was used to clamp skin temperature and control whole-body temperature.
Subjects were instrumented with two (Protocols IA, IC, IIA–IIC; see below) or three (Protocol IB) microdialysis fibres (MD 2000, Bioanalytical Systems, West Lafayette, IN, USA). The membrane of the microdialysis fibres were 10 mm in length with a 20-kDa molecular weight cut-off. Each fibre was placed in the dermal layer on the ventral side of the non-dominant forearm. To place the fibre, a 25-gauge needle was placed into the dermal layer of the skin. The fibre was threaded through the lumen of the needle and the needle was removed from the skin, leaving the fibre in place. The fibres were taped to the skin for a secure placement and infused with lactated Ringer solution at the rate of 2 μl min−1 via a microinfusion pump (CMA/102, CMA Microdialysis, Stockholm, Sweden). One microdialysis site was randomly selected as control and continuously infused with lactated Ringer solution throughout the local heating protocol. The other microdialysis sites were designated as experimental sites and treated accordingly (see protocols below). Following fibre placement, the trauma response was allowed to resolve (∼90–120 min).
After the trauma response, local heater devices (SH02 Skin Heaters, Moor Instruments, Devon, UK) and integrated laser-Doppler probes (MoorLAB, Moor Instruments) were placed directly over each microdialysis site. The local skin heaters cover approximately 700 mm2 of tissue and are used to control local skin temperature throughout the protocol. The laser-Doppler probes were placed in the centre of the local heater disc and used to measure red blood cell (RBC) flux as an index of skin blood flow.
Local heating protocol
Baseline skin blood flow was recorded for 20 min with the local heating devices set at 33°C. After the baseline period, the local heaters were raised 0.1°C min−1 until the local heater temperature reached 40°C. At 40°C, the temperature was clamped for a 10-min period. This standard local heating protocol was used for each study. At the end of the protocol, the local heaters were raised to 43°C, and 28 mm sodium nitroprusside (SNP; Nitropress, Abbot Laboratories, North Chicago, IL, USA) was infused to attain maximal skin blood flow.
Study I
The rationale of study I was to investigate the role of NO on the axon reflex response during gradual local heating of the skin. This study consisted of three individual protocols and a total of 18 subjects, as shown in Table 1. In protocol IA (n = 8), the experimental site was infused with 10 mmNG-nitro-l-arginine methyl ester (l-NAME, Calbiochem, San Diego, CA, USA) dissolved in lactated Ringer solution for complete NO synthase inhibition. This site was used to determine the contribution of endogenous NO to the axon reflex response. Protocol IB (n = 8) utilized two experimental sites, one treated with l-NAME, and the other with l-NAME plus low-dose (1.0 μm) SNP dissolved in lactated Ringer solution. The additional site was used to determine the effect of exogenous NO on the axon reflex response with the blockade of endogenous NO production. In protocol IC (n = 2), the experimental site was infused with low-dose SNP. This site was used to determine the effect of an exogenous NO donor on the axon reflex response without the blockade of endogenous NO production. All infusions were continuous throughout the entire local heating protocol.
Table 1. List of experimental sites for gradual local heating protocol.
| Protocol | Experimental site | Number of subjects |
|---|---|---|
| IA | l-NAME | 8 |
| IB | l-NAME | 8 |
| l-NAME plus low-dose (1 μm) SNP | 8 | |
| IC | Low-dose SNP | 2 |
| IIA | 10 mm BT | 8 |
| IIB | 10 mm BT plus low-dose (0.1 μm) NA | 5 |
| IIC | Low-dose NA | 5 |
NO, nitric oxide; l-NAME, NG-nitro-l-arginine methyl ester; SNP, sodium nitroprusside; BT, Bretylium tosylate; NA, Noradrenaline.
Study II
The aim of study II was to investigate the role of noradrenaline on the axon reflex response during gradual local heating of the skin. This study also consisted of three protocols and a total of 18 subjects, as shown in Table 1. In protocol IIA (n = 8), the experimental site was infused with 10 mm bretylium tosylate (Sigma Pharmaceuticals, St Louis, MO, USA) dissolved in lactated Ringer solution for a 45-min period prior to the local heating protocol and was continuously present throughout local heating. This site was used to determine the effect of presynaptic adrenergic blockade on the axon reflex response. Protocol IIB (n = 5) was similar to protocol IA, except that infusion of bretylium tosylate in the experimental site was interrupted prior to the local heating protocol to infuse low-dose (0.1 μm) noradrenaline (Sigma Pharmaceuticals) for 5 min. This site was used to determine the effect of low-dose noradrenaline on the axon reflex response during presynaptic adrenergic blockade. All subjects in protocols IIA and IIB endured a 3-min period of cold stress after bretylium tosylate infusion to ensure a complete adrenergic block at the microdialysis site. In protocol IIC (n = 5), the experimental site was infused with low-dose noradrenaline alone for the same amount of time as in protocol IIB. This site was used to determine the effect of low-dose noradrenaline on the axon reflex response without presynaptic adrenergic blockade. Microdialysis fibres infused with noradrenaline in protocols IIB and IIC were not infused with lactated Ringer solution during the local heating protocol in order to prevent a washout of noradrenaline from the experimental site.
