Abstract
Insulin and glucagon are the major hormones of the islets of Langerhans that are stored and released from the B- and A-cells, respectively. Both hormones are secreted when the intracellular cytosolic Ca2+ concentration ([Ca2+]i) increases. The [Ca2+]i is modulated by mutual inhibition and activation of different voltage-gated ion channels. The precise interplay of these ion channels in either glucagon or insulin release is unknown, owing in part to the difficulties in distinguishing A- from B-cells in electrophysiological experiments. We have established a single-cell RT-PCR method to identify A- and B-cells from the mouse. A combination of PCR, RT-PCR, electrophysiology and pharmacology enabled us to characterize the different sodium and calcium channels in mouse islet cells. In both A- and B-cells, 60% of the inward calcium current (ICa) is carried by L-type calcium channels. In B-cells, the predominant calcium channel is CaV1.2, whereas CaV1.2 and CaV1.3 were identified in A-cells. These results were confirmed by using mice carrying A- or B-cell-specific inactivation of the CaV1.2 gene. In B-cells, the remaining ICa flows in equal amounts through CaV2.1, CaV2.2 and CaV2.3. In A-cells, 30 and 15% of ICa is due to CaV2.3 and CaV2.1 activity, respectively, whereas CaV2.2 current was not found in these cells. Low-voltage-activated T-type calcium channels could not be identified in A- and B-cells. Instead, two TTX-sensitive sodium currents were found: an early inactivating and a residual current. The residual current was only recovered in a subpopulation of B-cells. A putative genetic background for these currents is NaV1.7.
The peptide hormones insulin and glucagon efficiently regulate glucose homeostasis in humans and other mammalia. Impaired insulin secretion, the inability of the target organs to respond to insulin, or a combination of both leads to type 2 diabetes, a state of chronic hyperglycaemia. In the diabetic state, insulin levels are inadequately low, whereas glucagon levels are often elevated, which aggravates the disease (Unger, 1971). Insulin and glucagon are produced in the B- and A-cells of the pancreatic islets of Langerhans, respectively. These cells are electrically excitable and use electrical signals to couple changes in blood glucose concentration to hormone release. In both cell types, influx of extracellular Ca2+ through voltage-gated Ca2+ channels with resultant elevation of cytoplasmic free Ca2+ concentration triggers exocytosis of the hormone-containing secretory granules. The mechanisms involved in insulin secretion are well established (Ashcroft & Rorsman, 1989). Upon elevation of the plasma glucose level, the glucose metabolism leads to an elevated ATP-to-ADP ratio that causes the closure of ATP-sensitive K+ channels (KATP channels) and B-cell depolarization. The membrane depolarization in turn opens voltage-gated Ca2+ channels (Ashcroft et al. 1994). The resulting oscillatory increase in [Ca2+]i directly triggers insulin exocytosis. Glucose-stimulated insulin secretion is biphasic, with a rapid first phase that lasts approximately 10 min and a sustained second phase that can last for several hours (Curry et al. 1968). It has been generally accepted that [Ca2+]i regulates both first and second phase insulin secretion (Henquin et al. 2003) and that L-, P/Q-, N- and R-type calcium channels are involved (Komatsu et al. 1989; Ramanadham & Turk, 1994). The generation of various knockout mice helped to differentiate the roles of the different calcium channels in insulin secretion. Of the four genes that encode L-type calcium channels, CaV1.2 and CaV1.3 have been identified in B-cells of different species (Iwashima et al. 1993; Seino, 1995; Safayhi et al. 1997; Yang et al. 1999). In mice lacking the CaV1.3 subunit, neither the blood sugar level nor insulin secretion was affected (Platzer et al. 2000). Instead, deletion of CaV1.2 decreased the whole-cell calcium inward current (ICa) to the same extent as the calcium channel blocker isradipine, and almost abolished first phase of insulin secretion. The calcium channel agonist Bay K 8644 as well as isradipine were without effect in CaV1.2-deficient islet B-cells (Schulla et al. 2003). A cocktail of isradipine, the R-type calcium channel antagonist SNX 482 and the P/Q-type calcium channel inhibitors ω-conotoxin MVIIC or ω-agatoxin IVA reduced ICa of wild-type islets by 97%, demonstrating the presence of both L- and non-L-type channels (Schulla et al. 2003). In agreement with this result, studies on CaV2.3−/− islets and mice suggested that CaV2.3 is responsible for the second phase of insulin secretion (Jing et al. 2005). A study on CaV2.2-deficient mice revealed an increased glucose tolerance and a reduced weight gain on a high-fat diet (Takahashi et al. 2005).
In A-cells, low blood glucose levels enhance [Ca2+]i followed by glucagon secretion. The mechanisms coupling low blood sugar levels to glucagon secretion are unknown (Gromada et al. 1997). It was speculated that low-voltage-activated (LVA) T-type calcium channels could be key regulators, depolarizing the membrane to potentials necessary to activate high-voltage-activated (HVA) calcium channels (Gopel et al. 2000). T-type channel expression in pancreatic islet cells is species dependent, with little or no expression in rodents, but it is readily detectable in humans (Perez-Reyes, 2003; Yang & Berggren, 2005). Others reported that KATP channels and the inactivation of TTX-sensitive Na+ channels regulated glucagon release (Bokvist et al. 1999; Gopel et al. 2000). Two types of TTX-sensitive sodium channels have been distinguished in islet cells according to their voltage dependence. B-cells have a voltage-dependent sodium current (Pressel & Misler, 1990) which, owing to the voltage dependence of inactivation, is unlikely to play a major role in glucose-induced electrical activity (Plant, 1988). A-cells possess a prominent TTX-sensitive sodium current, which is activated at physiological membrane potentials (Gopel et al. 1999; Barg et al. 2000). Six different genes (NaV1.1, NaV1.2, NaV1.3, NaV1.4, NaV1.6 and NaV1.7) coding for TTX-sensitive sodium channel α-subunits have been described (Goldin et al. 2000). Yet the molecular background for the sodium channel currents in islet cells is unknown.
In the present study, the expression of different calcium channels and TTX-sensitive sodium channels was analysed in A- and B-cells of the mouse by patch-clamp, specific blocker, PCR and single-cell RT-PCR. A- and B-cells were clearly identified by RT-PCR using insulin- and glucagon-specific primers.
Methods
All experiments were conducted in accordance with the Guide for the use and care of laboratory animals (National Academy of Sciences, USA) and approved by the Regierung von Oberbayern.
Materials
Unless otherwise noted the materials were obtained from Sigma and were of the highest purity available. The snail venom ω-conotoxin GVIA and the spider venoms ω-agatoxin IVA and SNX 482 were obtained from Alomone Laboratories (Jerusalem, Israel). A 20 μm stock solution of the P/Q-type calcium channel blocker ω-agatoxin IVA was prepared in destilled water and diluted in extracellular solution to a final concentration of 0.2 μm. An 82 μm stock solution of the N-type calcium channel blocker ω-conotoxin GVIA was prepared in destilled water and diluted in extracellular solution to a final concentration of 1.6 μm. A 10 μm stock solution of the R-type calcium channel blocker SNX 482 was prepared in destilled water and diluted in extracellular solution to a final concentration of 0.1 μm. The dihydropyridine (DHP) isradipine (Pfizer) was prepared as a 10 mm stock solution in ethanol and diluted in extracellular solution to the indicated concentrations. The Na+ channel blocker TTX was prepared as a 1 mm stock in distilled H2O and used in a final concentration of 0.1 μm.
Generation of βCaV1.2−/− mice
The generation and maintaining of the mice was described earlier (Schulla et al. 2003). The mice lack the L-type calcium channel α1-subunit CaV1.2 specifically in B-cells.
Generation of αCaV1.2−/− mice
To generate A-cell-specific CaV1.2-deficient mice, the CaV1.2+/L2 mouse carrying one L2 allele and one wild-type allele (Seisenberger et al. 2000; Schulla et al. 2003) was crossed with a mouse expressing the Cre (cyclization recombination)-recombinase under the control of the rat glucagon promotor (GlucCre+/tg; Herrera, 2000). The resulting CaV1.2+/L2GlucCre+/tg mice were mated with CaV1.2L2/L2 mice homozygous for the L2 allele. The A-cell-specific knockout CaV1.2L2/L2GlucCre+/tg (αCaV1.2−/−) mice and control animals CaV1.2+/L2GlucCre+/tg (αCaV1.2+/−) were obtained. Genotyping was performed using primers ag2 (5′-CTGCTAACCATGTTCATGCCT-3′), Cre1 (5′-CCTGTTTTGCACCG-3′) and Cre3 (5′-ATGCTTCTGTCCGTTTGCCG-3′). The background mouse strain was C57BL/6(6J).
Isolation of pancreatic islets and single islet cells
Wild-type, control or different knockout mice were killed by cervical dislocation and the pancreas was quickly removed. Pancreatic islets were isolated by a standard collagenase digestion (Barg et al. 2001; Schulla et al. 2003). Islets were transferred to plastic coverslips in a 24-well plate, and single islet cells were obtained by shaking the islets in Ca2+-free solution. Cells were allowed to attach to the coverslips overnight.
