Abstract
The sympathetic nerves that maintain blood pressure are modulated by the central respiratory generator. Neurones in the rostral ventrolateral medulla (RVLM) that drive this sympathetic nerve activity (SNA) also display central respiratory drive (CRD)-related activity, suggesting integration of respiratory and cardiovascular regulatory systems within the brainstem. Whether CRD-related activity in the RVLM is due to direct inputs from central respiratory neurones or modulation of cardiovascular-related neurones that influence the RVLM is not known. The caudal ventrolateral medulla (CVLM) contains GABAergic neurones that tonically inhibit presympathetic RVLM neurones and are essential for the production of numerous cardiovascular reflexes. The present study sought to determine whether cardiovascular-related GABAergic neurones in the CVLM display CRD-related activity. The firing patterns of individual barosensitive CVLM neurones were examined in relation to phrenic nerve activity in chloralose-anaesthetized, ventilated, neuromuscularly blocked, vagotomized rats. Histograms of phrenic-triggered CVLM neuronal activity showed that all baro-activated CVLM neurones displayed one of four patterns of CRD-related activity: (i) inspiratory peak (n = 15), (ii) inspiratory depression (n = 15), (iii) inspiratory peak with postinspiratory depression (n = 10), and (iv) postinspiratory peak (n = 9). A subset of each type of CVLM neurone was identified as GABAergic by individually filling the recorded neurone with biotinamide and observing expression of GAD67 mRNA by in situ hybridization (n = 10). These data suggest that the activity of GABAergic neurones in the CVLM is regulated by cardiovascular and respiratory inputs, and baro-activated GABAergic CVLM neurones may contribute to CRD-related modulation of presympathetic RVLM neurones and SNA.
The central respiratory pattern generator has a marked influence upon the sympathetic nerves that regulate cardiovascular function (Pilowsky, 1995; Malpas, 1998; Jänig & Häbler, 2003). Central respiratory drive (CRD)-related activity in sympathetic nerves has been observed in all species examined including rat (Numao et al. 1987; Guyenet et al. 1990; Häbler et al. 1996; Miyawaki et al. 1996), cat (Adrian et al. 1932; Cohen & Gootman, 1970; Häbler et al. 1994), rabbit (Adrian et al. 1932; Terui et al. 1986), dog (Okada & Fox, 1967), and human (Eckberg, 2003). The exact timing of the CRD-related activity varies with the species under study (Czyzyk et al. 1987; Häbler et al. 1994), from nerve to nerve within a subject (Numao et al. 1987; Boczek-Funcke et al. 1992a, b; Jänig & Häbler, 2003), or state of the preparation or individually recorded sympathetic neurones (Gilbey et al. 1986; Boczek-Funcke et al. 1992a; Koshiya & Guyenet, 1995; Dick et al. 2004). The predominant patterns include a peak in sympathetic nerve activity (SNA) coincident with inspiration, or a peak immediately after inspiration during the postinspiratory/early expiratory period. This CRD-related regulation of SNA appears to make a significant contribution to sympathetic vasomotor tone and arterial pressure (AP) in normocapnic states, and is likely to contribute to the sympatho-excitation and increase in AP observed with hypercapnia (Bachoo & Polosa, 1985; Haselton & Guyenet, 1989).
Some of the CRD-related activity observed in SNA may arise from presympathetic neurones in the brainstem. Indeed, in rats, cats and rabbits CRD-related patterns have been observed in the presympathetic neurones of the rostral ventrolateral medulla (RVLM; Terui et al. 1986; McAllen, 1987; Haselton & Guyenet, 1989; Miyawaki et al. 1995), which provide the major drive to sympathetic vasomotor tone under resting conditions in anaesthetized animals (Guyenet & Brown, 1986; McAllen, 1986; Schreihofer et al. 2000). Some of the observed CRD-related patterns are likely to be produced by direct inputs to these RVLM neurones from central respiratory neurones. In addition, some CRD-related patterns observed in presympathetic RVLM neurones may be due to the influence of central respiratory neurones upon cardiovascular-related neurones that regulate RVLM neuronal activity. For instance, inhibition of the caudal ventrolateral medulla (CVLM) alters CRD-related activity in presympathetic RVLM neurones and SNA (Miyawaki et al. 1996), and these changes in SNA can be mimicked by direct blockade of GABAA receptors in the RVLM (Guyenet et al. 1990; Miyawaki et al. 1996, 2002). If the GABAergic CVLM neurones that regulate the activity of presympathetic RVLM neurones are modulated by the central respiratory pattern generator, this cardiovascular-related input may be an important source of CRD-related activity observed in presympathetic RVLM neurones and SNA.
The CVLM contains GABAergic neurones that project to the RVLM and are essential for the production of many neural cardiovascular reflexes, including baroreflexes and the Bezold-Jarisch reflex (Gordon, 1987; Verberne & Guyenet, 1992). Extracellular unit recordings within the CVLM reveal neurones whose properties suggest they may be the critical inhibitory interneurones that convey this reflex information to the presympathetic RVLM neurones. These CVLM neurones are highly barosensitive (baro-activated) with activity that is modulated by each AP pulse (pulse-modulated), and their activity is inversely related to RVLM neuronal activity and SNA during the production of cardiovascular reflexes that require the CVLM (Terui et al. 1990; Agarwal & Calaresu, 1991; Gieroba et al. 1992; Jeske et al. 1993; Schreihofer & Guyenet, 2003). Neurones in the CVLM with these physiological properties have been shown to express GAD67 mRNA, suggesting that they are GABAergic, and have axons that project toward the RVLM (Terui et al. 1990; Agarwal & Calaresu, 1991; Gieroba et al. 1992; Jeske et al. 1993; Schreihofer & Guyenet, 2000c, 2003). To test the hypothesis that central respiratory and cardiovascular regulatory mechanisms interact within the CVLM, the present study determined whether baro-activated, pulse-modulated, GABAergic CVLM neurones display CRD-related activity. In addition, we recorded adjacent baro-inhibited neurones, which are likely to be rostrally projecting C1 adrenergic CVLM neurones (Verberne et al. 1999; Schreihofer & Guyenet, 2003), to determine whether they also display CRD-related activity as previously reported for the spinally projecting rostral C1 adrenergic neurones in the RVLM (Haselton & Guyenet, 1989). To ensure observation of CRD-related activity, the rats were vagotomized, and phrenic nerve discharge was not phase locked to the ventilation cycle.
Methods
All experiments were conducted in agreement with the regulations of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the Medical College of Georgia.
Surgical preparation
Male Sprague-Dawley rats (270–350 g; Harlan, http://www.harlan.com) were anaesthetized with 2.5% isoflurane in 100% oxygen for surgery. Catheters were inserted into the femoral vein to inject drugs and a brachial artery to measure upper body AP. Heart rate (HR) was monitored by triggering from the AP pulse. A tracheotomy was performed to provide artificial ventilation (Model 683, Harvard Apparatus, http://www.harvardapparatus.com). End tidal CO2 was monitored using infrared spectroscopy (100–130 mS response rate; CapStar-100, Charles Ward Electronics, http://www.cwe-inc.com) by sampling expired air as it exited the tracheal tube. The end-tidal CO2 levels ranged between 3.5 and 5.5% with a tidal volume of 1 ml (100 g body weight)−1 and a ventilation rate of 60 strokes min−1. If a prominent CRD-related activity was not observed in the SNA, ventilation rate was decreased slightly (∼55 strokes min−1) to raise CRD activity immediately prior to recording of CVLM neurones (Haselton & Guyenet, 1989). Core temperature was maintained at 37°C (Harvard). The rat was subjected to neuromuscular block (pancuronium, 1 mg kg−1i.v., Abbott Laboratories), and then cervical vagus nerves were cut bilaterally. A snare was placed around the abdominal aorta to rapidly increase upper body AP (Brown & Guyenet, 1985; Schreihofer & Guyenet, 2003). The rat was placed into a sterotaxic frame (David Kopf Instruments, http://www.kopfinstruments.com) in the supine position with the bite bar at −11 mm. A partial occipital craniotomy was performed to expose the dorsal surface of the brainstem. The spinal column was clamped at the mid-thoracic level to reduce ventilatory-related movements. Upon completion of the surgical preparation, α-chloralose (30 mg ml−1 solution in 3% sodium borate) was infused slowly (60 mg kg−1i.v., with hourly supplements of 20 mg kg−1 as needed) as isoflurane was eliminated. Animals were allowed recover for 30 min before extracellular recordings were performed. All recordings were performed under α-chloralose anaesthesia with 100% oxygen. Adequacy of anaesthesia was verified every 30 min and immediately before each supplement of pancuronium by evaluating the cardiovascular response to a firm toe pinch (< 10 mmHg increase in AP).