Noradrenaline was preserved in a solution of ascorbic acid (1 mg (ml Ringer solution)−1) to extend the half-life of noradrenaline in solution from 8.5 min to 180 min (Hughes & Smith, 1978). The ascorbic acid was obtained from Sigma Pharmaceuticals. The dosage of noradrenaline used in the protocol was based on a previous study (Thompson et al. 2005) and pilot work in our laboratory. In previous trials, doses of noradrenaline above 0.1 μm caused a profound vasoconstriction that prohibited an increase in RBC flux throughout the progressive local heating protocol. Although the local heating trials with the higher doses of noradrenaline were not used in the formal data analysis, the lack of change in RBC flux at 40°C confirmed that the potency of noradrenaline was maintained throughout the duration of the local heating protocol.
Data analysis
Data were digitized, stored on a computer at 40 Hz and analysed off-line with signal processing software (Windaq, Dataq Instruments, Akron, OH, USA). The axon reflex response was characterized as an immediate increase in RBC flux of at least 10 laser Doppler flux units(mV). Simultaneous ‘responses’ attributable to movement by the subject were not classified as true axon reflex responses. The temperature threshold was defined as the local heater temperature at the onset of the axon reflex response.
Skin blood flow was expressed as cutaneous vascular conductance (CVC), calculated by the quotient of RBC flux and mean arterial pressure (MAP):
All CVC values were expressed as a percentage of maximal vasodilatation (%CVCmax) in response to infusion of 28 mm SNP and local heating to 43°C. Baseline CVC values before and after drug infusions, prior to local heating, before and after cold stress, and at 40°C were averaged over a 10-min period.
Statistical analysis
Data are presented as means ± s.e.m. and analysed with statistical software (SigmaStat 2.03, SPSS Inc, Chicago, IL, USA). The temperature threshold for the axon reflex between control and experimental conditions within subject were compared by paired t tests. Within and between microdialysis sites for each subject, mean CVC values for baseline measurements 10 min before and after infusion prior to local heating, before and after whole-body cooling (protocols IIA and IIB), and at the local heater temperature of 40°C were compared by one-way repeated measures ANOVA, and Tukey's post hoc analysis was used for specific mean comparisons. Comparison of mean CVC values between different protocols at a specific time point was analysed by unpaired t tests. The comparison of temperature threshold data from the control and l-NAME sites (protocols IA and IB) and the mean CVC data during whole-body cooling at control sites and those treated with bretylium tosylate (protocols IIA and IIB) were pooled together for analysis because there was no difference between experimental procedures. The level of significance was set at P < 0.05.
Results
Study I
Temperature threshold data
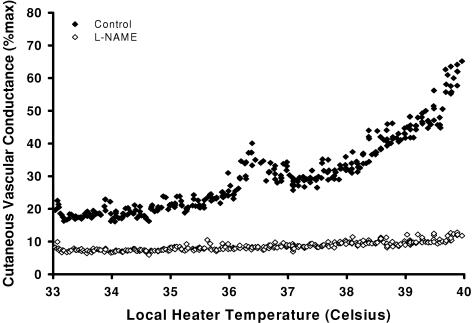
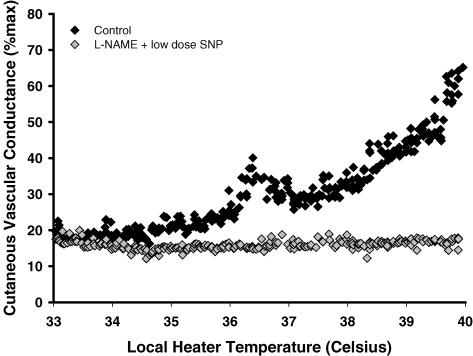
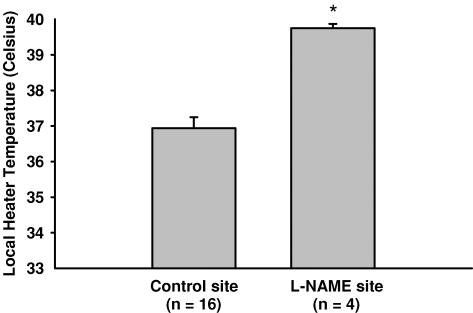
Figures 1 and 2 are representative tracings which show the difference in the skin blood flow response between the l-NAME (control) (protocol IA, Fig. 1) and l-NAME plus SNP (protocol IB, Fig. 2) sites. At the l-NAME site, the first axon reflex occurred at a higher local heater temperature in 4 subjects (control, 37.0 ± 0.3°C; l-NAME, 39.8 ± 0.1°C, P < 0.001, Fig. 3) and was absent in 12 subjects. The axon reflex response was also absent in all subjects at the l-NAME plus SNP site. In protocol IC, low-dose SNP alone did not alter the temperature threshold for the axon reflex compared to control sites (control, 38.1 ± 1.1°C; SNP alone, 38.9 ± 0.1°C, p = 0.586).