DNA isolation and PCR analysis
Genomic DNA from islet or heart tissue was isolated using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Briefly, the tissue was incubated overnight at 56°C in 180 μl ATL tissue lysis buffer plus 20 μl Proteinase K. The digest was purified with a QIAamp Spin Column and eluted with 100 μl buffer. Two microlitres were used for PCR analysis using the CaV1.2-specific primers VI4, VI8 and VI10 (Schulla et al. 2003).
RT-PCR on mRNA of islets
Freshly prepared islets were cultured overnight in RPMI 1640 medium (Gibco™) at 37°C. PolyA mRNA was isolated using Dynabeads Oligo (dT)25 (Dynal Biotech, Oslo, Norway). The following buffers were used: GTC buffer [4 m guanidine thiocyanate, 20 mm sodium acetate (pH 5.4), 0.1 mm dithiothreitol, 0.5% lauroyl sarcosinat (w/v), 6.5 ml ml−1 mercaptoethanol], binding buffer (100 mm Tris-HCl pH 8.0, 20 mm EDTA, 400 mm LiCl) and washing buffer (10 mm Tris-HCl pH 8.0, 0.15 m LiCl, 1 mm EDTA). The mRNA was eluted with diethylpyrocarbonate (DEPC)-treated water. Random hexamer primers and Superscript Reverse Transkriptase II (LifeTechnologies) were used for cDNA synthesis (Schulla et al. 2003). To distinguish glucagon-producing A- from insulin-producing B-cells, intron-spanning specific primer pairs were designed from sequences of the mouse insulin and gucagon gene, respectively (Table 1). For the amplification of the LVA calcium channels, degenerated primers, which bind to every known LVA calcium channel (CaV3.1, CaV3.2 and CaV3.3) were used, followed by a nested PCR with specific primers for each LVA calcium channel. For the amplification of the TTX-sensitive sodium channels, primers were designed from the appropriate mouse genes. A standard PCR protocol was used with optimal temperatures for every primer pair. Table 2 summarizes the primer pairs used for the amplification of HVA calcium channels (1), LVA calcium channels with degenerated primers (2) and with specific primers (3), and TTX-sensitive sodium channels (4).
Table 1. Sequences of insulin- and glucagon-specific primer pairs.
| Primer | Sequence |
|---|---|
| Ins 5′ | 5′-CAGCAAGCAGGTCATTGTTT-3′(Genbank reference sequence: NM_008386; start at nucleotide position 37) |
| Ins 3′ | 5′-CAGTAGTTCTCCAGCTGGTAGA-3′(start at nucleotide position 358) |
| Gluc 5′ | 5′-GACTTCCCAGAAGAAGTCGCCAT-3′(Genbank reference sequence: BC012975; start at nucleotide position 487) |
| Gluc-3′ | 5′-CTACGGTTACCAGGTGGTCATGT-3′ (start at nucleotide position 957) |
Table 2. Sequences of primer pairs used for the amplification of calcium and TTX-sensitive sodium channels.
| Primer | Sequence/comment |
|---|---|
| (1) HVA | |
| CaV1.1 (5′) | 5′-GCGAGGTCATGGACGTGGACGA-3′ |
| CaV1.1 (3′) | 5′-GATCACCAGCCAATAGAAGAC-3′ |
| CaV1.2 (5′) | 5′-CAGGAGGTGATGGAGAAGCCA-3′ |
| CaV1.2 (3′) | 5′-CTGCAGGCGGAACCTGTTGTT-3′ |
| CaV1.3 (5′) | 5′-GGGGTCCAGCTGTTCAAGGGGAA-3′ |
| CaV1.3 (3′) | 5′-GCATGATGAGGACGAACATCATG-3′ |
| dfor | 5′-ATTGGGAACCTTGAGCATGTG-3′ |
| drev | 5′-ATAGCCAGTGGGAGAGTCATC-3′ |
| CaV2.1 (5′) | 5′-CACCAACCCTGGTCCCGCCT-3′ |
| CaV2.1 (3′) | 5′-CATGGGCTTTGGGCCGTCCT-3′ |
| CaV2.2 (5′) | 5′-TGAAGACACACATGGACCG-3′ |
| CaV2.2 (3′) | 5′-AGTCCTGTGCATGCCGGTG-3′ |
| CaV2.3 (5′) | 5′-CCGATGTCTGCTCCCAACATG-3′ |
| CaV2.3 (3′) | 5′-CCTCCG ATAAAGGCTGGGGTG-3′ |
| (2) LVA | degenerated |
| T05 (5′) | 5′-GT(AG)GA(AG)GG(CT)TTCCAGGC(AGT)GAGG-3′ |
| T06 (3′) | 5′-GCTGTTCC(AG)GCTGGAGCG(AGC)C-3′ |
| (3) LVA | specific |
| CaV3.1 (5′) | 5′-CACCAAGTCTGAGTCAGAGC-3′ |
| CaV3.1 (3′) | 5′-TGATTTCATCTCATGATGGGC-3′ |
| CaV3.2 (5′) | 5′-AGAGGAAGATTTCGATAAGCT-3′ |
| CaV3.2 (3′) | 5′-GGCTGCTTCCTGCTCTGTT-3′ |
| CaV3.3 (5′) | 5′-AAGCTCC(AC)(AG)GA(AG)GGCCTGGA-3′ |
| CaV3.3 (3′) | 5′-GTAGTAGGAGCTCCGGGAGCT-3′ |
| (4) NaV | |
| NaV1.1 (5′) | 5′-CCAAGTTGAGTTCAAAGAGC-3′ |
| NaV1.1 (3′) | 5′-TACTGTTGCGTCGCTCTCC-3′ |
| NaV1.2 (5′) | 5′-GAAAGGCTTCCAGTTTTCCCT-3′ |
| NaV1.2 (3′) | 5′-TTCATGGGTAGAGTGGGTATC-3′ |
| NaV1.3 (5′) | 5′-GTGCTAAGGAGTGGAGGAA-3′ |
| NaV1.3 (3′) | 5′-AGTCTCTCCTGCTCTCGC-3′ |
| NaV1.4 (5′) | 5′-AATGAGGCTACCCTGGCC-3′ |
| NaV1.4 (3′) | 5′-AGCACTTTGTGTGCACACTTG-3′ |
| NaV1.6 (5′) | 5′-AGGCTCTCCGAGGAGCTCA-3′ |
| NaV1.6 (3′) | 5′-GTGCTGTTGCGTTTCACGCT-3′ |
| NaV1.7 (5′) | 5′-TCTTCAGAAACCTCCAGGCT-3′ |
| NaV1.7 (3′) | 5′-GGGGTCTATGGGGTACGAA-3′ |
Single-cell RT-PCR
Following recording, the single islet cells were aspirated with fine borosilicate glass pipettes and stored in a PCR tube with 18–20 μl 1× standard PCR buffer at −80°C after quick freezing in liquid nitrogen. An OneStep RT-PCR protocol (Qiagen) was used to check for the expression of the different ion channels in single islet cells and to distinguish glucagon-producing A- from insulin-producing B-cells. The cells were thawed and handled according to the protocol of the manufacturer. The reaction kit consisted of: RNase-free water, 5× Qiagen OneStep RT-PCR buffer, dNTP mix (containing 10 mm of each dNTP) and Qiagen OneStep RT-PCR enzyme mix (including Omniscript and Sensiscript reverse transcriptase and HotStarTaq polymerase). The RNase inhibitor RNasin Plus (Promega) was used to avoid any damage of the mRNA, and the appropriate primer pairs were added at a final concentration of 0.6 μm to the reaction mix. The reactions were amplified for 30 or 40 cycles. The PCR products were examined on a 2% agarose gel.
Electrophysiology
Currents were recorded at room temperature from single islet cells using the standard whole-cell technique. The measurements were performed using an EPC-9 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pflaz, Germany) and Pulse (version 8.54) software. Currents were compensated for capacitive transients with the built-in compensation. No leak compensation was done. Patch pipettes were pulled from borosilicate glass with a resistance of 2.8–4.0 MΩ when filled with the pipette solution. The pipette solution contained (mm): 125 CsCl, 1 MgCl2, 10 EGTA, 3 Mg2ATP and 10 Hepes, pH 7.15. The standard extracellular medium consisted of (mm): 118 NaCl, 20 tetraethylammonium-Cl (TEA-Cl), 5.6 KCl, 1.2 MgCl2, 5 Hepes and either 2.6 CaCl2 or BaCl2, pH 7.4.
The steady-state inactivation properties of sodium currents were studied using a conventional two-pulse protocol in which a 50 ms test depolarization to −10 or 0 mV was preceded by a 500 ms conditioning pulse (Vpre) to voltages between −150 and −30 mV or between −100 and −10 mV. Data for steady-state inactivation were fitted by a Boltzmann equation:
where I/Imax is the relationship between the current amplitudes (I) and the maximal current amplitude at the most negative Vpre (Imax), A is the saturated level of I/Imax, V0.5 is the potential at which I/Imax is half-maximal, and k represents the slope factor.
Data analysis
The electrophysiological data were analysed with Origin 6.1 (OriginalLab Corp., Northampton, MA, USA). Data are given as mean values ± s.e.m. Statistical significances were evaluated by unpaired Student's t test.