Peripheral nerve recordings
Raw splanchnic SNA (sSNA) was measured as previously reported (Schreihofer & Guyenet, 2000a, b; Schreihofer et al. 2000). The left splanchnic nerve was exposed by a retroperitoneal approach and placed on two Teflon-coated silver wires that were bared at the tips (250 μm bare diameter, A-M Systems, http://www.a-msystems.com). The nerve and wires were embedded in polyvinylsiloxane impression material (http://www.darbydental.com), and the incision was closed around the recording wires. The sSNA was amplified and filtered (10 Hz to 3 kHz band pass with a 60 Hz notch filter; Differential AC amplifier 1700, A-M Systems). The baseline sSNA (100%) was arbitrarily defined as the activity during the resting state immediately preceding each physiological test, and the minimum sSNA (0%) was determined after injection of clonidine at the end of the experiment (10 μg kg−1i.v., Sigma Chemical Co., http://www.sigma.com; Schreihofer & Guyenet, 2000a).
The left phrenic nerve was dissected using a dorsolateral approach as previously described (Schreihofer et al. 1999). The nerve was cut distally, placed on a bipolar silver electrode, and coated with polyvinylsiloxane. The raw phrenic nerve discharge (PND) was amplified and filtered (30 Hz to 3 kHz band pass with a 60-Hz notch filter; Differential AC amplifier 1700, A-M Systems). Artificial ventilation was offset from the PND to desynchronize CRD from ventilatory-related changes in AP (Fig. 1).
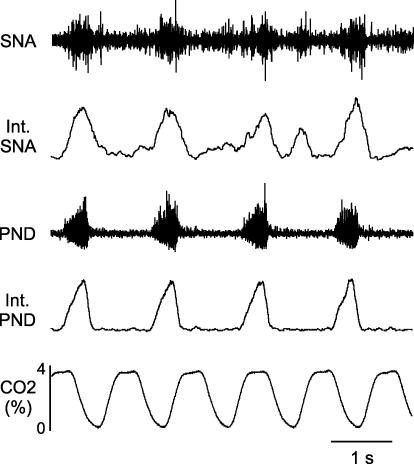
Figure 1. Example of relationships of phrenic nerve discharge (PND), end tidal CO2 and splanchnic sympathetic nerve activity (SNA).
The integrated SNA (int SNA) has prominent respiratory-related rhythm with most of the bursts occurring with the PND. In this vagotomized rat, the PND desynchronized with ventilation, as shown by a lack of relationship with changes in end tidal CO2.
Extracellular recording and juxtacellular labelling of CVLM neurones
Extracellular recordings from individual neurones in the CVLM were performed as previously described (Schreihofer & Guyenet, 2000c, 2003) using glass electrodes (World Precision Instruments; http://www.wpiinc.com) filled with 1.5% biotinamide (Molecular Probes, http://www.probes.invitrogen.com) in 0.5 m sodium acetate. The optimal electrode resistance was 10–20 MΩ measured in vivo. Recordings were made with an intracellular amplifier in bridge mode (Neuroprobe 1600, A-M Systems) to allow monitoring of the recording during injection of current through the recording electrode. The neuronal activity was amplified and filtered (300 Hz to 5 kHz, NeuroLog System, Digitmer Ltd, http://www.digitimer.com). The CVLM was located using stereotaxic coordinates in relation to calamus scriptorius: 1.2–1.5 mm rostral, 1.8–2.1 mm lateral and 2.5–2.9 mm ventral to the dorsal surface of the brainstem. We selected cardiovascular-related, baro-activated CVLM neurones based upon the following criteria: (1) spontaneous activity under resting conditions with significant baroreceptor tone (decreasing AP evoked increased sSNA), (2) discharge rate briskly increased by raising AP with constriction of the aortic snare (at least 3 times above baseline firing rate), (3) discharge pattern strongly modulated by the AP pulse, especially when AP was raised, (4) lack of obvious respiratory-related activity in the raw data, and (5) location within the CVLM often immediately ventral to cells with ON–OFF respiratory-related activity. For comparison, we selected cardiovascular-related, baro-inhibited CVLM neurones using the same criteria except that they were silenced by raising AP with constriction of the aortic snare.
A subset of individually recorded CVLM neurones was filled with biotinamide using a previously described juxtacellular labelling method (Pinault, 1996; Schreihofer & Guyenet, 1997, 2003; Schreihofer et al. 1999, 2000). Positive current pulses were delivered through the recording pipette (200 ms pulses of 1.0–3.0 nA, 2.5 Hz) while monitoring the activity of the isolated neurone. Successful entrainment (1–3 min) of the cell's activity to the current pulses produces the label of a single cell in the vast majority of cases (Schreihofer & Guyenet, 1997, 2003). One to three neurones were recorded from each rat, but only one attempt to label a neurone was performed on each side of the medulla.
Physiological data analyses
All analog physiological variables (AP, end-tidal CO2, sSNA, PND and CVLM unit activity) were converted to digital signals (Micro 1401, Cambridge Electronic Design, http://www.ced.co.uk) and viewed on-line (Spike2 software, Cambridge Electronic Design). The raw PND and SNA were full-wave rectified using the Spike2 software rectify function and were averaged using the Spike2 smooth function with 0.1 s bins. The CVLM unit activity was also counted in 0.1 s bins (Spike trigger, Neurolog). All triggered histograms were constructed off-line using 180–300 s of recorded data (Spike2 software). Event channels were created in Spike2 to trigger initiation of PND, peaks of CO2 and AP. The AP-triggered histograms were constructed to confirm pulse-modulated activity of CVLM neurones. We evaluated 1000–1500 sweeps of a 1 s window in 0.01 s bins, which usually contained six to eight AP cycles. The PND-triggered histograms were constructed to determine CRD-related activity in CVLM units and sSNA and to show a desynchronization with ventilation. End tidal CO2 wave-triggered histograms were constructed to determine whether CVLM neurones displayed ventilatory-related modulation. For PND and CO2 we evaluated 100–150 sweeps of a 4 s window in 0.1 s bins, which usually contained two to three cycles.
Histology of labelled CVLM neurones
At the end of the experiment, the rat was perfused transcardially with phosphate-buffered saline (pH 7.4, 250 ml) followed by 4% paraformaldehyde (pH 7.4, 500 ml). The brain was removed and stored in the same fixative for 48 h at 4°C. The brainstem was cut coronally into 30 μm sections using a Vibratome (The Vibratome Company, http://www.vibratome.com) and stored in a cryoprotectant solution at −20°C (Schreihofer & Guyenet, 1997).
Individual biotinamide-labelled neurones were processed to identify phenotype and confirm location within the CVLM. The baro-activated CVLM neurones were examined for expression of GAD67 mRNA to verify that they were GABAergic (Schreihofer & Guyenet, 2000c, 2003). Adjacent vagal motor neurones are also baro-activated, but do not express GAD67 mRNA (Schreihofer & Guyenet, 2003). The baro-inhibited CVLM neurones have previously been shown to be catecholaminergic (caudal C1 cells), so they were examined for tyrosine hydroxylase (TH) or phenylethanolamine N-methyl transferase (PNMT) immunoreactivity (Stornetta et al. 1999; Verberne et al. 1999; Schreihofer & Guyenet, 2003).
The biotinamide-filled neurones were revealed by incubating the tissue with strept-avidin Alexa 488 (Molecular Probes, http://www.probes.invitrogen.com 1: 200 with 0.1% Triton X-100, 2 h) and mounting the sections onto sterile slides in sterile phosphate buffer (pH 7.4). Sterile coverslips were placed onto the sections and the biotinamide-labelled neurone was located using an epifluorescence microscope (Olympus BX50, Olympus, http://www.olympusamericas.com). The section containing the labelled neurone was drawn, and the location of the labelled neurone was plotted using the Neurolucida system (Microbrightfield, http://www.microbrightfield.com). The section was gently removed from the slide and processed to reveal phenotype.
