Fifteen years ago Bading and Greenberg reported that Ca2+ influx through the NMDA receptor (NMDAR) was coupled to activation of the extracellular regulated kinases (ERK1 and 2; Bading & Greenberg, 1991). This is a Ras-dependent process: the Ca2+-dependent activation of the Ras occurs via several mechanisms, principally via the GTP/GDP exchange factor RasGRF. Ras then signals to ERK via MEK1.
Since its discovery, the NMDAR–Ras–ERK signalling cassette has become established as a key mediator of neuronal responses to synaptic activity. Many synaptic plasticity paradigms rely on NMDAR-dependent ERK activation, and ERK signalling also has an anti-apoptotic effect in neurons. Moreover, NMDAR-dependent ERK signalling targets the nucleus to trigger new gene expression, which in turn mediates long-lasting responses, such as the late phase of LTP and activity-dependent neuroprotection.
However, the NMDAR is not exclusively an activator of ERK signalling. Synaptic activity (activating synaptic NMDARs) promotes sustained ERK activity (Chandler et al. 2001; Papadia et al. 2005) whereas activation of all NMDA receptors by bath application of NMDA promotes activation followed by inactivation (Bading & Greenberg, 1991; Kim et al. 2005). Furthermore, synaptic NMDAR-dependent ERK activity can be turned off by the bath application of NMDA (Chandler et al. 2001). This begged the question: what is the basis for these antagonizing signals, given that they are both mediated by NMDARs?
In this issue of The Journal of Physiology, Ivanov et al. (2006) demonstrate that NMDAR signalling to ERK depends on location: synaptic NMDARs couple to activation, while extrasynaptic NMDARs promote ERK dephosphorylation and inactivation. They use network disinhibition to promote selective synaptic NMDAR signalling, while a pharmacological protocol (Hardingham et al. 2002) involving the use-dependent blockade of synaptic NMDARs enables extrasynaptic NMDARs to be selectively activated. They show that extrasynaptic NMDAR currents are unable to activate ERK, while equivalent currents carried partly by synaptic NMDARs do activate ERK. However, far from being neutral with regard to ERK activity, they show that extrasynaptic NMDARs couple to ERK inactivation. Consistent with this, Kim et al. (2005) recently showed that extrasynaptic NMDARs strongly inactivate Ras. Thus synaptic and extrasynaptic NMDARs are mutually antagonistic with regard to Ras–ERK signalling.
The laboratories of Medina/Clapham have shown that synaptic NMDARs couple to ERK activation via the interaction of the NMDAR subunit NR2B with RasGRF1 (Krapivinsky et al. 2003). NR2A, the other major NR2 subunit in cortical/hippocampal synapses, did not associate with RasGRF1 and was not a major activator of ERK1/2 (however, see Kim et al. 2005). Since extrasynaptic NMDARs are predominantly NR2B-containing, they conclude that synaptic versus extrasynaptic NMDAR signalling to ERK is not a function of different subunit composition of the receptors: NR2B-containing NMDARs have opposing effects on the ERK pathway, depending on their location.
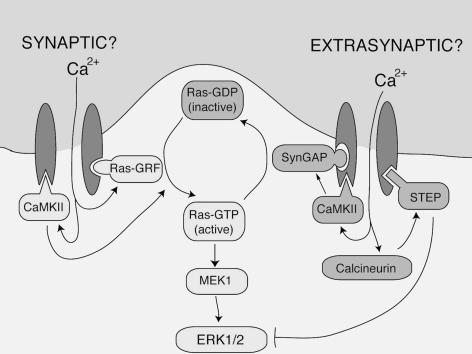
Synaptic NMDAR-dependent activation of ERK is triggered by Ca2+ increases in the immediate vicinity of the NMDAR (Hardingham et al. 2001), indicating that highly localized signalling events control the Ras–ERK pathway. One can currently only speculate as to differences that may exist between synaptic and extrasynaptic NMDAR signalling complexes to explain their opposing effects (Fig. 1). For example, the Ras activator RasGRF1 may be absent/depleted from the extrasynaptic NMDAR complex, while ERK-inactivating phosphatases may preferentially associate with extrasynaptic NMDARs. The NMDAR-associated striatal-enriched tyrosine phosphatase (STEP) is an attractive candidate, since it is activated by the Ca2+-dependent phosphatase calcineurin, whereupon it directly inactivates ERK (Paul et al. 2003). However, ERK suppression may also occur further upstream. Kim et al. (2005) showed that NR2B associates with the Ras-inactivating SynGAP, which they implicate in the inactivation of ERK by NMDARs.