Results
Isolation and identification of A-cells from pancreatic islets of the mouse
A-cells are easily lost during the preparation process. Therefore, most of the washing steps were omitted and, after the enzyme treatment, islets were transferred directly to coverslips in a 24-well plate. They were shaken in the 24-well plate to obtain single A- and B-cells. The next day the coverslips were transferred to the recording chamber. After the recording, single cells were aspirated with a fine borosilicate glass pipette and transferred to a PCR tube containing ice-cold PCR buffer. The tubes were stored at −80°C. A standard RT-PCR protocol was used with insulin- and glucagon-specific primers, which resulted in amplicons of 344 and 493 bp (Fig. 1A). Sequencing confirmed the correct identity of the amplified products. The identification process for A-cells was further substantiated by using islet cells from βCaV1.2−/− mice (Schulla et al. 2003). These mice lack the CaV1.2 L-type calcium channel only in B-cells. The L-type calcium current was inhibited by 1 μm isradipine, and subsequently the cells were genotyped by RT-PCR with insulin- and glugacon-specific primers. Fifteen out of 21 DHP-sensitive cells showed the 493 bp glucagon band. Fourteen of the DHP-insensitive cells were tested; all of them lacked the glucagon band. Thus, the expression of the glugacon band is sufficient to distinguish A- from B-cells. Altogether, 79 (54%) out of 145 cells were genotyped as B-cells and 42 (29%) as A-cells. The remaining cells (17%) could be D- or F-cells, although it cannot be excluded that the genotyping of some of these cells failed.
Figure 1. Single pancreatic A- and B-cells of the mouse do not express LVA calcium channels.
A, A- and B-cells can be distinguished by RT-PCR with glucagon- and insulin-specific primers. Lane 1, kb-ladder; lanes 2–6, RT-PCR of single islet cells with primer pairs Ins 5′, Ins 3′ and Gluc 5′, Gluc-3′; glucagon-specific band, 493 bp; insulin-specific band, 344 bp. B, PCR analysis of cDNA obtained from undissociated complete islets with LVA channel-specific primer pairs (see Methods). Lane 1, kb-ladder; lane 2, CaV3.1 (271 bp); lane 3, CaV3.2 (305 bp); and lane 4, CaV3.3 (258 bp). C, families of currents of a representative A-cell from holding potential (Vh) −80 (left) or −40 mV (middle) to potentials between −80 and +10 mV; right panel shows difference currents obtained by subtraction of the current measured at Vh−40 mV from the respective current at Vh−80 mV. D, mean current–voltage relationships (peak current versus voltage) measured in B-cells from a Vh of −80 mV (▪) and −40 mV (•); current–voltage relationship from difference currents obtained as described in Fig. 1C (▵); n = 6. E, RT-PCR analysis with LVA channel-specific primer pairs (CaV3.1, CaV3.2 and CaV3.3) on a single islet. Lane 1, kb-ladder. F, RT-PCR analysis with LVA channel-specific primer pairs on a single representative A-cell from a βCaV1.2−/− mouse. Lane 1, kb-ladder.
Molecular and electrophysiological characterization of LVA calcium channels
The expression of T-type calcium channels in pancreatic islets of the mouse was investigated by PCR analysis with a standard PCR protocol with degenerated primers for all T-type calcium channels (T05 (5′) and T06 (3′)) followed by a nested PCR with specific primers for CaV3.1, CaV3.2 and CaV3.3 (Table 2). Amplicons for each of the three LVA calcium channels were found in complete mouse islet cDNA (Fig. 1B) and in a single islet (Fig. 1E). Islets were dissociated into single cells by shaking in Ca2+-free medium. Although the cycle number was increased up to 40, no transcripts for CaV3.1, CaV3.2 or CaV3.3 were found in 47 islet cells analysed by single-cell RT-PCR and nested PCR (see also supplementary Fig. 1).
The expression of T-type calcium channels was studied further by whole-cell patch-clamp recordings in single isolated islet cells. Experiments were done in the presence of 0.1 μm TTX in the bath solution to inhibit sodium currents and with 2.6 mm Ba2+ as the charge carrier. Potassium channels were blocked using 20 mm TEA-Cl in the bath solution plus 125 mm CsCl instead of KCl in the pipette solution. In A-cells, only HVA currents could be seen in single-current traces elicited by depolarizing pulses from a holding potential (Vh) of −100 or −80 mV to voltages from −80 to +10 mV. Similar results were found in B-cells (Fig. 1C and D). In neuronal and other T-type-expressing cells the LVA component appears as a hump on the back of a larger HVA component in the I–V relationship. This hump was not found in I–V relationships from A- or B-cells. It was postulated that the LVA currents were hidden by the large HVA currents. T-type calcium channels inactivate at lower membrane potentials than L- and non-L-type channels (Bean, 1985). The activity of both channels was recorded from a hyperpolarized potential of −100 or −80 mV. Thereafter, the activity of HVA currents was measured at a Vh of −40 mV, where T-type but not HVA channels are inactivated. The HVA currents were subtracted from the currents at hyperpolarized potentials to isolate the T-type current. Subtraction of every single-current trace elicited from a Vh of −40 mV from the corresponding current trace elicited from a Vh of −100 or −80 mV showed that negative to −40 mV no inward current was detectable. The same results were found in B-cells. Current–voltage relationships for the currents elicited at Vh−80 mV, Vh−40 mV and the difference currents were drawn. They differed only in the current amplitude but not in the voltage dependence. Still no hump and thus no T-type was found. After the experiment the cells were further analysed by single-cell RT-PCR with insulin- and glucagon-specific primers. Nine cells were identified as A-cells and 21 as B-cells. None of them showed the hump in the individual I–V relationship. Figure 1C shows a representative example for an A-cell. Figure 1D summarizes the data on B-cells (for Vh−80 versus−40 mV). To confirm the result, single cells from βCaV1.2−/− islets were isolated and identified electrophysiologically by their isradipine sensitivity. Seven A- and 16 B-cells were subsequently analysed by single-cell RT-PCR with CaV3.1-, CaV3.2- and CaV3.3-specific primers. Transcripts could not be amplified in any of them with any primer pair. A representative result for an A-cell is shown in Fig. 1F. Thus, we conclude that LVA calcium channels are absent in pancreatic islet A- and B-cells of the mouse.
Molecular and electrophysiological characterization of TTX-sensitive sodium channels
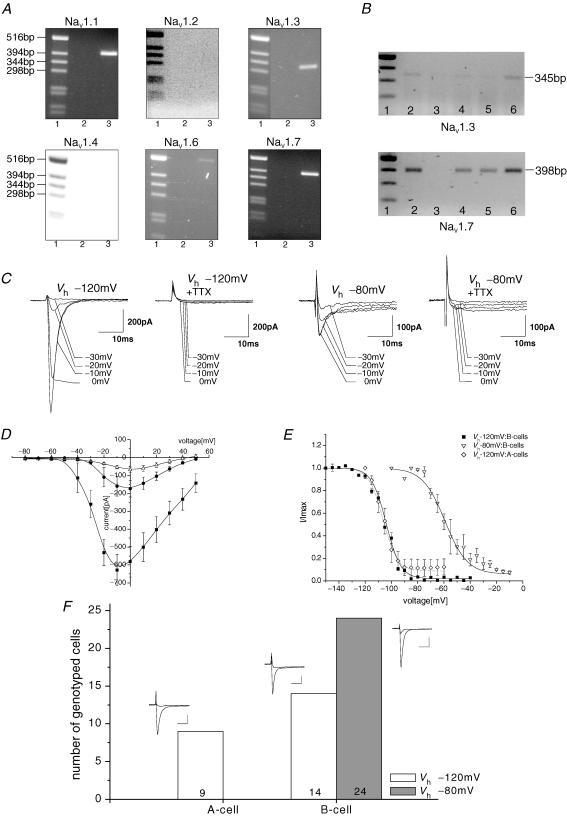
Similar to the experiments on LVA calcium channels, TTX-sensitive sodium channels were analysed by PCR in whole islet tissue. Specific primers for all known TTX-sensitive sodium channels (NaV1.1, NaV1.2, NaV1.3, NaV1.4, NaV1.6 and NaV1.7) were designed and used with a standard PCR protocol (Table 2). The primers were tested on brain and skeletal muscle cDNA from the mouse. High copy numbers of NaV1.1, NaV1.2, NaV1.3, NaV1.6 and NaV1.7 were identified in the brain cDNA, and amplicons for NaV1.4 and NaV1.2 were found in skeletal muscle. Using optimized temperature for each primer pair, amplicons for NaV1.1, NaV1.3, NaV1.7 and a tiny band of NaV1.6 were detected in complete mouse islet cDNA (Fig. 2A). In single cells, only transcripts for the NaV1.7 could be clearly amplified, although the cycle number was increased up to 40 (Fig. 2B).
Figure 2. Two different sodium channels are expressed in pancreatic islet cells of the mouse.
