Abstract
Background
Recent studies have reported high frequencies of somatic mutations in the phosphoinositide-3-kinase, catalytic, alpha (PIK3CA) gene in various human solid tumors. More than 75% of those somatic mutations are clustered in the helical (exon 9) and kinase domains (exon 20). The three hot-spot mutations, E542K, E545K, and H1047R, have been proven to elevate the lipid kinase activity of PIK3CA and activate the Akt signaling pathway. The mutational status of PIK3CA in intraductal papillary mucinous neoplasm/carcinoma (IPMN/IPMNC) has not been evaluated previously.
Methods
To evaluate a possible role for PIK3CA in the tumorigenesis of IPMN and IPMNC, exons 1, 4, 5, 6, 7, 9, 12, 18, and 20 were analyzed in 36 IPMN/IPMC and two mucinous cystadenoma specimens by direct genomic DNA sequencing.
Results
We identified four missense mutations in the nine screened exons of PIK3CA from 36 IPMN/IPMC specimens (11%). One of the four mutations, H1047R, has been previously reported as a hot-spot mutation. The remaining three mutations, T324I, W551G, and S1015F, were novel and somatic.
Conclusion
This is the first report of PIK3CA mutation in pancreatic cancer. Our data provide evidence that oncogenic properties of PIK3CA contribute to the tumorigenesis of IPMN/IPMC.
The abbreviations used are: IPMN/IPMC, intraductal papillary mucinous neoplasm/carcinoma; PCR, polymerase chain reaction; PIK3CA, phosphoinositide-3-kinase, catalytic, alpha; PIP3, phosphatidylinositol-3,4,5-triphosphate; LOH, loss of heterozygosity
Introduction
Intraductal papillary mucinous neoplasm of the pancreas (IPMN) is an increasingly recognized noninvasive cystic neoplasm of the pancreas. It is characterized by unique clinical, pathologic, and molecular features (1–7). These neoplasms are subdivided into three groups based on increasing nuclear and architectural atypia: adenoma, borderline, and intraductal papillary mucinous carcinoma (IPMC) (5). According to the absence or presence of neoplastic cells invading the pancreatic tissue surrounding the involved ducts, IPMC are separated into invasive and noninvasive types. The overall incidence of invasive carcinoma associated with an IPMN is 20% to 40% (8).
Recently, there has been an increase in the number of IPMN cases reported, although it is not clear if this represents a true increase in incidence or a manifestation of increased recognition and detection of these lesions (9). Most IPMN are slow growing and less aggressive compared with conventional ductal adenocarcinoma. An infiltrating adenocarcinoma, however, is frequently identified in pancreata affected by IPMN, suggesting that IPMN can also evolve into invasive ductal adenocarcinomas (1, 2, 7, 10). Although the overall outcome for IPMN is good, even with associated invasive carcinoma, a significant proportion of the patients with completely resected noninvasive IPMN may develop pancreatic adenocarcinoma in the pancreatic remnant and die of disseminated disease. A 4-year survival of only 64% for noninvasive IPMN was reported in one large series- a survival rate almost equal to that of IPMN with invasive carcinoma (11). Other studies have also reported recurrences of invasive carcinoma in completely resected noninvasive IPMN (1, 10), some of which demonstrated only moderate dysplasia (borderline IPMN). Although the majority of invasive carcinomas are associated with IPMC (intraductal carcinoma), invasive carcinoma coexisting with adenoma and borderline IPMN can occur (12). In addition invasive carcinoma is sometimes found distant from an IPMN, and small IPMNs have been detected incidentally in pancreata resected for conventional ductal pancreatic cancer (9).
Reported genetic alterations in IPMNs include mutations in the KRAS (13), TP53 (14), and STK11/LKB1 genes (15, 16), as well as loss of heterozygosity (LOH) of several chromosomal loci (15, 17). Recent evidence suggests that in addition to these genetic alterations, aberrant DNA methylation may contribute to the inactivation of a subset of tumor-suppressor genes in IPMNs (18, 19). Furthermore, two recent studies have evaluated gene expressing profiling in IPMNs mainly focusing on genes that are preferentially expressed in IPMNs (20, 21). To-date, no study has evaluated the mutational status of the PIK3CA gene in IPMNs/IPMCs.