Unless otherwise noted, all incubations and rinses were performed on free-floating sections at room temperature on a shaker in Tris-buffered saline (TBS, pH 7.4). Immunoreactivity for TH was revealed by incubation with a monoclonal mouse anti-TH antibody (1: 1000, 48 h, 4°C, Immunostar, http://www.immunostar.com) followed by incubation with donkey anti-mouse IgG-Cy3 (1: 200, 1 h, Jackson ImmunoResearch, http://www.jacksonimmuno.com). Immunoreactivity for PNMT was revealed by incubation with a polyclonal rabbit anti-PNMT antibody (1: 2000, 48 h, Chemicon, http://www.chemicon.com) followed by incubation with donkey anti-rabbit IgG-Cy3 (1: 200, 1 h, Jackson). Sections were mounted onto uncoated slides, and coverslips were applied with Krystalon.
In situ hybridization histochemistry for GAD67 mRNA
Expression of GAD67 mRNA was detected using antisense digoxigenin-labelled cRNA probes as previously described (Schreihofer et al. 1999; Schreihofer & Guyenet, 2003). The riboprobes were generated from a full-length cDNA encoding GAD67 (2.7 kb, generously supplied by Dr A. J. Tobin, University of California, Los Angeles, CA, USA; Erlander et al. 1991) cloned into pBluescript SK+ (Stratagene, http://www.stratagene.com). Plasmids were linearized with SalI (Promega, http://www.promega.com) and transcribed using T3 polymerase (Promega) in the presence of digoxigenin-11-UTP (Roche Applied Science, http://www.ibuybiochem.com). The template DNA was digested with RQ1 DNase (Promega), and unincorporated nucleotides were removed by Probe Quant G-50 Micro Columns (GE Healthcare, http://www1.amershambiosciences.com).
The section containing the biotinamide-labelled soma was rinsed in RNAse and DNAse-free saline and placed in a prehybridization solution (Schreihofer et al. 1999) at room temperature for 30 min and then at 37°C for 1 h. Then, the riboprobe (50–100 pg ml−1) was added directly to the solution containing the section to hybridize for 16 h at 55°C. Sections were rinsed through decreasing concentrations of sodium citrate (SSC) at 37°C, treated with a solution of RNAase A (1 h, 37°C), and then rinsed in 0.1 × SSC at 55°C for 1 h.
The digoxigenin-labelled riboprobe was revealed by incubation with a sheep polyclonal antidigoxigenin antibody conjugated to alkaline phosphatase (1: 1000, overnight, 4°C, Roche) with 10% heat inactivated horse serum (Gibco, http://www.invitrogen.com) and 0.1% Triton X-100. The next day sections were rinsed and incubated in NMT (0.1 m NaCl, 50 mm MgCl2, and 0.1 m Tris, 10 min). The blue–brown reaction product was produced by incubation in NMT with nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl-phosphate, 4-toluidine salt (Roche) in the dark for 1–3 h (Schreihofer et al. 1999; Schreihofer & Guyenet, 2003).
Mapping and imaging of biotinamide-labelled neurones
Epifluorescence was used to visualize biotinamide-labelled CVLM neurones incubated with strept-avidin Alexa 488, or TH immunoreactivity revealed with Cy3 as previously described (Schreihofer & Guyenet, 2003). Brightfield illumination was used to visualize the GAD67 mRNA hybridization reaction product. Examples of biotinamide-labelled CVLM neurones were captured using a digital camera (MagnaFire, Optronics, http://www.olympusamerica.com). The resulting files were imported into Adobe Photoshop (6.0, Adobe Systems, http://www.adobe.com) where they were converted to grayscale with the levels and sharpness adjusted to optimize visualization of the labelled neurones.
Results
CRD-related modulation of sSNA of vagotomized, chloralose-anaesthetized rats
To ensure that the preparation yielded a prominent CRD-related modulation of sSNA, an ultimate target of recorded CVLM neurones, we recorded splanchnic nerve activity in a subset of rats (28 of the 48 rats). The ventilation, as indicated by end tidal CO2, was not phase locked with the discharge of the phrenic nerve to ensure observation of CRD-related sSNA (Figs 1 and 4). The average length of the ventilation cycle (1.10 ± 0.02 s; peak to peak of end-tidal CO2) was shorter than the average length of the centrally driven respiratory cycle (1.68 ± 0.02 s; peak to peak of PND, P < 0.05). In every case, sSNA had a prominent peak during inspiration, coincident with the burst of PND (Figs 1 and 4Ac and Bc). No postinspiratory bursts were observed in the sSNA of any rats.
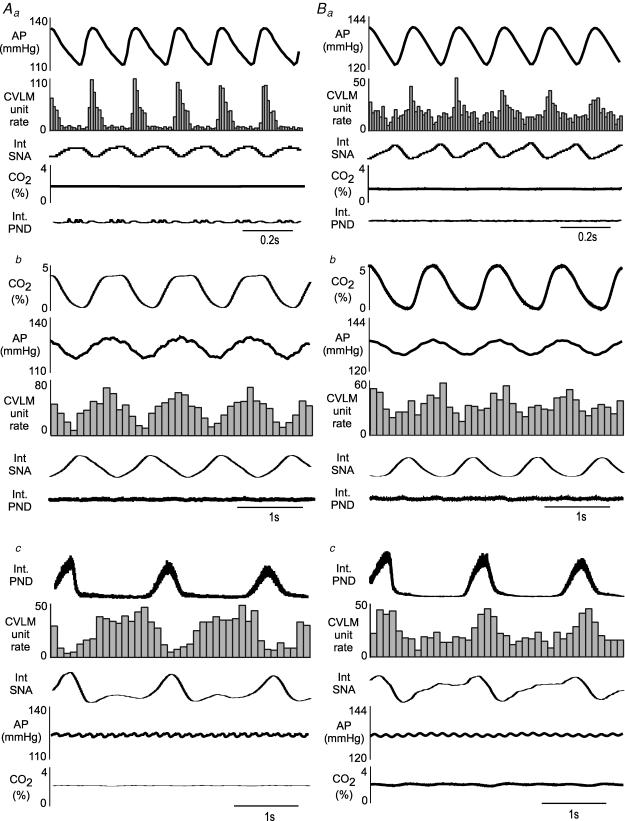
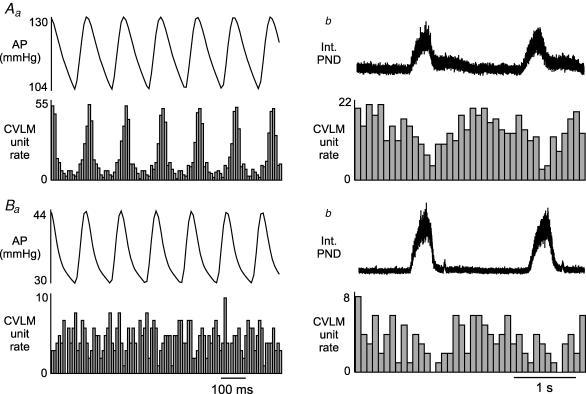
Figure 4. Examples of pulse modulation, ventilation-related modulation, and CRD-related activity in baro-activated CVLM neurones.
Aa, histogram of arterial pulse-triggered activity reveals a strong pulse modulation of CVLM neuronal activity, with highest firing occurring during systole. PND and end tidal CO2 waves were not related to the AP pulse. Ab, in the same neurone shown in Aa, a histogram of CO2-triggered activity reveals correlated waves in AP and CVLM neuronal activity, with the highest CVLM neuronal activity occurring with the coincident peaks in CO2 and AP. PND was unrelated to ventilation-related waves in end tidal CO2, AP, or CVLM neuronal activity. Ac, histogram of phrenic-triggered activity of same neurone shown in Aa and Ab reveals inspiratory-depression, type II CRD-related CVLM neuronal activity that was inversely related to SNA but unrelated to AP or end tidal CO2. Ba, histogram of pulse-triggered activity of another baro-activated CVLM neurone reveals pulse modulation of CVLM neuronal activity, which is weaker than the neurone shown in Aa. Bb, histogram of CO2-triggered activity of same neurone shown in Ba reveals ventilation-related waves in AP, CVLM neuronal activity, and SNA, which is also weaker than the neurone shown in Ab. Bc, histogram of phrenic-triggered activity of same neurone shown in Ba and Bb reveals inspiratory-peak type I CRD-related CVLM neuronal activity. Histograms of phrenic- and CO2-triggered activity represent 150 sweeps with a bin size of 0.1 s. The histograms of AP-triggered activity represent 1500 sweeps with a bin size of 0.01 s.