A, PCR analysis of cDNA obtained from undissociated complete islets with primer pairs specific for the known TTX-sensitive sodium channels (see Methods): lane 1, kb-ladder; lane 2, control (no cDNA); lane 3, islet cDNA. Primer specificity is given above each panel. B, RT-PCR on single islet cells. Cells were isolated and processed as described in the Methods. Single-cell RT-PCR was done with NaV1.3- (upper) and NaV1.7-specific primers (lower). Five representative cells are shown for each primer pair; lane 1, kb-ladder. C, representative current traces for the early inactivating and the residual sodium current from two different B-cells. One hundred millisecond currents were measured from a Vh of −120 or −80 mV to potentials from −30 to 0 mV from two different B-cells in the absence or presence of 0.1 μm TTX. D, mean current–voltage relations measured from a Vh of −120 mV (▪), a Vh of −80 mV (•) or a Vh of −120 mV in the presence of 0.1 μm TTX (▵; n = 8–11 each). Currents were measured as described above, averaged and plotted versus the appendant voltage. E, steady-state inactivation of the early inactivating and the residual sodium currents. Results are means ± s.e.m. The data were fitted by the Boltzmann equation. The smooth curve represents the averaged Boltzmann fit. Early inactivating sodium current: V0.5=−106 ± 1.7 mV, k = 5.4 ± 0.9, n = 4 (A-cells); V0.5=−104 ± 1.2 mV, k = 5.5 ± 0.3, n = 13 (B-cells); and residual sodium current: V0.5=−59 ± 3.3 mV, k = 8.5 ± 1.3, n = 6 (B-cells). F, identification of A- and B-cells expressing early inactivating (open bars) and residual sodium current (filled bar) with insulin- and glucagon-specific primers. Representative current traces from Vh−120 mV and Vh−80 mV to −10 or 0 mV are shown as insets for each type of cell. Scale bars, 10 ms, 200 pA.
Whole-cell patch-clamp experiments were done with standard pipette and bath solutions (2.6 mm Ca2+, no TTX). As described before, two types of Na+ currents were found (Gopel et al. 1999; Barg et al. 2000). One was only active when the membrane potential was held at −120 mV (early inactivating current); the other persisted at a physiological membrane potential of −80 mV (residual current; Fig. 2C and D). The early inactivating current was present in about 100% of the cells, whereas the residual current was visible in less than 50% of the cells. Currents measured from a Vh of −120 mV activated at −60 mV. Depolarizations to more positive voltages evoked progressively larger inward currents. The maximum current was measured at a potential of −10 mV. Currents measured from a Vh of −80 mV activated positive from −40 mV. The maximum current was observed at 0 mV (Fig. 2D). Both the early inactivating and the residual sodium current could be completely inhibited by 0.1 μm TTX (Fig. 2C and D). The IC50 values for TTX were 1.5 ± 0.5 and 3.2 ± 0.4 nm (P < 0.05) for the residual and early inactivating INa determined in five and eight different cells, respectively. After the inhibition of the sodium current by 0.1 μm TTX, only HVA calcium currents were identified. Steady-state inactivation of the early inactivating and the residual Na+ current were measured (Fig. 2E). The early inactivating currents were inhibited half-maximally at −106 ± 1.7 mV (n = 4) with a slope factor, k, of 5.4 ± 0.9 in A-cells and at −104 ± 1.2 mV (n = 13) with a slope factor, k, of 5.5 ± 0.3 in B-cells. The V0.5 of the B-cell residual current was −59 ± 3.3 mV (n = 6) with k = 8.5 ± 1.3. In two cells only the residual current was measured; the remaining four cells expressed both sodium currents.
The cells were genotyped by single-cell RT-PCR with insulin- and glucagon-specific primers. The result was highly surprising. Nine out of 23 cells which showed a sodium current at a holding potential of −120 mV were identified as A-cells, and 14 were recognized as B-cells. The 24 cells that showed the residual sodium current were identified without exception as B-cells (Fig. 2F). Thus, murine A-cells express only the early inactivating sodium current. B-cells can be divided in two populations: 37% of the cells express only the early inactivating sodium channel, and the other 63% express the residual or both types of sodium channels. Single-cell RT-PCR was performed with NaV1.7- and NaV1.3-specific primers. NaV1.7 was identified in cells expressing early inactivating and residual current (5 of 10 cells) as well as in cells with only early inactivating current (8 of 10 cells). Amplicons were not detected in each lane, but it cannot be excluded that this is due to the complexity of the single-cell RT-PCR.
Molecular and electrophysiological characterization of HVA calcium channels
High-voltage-activated calcium channels of islets tissue were analysed by PCR. Specific primer pairs (Table 2) were used to identify amplicons for CaV1.1, CaV1.2, CaV1.3, CaV2.1, CaV2.2 and CaV2.3. CaV1.4 was not analysed because this channel is only expressed in the retina and in T-lymphocytes (Bech-Hansen et al. 1998; Strom et al. 1998; Kotturi & Jefferies, 2005). The primers were tested on brain cDNA from the mouse with a standard PCR protocol (not shown). Amplicons for CaV1.2, CaV1.3, CaV2.1, CaV2.2 and CaV2.3 were detected in complete mouse islet cDNA (Fig. 3A) and in single islet cells (Fig. 3B). Electrophysiological experiments were done with standard pipette and bath solutions in the presence of 0.1 μm TTX, with 2.6 mm Ba2+ as the charge carrier. Potassium channels were blocked using 20 mm TEA-Cl in the bath solution plus 125 mm CsCl instead of KCl in the pipette solution. After the experiments, the cells were genotyped by single-cell RT-PCR with insulin- and glucagon-specific primers. In wild-type and control αCaV1.2+/− A- and B-cells, maximal calcium current amplitudes of −138 ± 16 (n = 28) and −106 ± 12 pA (n = 31), respectively, were measured from a Vh of −100 mV. On average, A-cells were smaller than B-cells with mean cell capacitances of 4.4 ± 0.6 (n = 28) versus 5.8 ± 0.4 pF (n = 31; P < 0.05). The difference was significant because a large number of cells were averaged, but when individual cells were measured it was difficult to identify them as an A- or a B cell by their capacitance only. Owing to the smaller size of the A-cells, their mean calcium current density was significantly higher compared to that of the B-cells (−37 ± 4.4 versus−22 ± 3.2 pA pF−1).
Figure 3. CaV1.2 carries most of the IBa in mouse pancreatic A- and B-cells.
A, RT-PCR analysis of cDNA obtained from undissociated complete islets with specific primers for L-type Ca2+ channels CaV1.1 (200 bp), CaV1.2 (316 bp) and CaV1.3 (556 bp) (lanes 2–4), and non-L-type Ca2+ channels CaV2.1 (333 bp), CaV2.2 (381 bp) and CaV2.3 (279 bp) (lanes 5–7), as well as kb-ladder (lane 1). B, RT-PCR analysis on single cells of pancreatic islets with specific primers for CaV1.2 (316 bp; Ba, lane 2), CaV2.1 (333 bp; Ba, lane 4), CaV2.2 (381 bp; Bb, lane 3) and CaV2.3 (279 bp; Bc, lane 3). More specific primers were designed for the identification of CaV1.3 with single-cell RT-PCR (dfor, drev; Table 2). A 380 kb piece was amplified (Bd). Lanes Ba1, Bb2, Bc2 and Bd2 show kb-ladder; lanes Ba3, Ba5, Bb1, Bc1 and Bd1 are controls (no DNA). C, isradipine block of IBa from single mouse A- (Ca) and B-cells (Cb). Peak IBa was measured during 200 ms depolarizations from −100 to +10 mV with 0.2 Hz under control conditions (▪) and in the presence of 0.03 (○), 0.1 (▵) and 1 μm isradipine (⋄). Cc shows a concentration–inhibition curve of the IBa inhibition by isradipine. The results for A- and B-cells were pooled because isradipine has similar effects on A- and B-cells. The smooth line is the fit of the experimental data calculated with the Hill equation. Isradipine blocked the IBa with an IC50 of 211 nm. The points are means ± s.e.m. (n = 4–14). D, A-cell-specific inactivation of the CaV1.2 gene. PCR analysis of genomic DNA from αCaV1.2−/− heart (lane 3), αCaV1.2−/− islets (lane 4) or αCaV1.2+/− islets (lane 5). Lane 1, kb-ladder; lane 2, no cDNA. E, IBa density in wild-type (wt; open bars) and αCaV1.2−/− (ko; hatched bars) pancreatic A-cells in the absence and presence of 1 μm isradipine (DHP). The results of wild-type and αCaV1.2+/− cells were combined. IBa was measured as indicated above. Data are given as means ± s.e.m. The values are significantly different (P < 0.001).
The distribution of HVA calcium channels in A- and B-cells was studied by inhibiting the whole-cell Ba2+ current (IBa) of islet cells with a specific HVA calcium channel blocker. Figure 3C shows IBaversus time for an A- and a B-cell under control conditions and in the presence of 0.03, 0.1 and 1 μm isradipine. Isradipine blocked IBa of the A- and B-cells with similar potency. The maximum inhibition was seen in the presence of 1 μm isradipine, and application of higher concentrations did not further increase the effect. Isradipine exerted a comparable maximum block of 59 ± 3.4 (n = 6) versus 58.6 ± 3% (n = 5) of the IBa in A- and B-cells, of respectively. To characterize the effect of isradipine on islet cells in more detail, concentration–inhibition curves were calculated. Since the drug effect on both cell types was comparable, it was not distinguished between A- and B-cells. The values could easily be fitted by a simple Hill equation. Isradipine blocked the IBa of islet cells with an IC50 of 211 nm (Fig. 3C). This value is similar to that determined for CaV1.3 calcium channels by Koschak et al. (2001). Thus, in islet cells CaV1.2 and CaV1.3 cannot be distinguished pharmacologically. But it has been shown previously that CaV1.2 is the only important L-type calcium channel in B-cells and that isradipine is without effect in βCaV1.2−/− mice (Koschak et al. 2001; Schulla et al. 2003). Therefore, we speculated that CaV1.3 is expressed in A-cells.
