Abstract
The potassium channels TASK-1 and TASK-3 show high sequence homology but differ in their sensitivity to extracellular divalent cations. Heterologous expression in HEK293 cells showed that the single-channel conductance of TASK-3 increased approximately four-fold after removal of external divalent cations, whereas the conductance of TASK-1 was unaffected. Replacing the glutamate at position 70 of TASK-3 by a lysine or arginine residue abolished the sensitivity to divalent cations. The reverse mutation in TASK-1 (K70E) induced sensitivity to divalent cations. The organic polycations spermine and ruthenium red modulated the conductance of TASK-3 in a similar way as Ca2+ or Mg2+. Our data suggest that these effects were mediated by shielding of the negative charges in the extracellular loops of TASK-3. Whole-cell currents carried by TASK-3 channels were inhibited by spermine and ruthenium red even in the presence of external divalent cations. These data suggest that, in addition to their effect on single-channel conductance, spermine and ruthenium red decreased the open probability of TASK-3 channels, probably by binding to residue E70. The standing outward current in thalamocortical relay neurons, which is largely carried by TASK channels, was also inhibited by divalent cations and spermine. Using the differential sensitivity of TASK-1 and TASK-3 to divalent cations and spermine we found that about 20% of the standing outward current in thalamocortical relay neurons flows through TASK-3 channels. We conclude from our results that inhibition of TASK-3 channels may contribute to the neuromodulatory effect of spermine released from neurons during repetitive activity or during hypoxia.
Two-pore-domain K+ channels (K2P channels) are characterized by four transmembrane regions (M1–M4), two pore-forming domains (P1 and P2) and a large extracellular loop between M1 and P2. Since the first K2P channels discovered gave rise to outwardly rectifying currents with no obvious voltage dependence, K2P channels have long been regarded as passive leak channels or ‘open rectifiers’ that merely contribute to setting the resting potential. However, recent studies indicate that the functional properties of K2P channels are far more diverse than initially expected (Patel & Honore, 2001; Bayliss et al. 2003; Kim, 2003; Talley et al. 2003). Homology analysis indicates that the K2P channels can be subdivided into six structurally related subfamilies. The potassium channels TASK-1, TASK-3 and TASK-5 (which has not been functionally expressed so far) form a separate subfamily (Duprat et al. 1997; Kim et al. 2000; Rajan et al. 2000; Ashmole et al. 2001; Karschin et al. 2001; Kim & Gnatenco, 2001). These two-pore-domain acid-sensitive K+ (TASK) channels show a very complex regulation: (i) they can be inhibited by extracellular acidification (Duprat et al. 1997; Kim et al. 2000; Rajan et al. 2000); (ii) they can be inhibited via Gq coupled receptors (Talley et al. 2000); (iii) they can be activated by depolarization (Rajan et al. 2000) (iv) they are upregulated after knockout of extra-junctional postsynaptic receptors (Brickley et al. 2001); (v) they can be activated by volatile anaesthetics (Patel et al. 1999; Sirois et al. 2000; Meuth et al. 2003); and (vi) they are sensitive to oxygen tension (Buckler et al. 2000; Hartness et al. 2001). These features indicate that TASK-1 and TASK-3 may be functionally important in the central nervous system (Bayliss et al. 2003), in cardiac muscle (Kim et al. 1999; Jones et al. 2002), and in other cell types (Czirjak & Enyedi, 2002; Gurney et al. 2003).
Recently, a rather unusual property of the K2P channel TASK-3 has been described: its conductance is extraordinarily sensitive to extracellular divalent cations (Rajan et al. 2000); removal of external Ca2+ and Mg2+ increased single-channel conductance about four-fold. In this paper we analyse the molecular mechanisms underlying the modulation of the permeation properties of the channel by extracellular Ca2+and Mg2+ and by the organic polycations spermine and ruthenium red. Our results suggest that extracellular divalent cations and polycations modulate the single-channel conductance of TASK-3 by shielding of negatively charged residues in the outer vestibule of the pore and that, in addition, spermine and ruthenium red can inhibit whole-cell currents by reducing the open probability of TASK-3 channels.
We then applied the insights gained by mutational analysis of the channels to the study of thalamocortical relay neurons, which are known to express TASK-1 and TASK-3. Our data suggest that at depolarized potentials a substantial ‘standing outward current’ is flowing through TASK-3 channels in these neurons. This current can be inhibited by spermine at concentrations between 100 and 500 μm. The resulting increase in neuronal excitability may contribute to the neuromodulatory effects of spermine released into the synaptic cleft from nerve terminals. A preliminary report of some of our findings has been published (Derst et al. 2002).
Methods
Cloning, mutagenesis and heterologous expression of TASK channels
The coding regions of mouse TASK-1 (accession number, AF065162) and human TASK-3 (accession number, AF212829) were cloned in the expression vector pcDNA3.1(+) (Rajan et al. 2000). Chimeric constructs were made using restriction fragment exchanges, either with internal restrictions sites of the TASK genes or with restriction sites introduced by site-directed mutagenesis as previously described (Karschin et al. 2001). Point mutations were introduced using the QuikChange mutagenesis kit (Stratagene). The sequences of all chimeric constructs and point mutations were verified by automated sequencing. For expression in HEK293 cells, ∼1 μg plasmid DNA was transfected using lipofectamine 2000 (Gibco).
Patch-clamp experiments in HEK293 cells were carried out as previously described (Rajan et al. 2000). In brief, single-channel currents were recorded using an Axopatch 200B amplifier and pCLAMP8 software (Axon Instruments). Cell capacitance and pipette series resistance were compensated. The pipette resistance was in the range 8–12 MΩ. The standard pipette solution contained (mm): 145 KCl, 1 CaCl2, 1 MgCl2, 5 Hepes, pH 7.4; CaCl2, MgCl2 or LaCl3 was added or omitted as required. Divalent-cation-free pipette solution contained, in addition, 5 mm EGTA. The bath solution contained (mm): 140 KCl, 2 MgCl2, 5 Hepes, 10 glucose; pH was adjusted to 7.4 with NaOH. All chemicals, including spermine and ruthenium red, were from Sigma (Deisenhofen, Germany). The data were analysed with software, PC.DAQ1.1, developed in our laboratory using LabView (National Instruments). Single-channel currents from cell-attached patches were sampled at 2.5 or 5 kHz and filtered with a −3 dB cut-off frequency of 0.5 or 1 kHz. The data were not corrected for the liquid junction potential (Neher, 1992).
Whole-cell measurements in thalamocortical relay neurons
Patch-clamp recordings were performed on thalamocortical relay (TC) neurons of the dorsal lateral geniculate nucleus (dLGN). Long Evans rats (postnatal days 12–20) were anaesthetized with halothane and decapitated. A block of tissue containing the thalamus was removed and placed in ice-cold saline, containing (mm): 200 sucrose, 20 Pipes, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, 0.5 CaCl2, 10 dextrose; pH was adjusted to 7.35 with NaOH. Thalamic slices were prepared as coronal sections on a vibratome. Prior to recording, slices were kept submerged in standard artificial cerebrospinal fluid (ACSF) containing (mm): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 24 NaHCO3, 2 MgSO4, 2 CaCl2, 10 dextrose; pH adjusted to 7.35 by bubbling with a mixture of 95% O2 and 5% CO2. Individual cells were visually identified by infrared differential interference contrast video-microscopy (Dodt & Zieglgänsberger, 1990). For whole-cell current-clamp recording, slices were continuously superfused with a solution containing (mm): 120 NaCl, 2.5 KCl, 1.25 NaH2PO4, 30 Hepes, 2 MgSO4, 2 CaCl2, 10 dextrose; pH was adjusted to 7.3 with HCl. For whole-cell voltage-clamp recording, slices were superfused with a solution containing (mm) 120 NaCl, 2.5 KCl, 1.25 NaH2PO4, 30 Hepes, 4 MgSO4, 10 glucose; pH was adjusted to 7.3 or 6.0 with HCl (control solution). For divalent-cation-free conditions we switched from control solution to a solution containing 0 mm Mg2+ and 0 mm Ca2+; the osmolality was kept constant at 305 mosmol kg−1 by adding mannitol.