Baro-activated CVLM neurones display CRD-related activity
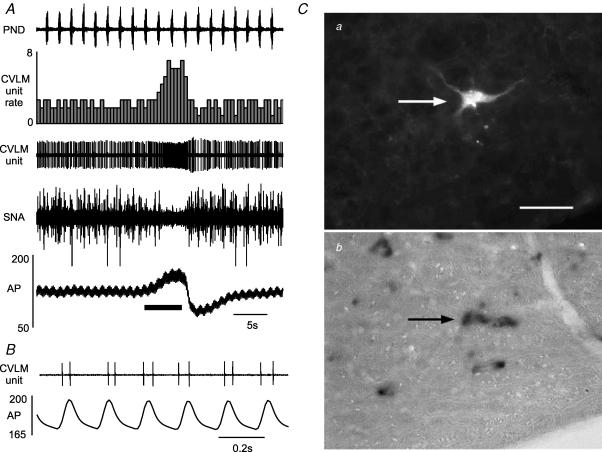
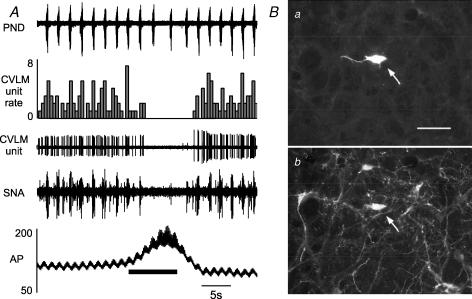
In 35 rats, we recorded 49 neurones in the CVLM that were briskly activated by raising upper body AP (baro-activated, Fig. 2A) and displayed no obvious respiratory-related activity in the raw data (Fig. 2A). These CVLM neurones, which were exquisitely sensitive to AP, showed activity that was modulated by each pulse of AP (pulse-modulated, Fig. 2B). As shown previously (Schreihofer & Guyenet, 2000c, 2003), CVLM neurones with these physiological characteristics express GAD67 mRNA (Fig. 2C), demonstrating they are GABAergic neurones. In this group of rats the mean AP was 123 ± 2 mmHg and the average end tidal CO2 was 5.7 ± 0.2% (4.8–6.5%) at the time of CVLM unit recordings. The average basal firing rate of the CVLM neurones was 2.3 ± 0.3 spikes s−1, and no differences in basal firing rate were observed when CVLM neurones were grouped by CRD-related pattern.
Figure 2. Example of a typical baro-activated GABAergic CVLM neurone.
A, elevating arterial pressure (AP) by constricting the abdominal snare (at bar under AP trace) increases the firing of this baro-activated neurone and reduces the splanchnic SNA. B, at high AP this baro-activated neurone displayed pulse synchrony, with firing occurring with each systole. Ca, the baro-activated neurone was filled with biotinamide revealed with strept-avidin Alexa 488 (at arrow) Cb, the same area shown in Ca but under brightfield to reveal the presence of GAD67 mRNA in the biotinamide-labelled neurone (at arrow). Scale bar, 25 μm.
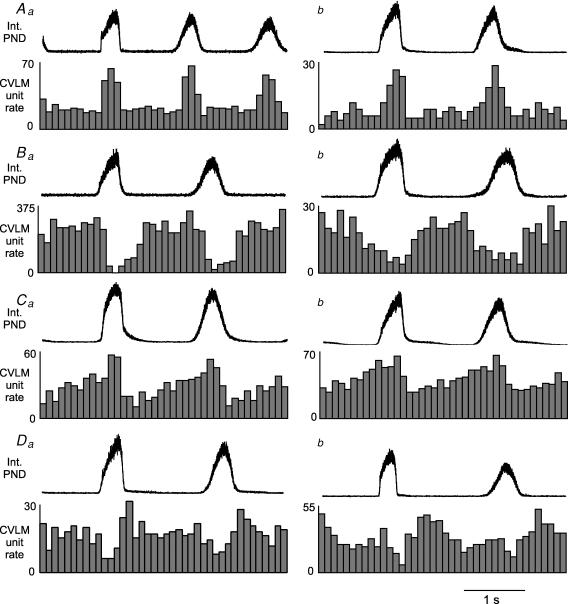
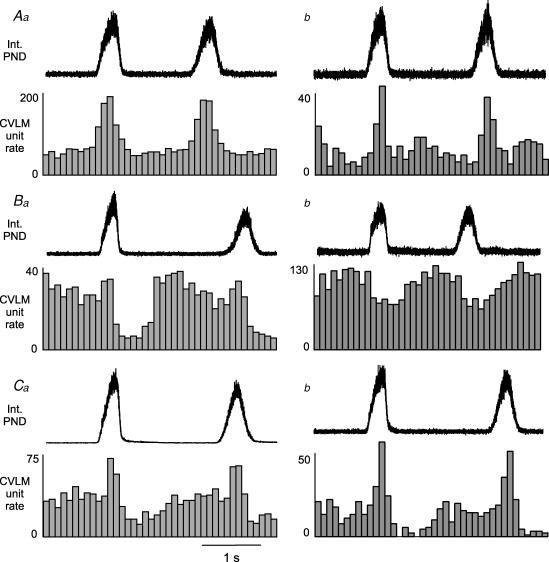
Phrenic-triggered unit histograms revealed four general patterns of CRD-related activity in baro-activated CVLM neurones. All baro-activated CVLM neurones displayed some pattern of CRD-related activity when triggered from the PND. Each neurone was categorized by its most prominent pattern feature. Type 1 (inspiratory peak) neurones were sharply activated at the onset of the PND and abruptly inhibited at the end of the phrenic burst. The activity between phrenic bursts was fairly stable, and considerably lower (n = 15, Fig. 3Aa and b). Three of these neurones were filled with biotinamide and found to express GAD67 mRNA (see example in Fig. 2C). Type II (inspiratory depression) neurones showed a decrease in activity during the PND, which was either abrupt in onset (Fig. 3Ba) or gradually decreasing with a nadir during the PND (Fig. 3Bb). There were no obvious peaks of activity in between phrenic bursts. (n = 15). Two of these neurones were filled with biotinamide and found to express GAD67 mRNA. Type III (inspiratory peak, postinspiratory depression) neurones were activated during the PND, usually with a ramp of increasing activity. In addition these neurones showed a decrease in firing rate at the end of the PND, which was the lowest period of activity (n = 10, Fig. 3Ca and b). Three of these neurones were filled with biotinamide and found to express GAD67 mRNA. Type IV (postinspiratory peak) neurones showed an increase in activity at the end of the PND or immediately following the burst, which ended with or soon after the termination of the phrenic burst. (n = 9, Fig. 3Da and b). In addition some type IV neurones showed a slight decrease in activity during the PND. Two of these neurones were filled with biotinamide and found to express GAD67 mRNA.
Figure 3. Four patterns of CRD-related activity in baro-activated CVLM neurones.
Aa and b, type I: histograms of phrenic-triggered activity reveal a clear peak of CVLM neuronal activity during the PND. Ba and b, type II: histograms of phrenic-triggered activity reveal a depression of CVLM neuronal activity during the PND, which often persisted after the termination of the phrenic burst (Ba). Ca and b, type III: histograms of phrenic-triggered activity reveal increased CVLM neuronal activity during the PND that was followed by a depression at the termination of the phrenic burst. Da and b, type IV: histograms of phrenic-triggered activity reveal an increase in CVLM neuronal activity immediately after the termination of the phrenic burst. Each histogram represents 150 sweeps with a 0.1 s bin size.