Figure 1. Representative tracing of the skin blood flow response as a function of local heater temperature between the control (♦) and l-NAME (⋄) sites.
The axon reflex response was absent in 12 out of 16 subjects at the l-NAME site.
Figure 2. Representative tracing of the skin blood flow response as a function of local heater temperature between the control (♦) and l-NAME plus low-dose SNP  sites.
sites.
Figure 3. Temperature threshold (mean ± s.e.m.) for the onset of the axon reflex between the control and l-NAME sites.
The threshold for the axon reflex was shifted to a higher temperature at the l-NAME site compared to the control site in 4 subjects (P < 0.05).
Mean CVC data
Mean CVC values at the experimental sites prior to local heating were not significantly different after l-NAME, l-NAME plus SNP, or SNP infusion (P > 0.05). The CVC values for all sites before and after infusion are presented in Table 2. However, mean CVC values at the l-NAME plus SNP site (pre infusion, 8 ± 1%CVCmax; post infusion, 11 ± 2%CVCmax, p = 0.08) and SNP alone site (pre infusion, 5 ± 1%CVCmax; post infusion, 17 ± 9%CVCmax, p = 0.38) tended to be higher after the infusion. Regardless of a visible change in CVC after each infusion, the axon reflex response in each subject was still altered in protocols IA and IB and did not change in protocol IC.
Table 2. Cutaneous vascular conductance prior to local heating, before and after infusion.
| Protocol (no. of subjects) | Control site | Experimental site | ||
|---|---|---|---|---|
| Experimental site | Before infusion | After infusion | Before infusion | After infusion |
| IA and IB (n = 16) | ||||
| l-NAME | 10 ± 1 | 12 ± 2 | 10 ± 1 | 9 ± 1 |
| IB (n = 8) | ||||
| l-NAME plus SNP | 8 ± 1 | 10 ± 2 | 8 ± 1 | 11 ± 2 |
| IC (n = 2) | ||||
| SNP | 9 ± 1 | 9 ± 1 | 5 ± 1 | 17 ± 9 |
| IIA (n = 8) | ||||
| BT | 11 ± 2 | 10 ± 2 | 7 ± 1 | 8 ± 1 |
| IIB (n = 5) | ||||
| BT plus NA | 8 ± 2 | 11 ± 2 | 9 ± 2 | 7 ± 1 |
| IIC (n = 5) | ||||
| NA | 6 ± 1 | 8 ± 2 | 7 ± 1 | 6 ± 1 |
Values are means ± s.e.m. expressed as a percentage of maximal cutaneous vascular conductance (%CVCmax) in response to infusion of 28 mm sodium nitroprusside (SNP). l-NAME, NG-nitro-l-arginine methyl ester; BT, bretylium tosylate; NA, noradrenaline.
At the end of the heating protocol when local heater temperature was clamped at 40°C, mean CVC increased in all sites (P < 0.05 versus before local heating). The CVC values before and after local heating are presented in Table 3. In comparison to the control site, combined mean CVC from protocols IA and IB was significantly attenuated at the l-NAME (control, 63 ± 4%CVCmax; l-NAME, 19 ± 2%CVCmax, P < 0.001) and l-NAME plus SNP (control, 55 ± 4%CVCmax; l-NAME plus SNP, 16 ± 2%CVCmax, P < 0.001) sites. In protocol IC, mean CVC at 40°C was not different between the SNP alone and control sites (control, 55 ± 10%CVCmax; SNP, 63 ± 10%CVCmax, p = 0.773).