In all experiments the pipette solution contained (mm): 95 potassium gluconate, 20 K3-citrate, 10 NaCl, 10 Hepes, 1 MgCl2, 0.5 CaCl2, 3 BAPTA, 3 Mg-ATP, 0.5 Na-GTP, 0.1 Na3VO4; the pH was adjusted to 7.25 with KOH, the osmolality was 295 mosmol kg−1. The patch pipettes were pulled from borosilicate glass (GC150T-10, Clark Electromedical Instruments, Pangbourne, UK). The patch electrodes had a resistance of 2–3 MΩ, the access resistance was in the range of 5–15 MΩ. The electrodes were connected to an EPC-10 amplifier (HEKA Elektronik, Lamprecht, Germany). Series resistance compensation of more than 40% was routinely used. Voltage-clamp experiments were controlled by Pulse software (HEKA Elektronik). A liquid junction potential of 8 ± 1 mV (n = 6) was measured (Neher, 1992) and taken into account. Current–voltage relations of TC neurons were studied by applying voltage ramps of 800 ms duration from −28 to −138 mV; the ramps were applied at intervals of 20 s. All experiments were carried out at room temperature.
Statistics
Results are presented as means ± s.d. Statistical significance was determined using the non-parametric Mann–Whitney test (GraphPad Prism software) or Student's t test. Differences were considered significant if P < 0.05. In the bar graphs presented, statistically significant differences are marked by an asterisk (*).
Results
Reduction of TASK-3 single-channel conductance by extracellular divalent cations
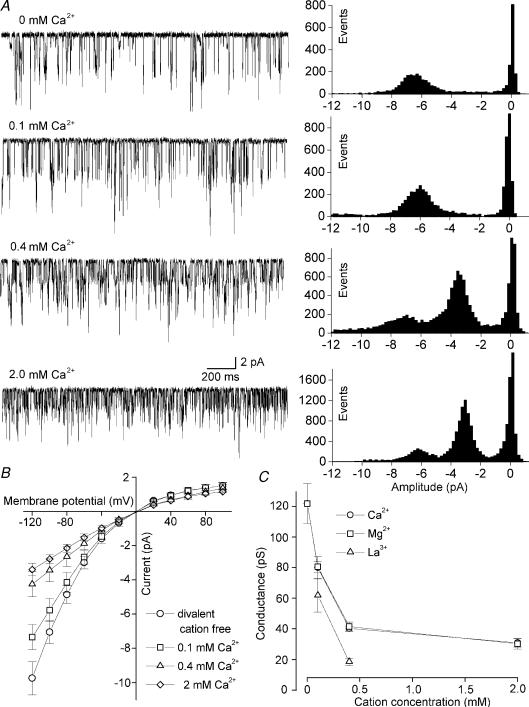
Removal of extracellular divalent cations causes an approximately four-fold increase in the conductance of TASK-3 channels (Rajan et al. 2000). To analyse the molecular mechanisms underlying this effect we carried out cell-attached recordings of TASK-3 channels expressed in HEK293 cells. Typical recordings and the corresponding amplitude histograms are shown in Fig. 1A. It can be seen that the amplitude of the single-channel currents measured at a transmembrane potential of −100 mV decreased with increasing Ca2+ concentration in the pipette solution. Since the single-channel current-voltage relation was curved (Fig. 1B) we determined γ−100, the mean slope conductance at a transmembrane potential of −100 mV, by linear interpolation of the currents measured between −120 and −80 mV (n = 3–8 patches). The dependence of γ−100 on the concentration of extracellular Ca2+, Mg2+ and La3+ is shown in Fig. 1C. In divalent-cation-free solution, γ−100 of TASK-3 was 122 ± 14 pS (n = 8); addition of 100 μm Ca2+ to the pipette solution decreased γ−100 to 80 ± 7 pS. In the presence of 0.4 and 2.0 mm Ca2+ the value of γ−100 was further decreased (40 ± 2 and 31 ± 3 pS, respectively). There was no significant difference between the effects of Ca2+ and Mg2+. In a solution containing 1 mm Ca2+ plus 1 mm Mg2+, the value of γ−100 was 29 ± 3 pS (n = 8; not shown). The trivalent cation lanthanum was even more potent than Ca2+ and Mg2+ in decreasing γ−100 (P < 0.01). With low concentrations of external divalent cations the single-channel current–voltage relation showed strong inward rectification (Fig. 1B). Due to the low signal-to-noise ratio, the effects of divalent cations on the conductance at positive potentials could not be analysed with sufficient accuracy.
Figure 1. Ca2+ dependence of single-channel conductance of TASK-3.
A, typical cell-attached recordings of TASK-3 channels at a transmembrane potential of −100 mV (inside – outside); inward currents are downward. The K+ concentration in the pipette solution was 145 mm; the Ca2+ concentration in the pipette solution is indicated above the traces. On the right the corresponding amplitude histograms are shown. B, single-channel current–voltage relation at external Ca2+ concentrations of 0, 0.1, 0.4 and 2 mm. C, plot of the mean single-channel slope conductance of TASK-3 at −100 mV (calculated by linear regression from the measurements between −120 and −80 mV) as a function of the external Ca2+, Mg2+ and La3+ concentration. The error bars represents standard deviation.
Comparison of the sensitivities of TASK-1 and TASK-3 to divalent cations
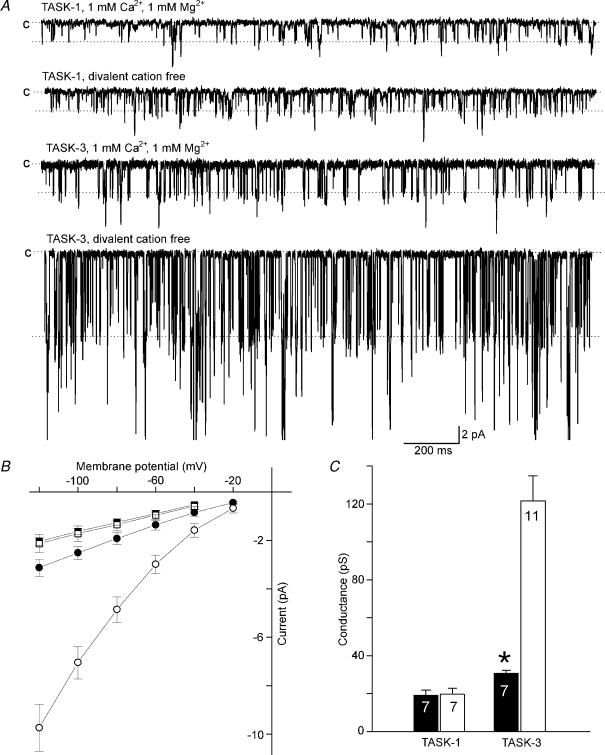
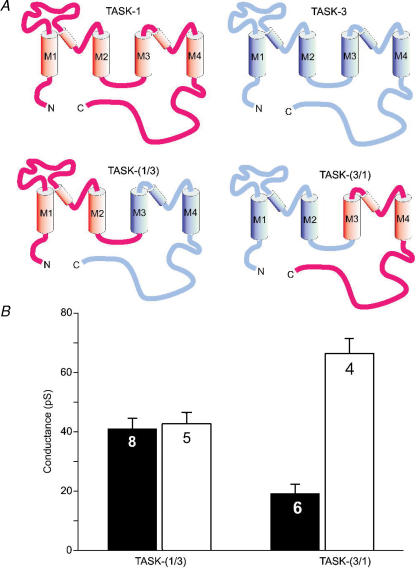
As a first step in the identification of the amino acid residues responsible for these effects we compared the characteristics of TASK-3 with those of the closest homologue, TASK-1. Surprisingly, the single-channel conductance of TASK-1 was found to be insensitive to extracellular divalent cations (Fig. 2). The value of γ−100 was 19 ± 3 pS in the presence of 1 mm Ca2+ plus 1 mm Mg2+ (n = 7) and 20 ± 3 pS (n = 7) in the absence of divalent cations. To localize the putative divalent-cation sensor we constructed two chimeras of TASK-1 and TASK-3 (denoted TASK-(1/3) and TASK-(3/1)) by fusing the N-terminal part of one channel (including M1, the large extracellular loop, P1, M2 and the intracellular M2–M3 linker, see Fig. 3A) to the C-terminal part of the other channel (including M3, P2, M4 and the cytosolic C-terminus). The TASK-(1/3) chimera showed a conductance (γ−100) around 40 pS both in the presence of 2 mm external Ca2+ and in divalent-cation-free solution (Fig. 3B). In contrast, the conductance of the TASK-(3/1) chimera was strongly dependent on external divalent cations; γ−100 increased more than three-fold (from 19 ± 3 to 66 ± 5 pS) after removal of external Ca2+. These results indicate that the amino acids responsible for the sensitivity to external divalent cations reside in the N-terminal part of TASK-3.