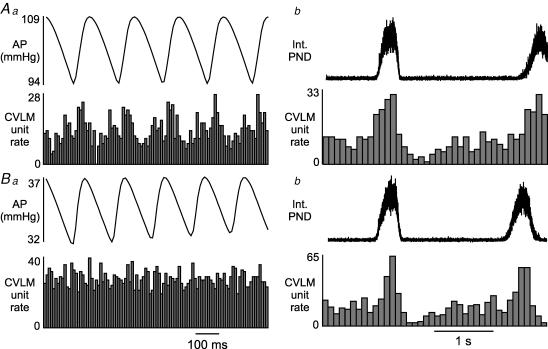
In addition to their pulse-modulated and CRD-related activity, the baro-activated CVLM neurones also displayed a ventilatory-related modulation of their firing rate. This pattern has previously been observed in the SNA of vagotomized, artificially ventilated rats and is produced by arterial baroreceptors sensing ventilation-mediated waves in AP (Häbler et al. 1996). Baro-activated CVLM neurones are extremely sensitive to changes in AP, and they fire more during each systole (Fig. 4Aa and Ba) and when AP increases on the expiratory phase of the ventilation cycle (Fig. 4Ab and Bb). These patterns related to AP were the same in all baro-activated CVLM neurones, and were not related to PND (Fig. 4A and B). The CRD-related patterns observed in baro-activated CVLM neurones were also independent from the pulse- and ventilation-modulation of activity (Figs 4Ac and 4Bc). In agreement, unloading arterial baroreceptors with infusion of nitroprusside abolished pulse-modulated activity (n = 13; Fig. 5Aa versus Ba), but did not alter the CRD-related pattern in baro-activated CVLM neurones (n = 13; Fig. 5Ab versus Bb).
Figure 5. Example of the effects of unloading arterial baroreceptors with nitroprusside (5 mg kg−1, i.v.) on the activity of a baro-activated CVLM neurone.
Aa, histogram of AP pulse-triggered activity reveals strong pulse-modulation of CVLM neuronal activity. Ab, a histogram of phrenic-triggered activity reveals inspiratory-depression CRD-related activity in the same CVLM neurone shown in Aa. Ba, AP-triggered histogram after nitroprusside-induced hypotension reveals an abolition of pulse-modulation of CVLM neuronal activity. Bb, histogram of phrenic-triggered activity of same neurone shown in Ba reveals nitroprusside does not alter inspiratory-depression in the CVLM neuronal activity. Histograms of phrenic-triggered activity represent 100 sweeps with a bin size of 0.1 s. The histograms of AP pulse-triggered activity represent 1000 sweeps with a bin size of 0.01 s.
Baro-inhibited CVLM neurones display CRD-related activity
In 22 rats, we recorded 30 neurones in the CVLM that were silenced by raising upper body AP (Fig. 6A). Baro-activated neurones were also recorded in nine of these rats. The baro-inhibited CVLM neurones displayed no obvious respiratory-related activity in the raw data (Fig. 6A). As shown previously (Schreihofer & Guyenet, 2003), CVLM neurones with these physiological characteristics express TH immunoreactivity (Fig. 6B), indicating they are catecholaminergic. The TH-immunoreactive neurones at this rostro-caudal level of the medulla also display PNMT immunoreactivity, suggesting they are C1 adrenergic neurones. These C1 neurones are unlikely to project to the spinal cord, but instead probably project to the forebrain (Verberne et al. 1999). In this group of rats the mean AP was 112 ± 2 mmHg and the average end tidal CO2 was 5.2 ± 0.1%. The average basal firing rate of the CVLM neurones was 1.8 ± 0.2 spikes s−1, and no differences in basal firing rate were observed when CVLM neurones were grouped by CRD-related pattern.
Figure 6. Example of a typical baro-inhibited catecholaminergic CVLM neurone.
A, elevating arterial pressure (AP) by constricting the abdominal snare (at bar under AP trace) silences the firing of this baro-inhibited neurone and splanchnic SNA. B, the baro-inhibited neurone was filled with biotinamide revealed with strept-avidin Alexa 488 (at arrow) C, the same area shown in B to reveal the presence of tyrosine hydroxylase immunoreactivity (at arrow), demonstrating this neurone is catecholaminergic. Scale bar, 25 μm.
All baro-inhibited CVLM neurones displayed some pattern of CRD-related activity, and phrenic-triggered unit histograms revealed 3 general patterns. Type 1 (inspiratorypeak) neurones were activated at the onset of the PND and had decreased activity at the end of the phrenic burst. The CVLM neuronal activity between phrenic bursts was fairly stable, but considerably lower (n = 7, Fig. 7Aa and b). Type II (late inspiratory–early expiratory depression) neurones had a decrease in activity during the end of the PND, which continued briefly after the termination of the phrenic burst (n = 11, Fig. 7Ba and b). Type III (inspiratory peak, postinspiratory depression) neurones were activated during the PND, with a decrease in activity immediately after the phrenic burst (n = 12, Fig. 7Ca and b). A catecholaminergic phenotype was confirmed in a subset of each type of baro-inhibited CVLM neurone (5 TH- and 3 PNMT-immunoreactive neurones).
Figure 7. Three patterns of CRD-related activity in baro-inhibited CVLM neurones.
Aa and b, type I: histograms of phrenic-triggered activity reveal a clear peak of CVLM neuronal activity during the PND. Ba and b, type II: histograms of phrenic-triggered activity reveal a depression of CVLM neuronal activity during the PND, which often persisted after the termination of the phrenic burst. Ca and b, type III: histograms of phrenic-triggered activity reveal increased CVLM neuronal activity during the PND that was followed by a depression at the termination of the phrenic burst. Each histogram represents 150 sweeps, and the bin size for PND-triggered histograms was 0.1 s.
At resting AP, many baro-inhibited CVLM neurones displayed some pulse-modulated activity, with activation occurring during the troughs of the AP pulses (Fig. 8Aa). Unloading arterial baroreceptors with infusion of nitroprusside increased firing rate and abolished pulse-modulated activity (Fig. 8Ba), but did not alter the CRD-related pattern in baro-inhibited neurones (Fig. 8Ab versus Bb).
Figure 8. Example of the effects of unloading arterial baroreceptors with nitroprusside (5 mg kg−1i.v.) on the activity of a baro-inhibited CVLM neurone.
Aa, histogram of AP pulse-triggered activity reveals pulse modulation of CVLM neuronal activity. Ab, a histogram of phrenic-triggered activity reveals inspiratory-peak CRD-related activity in the same CVLM neurone shown in Aa. Bb, histogram of AP-triggered activity after nitroprusside-induced hypotension reveals an abolition of pulse modulation of CVLM neuronal activity. Bb, histogram of phrenic-triggered activity of same neurone shown in Ba reveals nitroprusside does not alter inspiratory peak in the CVLM neuronal activity. Histograms of phrenic-triggered activity represent 100 sweeps with a bin size of 0.1 s. The histograms of AP pulse-triggered activity represent 1000 sweeps with a bin size of 0.01 s.
Location of recorded CVLM neurones
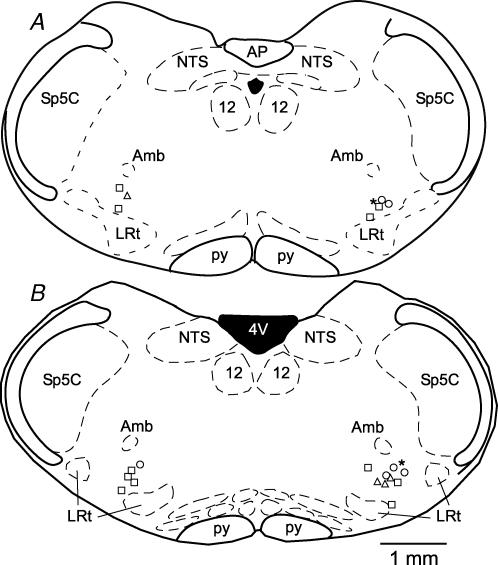
All recorded barosensitive neurones were found where expected within the CVLM. The neurones were ventral to nucleus ambiguus, and in between the lateral wings of the rostral tips of the lateral reticular nucleus (Fig. 9). There was no difference in the locations of the baro-activated (Fig. 9, right) and the baro-inhibited (Fig. 9, left) neurones, or locations examined by CRD-related firing pattern.
Figure 9. Location of recorded CVLM neurones that were filled with biotinamide.
Two representative coronal brainstem sections contain the plotted locations of recorded barosensitive CVLM neurones. The more rostrally located neurones are shown in A (bregma − 13.68 mm) and the more caudally located neurones are shown in B (bregma −13.24 mm). The baro-inhibited neurones are on the left side of the sections (n = 8 catecholaminergic neurones) and the baro-activated neurones are on the right (n = 10 GABAergic neurones, n = 5 filled with biotinamide, but not successfully processed for GAD67 mRNA). There was no difference in the location of baro-activated versus baro-inhibited neurones. Symbols represent location of barosensitive CVLM neurones categorized by CRD-related activity: ▵, type I, inspiratory-peak; ○, type II, inspiratory-depression; □, type III; inspiratory-peak with postinspiratory depression; *, type IV, postinspiratory peak.