Phosphatidylinositol-3 kinases (PI3Ks) constitute a large and complex family of lipid kinases encompassing three classes with multiple subunits and isoforms (22–24). PI3Ks play an important role in several cellular functions, such as proliferation, differentiation, chemotaxis, survival, trafficking, and glucose homeostasis (22). Class IA PI3Ks are heterodimeric proteins composed of a p110 catalytic subunit and a p85 regulatory subunit (25), which can be activated through interaction with phosphotyrosine residues of receptor tyrosine kinases (RTK) (26, 27) or through the binding of active RAS to the p110 catalytic subunit (24, 27–29). P85 lacks kinase activity and acts as an adaptor, coupling with the p110 subunit to activate protein tyrosine kinases (30). Activated PI3Ks phosphorylate the inositol ring 3’-OH group in inositol phospholipids to generate the second messenger phosphatidylinositol-3,4,5-triphosphate (PIP3) (31), which in turn activates diverse cellular target proteins such as the survival signaling kinase AKT/PKB (22, 23, 32). A tumorigenic role has been proposed for the PIK3CA gene that encodes the catalytic p110alpha subunit of phosphatidylinositol 3-kinase belonging to the class IA of PI3Ks (22, 24). One recent study reported mutations in PIK3CA in different tumor types, namely colorectal cancer, gastric cancer, glioblastoma, breast and lung cancer (33). Several other independent studies in hepatocellular carcinomas, breast carcinomas, lung cancers, ovarian carcinomas, brain tumors, acute leukemias, and head and neck squamous cell carcinomas have since supported and emphasized the oncogenic potential of PIK3CA in the development of cancer (34–38).
In the study by Samuels et al. (33), two PIK3CA mutational hot-spots were described and found to affect the helical (exon 9) and catalytic (exon 20) protein domains. In addition, exons 9 and 20 of PIK3CA were preferentially mutated in colon carcinomas (33). Mutations were also described in exons 1, 2, 4, 7, 12, 14 and 18 of PIK3CA, but only in a minority of cases (33, 34). Similar to colon tumors, PIK3CA mutations also clustered in the two hotspot regions (exons 9 and 20) in gastric carcinomas (33, 35, 39). No PIK3CA mutations have been previously reported in IPMN, IPMC, or conventional pancreatic ductal adenocarcinoma (33).
Materials and Methods
Patients and Tissue Samples
Surgical paraffin-embedded IPMN/IPMC and mucinous cystadenoma samples from 38 patients (female n=14, male n= 24, median age 68.1 years, range 41–84 years) were obtained from the archival tissue collection of the Columbia University Medical Center. Acquisition of the tissue specimens was approved by the Institutional Review Board of Columbia University Medical Center and performed in accordance with Health Insurance Portability and Accountability Act (HIPAA) regulations. In detail, we analyzed three IPMN, adenoma (female n= 1, male n = 2, median age 62.7 years, range 53–77 years); four IPMN, borderline (female n= 1, male n= 3, median age 66.3 years, range 62–72 years), five IPMC without invasion (male n= 5, median age 69.2 years, range 59–81), 24 IPMC with invasive carcinoma (male n= 14, female= 10, median age 68.9 years, range 41–84 years), and two mucinous cystadenomas (female n=2, median age 57.5 years, range 53–62 years). Thirty-two of these lesions arose in the pancreatic head, one in the uncinate process, four within the transition from pancreatic head to the body, one within the body, and one diffusely involving the entire gland. The maximum diameter of the lesions ranged from 0.4 to 7cm (mean: 4.2 cm). For a more detailed register, see Table 1.
Table 1.
Summary reports of the 38 patient samples
| Case No. | Sex | Age | Lesion analyzed | IPMN Nuclear Grade | Differentiation of invasive carcinoma, if present in resection | Location within pancreas | Maximum Dimension** (cm) |
|---|---|---|---|---|---|---|---|
| 1 | M | 62 | IPMN, borderline | 2 | N/A | body | - |
| 2 | M | 73 | IPMC with invasion | 3 | moderate | head and body | 4 |