Figure 2. Comparison of the Ca2+ sensitivity of TASK-1 and TASK-3 channels.
A, typical recordings of TASK-1 (top) and TASK-3 (bottom) in the presence and absence of divalent external cations. The level of the closed state (c) and the open state (derived from amplitude histograms) are indicated by broken lines. The transmembrane potential was −120 mV. B, single-channel current–voltage relation of TASK-1 (filled symbols) and TASK-3 (open symbols) in the presence (circles) and absence (squares) of external divalent cations. C, the mean slope conductance at −100 mV of TASK-1 and TASK-3 in the presence (filled bars) and absence (open bars) of external divalent cations. The error bars represent standard deviation. The number of different channels analysed in each case is indicated.
Figure 3. Analysis of TASK-1/TASK-3 chimeras.
A, schematic diagram of the constructs used; red represents TASK-1 and blue represents TASK-3. B, bar graph of the slope conductance at −100 mV of the TASK-(1/3) and the TASK-(3/1) chimeras. The data obtained in the presence (filled bars) and absence (open bars) of external divalent cations are compared. The error bars represent standard deviation.
The importance of the residue at position 70
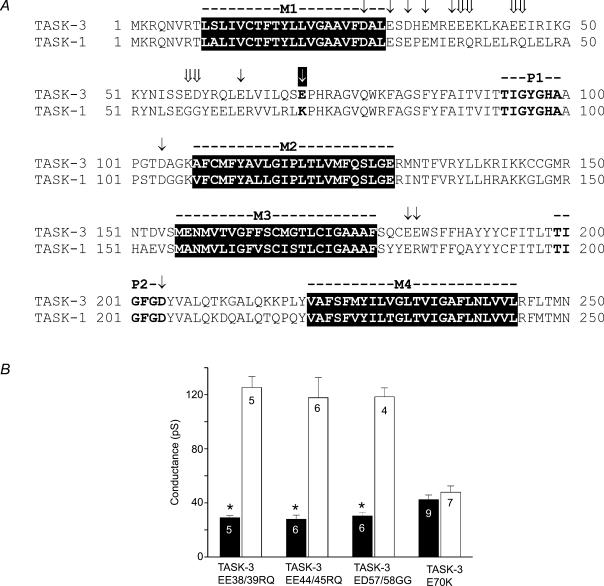
Since the two N-terminal transmembrane regions and the first pore region of TASK-1 and TASK-3 are highly homologous we speculated that the less conserved M1–P1 extracellular loop may determine the sensitivity to divalent cations. This part of the channel harbours many charged residues that may influence binding of divalent cations (Fig. 4A). To identify residues possibly involved in the modulation of single-channel conductance we selected seven negatively charged amino acids of TASK-3 that are not conserved in TASK-1, and mutated them to the corresponding amino acid of the TASK-1 sequence.
Figure 4. Mutagenesis of negatively charged residues in TASK-3.
A, alignment of the core region (M1–M4) of TASK-1 and TASK-3. The transmembrane regions M1 to M4 are shown in white on black and the pore regions are shown in bold letters. The simultaneous exchange of two adjacent residues is indicated by double arrows. The exchanged residues conserved between TASK-1 and TASK-3 are indicated by arrows (↓). The critical residues at position 70 are shown in bold and are marked by a white arrow. B, bar graph of the slope conductance at −100 mV of the mutants indicated. The data obtained in the presence (filled bars) and absence (open bars) of external divalent cations are compared. The error bars represent standard deviation.
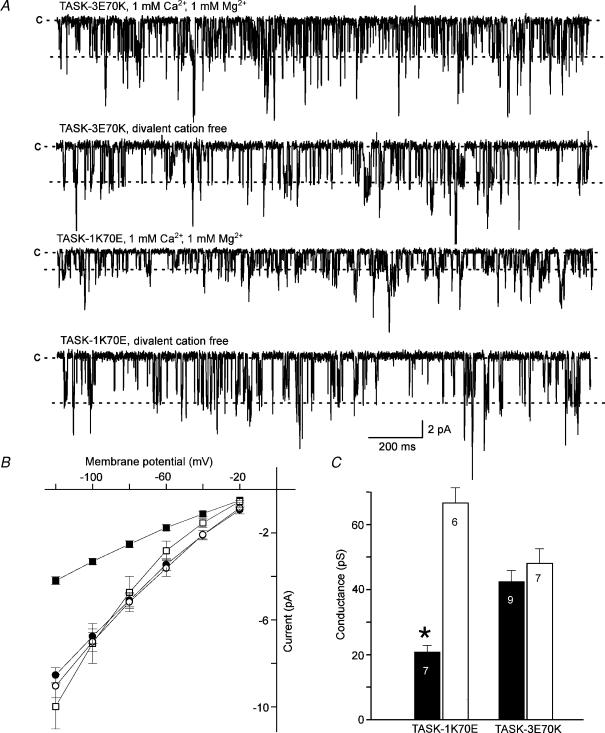
Three double mutants (indicated by the double arrows in Fig. 4A), TASK-3 EE38/39RQ, EE44/45RQ and ED57/58GG, showed Ca2+ sensitivities comparable to TASK-3 wild-type, which makes it unlikely that one of these six negatively charged residues is essential for the modulation of single-channel conductance by divalent cations. However, the TASK-3 mutation E70K (⇓, while arrow on black background) greatly reduced the sensitivity to external Ca2+: the value of γ−100 was 43 ± 4 pS in the presence and 48 ± 5 pS in the absence of 2 mm Ca2+. These data suggest that the glutamate residue at position 70 plays an essential role in the modulation of the conductance of TASK-3. Furthermore, the reverse mutation in TASK-1 channels, K70E, induced Ca2+ and Mg2+ sensitivity in this channel (Fig. 5). In this mutant, omission of Ca2+ and Mg2+ from the pipette solution increased γ−100 from 21 ± 2 to 67 ± 5 pS, indicating that TASK-1 K70E was almost as sensitive to external Ca2+ and Mg2+ as wild-type TASK-3. Taken together, the data presented in Figs 4 and 5 unambiguously show that the difference in the sensitivity of TASK-3 and TASK-1 channels to external divalent cations is related to the amino acid at position 70 in the extracellular loop.
Figure 5. Interconversion of the properties of TASK-1 and TASK-3.
A, typical recordings of the TASK-3E70K and the TASK-1K70E mutants in the presence and absence of external divalent cations. The transmembrane potential was −120 mV. B, current–voltage relation of TASK-3E70K (open symbols) and TASK-1K70E (filled symbols) in the presence (squares) and absence (circles) of divalent cations. C, bar graph of the slope conductance at −100 mV of the mutants indicated. The data obtained in the presence (filled bars) and absence (open bars) of external divalent cations are compared. The error bars represent standard deviation.
A positive charge at position 70 abolishes the sensitivity to divalent cations
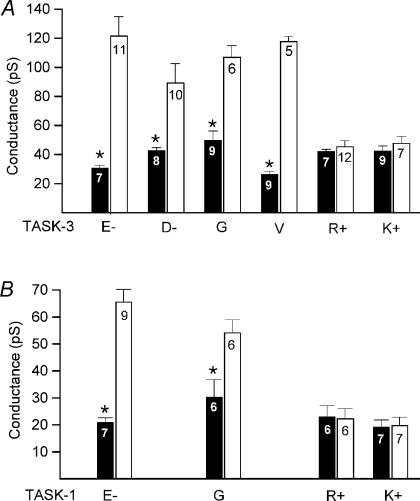
To clarify whether the glutamate at position 70 of TASK-3 acts as a Ca2+ sensor and directly binds divalent cations we exchanged E70 for different charged and uncharged residues (Fig. 6). As might have been expected, the conservative change to the negatively charged aspartate preserved the sensitivity to divalent cations. Surprisingly, the sensitivity to divalent cations was also preserved when glutamate was replaced by amino acids possessing a neutral side chain (E70G and E70V). This suggests that binding of divalent cations to glutamate at position 70 is not required for the change in single-channel conductance. Apparently, it is not the presence of a negative charge at residue 70 in TASK-3 that introduces the sensitivity, but it is the presence of a positive charge at position 70 in TASK-1 that abolishes the sensitivity to extracellular divalent cations. To test this hypothesis we constructed further mutants of TASK-1. Indeed, replacement of the wild-type lysine at position 70 by arginine (K70R) failed to introduce sensitivity to divalent cations, whereas the introduction of the neutral glycine (K70G) was associated with a substantial sensitivity to external cations (Fig. 6).