Discussion
The SNA that contributes to resting AP is prominently modulated by the central respiratory pattern generator (Adrian et al. 1932; Pilowsky, 1995; Malpas, 1998; Jänig & Häbler, 2003). Pre-sympathetic neurones in the RVLM that drive sympathetic vasomotor tone also display CRD-related activity (McAllen, 1987; Haselton & Guyenet, 1989; Miyawaki et al. 1995), indicating that the RVLM is a site of integration of brainstem regulatory systems for respiratory and cardiovascular functions. The principal observation of the present study is that integration between these two regulatory systems also occurs within the CVLM. Baro-activated GABAergic CVLM neurones display one of four patterns of CRD-related activity, namely inspiratory peak (type I), inspiratory depression (type II), inspiratory peak with postinspiratory depression (type III), and postinspiratory peak (type IV). In addition, many of these patterns appear to be inversely related to those observed in presympathetic RVLM neurones. These data suggest that CRD-related activity observed in presympathetic RVLM neurones and SNA may be shaped by GABAergic neurones in the CVLM.
Role of baro-activated GABAergic CVLM neurones in the regulation of SNA
An assumption of the present study is that at least some of the individually recorded CVLM neurones target the presympathetic RVLM neurones to alter SNA and AP. One issue at hand is that adjacent vagal motor neurones in the CVLM display some physiological properties that are indistinguishable from GABAergic CVLM neurones (Schreihofer & Guyenet, 2003). Vagal motor neurones, which are immediately dorsal to the baro-activated GABAergic neurones, are also baro-activated and display CRD-related activity (Jordan et al. 1982; Gilbey et al. 1984; Rentero et al. 2002). However, vagal motor neurones are cholinergic and do not express GAD67 mRNA, making the phenotypic identification of the CVLM neurones critical in the present study. Furthermore, whereas multiple CRD-related patterns are observed in cardiovascular-related CVLM and RVLM neurones, cardiac vagal motor neurones display a consistent CRD-related pattern from neurone to neurone within a preparation (Jordan et al. 1982; Gilbey et al. 1984; Rentero et al. 2002; Neff et al. 2003). The uniform CRD-related pattern observed in vagal motor neurones likely contributes to respiratory sinus arrhythmia. In contrast, the variety of patterns observed in barosensitive presympathetic CVLM and RVLM neurones probably reflects the diversity of their sympathetic targets and physiological functions. Although some of the CVLM neurones in the present study that were not identified as GABAergic may be vagal motor neurones, a GABAergic phenotype was conclusively established for CVLM neurones displaying each of the four CRD-related patterns.
In addition, we cannot conclusively demonstrate that the recorded CVLM neurones make synaptic contacts with presympathetic RVLM neurones. However, evidence from microinjection studies strongly suggests that the CVLM contains GABAergic neurones that tonically inhibit presympathetic RVLM neurones to reduce SNA (Willette et al. 1984; Blessing, 1988; Schreihofer et al. 2005) and mediate sympathetic cardiovascular reflexes such as the arterial baroreflex and Bezold-Jarisch reflex (Gordon, 1987; Verberne & Guyenet, 1992). In agreement, GABAergic neurones in the CVLM that project to the RVLM express Fos after increased AP, suggesting they are baro-activated (Minson et al. 1997; Chan & Sawchenko, 1998). Extracellular recordings in the CVLM reveal neurones whose activity is inversely related to SNA and expected RVLM neuronal activity. Namely, these GABAergic CVLM neurones are activated by increased AP and phenyl biguanide (Fig. 2; Schreihofer & Guyenet, 2003) and show strong pulse modulation with firing occurring only during systole at higher pressures (Fig. 2; Schreihofer & Guyenet, 2003). Thus, although their role in cardiovascular regulation is putative, these recorded CVLM neurones are excellent candidates for the critical GABAergic interneurones that regulate the RVLM and SNA.
Role of the CVLM in the generation of CRD-related activity in the RVLM and SNA
Some CRD-related patterns of activity in presympathetic RVLM neurones may be produced by baro-activated GABAergic CVLM neurones. Accordingly, in many cases CRD-related patterns observed in the CVLM appeared to be inversely related to previously reported patterns in presympathetic RVLM neurones (Haselton & Guyenet, 1989; Miyawaki et al. 1995) and SNA. For example, type I (inspiratory-peak) CVLM neurones could yield the inspiratory depression pattern observed in RVLM neurones and SNA (Haselton & Guyenet, 1989; Miyawaki et al. 1995). In agreement, inhibition of the CVLM abolishes the inspiratory depression pattern in presympathetic RVLM neurones (Miyawaki et al. 1996). Likewise, type II (inspiratory depression, a.k.a. expiratory-related activity) CVLM neurones could permit the inspiratory peak observed in RVLM neurones by a reduction of GABA, and maintain low activity of RVLM neurones and SNA during expiration. Consistent with this notion, inhibition of glutamatergic receptors in the RVLM does not affect the inspiratory peak in sSNA (Miyawaki et al. 1996), suggesting it may be mediated by disinhibition of the RVLM neurones. Furthermore, inhibition of the CVLM increases expiratory-related activity in some presympathetic RVLM neurones and SNA (Miyawaki et al. 1996). These effects on lumbar SNA were mimicked by blockade of GABAergic receptors in RVLM (Guyenet et al. 1990), suggesting expiration-related activity in CVLM neurones actively suppresses such activity in the RVLM and SNA to ostensibly enhance inspiratory-related activity.
Although some of the CRD-related activity in presympathetic RVLM neurones may be derived from the CVLM, other patterns may be produced by excitatory inputs to the RVLM but modulated simultaneously by the CVLM. The postinspiratory peak in sSNA is abolished by antagonizing glutamate receptors within the RVLM (Miyawaki et al. 1996), suggesting glutamatergic inputs to RVLM may produce the postinspiratory peak of presympathetic RVLM neurones and sSNA. In contrast, blockade of GABAergic receptors within the RVLM or the inhibition of the CVLM, a major source of GABAergic input, enhances the postinspiratory peak of individually recorded RVLM neurones and SNA (Guyenet et al. 1990; Miyawaki et al. 1996), and yields a greater CRD-related modulation of sSNA (Miyawaki et al. 1996). These data suggest that the CVLM could act to limit the amplitude of the postinspiratory peak of presympathetic RVLM neurones and SNA. Indeed, the activity of some baro-activated CVLM neurones displayed a postinspiratory peak (Fig. 3D). Interestingly, this was the only CRD-related pattern in CVLM neurones with CVLM neuronal activity coincident with the peak in sSNA (Fig. 4Bc), suggesting this particular pattern may restrain CRD-related fluctuation of sSNA.
CRD-related activity in baro-inhibited catecholaminergic CVLM neurones
In the present study baro-inhibited, catecholaminergic CVLM neurones also displayed CRD-related activity. Whether the adjacent baro-activated GABAergic CVLM neurones are the source of baroreceptor-mediated inhibition or their CRD-related activity is not clear. The three CRD-related patterns observed in the baro-inhibited CVLM neurones could either be described as similar or inverse to patterns observed in baro-activated CVLM neurones, depending upon which neurones are compared. For example, the baro-inhibited, inspiratory-depression neurones (Fig. 7Bb) could be viewed as inversely related to the baro-activated type I (inspiratory-peak) neurones (Fig. 3Ab), or as similar to the baro-activated type II (inspiratory-depression) neurones (Fig. 3Ba). The patterns observed in the baro-inhibited neurones are comparable to those observed in the spinally projecting rostral C1 cells of the RVLM, except that no baro-inhibited neurones with clear postinspiratory peaks were observed. This difference may be the result of a small sample size, a true distinction between caudal and rostral C1 cells, or the state of the preparation. Given that caudal C1 neurones project to autonomic regions of the forebrain (Verberne et al. 1999), they could provide respiratory-related modulation of long-loop autonomic responses.