Table 3. Cutaneous vascular conductance before and after gradual local heating.
| Protocol (no. of subjects) | Control site | Experimental site | ||
|---|---|---|---|---|
| Experimental site | Before LH | After LH | Before LH | After LH |
| IA and IB (n = 16) | ||||
| l-NAME | 12 ± 2 | 63 ± 4* | 9 ± 1 | 19 ± 2*† |
| IB (n = 8) | ||||
| l-NAME plus SNP | 10 ± 2 | 55 ± 4* | 11 ± 2 | 16 ± 2*† |
| IC (n = 2) | ||||
| SNP | 9 ± 1 | 56 ± 10* | 17 ± 9 | 63 ± 10* |
| IIA (n = 8) | ||||
| BT | 10 ± 2 | 54 ± 4* | 8 ± 1 | 21 ± 2*† |
| IIB (n = 5) | ||||
| BT plus NA | 11 ± 2 | 66 ± 9* | 7 ± 1 | 25 ± 13*† |
| IIC (n = 5) | ||||
| NA | 8 ± 2 | 63 ± 11* | 6 ± 1 | 48 ± 12* |
Values are means ± s.e.m. as a percentage of maximal cutaneous vascular conductance (%CVCmax) in response to infusion of 28 mm sodium nitroprusside (SNP).
Significant difference from pre local heating measurements
significant difference from control condition within same stage of heating protocol (P < 0.05). Before local heating (LH), temperature was 33°C; after local heating, temperature was 40°C. l-NAME, NG-nitro-l-arginine methyl ester; BT, bretylium tosylate; NA, noradrenaline.
Study II
Temperature threshold data
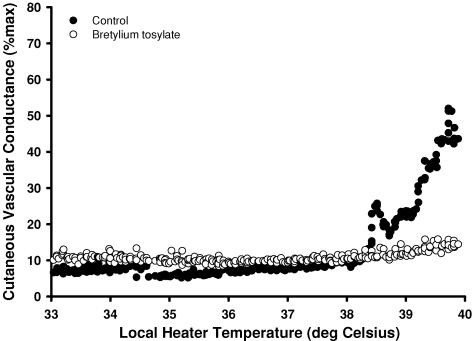
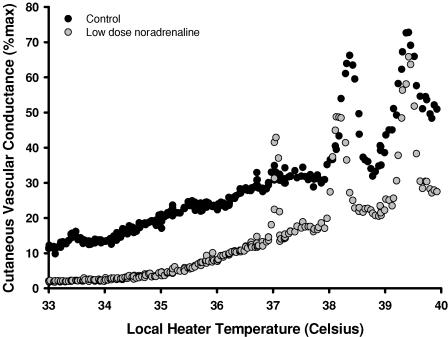
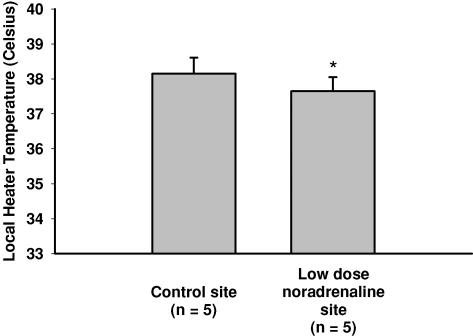
Figures 4 and 5 representative tracings that show the difference in the skin blood flow response at the control, bretylium tosylate (Fig. 4) and low-dose noradrenaline (Fig. 5) sites. In protocols IIA and IIB, the temperature threshold for the axon reflex at the control site was observed at 37.8 ± 0.5°C. In all subjects, the axon reflex response was abolished at the bretylium tosylate site (protocol IIA) and by bretylium tosylate followed by low-dose noradrenaline infusion (protocol IIB). However, low-dose noradrenaline infusion alone (protocol IIC) shifted the onset of the axon reflex to a significantly lower local heater temperature (control, 38.2 ± 0.5°C; low-dose noradrenaline, 37.7 ± 0.4°C, P < 0.05; Fig. 6).
Figure 4. Representative tracing of the skin blood flow response as a function of local heater temperature between the control (•) and bretylium tosylate (○) sites.
The axon reflex response was absent in all subjects at sites infused with bretylium tosylate alone or with low-dose noradrenaline.
Figure 5. Representative tracing of the skin blood flow response as a function of local heater temperature between the control (•) and low-dose noradrenaline (○) sites.
Exogenous noradrenaline without adrenergic blockade shifted the axon reflex response to a lower temperature in comparison to control conditions (P < 0.05).