Figure 6. Replacement of the residue at position 70 by different amino acids.
The data obtained in the presence (filled bars) and absence (open bars) of external divalent cations are compared. The residue at position 70 of TASK-3 (top) and TASK-1 (bottom) was exchanged for the amino acids indicated. The error bars represent standard deviation.
Other negative charges of TASK-3 are not essential
We hypothesized that some of the other negatively charged residues might play an auxiliary role in the interaction of divalent cations with the channel (by modifying the local electric field). Therefore we exchanged individually all negatively charged residues conserved between TASK-3 and TASK-1 (D-27, E-30, D-32, E-34, E-37, E-63) to alanine (n = 5 measurements for each mutant), as indicated in Fig. 4A ( ). However, none of these mutations resulted in a significant change in Ca2+ sensitivity. Replacement of one of the negatively charged amino acids at positions 104, 182 or 183 by alanine did not produce functional channels. Replacement with structurally more conserved amino acids (D104N; E182Q and E183Q; ↓ in Fig. 4A) did produce functional channels but had no effect on sensitivity to divalent cations (n = 5–8 for each mutant). Exchange of the aspartate residue at position 204 (↓), part of the second pore motif (GYGD), to alanine or asparagine residues did not result in functional channels; therefore no functional prediction can be made for D204. Taken together, these findings suggest that, apart from E70, none of the negatively charged residues in the extracellular loops appears to be essential for the effect of extracellular divalent cations on single-channel conductance.
). However, none of these mutations resulted in a significant change in Ca2+ sensitivity. Replacement of one of the negatively charged amino acids at positions 104, 182 or 183 by alanine did not produce functional channels. Replacement with structurally more conserved amino acids (D104N; E182Q and E183Q; ↓ in Fig. 4A) did produce functional channels but had no effect on sensitivity to divalent cations (n = 5–8 for each mutant). Exchange of the aspartate residue at position 204 (↓), part of the second pore motif (GYGD), to alanine or asparagine residues did not result in functional channels; therefore no functional prediction can be made for D204. Taken together, these findings suggest that, apart from E70, none of the negatively charged residues in the extracellular loops appears to be essential for the effect of extracellular divalent cations on single-channel conductance.
Spermine and ruthenium red also decrease the conductance of TASK-3 channels
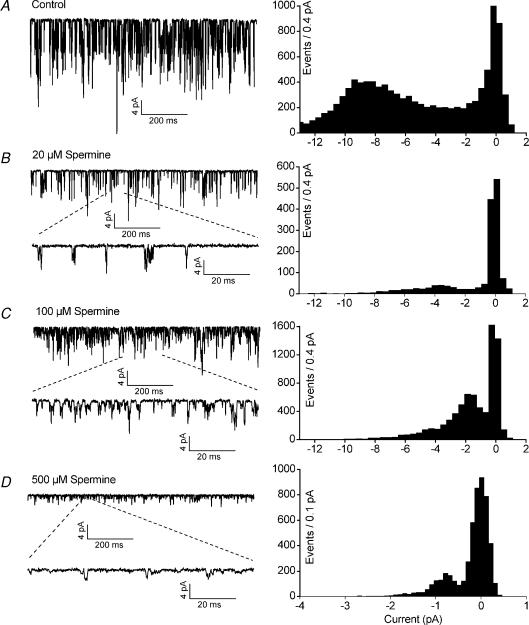
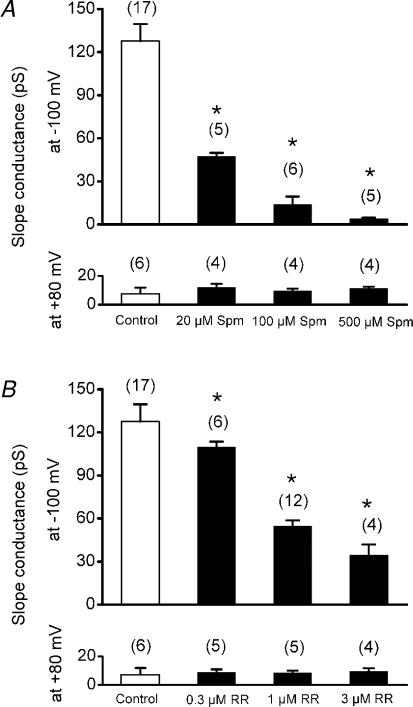
The indistinguishable effects of Ca2+ and Mg2+ on single-channel conductance and the even more pronounced effect of La3+ suggest that the screening of fixed negative charges in the outer vestibule of TASK-3 may be involved. To test this hypothesis we studied the effects of the organic polycations spermine and ruthenium red (RR). Addition of spermine to the (Ca2+-and Mg2+-free) pipette solution caused a concentration-dependent reduction in the amplitude of elementary TASK-3 inward currents (Fig. 7). The slope conductance of human TASK-3 at −100 mV (γ−100) was reduced to 47.2 ± 7.2 pS in the presence of 20 μm spermine, to 13.9 ± 5.6 pS in the presence of 100 μm spermine, and to 3.8 ± 0.8 pS in the presence of 500 μm spermine (Fig. 8A). γ−100 of rat TASK-3 was reduced from 20 ± 3 pS (n = 7) to 13.1 ± 2.4 pS (n = 9) in the presence of 100 μm spermine. The conductance at +80 mV was not significantly changed by application of spermine (Fig. 8A).
Figure 7. The effects of extracellular spermine on TASK-3 channels.
Representative cell-attached recordings at a transmembrane potential of −100 mV. The corresponding amplitude histograms are shown on the right. The cells were superfused with high-K+ bath solution; the pipette solution contained no divalent cations. A, control. B–D, with different concentrations of spermine in the pipette solution.
Figure 8. Effects of spermine and RR on the slope conductance of TASK-3 channels.
A, effects of spermine. B, effects of ruthenium red. The measurements were carried out with symmetrical K+. The number of experiments for each condition is shown in brackets. The slope conductance at a transmembrane potential of −100 mV (γ−100) for each channel was estimated by linear regression of the single-channel currents at −120, −100 and −80 mV; the slope conductance at +80 mV (γ+80) was estimated by linear regression of the single-channel currents at +60, + 80 and + 100 mV. The error bars represent standard deviation.
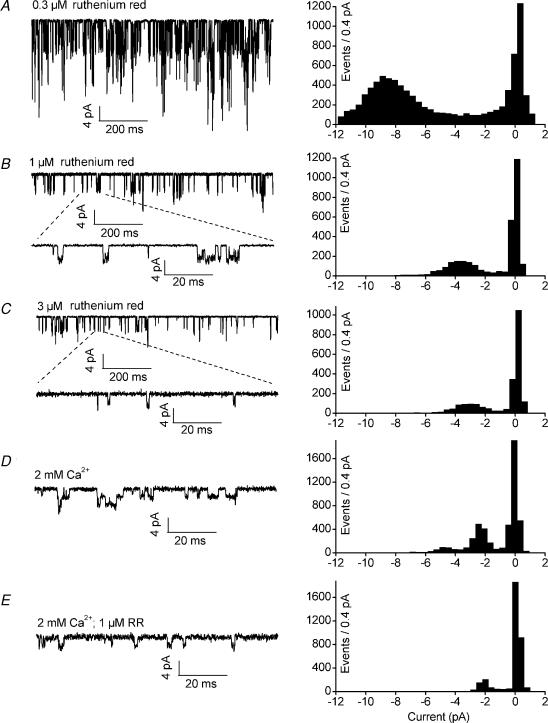
Addition of RR to the pipette solution also caused a concentration-dependent reduction in single-channel conductance of TASK-3 (Fig. 9A-C). γ−100 was decreased to 109.7 pS in the presence of 0.3 μm RR, to 53.1 ± 3.5 pS in the presence of 1 μm RR, and to 34.7 ± 3.9 pS in the presence of 3 μm RR (Fig. 8B). The conductance at +80 mV was not significantly changed by application of RR (Fig. 8B). The similarity of the effects of inorganic divalent cations (Fig. 1) and the organic polycations spermine and RR suggests that screening of negative charges in the outer vestibule of the channel reduces permeation of K+ ions in the inward direction.