| 3 | M | 67 | IPMC with invasion | 3 | moderate | head | - |
| 4 | M | 69 | IPMC | 3 | N/A | head | 3.5 |
| 5 | F | 75 | IPMC with invasion | 3 | moderate | head | 5.5 |
| 6 | F | 68 | IPMC with invasion | 3 | moderate | head | 5 |
| 7 | M | 65 | IPMN, borderline | 2 | poor (*) | head | 5 |
| 8 | F | 66 | IPMN, borderline | 2 | N/A | uncinate process | 2 |
| 9 | M | 84 | IPMC with invasion | 3 | moderate | head | 5 |
| 10 | M | 53 | IPMN, adenoma | 1 | N/A | head | 3.5 |
| 11 | M | 71 | IPMC with invasion | 2–3 | N/A | head | - |
| 12 | M | 81 | IPMC | 3 | N/A | head | 2.5 |
| 13 | M | 63 | IPMC | 3 | moderate to poor (*) | head | 2.3 |
| 14 | M | 66 | IPMC with invasion | 3 | moderate to poor | head | 6 |
| 15 | F | 70 | IPMC with invasion | 3 | moderate to poor | head and body | 7 |
| 16 | F | 70 | IPMC with invasion | 3 | moderate | head | 1.5 |
| 17 | M | 72 | IPMN, borderline | 2 | N/A | head | 0.4 |
| 18 | F | 53 | mucinous cystadenoma | 1 | N/A | head | 3 |
| 19 | M | 79 | IPMC with invasion | 3 | moderate to poor | head | 6 |
| 20 | M | 63 | IPMC with invasion | 3 | moderate to poor | head | 3.5 |
| 21 | M | 77 | IPMN, adenoma | 1 | N/A | head and body | 2.2 |
| 22 | F | 62 | mucinous cystadenoma | 1 | N/A | head | 2 |
| 23 | M | 41 | IPMC with invasion | 3 | moderate to poor | head | 5 |
| 24 | M | 71 | IPMC with invasion | 3 | moderate to poor | head | 1.5 |
| 25 | F | 58 | IPMN, adenoma | 1 | N/A | head | 1.5 |
| 26 | M | 49 | IPMC with invasion | 3 | moderate | head | 4.5 |
| 27 | M | 71 | IPMC with invasion | 3 | moderate to poor | head | 5.5 |
| 28 | M | 74 | IPMC | 3 | well (*) | head and body | - |
| 29 | M | 59 | IPMC | 3 | poor (*) | head | 7 |
| 30 | M | 81 | IPMC with invasion | 3 | moderate to poor | head | 3 |
| 31 | F | 80 | IPMC with invasion | 3 | moderate to poor | head | 5 |
| 32 | F | 66 | IPMC with invasion | 3 | poor | head | 3 |
| 33 | F | 77 | IPMC with invasion | 3 | poor | head | 3 |
| 34 | M | 73 | IPMC with invasion | 3 | poor | head | 5.5 |
| 35 | F | 77 | IPMC with invasion | 3 | well | head | 3.2 |
| 36 | F | 61 | IPMC with invasion | 3 | well | head | 1 |
| 37 | M | 62 | IPMC with invasion | 3 | moderate | head | 2.2 |
| 38 | F | 59 | IPMC with invasion | 3 | moderate | head | 3.4 |
N/A, not applicable.
Invasive carcinoma was associated with IPMN/IPMC in pancreatic resection, but lesion analyzed did not sample invasive carcinoma.
Maximum tumor size includes both invasive and non-invasive components of tumor; -, not available.
DNA Samples for Mutation Analysis
All pre-polymerase chain reaction (PCR) tissue samples were handled in an environment free of PCR products. All samples were coded, and the investigator was unaware of all patients’ clinical data. Paraffin-embedded tumor samples were micro-dissected by hand to ensure the highest possible amount of tumor cells. Surrounding non-tumorous tissue or tissue derived from a tumor-free block of each case served as the corresponding normal control. Genomic DNA was extracted using QIAmp DNA Mini Kit (Qiagen, CA). The procedures were performed according to the manufacturer’s instructions for paraffin-embedded tissues.
Exons 1, 4, 5, 6, 7, 9, 12, 18, and 20 of PIK3CA were analyzed by PCR amplification of genomic DNA, and the purified PCR products were directly sequenced. Genomic DNA (40ng per sample) was amplified with primers that had been designed to amplify each exon and its exon/intron boundaries (33, 38). All PCR products were purified using QIAquick PCR Purification Kit according to the manufacturer’s instructions prior to sequencing. Sequencing was performed with ABI’s 3100 capillary automated sequencer at the DNA Core Facility of Columbia University Medical Center. Each sample found to have a genetic alteration in the target gene was subsequently sequenced in the reverse direction to confirm the mutation. The mutation was then further verified by sequencing of a second PCR product derived independently from the original template.