Ventilatory-related modulation of CVLM neuronal activity
In addition to their pulse-modulated and CRD-related activity, the baro-activated CVLM neurones also displayed a ventilation-related modulation of their firing rate. This pattern has been observed in the SNA of vagotomized, artificially ventilated rats (Häbler et al. 1996). These ventilation-related fluctuations in SNA are eliminated by transecting arterial baroreceptor afferent nerves, suggesting ventilation-related fluctuations in AP are sensed by baroreceptors and transmitted to the SNA (Boczek-Funcke et al. 1992a; Häbler et al. 1996). All baro-activated CVLM neurones had increased neuronal activity coincident with rises in end-tidal CO2 and AP (Fig. 4Ab and Bb). The strength of this modulation appeared to coincide with the degree of pulse modulation (Fig. 4Aa and bversus 4Ba and b), in agreement with the notion that both patterns arise from the same afferent signals. The ventilation-related modulation was independent of the CRD-related activity, which was not synchronized with ventilation (Fig. 4Ac and Bc). However, in animals with intact vagus nerves, where ventilation and the central respiratory pattern generator are synchronized, this baroreceptor-mediated modulation is likely to enhance respiratory-related activity of barosensitive brainstem neurones and SNA.
Modulation of SNA by central respiratory generator
In the present study we recorded sSNA to ensure the preparation had significant CRD-related modulation of autonomic function. In all cases sSNA peaked during inspiration, with no postinspiratory peak observed in any animals. This pattern has also been observed in splanchnic nerve of vagotomized, ventilated, neuromuscularly blocked rats anaesthetized with halothane (Numao et al. 1987), urethane after clonidine (Koshiya & Guyenet, 1995), or pentobarbital (Miyawaki et al. 1996), and in cats under urethane or after decerebration (Cohen & Gootman, 1970). Under comparable experimental conditions, sympathetic nerves to other targets, such as renal, adrenal, muscle constrictor, or cardiac, also display a peak in activity during inspiration (Connelly & Wurster, 1985; Numao et al. 1987; Jänig & Häbler, 2003). In contrast, under other experimental conditions sSNA displays a prominent postinspiratory peak (Koshiya & Guyenet, 1995; Miyawaki et al. 1996). In addition, within the same preparation different sympathetic nerves may simultaneously display diverse CRD-related patterns. For example, in halothane-anaesthetized rats while splanchnic nerve activity peaks during inspiration, cervical and lumbar nerve activities peak only during postinspiration (Numao et al. 1987). These observations suggest that CRD-related activity observed in SNA is differentially regulated to distinct autonomic targets and the patterns observed in whole nerves are state dependent. Whether different states also change the CRD-related patterns observed in individual medullary cardiovascular-related neurones or simply alter which neurones are active remains to be determined.
Summary
In summary, the present study provides evidence for the integration of cardiovascular and respiratory controls within the CVLM in rats. GABAergic CVLM neurones are believed to play a major role in regulating the activity of presympathetic RVLM neurones and to have an essential role in the production of many autonomic reflexes. Data from previous microinjection studies and recordings from individual CVLM neurones in the present study suggest that the CVLM may also provide CRD-related modulation of presympathetic RVLM neurones, SNA and AP. The present study suggests the CVLM may shape CRD-related patterns in SNA by providing inspiratory- and expiratory-related depressions and restraint of prominent postinspiratory peaks in SNA. In addition, the absence of CVLM neuronal activity may allow inspiratory-related peaks in SNA. Further study will be necessary to determine the mechanisms underlying CRD-related modulation of baro-activated GABAergic CVLM neurones and to understand how or whether CVLM neurones with particular CRD-related patterns are connected to baro-inhibited neurones within the ventrolateral medulla.
Acknowledgments
This work was supported by NIH grant HL-075174 to A.M.S. The authors thank Ms Sherita James for her invaluable technical assistance in the histological processing.
References
- Adrian ED, Bronk DW, Philips G. Discharges in mammalian sympathetic nerves. J Physiol. 1932;74:115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal SK, Calaresu FR. Monosynaptic connection from caudal to rostral ventrolateral medulla in the baroreceptor reflex pathway. Brain Res. 1991;555:70–74. doi: 10.1016/0006-8993(91)90861-o. [DOI] [PubMed] [Google Scholar]
- Bachoo M, Polosa C. Properties of a sympatho-inhibitory and vasodilator reflex evoked by superior laryngeal nerve afferents in the cat. J Physiol. 1985;364:183–198. doi: 10.1113/jphysiol.1985.sp015738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW. Depressor neurons in rabbit caudal medulla act via GABA receptors in rostral medulla. Am J Physiol Heart Circ Physiol. 1988;254:H686–H692. doi: 10.1152/ajpheart.1988.254.4.H686. [DOI] [PubMed] [Google Scholar]
- Boczek-Funcke A, Dembowsky K, Häbler H-J, Jänig W, Michaelis M. Respiratory-related activity patterns in preganglionic neurons projecting into the cat cervical sympathetic trunk. J Physiol. 1992a;457:277–296. doi: 10.1113/jphysiol.1992.sp019378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczek-Funcke A, Häbler HJ, Jänig W, Michaelis M. Respiratory modulation of the activity in sympathetic neurones supplying muscle, skin and pelvic organs in the cat. J Physiol. 1992b;449:333–361. doi: 10.1113/jphysiol.1992.sp019089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res. 1985;56:359–369. doi: 10.1161/01.res.56.3.359. [DOI] [PubMed] [Google Scholar]
- Chan RK, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci. 1998;18:371–387. doi: 10.1523/JNEUROSCI.18-01-00371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MI, Gootman PM. Periodicities in efferent discharge of splanchnic nerve of the cat. Am J Physiol. 1970;218:1092–1101. doi: 10.1152/ajplegacy.1970.218.4.1092. [DOI] [PubMed] [Google Scholar]
- Connelly CA, Wurster RD. Sympathetic rhythms during hyperventilation-induced apnea. Am J Physiol Regul Integr Comp Physiol. 1985;249:R424–R431. doi: 10.1152/ajpregu.1985.249.4.R424. [DOI] [PubMed] [Google Scholar]
- Czyzyk MF, Fedorko L, Trzebski A. Pattern of the respiratory modulation of the sympathetic nerve activity is species dependent: synchronization of the sympathetic outflow over the respiratory cycle in the rat. In: Ciriello J, Calaresu FR, Renaud LP, Polosa C, editors. Organization of the Autonomic Nervous System: Central and Peripheral Mechanisms. New York: Liss; 1987. pp. 143–152. [Google Scholar]
- Dick TE, Hsieh Y-H, Morrison S, Coles SK, Prabhakar N. Entrainment pattern between sympathetic and phrenic nerve activities in the Sprague-Dawley rat: hypoxia-evoked sympathetic activity during expiration. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1121–R1128. doi: 10.1152/ajpregu.00485.2003. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. The human respiratory gate. J Physiol. 2003;548:339–352. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tillakaratne NJ, Feldblum S, Patel N, Tobin AJ. Two genes encode distinct glutamate decarboxylases. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- Gieroba ZJ, Li Y-W, Blessing WW. Characteristics of caudal ventrolateral medullary neurons antidromically activated from rostral ventrolateral medulla in the rabbit. Brain Res. 1992;582:196–207. doi: 10.1016/0006-8993(92)90133-t. [DOI] [PubMed] [Google Scholar]