Figure 9. The effects of ruthenium red on TASK-3 channels.
Representative cell-attached recordings at a transmembrane potential of −100 mV; the corresponding amplitude histograms are shown on the right. The cells were superfused with high-K+ bath solution. A–C, with different concentrations of RR and no divalent cations in the pipette solution. D, with 2 mm Ca2+ in the pipette solution. E, with 2 mm Ca2+ and 1 μm RR in the pipette solution.
In the presence of 2 mm extracellular Ca2+, 1 μm RR had no significant effect on single-channel conductance; the single-channel current at −100 mV was 2.34 ± 0.05 pA (n = 7) in the presence of 2 mm Ca2+ and 2.37 ± 0.09 pA (n = 5) in the presence of 2 mm Ca2+ plus 1 μm RR (Fig. 9D and E). Similar occlusion was observed for the effects of Ca2+ and spermine on single-channel conductance (not illustrated), consistent with a common mechanism of action of divalent inorganic cations and organic polyvalent cations. On the other hand, NPo, the number of channels in the patch × open probability, appeared to be lower in cell-attached patches containing spermine or RR (see below). However, due to the variability of the number of channels per patch this was difficult to verify in single-channel recordings.
Effects of spermine and ruthenium red on TASK-3 and TASK-1 whole-cell currents
To test whether spermine and RR may affect the open probability of TASK-3 channels we analysed the effects of these polycations on whole-cell currents in transfected HEK293 cells superfused with physiological salt solution (5 mm K+). Figure 10A illustrates that the whole-cell current carried by TASK-3 channels showed outward rectification, which is attributable to the asymmetrical K+ concentration and to an increase in open probability with depolarization (Rajan et al. 2000). In Ca2+- and Mg2+-free bath solution, the mean inward current at −100 mV was − 736 ± 194 pA, and the mean outward current at −20 mV was 1576 ± 266 pA (n = 12). Application of 20, 100 or 500 μm spermine caused a significant inhibition of both inward and outward currents in the absence of external divalent cations (Fig. 10B). The reversal potential of the spermine-sensitive current was near the calculated K+ equilibrium potential (taking into account the liquid junction potential; see Methods). Spermine at a concentration of 100 μm reduced the TASK-3 current by more than 50%. Application of 0.1 μm RR also reduced TASK-3 inward and outward currents by more than 50%, and 3 μm RR had an even larger effect.
Figure 10. Whole-cell currents in HEK293 cells transfected with TASK-3.
The cells were superfused with physiological extracellular solution (5 mm K+). A, whole-cell current measured in divalent-cation-free external solution and after addition of 100 or 500 μm spermine. B, concentration dependence of the effects spermine on steady-state outward current at −20 mV and on steady-state inward current at −100 mV. C, concentration dependence of the effects RR on steady-state currents at −20 and −100 mV. The number of experiments is indicated in brackets. The error bars represent standard deviation.
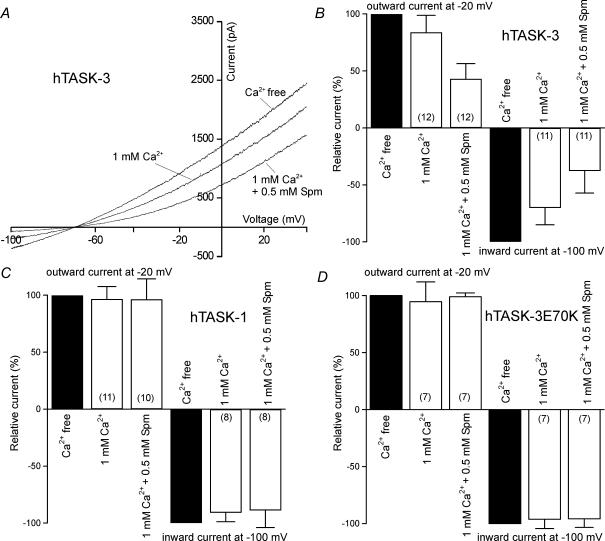
Since our single-channel measurements had suggested that organic polycations had no additive effect on single-channel conductance in the presence of external Ca2+ (Fig. 9D and E), we also tested the effects of successive application of Ca2+ and spermine on whole-cell currents. Application of 1 mm Ca2+ produced a significant decrease in both the inward current at −100 mV and the outward current at −20 mV (Fig. 11A and B). Application of 500 μm spermine in the presence of Ca2+ induced an additional inhibition of inward and outward currents (Fig. 11A and B). Taken together, our whole-cell measurements suggest that spermine and RR may cause a reduction in the open probability of wild-type TASK-3 channels. When the cells were transfected with TASK-1 (Fig. 11C) or TASK-3 E70K (Fig. 11D), application of 1 mm Ca2+ or 1 mm Ca2+ plus 500 μm spermine had no significant effect on whole-cell currents.
Figure 11. Effects of spermine in the presence of external Ca2+ on TASK-3, TASK-1 and the TASK-3 mutant E70K.
A, whole-cell hTASK-3 current measured in HEK293 cells with divalent-cation-free external solution and after addition of 1 mm Ca2+, or 1 mm Ca2+ plus 500 μm spermine. B, effects of Ca2+ and spermine on TASK-3 channels. C, effects of Ca2+ and spermine on TASK-1 channels. D, effects of Ca2+ and spermine on TASK-3E70K channels. The error bars represent standard deviation.
TASK currents in thalamocortical relay neurons
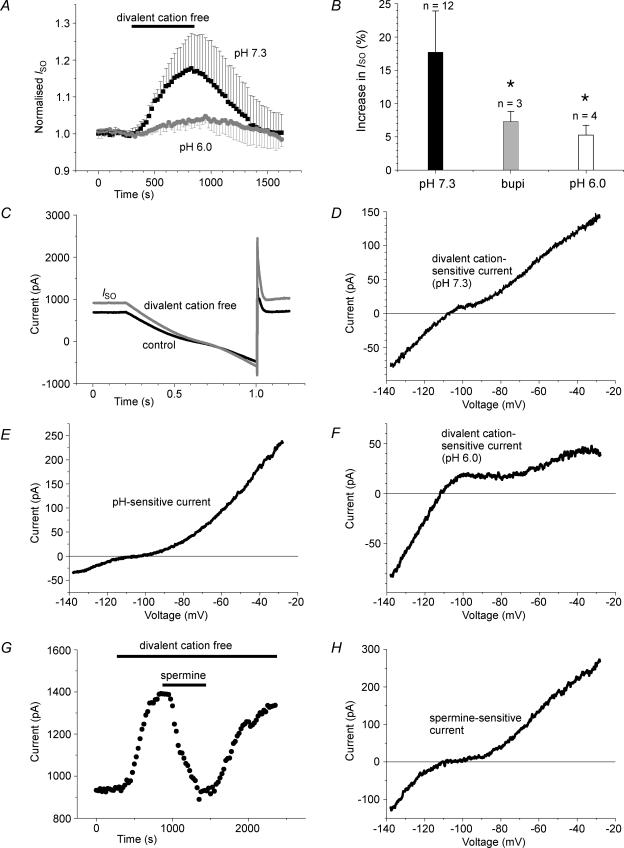
In order to assess the role of divalent and polyvalent cations on native TASK-mediated currents we have made use of thalamocortical relay (TC) neurons, in which the contribution of TASK channels to a standing outward current (ISO) is well documented (Meuth et al. 2003). Here we sought to identify the relative contribution of TASK-1 and TASK-3 channels to ISO by exploiting the differential sensitivity of the two isoforms to external divalent cations and spermine. A typical experiment is illustrated in Fig. 12. TC neurons were superfused with Ca2+-free solution containing 4 mm Mg2+ (pH 7.3) and, under these conditions, displayed an ISO of 626 ± 226 pA at −28 mV (n = 19). Upon removal of Mg2+, ISO increased by 18 ± 6% (n = 12; P < 0.001) (Fig. 12A). The current–voltage relation of the divalent-cation-sensitive current was obtained by applying voltage ramps between −28 and −138 mV (Fig. 12C) and subtracting the currents measured after removal of divalent cations from those obtained under control conditions. The subtraction current (Fig. 12D) displayed a nearly linear current–voltage relation and a reversal potential of −105 ± 8 mV (n = 8), which is close to the expected K+ equilibrium potential of −104 mV. In 4 out of 23 tested cells the subtraction current reversed at ∼−50 mV. These cells were omitted from the analysis. Pre-incubation with bupivacaine (20 μm), which inhibits K2P channels (Kindler et al. 1999), resulted in a significantly decreased effect of divalent-cation-free solution (7 ± 2%; n = 3; Fig. 12B).