Figure 6. Temperature threshold (mean ± s.e.m.) for the onset of the axon reflex between the control and low-dose noradrenaline sites.
Noradrenaline infusion alone significantly shifted the threshold for the axon reflex to a lower temperature compared to control conditions (P < 0.05).
Mean CVC values
Prior to the local heating protocol, baseline mean CVC values were not significantly different after the infusion of bretylium tosylate, bretylium tosylate followed by low-dose noradrenaline, and low-dose noradrenaline alone (P > 0.05 for all comparisons). The mean CVC values prior to local heating at all sites before and after infusion are displayed in Table 2. In protocols IIA and IIB (n = 13), the skin blood flow response to whole-body cooling at the control site significantly decreased from baseline measurements (before cold stress, 10 ± 1%CVCmax; cold stress, 6 ± 1%CVCmax, P < 0.05) whereas the sites treated with bretylium tosylate remained unchanged (before cold stress, 9 ± 1%CVCmax; cold stress, 8 ± 1%CVCmax, p = 0.09), which verified complete adrenergic inhibition. Baseline mean CVC significantly recovered within 10 min after whole-body cooling in the control site (cold stress, 6 ± 1%CVCmax; after cold stress, 10 ± 2%CVCmax, P < 0.05) and was not significantly different from baseline measurements prior to whole-body cooling (after cold stress, 10 ± 2%CVCmax; before cold stress, 10 ± 1%CVCmax, p = 0.38).
Compared to baseline values, mean CVC increased in all sites at the end of the heating protocol (P < 0.05). The mean CVC values before and after local heating are shown in Table 3. At 40°C, mean CVC was significantly higher at the control site compared to the bretylium tosylate (control, 54 ± 4%CVCmax; bretylium tosylate, 21 ± 2%CVCmax, P < 0.001) and bretylium tosylate plus low-dose noradrenaline sites (control, 66 ± 9%; bretylium tosylate, 25 ± 13%, P < 0.05), but not different in comparison to the low-dose noradrenaline site (control, 63 ± 11%CVCmax; 48 ± 12%CVCmax, p = 0.21). Mean CVC values at 40°C were not significantly different between sites treated with bretylium tosylate alone and bretylium tosylate followed by low-dose noradrenaline infusion (P < 0.05).
Discussion
Our investigation was the first to examine the role of NO (study I) and noradrenaline (study II) during the onset of the axon reflex with the use of a progressive local heating protocol within the range of 33°C to 40°C. The main findings of the present investigation were as follows. In study I, the axon reflex was either absent or shifted to a higher temperature threshold with NO synthase inhibition. A low dose of exogenous NO did not restore the axon reflex response during NO synthase inhibition or alter the response in the presence of endogenous NO. In study II, the axon reflex was absent during adrenergic blockade, and this response was not restored with infusion of a low dose of noradrenaline. However, low-dose noradrenaline alone shifted the threshold for the axon reflex to a lower temperature compared to control conditions. Together, these results are suggestive of roles for NO and noradrenaline during the onset of the axon reflex response to progressive local heating from 33°C to 40°C in human skin.
The contribution of NO to the skin blood flow response to direct changes in temperature may involve various mechanisms. Previous work from our laboratory has shown that NO synthase inhibition during rapid local heating slightly attenuated the axon reflex response, but reduced the secondary plateau response by approximately 70% (Minson et al. 2001, 2002). This suggested a greater role for NO during the prolonged secondary plateau phase than during the axon reflex. However, the findings from our current study introduced a new role for NO during this thermal hyperaemia. The absence or shift in the onset of the axon reflex to a higher temperature during l-NAME infusion may indicate a sensitizing role for endogenous NO earlier in the response.
The axon reflex in human skin is a response that may be mediated by the onset of antidromic vasodilatation via the release of CGRP and substance P from C-fibre nociceptors. Previous investigations showed that NO aided the release of CGRP in the skin (Hughes & Brain, 1994; Klede et al. 2003) and contributed to dilatation in response to substance P (Klede et al. 2003; Wong et al. 2005). If CGRP and substance P are involved in this response, then the observed rightward shift in temperature threshold of the axon reflex with NO synthase inhibition may be explained by a desensitization of C-fibre nociceptors and antidromic vasodilatation.
The data from protocols IB and IC showed that low-dose SNP did not restore the axon reflex response when NO synthase was inhibited, or alter the axon reflex response in comparison to control conditions. Although these results did not support our hypothesis, the data combined with the results from protocol IA suggest a role for NO during the onset of the axon reflex response. Our results showed that during NO synthase inhibition, the axon reflex was either absent or shifted to a higher temperature, which suggests that basal levels of endogenous NO must be present for the axon reflex response to occur within the temperature range studied.