Figure 1. Positive and negative NMDA receptor signalling to the Ras–ERK pathway: could differential recruitment of these pathways underlie synaptic versus extrasynaptic signalling to Ras and ERK?
Synaptic NMDARs couple strongly to Ras activation, with Ca2+-dependent activation of RasGRF being a prime mediator of this (although other mechanisms are also likely to contribute, such as activation of proline-rich tyrosine kinase 2 (PYK2) by Ca2+–calmodulin-dependent protein kinase II (CaMKII)). In contrast, extrasynaptic NMDARs cause inactivation of Ras and ERK. While the molecular basis of this is unclear, several Ca2+-dependent, NMDAR-associated signalling molecules are known to inhibit Ras and ERK. The ERK-inactivating phosphatase STEP is activated by the Ca2+-dependent phosphatase calcineurin, while the Ras-inactivating synGAP is activated by CaMKII (not inhibited, as was previously thought). Differences between the synaptic and extrasynaptic NMDAR signalling complex may explain their opposing influence on the Ras–ERK signalling pathway.
The antagonistic signalling of extrasynaptic versus synaptic NMDARs to ERK bears striking similarity with the opposing actions of these receptors with regard to CREB-dependent gene expression and, crucially, neuronal survival/death (Hardingham et al. 2002). Synaptic NMDARs strongly activate CREB-dependent gene expression, and resultant neuroprotection (Papadia et al. 2005). In contrast, extrasynaptic NMDARs are not neuroprotective, and are efficiently coupled to mitochondrial dysfunction and also to a dominant CREB shut-off pathway (Hardingham et al. 2002). While Ras–ERK signalling does promote CREB phosphorylation, shutting down this pathway cannot alone explain CREB inactivation, since other CREB-activating pathways (principally nuclear CaM kinases) are still active and sufficient to phosphorylate CREB in the absence of ERK activity.
Efficient glutamate uptake mechanisms mean that chronic extrasynaptic NMDAR activation is unlikely to occur in vivo under normal physiological conditions. However, it may be highly relevant to the pathophysiology of hypoxia/ischaemia (H/I) and seizure-induced damage. The reversal of the glutamate transporter during H/I episodes causes extracellular glutamate to build up which would activate extrasynaptic NMDARs. Consistent with this, H/I episodes also trigger CREB inactivation in vulnerable neurons. During seizure activity, glutamate uptake capacity may be exceeded, causing glutamate to build up and spill over onto extrasynaptic NMDARs. Importantly, Ivanov et al. (2006) show that by inhibiting glutamate uptake, synaptically released glutamate can spill onto extrasynaptic NMDARs and induce ERK inactivation.
The net effect of extrasynaptic NMDAR activation is clearly pro-death (Hardingham et al. 2002), and ERK inactivation may contribute to this, given the known anti-apoptotic role of Ras–ERK signalling. Alternatively, inhibition of ERK-dependent AMPA receptor insertion (Kim et al. 2005) may protect vulnerable neurons by reducing the overall ionic load. What is less equivocal is that the extrasynaptic NMDAR will reveal many more secrets in the coming years.
References
- Bading H, Greenberg ME. Science. 1991;253:912–914. doi: 10.1126/science.1715095. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G, Dorairaj NR, Norwood D. J Biol Chem. 2001;276:2627–2636. doi: 10.1074/jbc.M003390200. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. Nat Neurosci. 2001;4:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Papadia S, Stevenson P, Hardingham NR, Bading H, Hardingham GE. J Neurosci. 2005;25:4279–4287. doi: 10.1523/JNEUROSCI.5019-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]