Figure 12. Effect of divalent cations and spermine on the standing outward current (ISO) in thalamocortical relay (TC) neurons.
A, normalized mean amplitude of ISO (at −28 mV) plotted against time, under control conditions (pH 7.3; ▪; n = 6) and during extracellular acidification (pH 6.0; □; n = 4). The period of divalent cation removal is indicated by the horizontal line. B, bar graph representation of ISO at pH 7.3, after preincubation with bupivacaine (20 μm) and at pH 6.0. C, representative currents traces evoked by ramping the membrane potential from −28 mV to −138 mV over 800 ms under control conditions (black trace) and during removal of divalent cations from the extracellular medium (grey trace). D–F, current–voltage relation of the divalent-cation-sensitive current at pH 7.3 (D, divalent cation-free–control), the pH-sensitive current (E, pH 7.3–pH 6.0), and the divalent-cation-sensitive current at pH 6.0 (F, divalent-cation-free, pH 6–control, pH 6.0) obtained by graphical subtraction. G, amplitude of ISO plotted against time in a TC neuron recorded under voltage-clamp conditions at −28 mV. Periods of removal of divalent cations and application of spermine are indicated by horizontal lines. H, current–voltage relation of the spermine-sensitive (500 μm; divalent cation-free–spermine) current obtained by graphical subtraction. The error bars represent standard deviation.
Next, the same set of experiments was performed at pH 6. The switch of pH from 7.3 to 6.0 was accompanied by a decrease in ISO of 35 ± 5% (n = 4). The pH-sensitive current (measured as subtraction current in voltage-ramp experiments) displayed outward rectification, as expected for TASK channels (Fig. 12E), and had a reversal potential of −100 ± 5 mV (n = 4). At pH 6, removal of Mg2+ from the bathing solution increased ISO by only 5 ± 1%, which was significantly (P < 0.002) smaller than the increase at pH 7.3 (Fig. 12A and B). The divalent-cation-sensitive currents at pH 6.0 displayed inward rectification and reversed at −106 ± 11 mV (n = 4; Fig. 12F), indicating a contribution of inward rectifier K+ channels (Meuth et al. 2003).
Effects of spermine on the standing outward current in thalamocortical relay neurons
The divalent-cation-sensitive ISO in TC neurons was further characterized by superfusion of brain slices with spermine (Fig. 12G). In Mg2+-free solution, application of spermine at a concentration of 100 and 500 μm reversibly decreased ISO by 14 ± 4% (n = 3) and 24 ± 6% (n = 3; Fig. 12G), respectively. The current–voltage relation of the spermine-sensitive current (Fig. 12H) was similar to that of the divalent-cation-sensitive current (Fig. 12D) and had a reversal potential of −103 ± 6 mV.
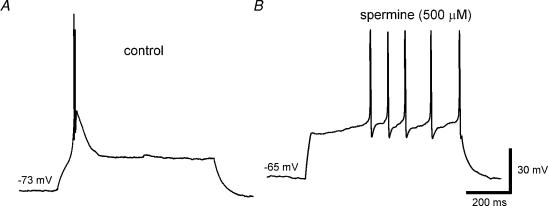
To demonstrate the functional impact of TASK channel modulation by spermine, the firing pattern of thalamocortical relay neurons submerged in normal ACSF was studied under current-clamp conditions. All recordings were obtained at slightly hyperpolarized values of the membrane potential (−72 ± 3 mV, n = 5) using constant current injection. Under these conditions a depolarizing current pulse (150 pA, 800 ms) evoked a typical low-threshold Ca2+ spike on which a brief burst of 2–4 action potentials was superimposed (mean frequency, ∼150 Hz), as illustrated in Fig. 13A. Application of spermine (500 μm) resulted in (i) a depolarization to −64 ± 4 mV (n = 4; P < 0.01); (ii) abolition of the low-threshold Ca2+ spike (due to inactivation of Ca2+ channels); and (iii) appearance of tonic firing (frequency, ∼20 Hz) in response to a depolarizing current step (Fig. 13B). The tonic firing was preceded by a quiescent period (duration 80–300 ms), which was probably due to activation of a transient outward current (Budde et al. 1992). In all experiments (n = 4), the phasic firing elicited by the current pulse under control conditions was converted to tonic firing in the presence of spermine.
Figure 13. Effect of spermine on the firing mode of TC neurons.
Representative whole-cell current-clamp recording from a TC neuron; the membrane potential was adjusted to −73 mV by constant current injection. The measurements were performed in normal ACSF containing 2 mm Ca2+ and 2 mm Mg2+. A, firing pattern produced by injection of a depolarizing current pulse before application of spermine. B, firing pattern produced by injection of the same current pulse (superimposed on the same hyperpolarizing holding current) 4 min after application of spermine (500 μm).
In conclusion, the pH-, divalent-cation- and spermine-sensitive ISO in thalamocortical relay neurons was in the range 100–300 pA at −28 mV (Fig. 12). Since TASK-1 channels were found to be insensitive to divalent cations and spermine, our results suggest that the major part of the pH-sensitive standing outward current in TC neurons is carried by TASK-3 channels. Inhibition of TASK-3 by spermine depolarized the neurons by about 8 mV and changed the firing pattern elicited by depolarizing current pulses.
Discussion
Shielding of surface charges at the outer vestibule of TASK-3 channels
The conductance of TASK-3 at negative potentials is highly sensitive to extracellular divalent cations, whereas the conductance of TASK-1 is insensitive. Mutational analysis showed that the residues at position 70, glutamate in TASK-3 and lysine in TASK-1, are crucial for the manifestation of cation sensitivity. Introducing a lysine at position 70 abolished cation sensitivity in TASK-3; introducing a glutamate at position 70 established cation sensitivity in TASK-1. Thus it might be supposed that this effect is related to binding of cations to the negatively charged glutamate. However, our experiments show that this is not the case. Replacement of glutamate-70 in TASK-3 by neutral amino acids did not affect the sensitivity to divalent cations. Positively charged amino acids, arginine or lysine, at position 70 of TASK-1 or TASK-3 were required to abolish cation sensitivity. Thus, mechanisms other than binding of divalent cations to residue 70 must be involved.
In all mutants, Mg2+ had the same effect on single-channel conductance as Ca2+. Furthermore, the polycations spermine and RR also caused a concentration-dependent conductance decrease in divalent-cation-free solution. The most likely explanation for these findings is shielding of negative charges in the outer vestibule of TASK-3. Charged groups on channel proteins can influence ion permeation by generating local surface potentials, which alters the local concentration of permeant ions (or their probability of being at a certain location) (Imoto et al. 1988; Wilson et al. 2000; Khan et al. 2002). Each TASK-3 subunit has 13 negatively charged residues in its M1–M2 loop. Mutation of individual negative charges other than E70, one or two at a time, did not significantly affect the sensitivity to divalent cations. This may be due to the fact that the increase in the local K+ concentration is brought about by the combined effect of several negative charges in the outer vestibule of the channel. Evidence for electrostatic potentials at the outer vestibule, caused by a ring of negatively charged amino acids, has previously been obtained for other ion channels (Wilson et al. 2000; Khan et al. 2002). The inward rectifier channel Kir2.1 also possesses a critical glutamate residue (E125), located near the outer channel vestibule, that is involved in K+ permeation (Alagem et al. 2001; Murata et al. 2002). In line with the present study, Murata et al. (2002) found that the conductance of Kir2.1 decreased with increasing divalent or trivalent cation concentration, and they also attributed this effect to shielding of fixed negative charges. However, in contrast to our results, mutation of the critical glutamate to a neutral residue abolished sensitivity to divalent cations. Thus, the mechanisms responsible for the effects of Ca2+ and Mg2+ on single-channel conductance of Kir2.1 differ from the effects on TASK-3 described here.