Results and Discussion
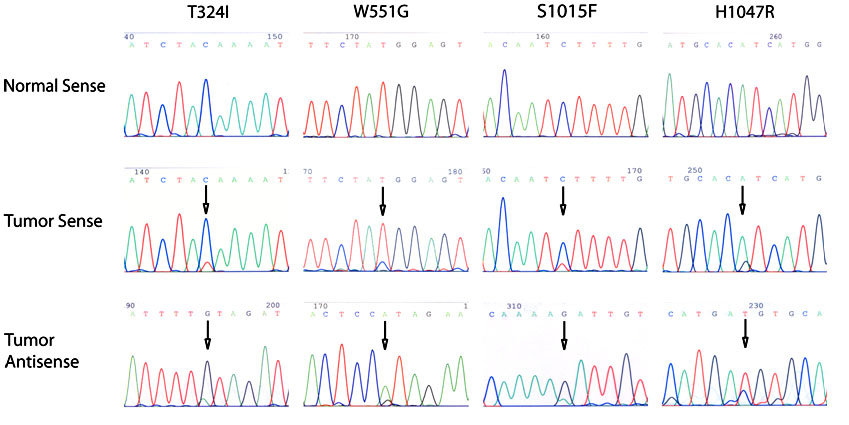
In the present study, four of the 36 specimens contained a somatic mutation of the PIK3CA gene (Figure 1 and Table 2)- one in exon 4 (T324I), one in exon 9 (W551G), and two in exon 20 (S1015F, H1047R). None of these mutations were detected in the corresponding normal tissues. One of the missense mutations in exon 20 of PIK3CA, H1047R, is a previously described hot-spot mutation (33). The other mutations in exons 4 and 9 are novel mutations.
Figure 1. Somatic PIK3CA mutations found in IPMN/IPMC.
One of the four mutations (H1047R) was a hot-spot mutation. The other three mutations were novel. All four mutations were confirmed to be somatic.
Table 2.
Nucleotide alterations within the coding exons of PIK3CA identified in the 36 IPMN/IPMC
| Case No. | Lesion analyzed | Exon | Nucleotide | Amino acid | Present in normal Tissue |
|---|---|---|---|---|---|
| 4 | IPMC | 20 | C3044T | S1015F | No |
| 14 | IPMC with invasion | 20 | A3140G | H1047R | No |
| 1 | IPMN, bordernline | 9 | T1654G | W551G | No |
| 5 | IPMC with invasion | 4 | C0971T | T324I | No |
| 3, 9, 19, 23 | 6 | A1173G | I391M | yes | |
| 4, 8, 24, 31, 33, 35 | 12 | T1929C | Y644H | yes |
Recently, much attention has been given to the significance of the PIK3CA gene mutations identified in several human tumors. Mutational analysis of the PIK3CA gene has revealed that genetic alterations at its locus occur in a wide spectrum of human neoplasms (33–38). PIK3CA mutations preferentially occur in exons 9 and 20, affecting the functionally important helical and kinase domains of the protein (33–35, 37, 39). Functional studies have showed that PI3Ks carrying any one of the three hot-spot mutations is able to induce transformation in cultures of chicken embryo fibroblasts, and that transforming activity of the mutant is correlated with increased lipid kinase activity and activation of the Akt signaling pathway (33, 40). Although two of our mutations in exons 9 and 20 are not hot-spot mutations, the mutations are likely to have affected the kinase activity of the PIK3CA. The mutation within exon 4, nucleotide 971 C → T, which leads to an alteration of codon 324 ACA (T) → ATA (I), has not been described before. Although the significance of the novel mutation T324I, which belongs to the C2 domain, is unclear, a recent study found that the C2 domain of PKC d could be a phosphotyrosine binding domain (41). Since 7% of PIK3CA mutations have been detected within the C2 domain (33), it might be of value to study whether the C2 domain also plays a critical role in PIK3CA activity in the future.
Two more nucleotide alterations were detected in the exons of the PIK3CA gene. One is located at exon 6 nucleotide 1173 A → G, leading to a change at codon 391 ATA (I) → ATG (M). This alteration, observed in four tumor specimens and their matching normal tissues, was subsequently identified as a known single-nucleotide polymorphism (SNP, rs2230461). The other is located at exon 12 nucleotide 1929 T → C, leading to a change at codon 644 TAT (Y) → CAT (H). This alteration was observed in six tumor cases and also present within their corresponding normal tissues. Although a search of the SNP database found no match, this alteration is most likely a polymorphism without carcinogenic pathologic value. This alteration has not been reported by previous publications probably because the majority of the PIK3CA studies focused only on exon 9 and 20, and polymorphisms are often omitted from the reports. No mutations were detected in the two mucinous cystadenoma cases.