We used islet cells from βCaV1.2−/− mice to validate this interpretation (see supplementary Fig. 2). The Ba2+ current of 20 cells from four different βCaV1.2−/− pancreatic islet preparations was tested for its isradipine sensitivity. After the patch-clamp experiment, single-cell RT-PCR was done with the CaV1.3-specific primers. Isradipine (1 μm) had no effect in 15 cells; in nine of these cells no transcripts for CaV1.3 were found, whereas in six cells CaV1.3 mRNA was detected. The averaged cell capacitances were 7.9 ± 1.4 and 7.4 ± 0.7 pF for the cells without and with transcripts, respectively, suggesting that the cells were B-cells. These results indicate that some B-cells have CaV1.3 mRNA without expressing a functional protein. The IBa of five cells was blocked by isradipine. The mean inhibition was 60 ± 7.6%, and the mean cell capacitance 4.6 ± 0.7 pF, suggesting that the cells were A-cells. These cells contained CaV1.3 transcripts.
We used islet cells from αCaV1.2−/− mice to confirm that IBa through CaV1.3 channels was only found in A-cells. A-cell-specific Cre/loxP recombination was achieved by expressing the Cre-recombinase under the control of the rat glucagon promotor (Herrera, 2000). The cell specificity of the Cre-recombinase was ascertained by PCR analysis using DNA isolated from islets of control and αCaV1.2−/− mice (Fig. 3D). The islets of αCaV1.2−/− mice still contain high amounts of the floxed CaV1.2 gene (L2 allele), because most of the islet cells are B-cells, which do not express the glucagon promotor. No Cre-mediated recombination was detectable in heart (Fig. 3D). Mice were viable and fertile and showed no gross abnormalities. Electrophysiological experiments showed that the IBa density of islet cells was decreased from −37 ± 4.4 pA pF−1 (n = 28) in wild-type to −19 ± 2.0 pA pF−1 (n = 34) in αCaV1.2−/− mice. Isradipine (1 μm) reduced IBa of both wild-type and αCaV1.2−/− A-cells to similar values of −8.9 ± 2.6 (n = 5) and −6.5 ± 1.0 pA pF−1(n = 16), respectively (Fig. 3E). In αCaV1.2−/− B-cells, IBa current density and isradipine inhibition were similar to those obtained in wild-type and αCaV1.2+/− B-cells.
The concentration–inhibition curve showed that about 40% of IBa in wild-type islet cells is an isradipine-resistant current that must be caused by non-L-type calcium channels, like CaV2.1, CaV2.2 or CaV2.3. The peptide neurotoxin ω-agatoxin IVA is a selective inhibitor of P/Q-type calcium currents. The affinity depends on the splice form of the CaV2.1 channel (P- or Q-type) and the β-subunit (Moreno et al. 1997; Bourinet et al. 1999). In A- and B-cells, ω-agatoxin IVA exerted similar effects on IBa. ω-Agatoxin IVA (200 nm) inhibited 16.1 ± 4.4% in A- and 15.5 ± 3.2% of IBa in B-cells (Fig. 4A). N-type Ca2+ channels can be specifically and irreversibly inhibited by ω-conotoxin GVIA (Olivera et al. 1984; McCleskey et al. 1987). In B-cells, 1.6 μm of the toxin irreversibly eliminated 15.1 ± 2.4% of IBa (Fig. 4B). The same concentration of ω-conotoxin GVIA was without effect in A-cells. Thus, it was concluded that mouse pancreatic A-cells do not express CaV2.2 calcium channels. We used SNX 482, the recently described potent and selective blocker of R-type calcium currents (Newcomb et al. 1998), to test for the expression of CaV2.3 calcium channels in islet cells. SNX 482 (100 nm) blocked 30 ± 4% of IBa in A-cells and 18.1 ± 1.8% of IBa in B-cells (Fig. 4C). The results are summarized in Fig. 5. The total amount of IBa blocked by the different L- and non-L-type calcium channel blockers was 104% in A-cells and 108% in B-cells.
Figure 4. IBa of mouse pancreatic A- and B-cells is inhibited by different snail and spider venoms.
Peak IBa was measured with 200 ms depolarization from −100 to +10 mV at a frequency of 0.2 Hz and plotted versus time. Time courses of the inhibition recorded in the absence (▪) or presence (□) of 0.2 μmω-agatoxin IVA (A), 1.6 μmω-conotoxin VIA (B) and 0.1 μm SNX 482 (C) in A-cells (a) and B-cells (b), respectively.
Figure 5. Mouse A- and B-cells express different HVA calcium channels.
The figure summarizes the data presented in Figs 3 and 4. The current inhibition is drawn as percentage of the maximum current for n = 4–14 experiments. Inhibition of IBa in A- and B-cells, respectively, was: isradipine, 59 ± 3.4 and 58.6 ± 3.0%; ω-agatoxin IVA, 16.1 ± 4.4 and 15.5 ± 3.2%; ω-conotoxin VIA, 15.1 ± 2.4 and 0.36 ± 0.36% (P = 0.001); and SNX 482, 30 ± 4 and 18.1 ± 1.8% (P = 0.05).
Discussion
Mouse pancreatic islet cells express different voltage-dependent calcium and sodium channels
Electrophysiological and molecular studies have identified multiple types of L- and non-L-type channel isoforms in islet cells and insulin-secreting cell lines (for review see Satin, 2000; Mears, 2004; Yang & Berggren, 2005). Most of the studies were rather indirect, using whole-animal or whole-islet assays. Electrophysiological studies were hampered by the fact that islet cells could not be clearly identified as A- or B-cells. Therefore, a clear allocation of the different voltage-dependent ion channels has not been possible to date. In our studies, we combined electrophysiological and pharmacological experiments with PCR and single-cell RT-PCR. Some results were substantiated by the utilization of αCaV1.2−/− and βCaV1.2−/− mice. Thus, it could be shown that A- and B-cells of the mouse are equipped with different calcium channels. In both A- and B-cells, 60% of the IBa is carried by L-type calcium channels. In B-cells only CaV1.2 current was identified, whereas in A-cells CaV1.2 and CaV1.3 currents are expressed. This could explain why it was believed previously that CaV1.3 represents the major L-type calcium channel in pancreatic islet cells (Iwashima et al. 1993; Seino, 1995; Safayhi et al. 1997; Yang et al. 1999). A- and B-cells also differ in the expression of non-L-type HVA calcium channels. In B-cells, similar fractions (about 15% each) of CaV2.1, CaV2.2 and CaV2.3 currents were found. In A-cells, 30% of the current runs through CaV2.3 and the remaining current traverses via CaV2.1. CaV2.2 current was absent in A-cells. Since the calculated and measured total inhibition of calcium currents in mouse A- and B-cells is complete or even exceeds 100%, we can exclude the possibility that additional HVA calcium channels are expressed in both cell types.
Our experiments clearly show that single pancreatic A- and B-cells of the mouse do not express any T-Type calcium current or T-Type calcium channel mRNA, whereas amplicons for each of the three LVA calcium channel subtypes could be found in whole islet cDNA. Two types of sodium current were detected, an early inactivating and a residual current, but the expression pattern was different from that described earlier (Gopel et al. 1999; Barg et al. 2000). In single A-cells, only the early inactivating type of current was found. B-cells could be devided into two populations: 40% of the B-cells only showed the early inactivating current, whereas the remaining 60% had an additional residual current. Thus 45% of the cells expressing exclusively the early inactivating current are A-cells. NaV1.7 mRNA was identified in both A- and B-cells by single-cell RT-PCR.
Comparison of the present results with other studies
Our results on L-type calcium channels are in agreement with earlier studies in different knockout mice. It has been shown that deletion of CaV1.3 in mice is without effect on serum glucose and insulin levels (Platzer et al. 2000). Accordingly, Schulla et al. (2003) showed that after selective disruption of CaV1.2 in mouse pancreatic B-cells, the entire DHP-sensitive Ca2+ current and the first phase of insulin secretion were lost. These results were confirmed by the use of the CaV1.2DHP−/− mice (Sinnegger-Brauns et al. 2004). The calcium channel agonist Bay K 8644 as well as isradipine were without effect on ICa of CaV1.2DHP−/− islet cells. The glucose-dependent insulin secretion was not DHP sensitive in this mouse model, substantiating the suggestion that CaV1.3 does not contribute to calcium channel current and insulin secretion in B-cells of the mouse. However, different CaV1.3 splice forms have been cloned from human B-cells, from rat RINm5F insulinoma cells and from the hamster insulin-secreting cell line HIT (Seino et al. 1992; Yaney et al. 1992; Ihara et al. 1995). In accordance with these findings, we found CaV1.3 transcripts in some of the B-cells, but no CaV1.3-related current. This could either be due to the absence of CaV1.3 protein or due to inactive CaV1.3 channels despite their expression in B-cells. Other groups demonstrated the presence of CaV1.3 subunit mRNAs and proteins in mouse pancreatic islet cells (Yang et al. 1999; Namkung et al. 2001). CaV1.3−/− mice had a decreased number and size of islets. It was speculated about a putative connection between the levels of CaV1.3 gene expression and a transcriptional regulation (Yang et al. 1999). Furthermore, mutant islets secreted less insulin than control islets only in the presence of 3 mm glucose, a concentration at which wild-type A-cells are highly active in secreting glucagon. Thus, the decreased insulin secretion could be explained by a loss of glucagon release owing to the deletion of CaV1.3 in A-cells. This is in agreement with our finding that CaV1.3 current is only found in A-cells.