To explain the observation that the cation sensitivity of the conductance of TASK-3 channels was eliminated by a positive charge at position 70, but not by a neutral one, we propose that (i) the large extracellular loops of TASK-3 form an outer vestibule of the channel that is lined by negative charged amino acid residues; (ii) in the absence of external divalent or trivalent cations this ring of negative charges increases the local concentration of K+ ions and thus increases single-channel conductance in the inward direction (Hille, 2001); (iii) divalent cations shield the negative charges inside the vestibule and thus prevent the increase in local K+ concentration; trivalent cations like La3+ are even more potent than divalent cations at shielding the charges (McLauchlin, 1989); (iv) the glutamate residue at position 70 is located at a critical position near the selectivity filter (and forms some kind of ‘bottleneck’); introducing a positive charge at this position perturbs the electric field at the entrance of the selectivity filter, which reduces single-channel conductance and makes the accumulation of K+ ions at more distant sites ineffective.
Effects of spermine and ruthenium red on the open probability of TASK-3 channels
In the presence of 2 mm extracellular Ca2+, RR and spermine had no effect on the conductance of TASK-3 channels. In contrast, whole-cell currents carried by TASK-3 were substantially reduced in the presence of spermine, both in the absence and in the presence of divalent extracellular cations. In particular the marked effect of 500 μm spermine on outward currents in the presence of 1 mm external Ca2+ strongly suggests that, in addition to its effect on single-channel conductance, spermine also reduced the open-state probability of TASK-3 channels. This effect was probably due to binding of spermine to the glutamate residue at position 70, since it was not observed in TASK-1 and in the TASK-3 E70K mutant. The inhibitory effect of RR on outward current was also abolished in the TASK-3 E70K mutant (Czirjak & Enyedi, 2003). Thus, spermine and RR appear to have two separate effects on TASK-3: (i) they can block TASK-3 channels by binding to residue E70; and (ii) in the absence of external divalent cations, they can decrease channel conductance by screening fixed negative charges in the outer vestibule of TASK-3. Interestingly, application of zinc also selectively inhibited TASK-3 (and not TASK-1) whole-cell currents, and this effect was abolished in the TASK-3E70K mutant (Clarke et al. 2004).
Effects of divalent cations and spermine on thalamocortical relay neurons
TASK-3 channels are expressed in many neurons (Talley et al. 2001) and play an important role in the regulation of neuronal excitability. Consistent with our whole-cell recordings in the heterologous expression system, we found that in thalamocortical relay neurons a component of the standing outward current was sensitive to external divalent cations. This component was much smaller at pH 6, where most of the TASK-3 current should be blocked. The remaining divalent-cation-sensitive inward current at pH 6 appeared to be carried by inward rectifier channels, probably of the Kir2.1 subtype, which are also inhibited by external Mg2+ ions (Murata et al. 2002). Spermine blocked a fraction of the ISO observed in the absence of external divalent cations, in a concentration-dependent manner. The spermine-sensitive current showed nearly the same voltage dependence as the divalent-cation-dependent current. These findings suggest that extracellular spermine can inhibit both TASK-3 and inward rectifier channels. At potentials between −30 and +30 mV (where the amplitude of the ISO is relatively large) the strongly rectifying channels of the Kir2 subfamily are closed, and the spermine- and pH-sensitive component should be largely carried by TASK-3. The sensitivity to spermine and divalent cations was used to assess the relative contribution of TASK-3 channels to the total membrane current in thalamocortical relay neurons, which express both TASK-1 and TASK-3 (Meuth et al. 2003). The outward current blocked by Mg2+ was ∼18%; the current blocked by 500 μm spermine was ∼24% of the ISO. Thus, our data suggest that in thalamocortical relay neurons about 20% of ISO is flowing through TASK-3 channels. Using selective inhibition of TASK-3 channels by zinc, Clarke et al. (2004) estimated that in cultured cerebellar granule neurons TASK-3 contributes about 60% to the standing outward current.
The possible functional role of spermine block of neuronal TASK-3 channels
Recently, there has been considerable interest in the role of extracellular polyamines as modulators of ion channels (Williams, 1997; Dingledine et al. 1999; Stromgaard & Mellor, 2004). The free cytosolic concentration of spermine is probably in the order of 10–50 μm (Bowie & Mayer, 1995; Yan et al. 2005). In contrast, the spermine content of synaptic vesicles has been estimated to be around 2 mm (Masuko et al. 2003). In neurons and glial cells, transporter-driven uptake and voltage-dependent as well as receptor-mediated release of spermine has been found (Harman & Shaw, 1981; Fage et al. 1992; Masuko et al. 2003), indicating that the concentration of spermine in the synaptic cleft depends on the dynamic equilibrium between uptake and release. During repetitive firing and during cerebral ischaemia (Dempsey et al. 1988; Paschen et al. 1992) the local concentrations in the synaptic cleft may be high enough for spermine to influence neuronal ion channels (Traynelis et al. 1995; Williams, 1997; Nevin et al. 2000; Masuko et al. 2003). We show here that spermine can inhibit the outward current flowing through TASK-3 channels, which are strongly expressed in various brain regions (Karschin et al. 2001; Talley et al. 2001). The decrease in standing outward current induced by application of 500 μm spermine changed the firing mode of thalamic neurons from burst to tonic generation of action potentials, and this may contribute to the neuromodulatory effects of spermine. A change from the burst mode to the tonic mode of discharge in these neurons may be associated with transition from sleep to the conscious state (Steriade, 2004).
It has recently been suggested that release of synaptic vesicles can lead to acidification of the synaptic cleft (DeVries, 2001; Traynelis & Chesler, 2001; Palmer et al. 2003), which would also inhibit postsynaptic TASK-3 channels. Thus, spermine and proton accumulation during repetitive activity of presynaptic nerves may act synergistically on postsynaptic TASK-3 channels to increase neuronal excitability. This may be of crucial significance in thalamocortical relay neurons, in which TASK-3 channels are major effectors of muscarinic receptor activation (Meuth et al. 2003). As part of the ascending activation system of the brainstem, these receptors control the firing mode of thalamic neurons (Steriade, 2004). More precisely, muscarinic receptor activation results in a decrease in TASK-mediated outward current, thereby resulting in a membrane depolarization associated with a shift in activity mode from rhythmic burst firing towards generation of single action potentials. Impairment of this regulatory balance may play an important role in thalamic dysrhythmias such as absence epilepsy and sleep disturbances (Crunelli & Leresche, 2002; Steriade, 2004).
Acknowledgments
We thank Andrea Schubert, Anette Hennighausen, Brigitte Burk, Roswitha Luzius, Robert Graf, Kersten Schneider, Mrs A Jahn and Mrs R. Ziegler for technical support. This work was supported by the Deutsche Forschungsgemeinschaft (grants Da 177/8-3, BU 1019/5-2, Pa 336/14) and the Ernst-and-Berta-Grimmke Stiftung.