The pharmacological properties of SNP as an exogenous NO donor may also contribute to the results from protocols IB and IC. Recently, Durand et al. (2005) reported that exogenous NO, specifically via SNP, inhibited sympathetically mediated vasoconstriction in human skin. If exogenous NO inhibits adrenergic nerves, it is possible that SNP may have an inhibitory effect on other nerves or vasoactive substances that normally contribute to the axon reflex. Furthermore, if noradrenaline is involved in the axon reflex response as suggested by the results in study II, the inhibitory influence of SNP on adrenergic nerves may also explain why the axon reflex was not restored in protocol IB.
In study II, the axon reflex response was absent in all subjects treated with bretylium tosylate (protocol IIA) and bretylium tosylate followed by low-dose noradrenaline infusion (protocol IIB). Although the results from protocol IIB may not support a role for noradrenaline during the axon reflex, the absence of the axon reflex with bretylium tosylate treatment should be recognized as an important pharmacological finding. Given that bretylium tosylate causes presynaptic adrenergic blockade, a possible role for endogenous noradrenaline during the axon reflex should not be discarded. However, the absence of the axon reflex in the presence of noradrenaline may also be indicative of a non-specific effect of bretylium tosylate in the vasculature. One possibility is that bretylium tosylate may also act as a postsynaptic α-adrenoreceptor antagonist, which may explain why the axon reflex was not restored with low-dose noradrenaline in the presence of bretylium tosylate.
Alternatively, the results from protocol IIB may also be explained by a possible role for neuropeptide Y during the axon reflex response. It has been shown that neuropeptide Y is coreleased with noradrenaline from sympathetic nerves (Lundberg et al. 1990) and contributes to sympathetically mediated cutaneous vasoconstrictor responses in human skin (Stephens et al. 2004). It has also been shown that neuropeptide Y and Y2 receptor agonists augment the flare response to intradermal administration of capsaicin (Lin et al. 2004). In protocol IIB, bretylium tosylate may have also blocked the release or action of neuropeptide Y, which also may have lead to the absence of the axon reflex.
The data from protocol IIC showed that low-dose noradrenaline infusion alone shifted the axon reflex to a lower temperature in comparison to control conditions, which supports a role for noradrenaline in the axon reflex response. As with NO, noradrenaline may also sensitize the onset of antidromic vasodilatation by C-fibre nociceptors. It was previously shown that the axon reflex response was enhanced by administration of noradrenaline (Drummond & Lipnicki, 1999) and other α-adrenoreceptor agonists (Drummond, 1995) via iontophoresis. Drummond & Lipnicki (1999) postulated that the enhanced response may be attributed to the stimulation of α1-adrenoreceptors on nociceptor afferents or the release of other nociceptor mediators such as prostaglandins (Holzer et al. 1995) or NO (Holzer & Jocic, 1994).
In contrast to these findings, a study by Zahn et al. (2004) showed that α-adrenoreceptor stimulation has no effect on C-fibre activation. However, the important difference between these two investigations is the first group delivered these pharmacological agents iontophoretically whereas the microdialysis technique was used in the second study. The electrical charge from iontophoresis may have provided the proper stimulus for C-fibres to elicit a response that was further enhanced by α-adrenergic agonists. The experimental protocol used for the present study in protocol IIC was similar to that of Drummond & Lipnicki (1999), except that heat was utilized to stimulate the axon reflex response rather than the electrical stimulus provided by iontophoresis. In agreement with their results, the data from protocol IIC highlighted a sensitization in the axon reflex response, because the threshold was shifted to a lower temperature in the presence of low-dose noradrenaline.
During this investigation we experienced two main pharmacological limitations. The first limitation concerns the potential pharmacological effects of low-dose SNP during the local heating protocol. The use of an alternative NO donor may provide additional information regarding the role of NO during the axon reflex response. The second limitation is the potential non-specific effects of bretylium tosylate on the vasculature. Investigating the role of specific adrenergic receptors, as well as the potential role for neuropeptide Y during the axon reflex, may further our understanding of noradrenaline during this response.
We also experienced two technical limitations during this study. The first limitation was that local heat only reached 40°C instead of 42°C. However, we chose to stop heating at 40°C because an axon reflex was observed in every subject below 40°C under control conditions. The second limitation was that we were unable to establish a direct link between NO, noradrenaline and C-fibre nociceptors, although the previous studies suggest that NO and noradrenaline may augment the release or action of CGRP and substance P, which are vasoactive substances that may contribute to antidromic vasodilatation. Investigating the specific action of NO and noradrenaline on C-fibre nociceptors, may provide insight into the specific mechanisms of the axon reflex response.