The expression of non-L-type calcium channels in islet cells has been discussed with controversy. The discrepances were raised, in part, by interspecies differences. Thus, for rat B-cells, P- and N-type calcium channels have been described (Komatsu et al. 1989; Ramanadham & Turk, 1994), whereas in humans, N-type calcium channels are either absent or represent a very minor component (Pollo et al. 1993; Davalli et al. 1996). In accordance with our findings, the expression of P/Q-, N- and R-type Ca2+ currents was postulated in mice B-cells by different authors (Schulla et al. 2003; Takahashi et al. 2005). For A-cells, very little is known about the expression of non-L-type HVA channels. Here we show that 30% of the A-cell IBa could be blocked by the CaV2.3 calcium channel blocker SNX 482. Thus, the R-type current represents the second dominant calcium channel in A-cells of mice, and one can assume that it plays a considerable role in glucagon secretion. Indeed in CaV2.3 deficient mice, the glucagon release was severely disturbed (Jing et al. 2005). In rat A-cells, an N-type calcium channel with an atypical reversible inhibition by ω-conotoxin GVIA was found (Gromada et al. 1997). In mice A-cells, the IBa and the glucagon secretion could be inhibited by the N-type calcium channel blocker (Barg et al. 2000; Gopel et al. 2004). In our hands, mouse A-cells do not express N-type calcium channels. This discrepancy could be explained by the different methods of distinguishing A- from B-cells. In the present work, A- and B-cells were unequivocally identified after the patch-clamp experiments by single-cell RT-PCR with glucagon-specific primer pairs. Others describe and base their identification on the expression of a TTX-sensitive voltage-gated sodium current in A-cells, which, in contrast to its counterpart in B-cells, remains able to be activated at physiological membrane potentials (Gopel et al. 1999, 2004; Barg et al. 2000; Lou et al. 2003; Jing et al. 2005). In an elegant recent study, B-cells that expressed GFP under the mouse insulin promotor were identified by green fluorescence (Leung et al. 2005). In agreement with our experiments, the residual sodium current was not found in single A-cells. In contrast to our experiments, fluorescent B-cells only expressed the early inactivating sodium current in single cells and whole islets. The residual sodium current was identified in non-green (probably A-cells) of whole islets by Leung et al. (2005). The discrepancies may be caused by the following facts. (1) The early inactivating sodium current of B-cells has a high amplitude that may make it difficult to detect the relative small residual sodium current. (2) Islet cells which exclusively express the residual current are rare. One can speculate that, similar to A-cells, they are located in the periphery of the islets and thus were easily mistaken for A-cells. (3) It cannot be excluded that A-cells also express a residual sodium current, which according to Leung et al. (2005) got lost during the isolation procedure. (4) The MIP-GFP mice from the study of Leung et al. (2005) are transgenic mice. One hundred per cent of green cells were positively stained for insulin, but it is not clear how many of the non-green fluorescent cells were insulin positive. (5) In addition, in whole islets it was difficult to distinguish the non-green from the green fluorescent cells (Leung et al. 2005). (6) The differences could be due to the use of different mouse strains. Differences in metabolism have been reported between C57BL6, DBA/2, 129T2, ICR, FVB/n, MRL/MP and BALB/c (Andrikopoulos et al. 2005; Burgess et al. 2005; Gurley et al. 2006).
Direct experimental data on LVA calcium channels in islet cells are sparse, owing to the large HVA currents hiding the LVA currents combined with the lack of highly specific T-type calcium channel blockers. In mice, T-type channels have only been measured in intact pancreatic islets with the perforated patch-clamp technique (Gopel et al. 2000), whereas in isolated cells they were absent (Barg et al. 2000). Otherwise, T-type channels have only been described in insulinoma cell lines like INS-1, HIT-T15 and NIT-1. In the cardiac system, T-type calcium channel expression shows a spatial and temporal dependence (for review see Perez-Reyes, 2003). The currents are detectable in neonatal animals, increasing slightly to a peak between postnatal days 4 and 8, and then decline slowly to a steady state. In pathological conditions, such as hypertrophy, they reappear (Richard et al. 1998). In coronary artery and aortic smooth muscle, LVA currents were only detected when cells were cultured and proliferating (Rodman et al. 2005). Thus, it could be speculated that in certain tissue LVA calcium currents are not expressed under normal physiological conditions, but might play a role in proliferation and/or pathological state. In accordance with these results, it has been reported that the expression of LVA currents in mouse B-cells could be stimulated by cytokine treatment (Wang et al. 1996). For an islet cell, accustomed to being surrounded by neighbours, an existence in isolation is likely to be as abnormal as it gets. A likely explanation would be that gene transcription is influenced by paracrine signalling, which only operates in the intact islet. This notion was supported by the fact that mRNA for all known T-type channels has been found in whole islet tissue and in insulinoma cell lines. Alternatively, it may simply reflect the expression of T-type channels in cells other than A- and B-cells in the islet of Langerhans, since islet tissue contains four types of endocrine cells as well as nerve endings and capillaries.
Secretion of insulin and glucagon depends on different ion channel interplay
A- and B-cells show a competely different behaviour depending on the serum glucose level. High blood sugar levels induce a bursting activity in B-cells and silence A-cells. Glucagon secretion and A-cell activity is highest in the presence of low blood glucose. In both cell types, influx of extracellular Ca2+ through L-type calcium channels triggers hormone secretion. Therefore, the glucose concentration must be signalled to the L-type calcium channels through different mechanisms. It is assumed that at least two or three voltage-gated calcium channel types with different activating thresholds co-operate during Ca2+-triggered insulin release and that glucagon release is controlled by different channels (Pereverzev et al. 2002; Barg, 2003; Schulla et al. 2003). According to this hypothesis, we found a different distribution of HVA calcium and sodium channels in mouse A- and B-cells. Thus, the model of Pereverzev et al. (2002) concerning the successive activation of the KATP channel, and low-, middle- and high-voltage-activated calcium channels to trigger insulin release, could be modified. In both A- and B-cells of the mouse, T-type channels are not involved in hormone secretion under normal conditions. Since the residual sodium channel was found in a subpopulation of B-cells, closure of KATP channels in response to elevated glucose levels may result in the activation of those channels. Thus, it seems possible that the sodium current contributes to the calcium current-induced action potentials which spread over the remaining B-cells. In addition, it was shown that the sodium channel agonist veratridine and a scorpion toxin, TsTx-V, modulated the electrical activity in mouse pancreatic B-cells (Eberhardson & Grapengiesser, 1999; Goncalves et al. 2003). Also the involvement of transient receptor potential (TRP) channels cannot be excluded, since the involvement of non-selective cation currents provides an excellent mechanism for oscillatory calcium response (Qian et al. 2002). In accordance with this, TRPC4 was detected in islets by RT-PCR analysis. Studies on TRPC4-deficient mice are on-going to reveal their possible functions in pancreatic islets (Freichel et al. 2005). The fractions of the CaV2.1, CaV2.2 and CaV2.3 currents in B-cells are low compared to the whole calcium current, and a direct participation of these channels in first phase insulin secretion has not been proven to date. Thus, in B-cells they seem to fulfill secondary tasks, such as refilling or mobilizing reserve granules (Schulla et al. 2003). Recently, it was found that CaV2.3 knockout mice lack the second phase of insulin secretion and show markedly reduced glucose tolerance, impaired insulin release and stress-induced hyperglycaemia (Matsuda et al. 2001; Pereverzev et al. 2002; Jing et al. 2005).
In A-cells, the same amount of current as in B-cells traverses via L-type calcium channels, but A-cells express CaV1.2 and CaV1.3. Similar to B-cells, CaV2.1 currents amount to 15%. CaV2.2 currents are not detectable, whereas 30% of the calcium current was inhibited by the CaV2.3 blocker SNX 482. R-type currents show a voltage dependence between T- and L-type channels (Randall & Tsien, 1995; Tottene et al. 1996). In A-cells, the number of KATP channels is low and the membrane potential is partly depolarized in the absence of glucose (Gopel et al. 2000). Therefore, we speculated that in the presence of low glucose, CaV2.3 depolarizes the membrane potential sufficiently to activate CaV1.3 and subsequently CaV1.2 calcium channels. Alternatively, it was demonstrated earlier that neither isradipine nor SNX 482 inhibited glucagon secretion in the presence of low glucose levels in mouse islets. Both compounds impaired the ability of glucose to suppress glucagon secretion (Gopel et al. 2004; Jing et al. 2005). These results suggest that CaV1.2 and CaV2.3 are more likely to be associated with the prevention of glucagon release than with the release itself. In agreement with this, it has been found that glucagon secretion from rat A-cells is mediated by GABA release from neighbouring B-cells. GABA receptors have only been identified in A- but not in B-cells. Thus, the paradoxical stimulation of glucagon secretion by isradipine reflects the inhibition of GABA secretion due to the block of L-type calcium channels in B-cells (Wendt et al. 2004).