- Gilbey MP, Jordan D, Richter DW, Spyer KM. Synaptic mechanisms involved in the inspiratory modulation of vagal cardio-inhibitory neurones in cat. J Physiol. 1984;356:65–78. doi: 10.1113/jphysiol.1984.sp015453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbey MP, Numao Y, Spyer KM. Discharge patterns of cervical sympathetic preganglionic neurones related to central respiratory drive in the rat. J Physiol. 1986;378:253–265. doi: 10.1113/jphysiol.1986.sp016218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon FJ. Aortic baroreceptor reflexes are mediated by NMDA receptors in caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 1987;252:R628–R633. doi: 10.1152/ajpregu.1987.252.3.R628. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Brown DL. Unit activity in nucleus paragigantocellularis lateralis during cerebral ischemia in the rat. Brain Res. 1986;364:301–314. doi: 10.1016/0006-8993(86)90843-7. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Darnall RA, Riley TA. Rostral ventrolateral medulla and sympathorespiratory integration in rats. Am J Physiol Regul Integr Comp Physiol. 1990;259:R1063–R1074. doi: 10.1152/ajpregu.1990.259.5.R1063. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Bartsch T, Jänig W. Two distinct mechanisms generate the respiratory modulation in fibre activity of the cervical sympathetic trunk. J Auton Nerv Syst. 1996;61:116–122. doi: 10.1016/s0165-1838(96)00066-5. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Jänig W, Michaelis M. Respiratory modulation of activity in sympathetic neurones. Prog Neurobiol. 1994;43:567–606. doi: 10.1016/0301-0082(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol Regul Integr Comp Physiol. 1989;256:R739–R750. doi: 10.1152/ajpregu.1989.256.3.R739. [DOI] [PubMed] [Google Scholar]
- Jänig W, Häbler H-J. Neurophysiological analysis of target-related sympathetic pathways – from animal to human: similarities and differences. Acta Physiol Scand. 2003;177:255–274. doi: 10.1046/j.1365-201X.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- Jeske I, Morrison SF, Cravo SL, Reis DJ. Identification of baroreceptor reflex interneurons in the caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 1993;264:R169–R178. doi: 10.1152/ajpregu.1993.264.1.R169. [DOI] [PubMed] [Google Scholar]
- Jordan D, Khalid ME, Schneiderman N, Spyer KM. The location and properties of preganglionic vagal cardiomotor neurones in the rabbit. Pflugers Arch. 1982;395:244–250. doi: 10.1007/BF00584817. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. Sympatholytic effect of clonidine depends on the respiratory phase in rat splanchnic nerve. J Auton Nerv Syst. 1995;53:82–86. doi: 10.1016/0165-1838(94)00181-i. [DOI] [PubMed] [Google Scholar]
- Malpas S. The rhythmicity of sympathetic nerve activity. Prog Neurobiol. 1998;56:65–96. doi: 10.1016/s0301-0082(98)00030-6. [DOI] [PubMed] [Google Scholar]
- McAllen RM. Identification and properties of sub-retrofacial bulbospinal neurones: a descending cardiovascular pathway in the cat. J Auton Nerv Syst. 1986;17:151–164. doi: 10.1016/0165-1838(86)90090-1. [DOI] [PubMed] [Google Scholar]
- McAllen RM. Central respiratory modulation of subretrofacial bulbospinal neurones in the cat. J Physiol. 1987;388:533–545. doi: 10.1113/jphysiol.1987.sp016630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson JB, Llewellyn-Smith IJ, Chalmers JP, Pilowsky PM, Arnolda LF. c-fos identifies GABA-synthesizing barosensitive neurons in caudal ventrolateral medulla. Neuroreport. 1997;8:3015–3021. doi: 10.1097/00001756-199709290-00005. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Goodchild AK, Pilowsky PM. Evidence for a tonic GABA-ergic inhibition of excitatory respiratory-related afferents to presympathetic neurons in the rostral ventrolateral medulla. Brain Res. 2002;924:56–62. doi: 10.1016/s0006-8993(01)03025-6. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Minson J, Arnolda Chalmers J, Llewellyn-Smith I, Pilowsky P. Role of excitatory amino acid receptors in cardiorespiratory coupling in ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1221–R1230. doi: 10.1152/ajpregu.1996.271.5.R1221. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Pilowsky P, Sun Q-J, Minson J, Suzuki S, Arnolda L, Llewellyn-Smith I, Chalmers J. Central inspiration increases barosensitivity of neurons in rat rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 1995;268:R909–R918. doi: 10.1152/ajpregu.1995.268.4.R909. [DOI] [PubMed] [Google Scholar]
- Neff RA, Wang J, Baxi S, Evans C, Mendelowitz D. Respiratory sinus arrhythmia: Endogenous activation of nicotinic receptors mediates respiratory modulation of brainstem cardioinhibitory parasympathetic neurons. Circ Res. 2003;93:565–572. doi: 10.1161/01.RES.0000090361.45027.5B. [DOI] [PubMed] [Google Scholar]
- Numao Y, Koshiya N, Gilbey MP, Spyer KM. Central respiratory drive-related activity in sympathetic nerves of the rat: the regional differences. Neurosci Lett. 1987;81:279–284. doi: 10.1016/0304-3940(87)90396-x. [DOI] [PubMed] [Google Scholar]
- Okada H, Fox IJ. Respiratory grouping of abdominal sympathetic activity in the dog. Am J Physiol. 1967;213:48–56. doi: 10.1152/ajplegacy.1967.213.1.48. [DOI] [PubMed] [Google Scholar]
- Pilowsky P. Good vibrations? Respiratory rhythms in the central control of blood pressure. Clin Exp Pharmacol Physiol. 1995;22:594–604. doi: 10.1111/j.1440-1681.1995.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Pinault D. A novel single-cell staining procedure performed in vivo under electrophysiological control: Morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or neurobiotin. J Neurosci Meth. 1996;65:113–136. doi: 10.1016/0165-0270(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Rentero N, Cividjian A, Trevaks D, Pequignot JM, Quintin L, McAllen RM. Activity patterns of cardiac vagal motoneurons in rat nucleus ambiguous. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1327–R1334. doi: 10.1152/ajpregu.00271.2002. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Role of presympathetic C1 neurons in the sympatholytic and hypotensive effects of clonidine in rats. Am J Physiol Regul Integr Comp Physiol. 2000a;279:R1753–R1762. doi: 10.1152/ajpregu.2000.279.5.R1753. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Sympathetic reflexes after depletion of bulbospinal catecholaminergic neurons with anti-DβH-saporin. Am J Physiol Regul Integr Comp Physiol. 2000b;279:R729–R742. doi: 10.1152/ajpregu.2000.279.2.R729. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. The baroreflex and beyond: Control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol. 2000c;29:514–521. doi: 10.1046/j.1440-1681.2002.03665.x. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Baro-activated neurons with pulse-modulated activity in the rat caudal ventrolateral medulla express GAD67 mRNA. J Neurophysiol. 2003;89:1265–1277. doi: 10.1152/jn.00737.2002. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Ito S, Sved AF. Brain stem control of arterial pressure in chronic baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1746–R1755. doi: 10.1152/ajpregu.00307.2005. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Evidence for glycinergic respiratory neurons: Botzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol. 1999;407:583–597. doi: 10.1002/(sici)1096-9861(19990517)407:4<583::aid-cne8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL, Guyenet PG. Regulation of sympathetic tone and arterial pressure by rostral ventrolateral medulla after depletion of C1 cells in rat. J Physiol. 2000;529:221–236. doi: 10.1111/j.1469-7793.2000.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Akey PJ, Guyenet PG. Location and electrophysiological characterization of rostral medullary adrenergic neurons that contain neuropeptide Y mRNA in rat medulla. J Comp Neurol. 1999;415:482–500. doi: 10.1002/(sici)1096-9861(19991227)415:4<482::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Terui N, Masuda N, Saeki, Kumada M. Activity of barosensitive neurons in the caudal ventrolateral medulla that send axonal projections to the rostral ventrolateral medulla in rabbits. Neurosci Lett. 1990;118:211–214. doi: 10.1016/0304-3940(90)90629-n. [DOI] [PubMed] [Google Scholar]
- Terui N, Saeki Y, Kumada M. Barosensory neurons in the ventrolateral medulla in rabbits and their responses to various afferent inputs from peripheral and central sources. Jap J Physiol. 1986;36:1141–1164. doi: 10.2170/jjphysiol.36.1141. [DOI] [PubMed] [Google Scholar]
- Verberne AJM, Guyenet PG. Medullary pathway of the Bezold-Jarisch reflex in the rat. Am J Physiol Regul Integr Comp Physiol. 1992;263:R1195–R1202. doi: 10.1152/ajpregu.1992.263.6.R1195. [DOI] [PubMed] [Google Scholar]
- Verberne AJM, Stornetta RL, Guyenet PG. Properties of C1 and other ventrolateral medullary neurones with hypothalamic projections in the rat. J Physiol. 1999;517:477–494. doi: 10.1111/j.1469-7793.1999.0477t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willette RN, Punnen S, Krieger AJ, Sapru H. Interdependence of rostral and caudal ventrolateral medullary areas in the control of blood pressure. Brain Res. 1984;321:169–174. doi: 10.1016/0006-8993(84)90696-6. [DOI] [PubMed] [Google Scholar]