In summary, our results show that below the local heater temperature of 40°C, NO and noradrenaline have a role in regulating the temperature threshold for the axon reflex response during gradual local heating. Based on these findings, we propose that NO and noradrenaline may regulate the onset for this response at the level of C-fibre nociceptors and may affect antidromic vasodilatation. The specific roles for NO and noradrenaline still remain unclear, but our observations add to the understanding of how rate of heating, NO and noradrenaline may influence the axon reflex response to direct heat in human skin.
Acknowledgments
The authors would like to express their gratitude to the subjects who volunteered for this series of studies. This study was conducted by Belinda L. Houghton in partial fulfilment of the requirements for the Masters of Science degree in the Department of Human Physiology at the University of Oregon. Funding for this study was provided by National Institutes of Health Grant HL-70928.
References
- Banik RK, Sato J, Giron R, Yajima H, Mizumura K. Interactions of bradykinin and norepinephrine on rat cutaneous nociceptors in both normal and inflamed conditions in vitro. Neurosci Res. 2004;49:421–425. doi: 10.1016/j.neures.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Banik RK, Sato J, Yajima H, Mizumura K. Differences between the Lewis and Sprague-Dawley rats in chronic inflammation induced norepinephrine sensitivity of cutaneous C-fiber nociceptors. Neurosci Lett. 2001;299:21–24. doi: 10.1016/s0304-3940(00)01770-5. [DOI] [PubMed] [Google Scholar]
- Boignard A, Salvat-Melis M, Carpentier PH, Minson CT, Grange L, Duc C, Sarrott-Reynauld F, Cracowski J. Local hyperemia to heating is impaired in secondary Raynaud's phenomenon. Arthritis Res Ther. 2005;7:R1103–R1112. doi: 10.1186/ar1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Williams TJ. Substance P regulates the vasodilator activity of calcitonin gene-related peptide. Nature. 1988;335:73–75. doi: 10.1038/335073a0. [DOI] [PubMed] [Google Scholar]
- Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- Drummond PD. Noradrenaline increases hyperalgesia to heat in skin sensitised by capsaicin. Pain. 1995;60:311–315. doi: 10.1016/0304-3959(94)00130-7. [DOI] [PubMed] [Google Scholar]
- Drummond PD. Enhancement of thermal hyperalgesia by α-adrenoreceptor in capsaicin-treated skin. J Auton Nerv Syst. 1998;69:96–102. doi: 10.1016/s0165-1838(98)00017-4. [DOI] [PubMed] [Google Scholar]
- Drummond PD, Lipnicki DM. Noradrenaline provokes axon reflex hyperaemia in the skin of the human forearm. J Auton Nerv Syst. 1999;77:39–44. doi: 10.1016/s0165-1838(99)00034-x. [DOI] [PubMed] [Google Scholar]
- Durand S, Davis S, Cui J, Crandall CG. Exogenous nitric oxide inhibits sympathetically mediated vasoconstriction in human skin. J Physiol. 2005;562:629–634. doi: 10.1113/jphysiol.2004.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Jocic M. Cutaneous vasodilatation induced by nitric oxide stimulation of afferent nerves in the rat. Br J Pharmacol. 1994;112:1181–1187. doi: 10.1111/j.1476-5381.1994.tb13208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P, Jocic M, Peskar B. Mediation by prostaglandins of the nitric oxide-induced neurogenic vasodilatation in rat skin. Br J Pharmacol. 1995;116:2365–2370. doi: 10.1111/j.1476-5381.1995.tb15081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes IE, Smith JA. The stability of Noradrenaline in physiological saline solutions. J Pharm Pharmacol. 1978;30:124–126. doi: 10.1111/j.2042-7158.1978.tb13179.x. [DOI] [PubMed] [Google Scholar]
- Hughes S, Brain S. Nitric oxide-dependent release of vasodilator quantities of calcitonin gene-related peptide from capsaicin-sensitive nerves in rabbit skin. Br J Pharmacol. 1994;111:425–430. doi: 10.1111/j.1476-5381.1994.tb14752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DL, Liu Y, IFK, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Pérgola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Klede M, Clough G, Lischetzki G, Schmelz M. The effect of the nitric oxide synthase inhibitor N-nitro-L-arginine-methyl ester on neuropeptide-induced vasodilation and protein extravasation in human skin. J Vasc Res. 2003;40:105–114. doi: 10.1159/000070707. [DOI] [PubMed] [Google Scholar]