Conclusion
An increase in intracellular [Ca2+] is caused, in part, by the influx of Ca2+ through voltage-dependent calcium channels. Numerous studies have shown that multiple voltage-gated calcium channels, but also sodium and potassium channels are expressed in insulin-secreting B- and glucagon-secreting A-cells. Most of these studies on hormone secretion in islet cells were rather indirect, and an unequivocal allocation of the effects of different ion channels was hampered by the fact that A- and B-cells could not be distinguished beyond doubt by electrophysiological methods. Our approach, combining electrophysiology with PCR, clearly shows that HVA calcium channels and sodium channels are distributed differently in A- and B-cells of mouse pancreatic islets. Thus, in B-cells, CaV1.2, CaV2.1, CaV2.2, CaV2.3 and NaV1.7 currents are found, whereas A-cells express CaV1.2, CaV1.3, CaV2.1, CaV2.3 and NaV1.7 currents. LVA currents could be identified in none of the cells. These results could form the basis for further analysis of hormone secretion in islets in both healthy and diseased states.
Acknowledgments
We are grateful to Dr Pedro Luis Herrera for the generous gift of the GlucCre+/tg mice. Thanks to reviewer 1 for his helpful comments. We thank Mrs Ulla Kremser for excellent technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 391), Graduierten Kolleg 333 der LMU and Fond der Chemischen Industrie.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2005.102368
http://jp.physoc.org/cgi/content/full/jphysiol.2005.102368/DC1 and contains supplemental material consisting of two figures
T-type primers are sensitive enough to amplify transcripts in single islet cells. Three to five mouse pancreatic islets (500–2000 cells per islet) were freeze-thawed and mRNA reverse transcribed.
Isradipine sensitivity and single-cell RT-PCR with CaV1.3-specific primers in B- and A-cells of βCaV1.2−/− mice.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Andrikopoulos S, Massa CM, Aston-Mourney K, Funkat A, Fam BC, Hull RL, et al. Differential effect of inbred mouse strain (C57BL/6, DBA/2, 129T2) on insulin secretory function in response to a high fat diet. J Endocrinol. 2005;187:45–53. doi: 10.1677/joe.1.06333. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Proks P, Smith PA, Ammala C, Bokvist K, Rorsman P. Stimulus-secretion coupling in pancreatic beta cells. J Cell Biochem. 1994;55(Suppl.):54–65. doi: 10.1002/jcb.240550007. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Barg S. Mechanisms of exocytosis in insulin-secreting B-cells and glucagon-secreting A-cells. Pharmacol Toxicol. 2003;92:3–13. doi: 10.1034/j.1600-0773.2003.920102.x. [DOI] [PubMed] [Google Scholar]
- Barg S, Galvanovskis J, Gopel SO, Rorsman P, Eliasson L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes. 2000;49:1500–1510. doi: 10.2337/diabetes.49.9.1500. [DOI] [PubMed] [Google Scholar]
- Barg S, Ma X, Eliasson L, Galvanovskis J, Gopel SO, Obermuller S, et al. Fast exocytosis with few Ca2+ channels in insulin-secreting mouse pancreatic B cells. Biophys J. 2001;81:3308–3323. doi: 10.1016/S0006-3495(01)75964-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Genl Physiol. 1985;86:1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, et al. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–267. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- Bokvist K, Olsen HL, Hoy M, Gotfredsen CF, Holmes WF, Buschard K, et al. Characterisation of sulphonylurea and ATP-regulated K+ channels in rat pancreatic A-cells. Pflugers Arch. 1999;438:428–436. doi: 10.1007/s004249900076. [DOI] [PubMed] [Google Scholar]
- Bourinet E, Soong TW, Sutton K, Slaymaker S, Mathews E, Monteil A, et al. Splicing of alpha 1A subunit gene generates phenotypic variants of P- and Q-type calcium channels. Nat Neurosci. 1999;2:407–415. doi: 10.1038/8070. [DOI] [PubMed] [Google Scholar]
- Burgess SC, Jeffrey FM, Storey C, Milde A, Hausler N, Merritt ME, et al. Effect of murine strain on metabolic pathways of glucose production after brief or prolonged fasting. Am J Physiol Endocrinol Metab. 2005;289:E53–E61. doi: 10.1152/ajpendo.00601.2004. [DOI] [PubMed] [Google Scholar]
- Curry DL, Bennett LL, Grodsky GM. Dynamics of insulin secretion by the perfused rat pancreas. Endocrinology. 1968;83:572–584. doi: 10.1210/endo-83-3-572. [DOI] [PubMed] [Google Scholar]
- Davalli AM, Biancardi E, Pollo A, Socci C, Pontiroli AE, Pozza G, et al. Dihydropyridine-sensitive and -insensitive voltage-operated calcium channels participate in the control of glucose-induced insulin release from human pancreatic beta cells. J Endocrinol. 1996;150:195–203. doi: 10.1677/joe.0.1500195. [DOI] [PubMed] [Google Scholar]
- Eberhardson M, Grapengiesser E. Role of voltage-dependent Na+ channels for rhythmic Ca2+ signalling in glucose-stimulated mouse pancreatic beta-cells. Cell Signal. 1999;11:343–348. doi: 10.1016/s0898-6568(99)00002-9. [DOI] [PubMed] [Google Scholar]
- Freichel M, Vennekens R, Olausson J, Stolz S, Philipp SE, et al. Functional role of TRPC proteins in native systems: implications from knockout and knock-down studies. J Physiol. 2005;567:59–66. doi: 10.1113/jphysiol.2005.092999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, et al. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Goncalves AA, Toyama MH, Carneiro EM, Marangoni S, Arantes EC, et al. Participation of Na+ channels in the potentiation by Tityus serrulatus alpha-toxin TsTx-V of glucose-induced electrical activity and insulin secretion in rodent islet beta-cells. Toxicon. 2003;41:1039–1045. doi: 10.1016/s0041-0101(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Gopel S, Kanno T, Barg S, Galvanovskis J, Rorsman P. Voltage-gated and resting membrane currents recorded from B-cells in intact mouse pancreatic islets. J Physiol. 1999;521:717–728. doi: 10.1111/j.1469-7793.1999.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P. Regulation of glucagon release in mouse -cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J Physiol. 2000;528:509–520. doi: 10.1111/j.1469-7793.2000.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopel S, Zhang Q, Eliasson L, Ma XS, Galvanovskis J, Kanno T, et al. Capacitance measurements of exocytosis in mouse pancreatic α-, β- and δ-cells within intact islets of Langerhans. J Physiol. 2004;556:711–726. doi: 10.1113/jphysiol.2003.059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding WG, Barg S, Buschard K, et al. Adrenaline stimulates glucagon secretion in pancreatic A-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. J Gen Physiol. 1997;110:217–228. doi: 10.1085/jgp.110.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM. Impact of genetic background on nephropathy in diabetic mice. Am J Physiol Renal Physiol. 2006;290:F214–F222. doi: 10.1152/ajprenal.00204.2005. [DOI] [PubMed] [Google Scholar]
- Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P. Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest. 2003;33:742–750. doi: 10.1046/j.1365-2362.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Ihara Y, Yamada Y, Fujii Y, Gonoi T, Yano H, Yasuda K, et al. Molecular diversity and functional characterization of voltage-dependent calcium channels (CACN4) expressed in pancreatic beta-cells. Mol Endocrinol. 1995;9:121–130. doi: 10.1210/mend.9.1.7760845. [DOI] [PubMed] [Google Scholar]
- Iwashima Y, Pugh W, Depaoli AM, Takeda J, Seino S, et al. Expression of calcium channel mRNAs in rat pancreatic islets and downregulation after glucose infusion. Diabetes. 1993;42:948–955. doi: 10.2337/diab.42.7.948. [DOI] [PubMed] [Google Scholar]
- Jing X, Li DQ, Olofsson CS, Salehi A, Surve VV, Caballero J, et al. CaV2.3 calcium channels control second-phase insulin release. J Clin Invest. 2005;115:146–154. doi: 10.1172/JCI22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Yokokawa N, Takeda T, Nagasawa Y, Aizawa T, Yamada T. Pharmacological characterization of the voltage-dependent calcium channel of pancreatic B-cell. Endocrinology. 1989;125:2008–2014. doi: 10.1210/endo-125-4-2008. [DOI] [PubMed] [Google Scholar]
- Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, et al. alpha 1D (CaV1.3) subunits can form L-type Ca2+ channels activating at negative voltages. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- Kotturi MF, Jefferies WA. Molecular characterization of L-type calcium channel splice variants expressed in human T lymphocytes. Mol Immunol. 2005;42:1461–1474. doi: 10.1016/j.molimm.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Leung YM, Ahmed I, Sheu L, Tsushima RG, Diamant NE, et al. Electrophysiological characterization of pancreatic islet cells in the mouse insulin promoter-green fluorescent protein mouse. Endocrinology. 2005;146:4766–4775. doi: 10.1210/en.2005-0803. [DOI] [PubMed] [Google Scholar]
- Lou XL, Yu X, Chen XK, Duan KL, He LM, Qu A, et al. Na+ channel inactivation: a comparative study between pancreatic islet β-cells and adrenal chromaffin cells in rat. J Physiol. 2003;548:191–202. doi: 10.1113/jphysiol.2002.034405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey EW, Fox AP, Feldman DH, Cruz LJ, Olivera BM, et al. Ω-conotoxin: direct and persistent blockade of specific types of calcium channels in neurons but not muscle. Proc Natl Acad Sci U S A. 1987;84:4327–4331. doi: 10.1073/pnas.84.12.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Saegusa H, Zong S, Noda T, Tanabe T. Mice lacking CaV2.3 (alpha 1E) calcium channel exhibit hyperglycemia. Biochem Biophys Res Commun. 2001;289:791–795. doi: 10.1006/bbrc.2001.6051. [DOI] [PubMed] [Google Scholar]
- Mears D. Regulation of insulin secretion in islets of Langerhans by Ca2+ channels. J Membr Biol. 2004;200:57–66. doi: 10.1007/s00232-004-0692-9. [DOI] [PubMed] [Google Scholar]
- Moreno H, Rudy B, Llinas R. beta subunits influence the biophysical and pharmacological differences between P- and Q-type calcium currents expressed in a mammalian cell line. Proc Natl Acad Sci U S A. 1997;94:14042–14047. doi: 10.1073/pnas.94.25.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung Y, Skrypnyk N, Jeong MJ, Lee T, Lee MS, Kim HL, et al. Requirement for the L-type Ca2+ channel alpha 1D subunit in postnatal pancreatic beta cell generation. J Clin Invest. 2001;108:1015–1022. doi: 10.1172/JCI13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb R, Szoke B, Palma A, Wang G, Chen X, Hopkins W, et al. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- Olivera BM, McIntosh JM, Cruz LJ, Luque FA, Gray WR. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984;23:5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Pereverzev A, Mikhna M, Vajna R, Gissel C, Henry M, Weiergraber M, et al. Disturbances in glucose tolerance, insulin-release, and stress-induced hyperglycemia upon disruption of the CaV2.3 (alpha 1E) subunit of voltage-gated Ca2+ channels. Mol Endocrinol. 2002;16:884–895. doi: 10.1210/mend.16.4.0801. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Plant TD. Na+ currents in cultured mouse pancreatic B-cells. Pflugers Arch. 1988;411:429–435. doi: 10.1007/BF00587723. [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, et al. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Pollo A, Lovallo M, Biancardi E, Sher E, Socci C, Carbone E. Sensitivity to dihydropyridines, omega-conotoxin and noradrenaline reveals multiple high-voltage-activated Ca2+ channels in rat insulinoma and human pancreatic beta-cells. Pflugers Arch. 1993;423:462–471. doi: 10.1007/BF00374942. [DOI] [PubMed] [Google Scholar]
- Pressel DM, Misler S. Sodium channels contribute to action potential generation in canine and human pancreatic islet B cells. J Membr Biol. 1990;116:273–280. doi: 10.1007/BF01868466. [DOI] [PubMed] [Google Scholar]
- Qian F, Huang P, Ma L, Kuznetsov A, Tamarina N, Philipson LH. TRP genes: candidates for nonselective cation channels and store-operated channels in insulin-secreting cells. Diabetes. 2002;51(Suppl. 1):S183–S189. doi: 10.2337/diabetes.51.2007.s183. [DOI] [PubMed] [Google Scholar]
- Ramanadham S, Turk J. omega-Conotoxin inhibits glucose- and arachidonic acid-induced rises in intracellular [Ca2+] in rat pancreatic islet beta-cells. Cell Calcium. 1994;15:259–264. doi: 10.1016/0143-4160(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S, Leclercq F, Lemaire S, Piot C, Nargeot J. Ca2+ currents in compensated hypertrophy and heart failure. Cardiovasc Res. 1998;37:300–311. doi: 10.1016/s0008-6363(97)00273-3. [DOI] [PubMed] [Google Scholar]
- Rodman DM, Reese K, Harral J, Fouty B, Wu S, West J, et al. Low-voltage-activated (T-type) calcium channels control proliferation of human pulmonary artery myocytes. Circ Res. 2005;96:864–872. doi: 10.1161/01.RES.0000163066.07472.ff. [DOI] [PubMed] [Google Scholar]
- Safayhi H, Haase H, Kramer U, Bihlmayer A, Roenfeldt M, Ammon HP, et al. L-type calcium channels in insulin-secreting cells: biochemical characterization and phosphorylation in RINm5F cells. Mol Endocrinol. 1997;11:619–629. doi: 10.1210/mend.11.5.9922. [DOI] [PubMed] [Google Scholar]
- Satin LS. Localized calcium influx in pancreatic beta-cells: its significance for Ca2+-dependent insulin secretion from the islets of Langerhans. Endocrine. 2000;13:251–262. doi: 10.1385/ENDO:13:3:251. [DOI] [PubMed] [Google Scholar]
- Schulla V, Renstrom E, Feil R, Feil S, Franklin I, Gjinovci A, et al. Impaired insulin secretion and glucose tolerance in beta cell-selective CaV1.2 Ca2+ channel null mice. EMBO J. 2003;22:3844–3854. doi: 10.1093/emboj/cdg389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S. CACN4, the major alpha 1 subunit isoform of voltage-dependent calcium channels in pancreatic beta-cells: a minireview of current progress. Diabetes Res Clin Pract. 1995;28(Suppl.):S99–S103. doi: 10.1016/0168-8227(95)01085-r. [DOI] [PubMed] [Google Scholar]
- Seino S, Chen L, Seino M, Blondel O, Takeda J, et al. Cloning of the alpha 1 subunit of a voltage-dependent calcium channel expressed in pancreatic beta cells. Proc Natl Acad Sci U S A. 1992;89:584–588. doi: 10.1073/pnas.89.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, Kuhbandner S, et al. Functional embryonic cardiomyocytes after disruption of the L-type alpha 1C (CaV1.2) calcium channel gene in the mouse. J Biol Chem. 2000;275:39193–39199. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renstrom E, Wietzorrek G, Berjukov S, et al. Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca2+ channels. J Clin Invest. 2004;113:1430–1439. doi: 10.1172/JCI20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, et al. An L-type calcium channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–263. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- Takahashi E, Ito M, Miyamoto N, Nagasu T, Ino M, Tanaka I. Increased glucose tolerance in N-type Ca2+ channel alpha 1B -subunit gene-deficient mice. Int J Mol Med. 2005;15:937–944. [PubMed] [Google Scholar]
- Tottene A, Moretti A, Pietrobon D. Functional diversity of P-type and R-type calcium channels in rat cerebellar neurons. J Neurosci. 1996;16:6353–6363. doi: 10.1523/JNEUROSCI.16-20-06353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger RH. Glucagon physiology and pathophysiology. N Engl J Med. 1971;285:443–449. doi: 10.1056/NEJM197108192850806. [DOI] [PubMed] [Google Scholar]
- Wang L, Bhattacharjee A, Fu J, Li M. Abnormally expressed low-voltage-activated calcium channels in beta-cells from NOD mice and a related clonal cell line. Diabetes. 1996;45:1678–1683. doi: 10.2337/diab.45.12.1678. [DOI] [PubMed] [Google Scholar]
- Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, et al. Glucose inhibition of glucagon secretion from rat alpha-cells is mediated by GABA released from neighboring beta-cells. Diabetes. 2004;53:1038–1045. doi: 10.2337/diabetes.53.4.1038. [DOI] [PubMed] [Google Scholar]
- Yaney GC, Wheeler MB, Wei X, Perez-Reyes E, Birnbaumer L, et al. Cloning of a novel alpha 1-subunit of the voltage-dependent calcium channel from the beta-cell. Mol Endocrinol. 1992;6:2143–2152. doi: 10.1210/mend.6.12.1337146. [DOI] [PubMed] [Google Scholar]
- Yang SN, Berggren PO. Beta-cell CaV channel regulation in physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2005;288:E16–E28. doi: 10.1152/ajpendo.00042.2004. [DOI] [PubMed] [Google Scholar]
- Yang SN, Larsson O, Branstrom R, Bertorello AM, Leibiger B, Leibiger IB, et al. Syntaxin 1 interacts with the L(D) subtype of voltage-gated Ca2+ channels in pancreatic beta cells. Proc Natl Acad Sci U S A. 1999;96:10164–10169. doi: 10.1073/pnas.96.18.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
T-type primers are sensitive enough to amplify transcripts in single islet cells. Three to five mouse pancreatic islets (500–2000 cells per islet) were freeze-thawed and mRNA reverse transcribed.
Isradipine sensitivity and single-cell RT-PCR with CaV1.3-specific primers in B- and A-cells of βCaV1.2−/− mice.