References
- Alagem N, Dvir M, Reuveny E. Mechanism of Ba2+ block of a mouse inwardly rectifying K+ channel: differential contribution by two discrete residues. J Physiol. 2001;534:381–393. doi: 10.1111/j.1469-7793.2001.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmole I, Goodwin PA, Stanfield PR. TASK-5, a novel member of the tandem pore K+ channel family. Pflugers Arch. 2001;442:828–833. doi: 10.1007/s004240100620. [DOI] [PubMed] [Google Scholar]
- Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Intervent. 2003;3:205–219. doi: 10.1124/mi.3.4.205. [DOI] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Buckler KJ, Williams BA, Honore E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde T, Mager R, Pape HC. Different types of potassium outward current in relay neurons acutely isolated from the rat lateral geniculate nucleus. Eur J Neurosci. 1992;4:708–722. doi: 10.1111/j.1460-9568.1992.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Clarke CE, Veale EL, Green PJ, Meadows HJ, Mathie A. Selective block of the human 2-P domain potassium channel, TASK-3, and the native leak potassium current, IKSO, by zinc. J Physiol. 2004;560:51–62. doi: 10.1113/jphysiol.2004.070292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. TASK-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol. 2002;16:621–629. doi: 10.1210/mend.16.3.0788. [DOI] [PubMed] [Google Scholar]
- Czirjak G, Enyedi P. Ruthenium red inhibits TASK-3 potassium channel by interconnecting glutamate 70 of the two subunits. Mol Pharmacol. 2003;63:646–652. doi: 10.1124/mol.63.3.646. [DOI] [PubMed] [Google Scholar]
- Dempsey RJ, Roy MW, Cowen DE, Combs DJ. Polyamine inhibition preserves somatosensory evoked potential activity after transient cerebral ischaemia. Neurol Res. 1988;10:141–144. doi: 10.1080/01616412.1988.11739831. [DOI] [PubMed] [Google Scholar]
- Derst C, Liu GX, Musset B, Rajan S, Preisig-Müller R, Daut J. Sensitivity of TASK channels to extracellular divalent cations. Biophys J. 2002;82:636a. [Google Scholar]
- DeVries SH. Exocytosed protons feedback to suppress the Ca2+ current in mammalian cone photoreceptors. Neuron. 2001;32:1107–1117. doi: 10.1016/s0896-6273(01)00535-9. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dodt HU, Zieglgänsberger W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 1990;537:333–336. doi: 10.1016/0006-8993(90)90380-t. [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fage D, Voltz C, Scatton B, Carter C. Selective release of spermine and spermidine from the rat striatum by N-methyl-d-aspartate receptor activation in vivo. J Neurochem. 1992;58:2170–2175. doi: 10.1111/j.1471-4159.1992.tb10960.x. [DOI] [PubMed] [Google Scholar]
- Gurney AM, Osipenko ON, MacMillan D, McFarlane KM, Tate RJ, Kempsill FE. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ Res. 2003;93:957–964. doi: 10.1161/01.RES.0000099883.68414.61. [DOI] [PubMed] [Google Scholar]
- Harman RJ, Shaw GG. The spontaneous and evoked release of spermine from rat brain in vitro. Br J Pharmacol. 1981;73:165–174. doi: 10.1111/j.1476-5381.1981.tb16786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartness ME, Lewis A, Searle GJ, O'Kelly I, Peers C, Kemp PJ. Combined antisense and pharmacological approaches implicate hTASK as an airway O2 sensing K+ channel. J Biol Chem. 2001;276:26499–26508. doi: 10.1074/jbc.M010357200. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer; 2001. [Google Scholar]
- Imoto K, Busch C, Sakmann B, Mishina M, Konno T, Nakai J, Bujo H, Mori Y, Fukuda K, Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988;335:645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Jones SA, Morton MJ, Hunter M, Boyett MR. Expression of TASK-1, a pH-sensitive twin-pore domain K+ channel, in rat myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H181–H185. doi: 10.1152/ajpheart.00963.2001. [DOI] [PubMed] [Google Scholar]
- Karschin C, Wischmeyer E, Preisig-Müller R, Rajan S, Derst C, Grzeschik KH, Daut J, Karschin A. Expression pattern in brain of TASK-1, TASK-3, and a tandem pore domain K+ channel subunit, TASK-5, associated with the central auditory nervous system. Mol Cell Neurosci. 2001;18:632–648. doi: 10.1006/mcne.2001.1045. [DOI] [PubMed] [Google Scholar]
- Khan A, Romantseva L, Lam A, Lipkind G, Fozzard HA. Role of outer ring carboxylates of the rat skeletal muscle sodium channel pore in proton block. J Physiol. 2002;543:71–84. doi: 10.1113/jphysiol.2002.021014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. Fatty acid-sensitive two-pore domain K+ channels. Trends Pharmacol Sci. 2003;24:648–654. doi: 10.1016/j.tips.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TBAK-1 and TASK-1, two-pore K+ channel subunits: kinetic properties and expression in rat heart. Am J Physiol. 1999;277:H1669–H1678. doi: 10.1152/ajpheart.1999.277.5.H1669. [DOI] [PubMed] [Google Scholar]
- Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K+ channel family. J Biol Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- Kim D, Gnatenco C. TASK-5, a new member of the tandem-pore K+ channel family. Biochem Biophys Res Commun. 2001;284:923–930. doi: 10.1006/bbrc.2001.5064. [DOI] [PubMed] [Google Scholar]
- Kindler CH, Yost CS, Gray AT. Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology. 1999;90:1092–1102. doi: 10.1097/00000542-199904000-00024. [DOI] [PubMed] [Google Scholar]
- Masuko T, Kusama-Eguchi K, Sakata K, Kusama T, Chaki S, Okuyama S, Williams K, Kashiwagi K, Igarashi K. Polyamine transport, accumulation, and release in brain. J Neurochem. 2003;84:610–617. doi: 10.1046/j.1471-4159.2003.01558.x. [DOI] [PubMed] [Google Scholar]
- McLauchlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Meuth SG, Budde T, Kanyshkova T, Broicher T, Munsch T, Pape HC. Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J Neurosci. 2003;23:6460–6469. doi: 10.1523/JNEUROSCI.23-16-06460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Fujiwara Y, Kubo Y. Identification of a site involved in the block by extracellular Mg2+ and Ba2+ as well as permeation of K+ in the Kir2.1 K+ channel. J Physiol. 2002;544:665–677. doi: 10.1113/jphysiol.2002.030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. In: Rudy B, Iverson LE, editors. Methods in Enzymology. Vol. 207. San Diego: Academic Press; 1992. pp. 123–131. [DOI] [PubMed] [Google Scholar]
- Nevin ST, Haddrill JL, Lynch JW. A pore-lining glutamic acid in the rat olfactory cyclic nucleotide-gated channel controls external spermine block. Neurosci Lett. 2000;296:163–167. doi: 10.1016/s0304-3940(00)01650-5. [DOI] [PubMed] [Google Scholar]
- Palmer MJ, Hull C, Vigh J, von Gersdorff H. Synaptic cleft acidification and modulation of short-term depression by exocytosed protons in retinal bipolar cells. J Neurosci. 2003;23:11332–11341. doi: 10.1523/JNEUROSCI.23-36-11332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschen W, Widmann R, Weber C. Changes in regional polyamine profiles in rat brains after transient cerebral ischemia (single versus repetitive ischemia): evidence for release of polyamines from injured neurons. Neurosci Lett. 1992;135:121–124. doi: 10.1016/0304-3940(92)90150-6. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Anesthetic-sensitive 2P domain K+ channels. Anesthesiology. 2001;95:1013–1021. doi: 10.1097/00000542-200110000-00034. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Xin Liu G, Preisig-Müller R, Daut J, Karschin A, Derst C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histidine as pH sensor. J Biol Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- Sirois JE, Lei Q, Talley EM, Lynch C, Bayliss DA. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anesthetics. J Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. 3rd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Acetylcholine systems and rhythmic activities during the waking-sleep cycle. Prog Brain Res. 2004;145:179–196. doi: 10.1016/S0079-6123(03)45013-9. [DOI] [PubMed] [Google Scholar]
- Stromgaard K, Mellor I. AMPA receptor ligands: synthetic and pharmacological studies of polyamines and polyamine toxins. Med Res Rev. 2004;24:589–620. doi: 10.1002/med.20004. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Talley EM, Sirois JE, Lei Q, Bayliss DA. Two-pore Domain (KCNK) potassium channels: dynamic roles in neuronal function. Neuroscientist. 2003;9:46–56. doi: 10.1177/1073858402239590. [DOI] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Chesler M. Proton release as a modulator of presynaptic function. Neuron. 2001;32:960–962. doi: 10.1016/s0896-6273(01)00549-9. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Hartley M, Heinemann SF. Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science. 1995;268:873–876. doi: 10.1126/science.7754371. [DOI] [PubMed] [Google Scholar]
- Williams K. Interactions of polyamines with ion channels. Biochem J. 1997;325:289–297. doi: 10.1042/bj3250289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GG, Pascual JM, Brooijmans N, Murray D, Karlin A. The intrinsic electrostatic potential and the intermediate ring of charge in the acetylcholine receptor channel. J General Physiol. 2000;115:93–106. doi: 10.1085/jgp.115.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan DH, Nishimura K, Yoshida K, Nakahira K, Ehara T, Igarashi K, Ishihara K. Different intracellular polyamine concentrations underlie the difference in the inward rectifier K+ currents in atria and ventricles of the guinea-pig heart. J Physiol. 2005;563:713–724. doi: 10.1113/jphysiol.2004.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]