- Lin Q, Zou X, Ren Y, Wang J, Fang L, Willis W. Involvement of peripheral neuropeptide Y receptors in sympathetic modulation of acute cutaneous flare induced by intradermal capsaicin. Neuroscience. 2004;123:337–347. doi: 10.1016/j.neuroscience.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lundberg J, Franco-Cereceda A, Lacroix J, Pernow J. Neuropeptide Y and sympathetic neurotransmission. Ann N Y Acad Sci. 1990;611:166–174. doi: 10.1111/j.1749-6632.1990.tb48930.x. [DOI] [PubMed] [Google Scholar]
- Magerl W, Treede RD. Heat-evoked vasodilation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol. 1996;497:837–848. doi: 10.1113/jphysiol.1996.sp021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medow M, Minson CT, Stewart JM. Decreased microvascular nitric oxide dependent vasodilation in postural tachycardia syndrome. Circulation. 2005;112:2611–2618. doi: 10.1161/CIRCULATIONAHA.104.526764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Nam TS, Yeon DS, Leem JW, Paik KS. Adrenergic sensitivity of uninjured C-fiber nociceptors in neurogenic rats. Yonsei Med J. 2000;41:252–257. doi: 10.3349/ymj.2000.41.2.252. [DOI] [PubMed] [Google Scholar]
- Sann H, Pierau FK. Efferent functions of C-fiber nociceptors. Z Rheumatol. 1998;57:8–13. doi: 10.1007/s003930050226. [DOI] [PubMed] [Google Scholar]
- Sato J, Yajima H, Banik RK, Kumazawa T, Mizumura K. Norepinephrine reduces heat responses of cutaneous C-fiber nociceptors in Sprague-Dawley rats in vitro. Neurosci Lett. 2005;378:111–116. doi: 10.1016/j.neulet.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Michael K, Weidner C, Schimidt R, Torebjörk HE, Handewerker HO. Which nerve fibers mediate the axon reflex flare in human skin? Neuroreport. 2000;11:645–648. doi: 10.1097/00001756-200002280-00041. [DOI] [PubMed] [Google Scholar]
- Stephens DP, Saad AR, Bennett LA, Koshiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol. 2004;287:H1404–H1409. doi: 10.1152/ajpheart.00061.2004. [DOI] [PubMed] [Google Scholar]
- Thompson C, Holowatz LA, Kenney WL. Cutaneous vasoconstrictor responses to norepinephrine are attenuated in older humans. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1108–R1113. doi: 10.1152/ajpregu.00839.2004. [DOI] [PubMed] [Google Scholar]
- Wallengren J, Hakanson R. Effects of substance P, neurokinin A and calcitonin gene-related peptide in human skin and their involvement in sensory nerve-mediated responses. Eur J Pharmacol. 1987;143:267–273. doi: 10.1016/0014-2999(87)90542-5. [DOI] [PubMed] [Google Scholar]
- Wallengren J, Wang Z. Interaction between tachykinins and CGRP in human skin. Acta Derm Venereol. 1993;73:259–261. doi: 10.2340/0001555573259261. [DOI] [PubMed] [Google Scholar]
- Weidner C, Klede M, Rukwied R, Grischa L, Neisius U, Skov PS, Petersen LJ, Schmelz M. Acute effects of substance P and calcitonin gene-related peptide in human skin – a microdialysis study. J Invest Dermatol. 2000;115:1015–1020. doi: 10.1046/j.1523-1747.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- Wigington G, Ngo B, Rendell M. Skin blood flow in diabetic dermopathy. Arch Dermatol. 2004;140:1248–1250. doi: 10.1001/archderm.140.10.1248. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Tublitz NJ, Minson CT. Neurokinin-1 receptor desensitisation to consecutive microdialysis infusions of substance P in human skin. J Physiol. 2005;568:1047–1056. doi: 10.1113/jphysiol.2005.095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong BJ, Williams SJ, Minson CT. Minimal role for H1 and H2 histamine receptors in cutaneous thermal hyperemia to local heating in humans. J Appl Physiol. 2006;100:535–540. doi: 10.1152/japplphysiol.00902.2005. [DOI] [PubMed] [Google Scholar]
- Zahn S, Leis S, Schick C, Schmelz M, Birklein F. No α-adrenoreceptor-induced C-fiber activation in healthy human skin. J Appl Physiol. 2004;96:1380–1384. doi: 10.1152/japplphysiol.00990.2003. [DOI] [PubMed] [Google Scholar]