The frequency of PIK3CA mutations has been reported to be 32% in colon cancer, 4–25% in gastric cancer, 8–40% in breast cancer, 5–27% in brain tumors, 4% in lung cancer, and 4–7% in ovarian cancer (33, 36, 39, 42). Samuels et al screened 11 pancreatic ductal adenocarcinoma cell lines and found no mutation in the entire coding region of the PIK3CA gene (33). A negative finding was also reported by Gallmeier et al who examined the exons 9 and 20 of PIK3CA for mutation using direct genomic sequencing on the genomic DNA from 91 pancreatic cancer xenografts (43). In the present study we report 11% (4/36) of IPMN/IPMC to have PIK3CA mutations. Two of these mutations (W551G and S1015F) were found in IPMN with nuclear grade 3 (IPMC) and nuclear grade 2 (IPMN, borderline), respectively, without associated invasive carcinoma. The other two (T324I and H1047R) were observed in IPMC with associated invasive carcinoma. The findings in colorectal cancers indicate that PIK3CA mutations generally arise just before or coincident with invasion (33). Our data show that in IPMN, mutations of the PIK3CA gene seem to be a rather late event on the transition of these lesions to malignancy. So far, genetic analyses of IPMN have disclosed abnormalities in many of the same genes altered in conventional ductal adenocarcinoma, including mutations of KRAS (13), TP53/p53 (44) and CDKN2A/p16 genes (45). In addition, as is true for pancreatic ductal carcinomas, a number of genes, including CDKN2A/p16, may be epigenetically inactivated in IPMNs through aberrant DNA methylation (18, 19, 46, 47). The Peutz-Jeghers gene STK11/LKB1 is inactivated more frequently in IPMN (up to one third) than in ductal adenocarcinoma (4%) (16, 48), and some patients with the Peutz-Jeghers Syndrome develop IPMNs (15). In contrast to ductal adenocarcinomas and PanIN-3 (pancreatic intraepithelial neoplasia-3) lesions, abnormalities in the MADH4/SMAD4/DPC4 gene seem to be rare in IPMNs (6). PIK3CA is the first gene to be found mutated in IPMN that had not been reported in ductal adenocarcinoma.
In summary, this is the first report of missense mutations of the PIK3CA gene in IPMN/IPMC (4/36, 11%). All four mutations were found to be somatic. Our data suggest that PIK3CA is important to IPMN/IPMC tumorigenesis. The knowledge of PIK3CA’s involvement in IPMC is important because specific kinase inhibitors could be considered as a future additional therapeutic option for more advanced IPMCs with PIK3CA mutations. Recently kinase inhibitors, such as Gleevec (Imatinib), Herceptin (Trastzumab), and Iressa (Gefitinib) have been successfully developed for therapies in some cancer types (49). Our finding may provide a potential target in IPMC for pathway-specific or kinase-inhibitor-based therapies, in addition to surgery.
Footnotes
This work was supported by the NCI Temin Award CA95434 and the NCI R01 CA109525.
References
- 1.Adsay NV, Conlon KC, Zee SY, Brennan MF, Klimstra DS. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer. 2002;94:62–77. doi: 10.1002/cncr.10203. [DOI] [PubMed] [Google Scholar]
- 2.D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–8. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukushima N, Mukai K, Kanai Y, et al. Intraductal papillary tumors and mucinous cystic tumors of the pancreas: clinicopathologic study of 38 cases. Hum Pathol. 1997;28:1010–7. doi: 10.1016/s0046-8177(97)90053-8. [DOI] [PubMed] [Google Scholar]
- 4.Nagai E, Ueki T, Chijiiwa K, Tanaka M, Tsuneyoshi M. Intraductal papillary mucinous neoplasms of the pancreas associated with so-called "mucinous ductal ectasia". Histochemical and immunohistochemical analysis of 29 cases. Am J Surg Pathol. 1995;19:576–89. doi: 10.1097/00000478-199505000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 6.Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, et al. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157:755–61. doi: 10.1016/S0002-9440(10)64589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–97. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–95. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 9.Adsay NV. The "new kid on the block": Intraductal papillary mucinous neoplasms of the pancreas: current concepts and controversies. Surgery. 2003;133:459–63. doi: 10.1067/msy.2003.127. [DOI] [PubMed] [Google Scholar]
- 10.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–85. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohn TA, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Lillemoe KD. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–21. doi: 10.1097/00000658-200109000-00005. discussion 321–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paal E, Thompson LD, Przygodzki RM, Bratthauer GL, Heffess CS. A clinicopathologic and immunohistochemical study of 22 intraductal papillary mucinous neoplasms of the pancreas, with a review of the literature. Mod Pathol. 1999;12:518–28. [PubMed] [Google Scholar]
- 13.Z'Graggen K, Rivera JA, Compton CC, et al. Prevalence of activating K-ras mutations in the evolutionary stages of neoplasia in intraductal papillary mucinous tumors of the pancreas. Ann Surg. 1997;226:491–8. doi: 10.1097/00000658-199710000-00010. discussion 498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sessa F, Solcia E, Capella C, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425:357–67. doi: 10.1007/BF00189573. [DOI] [PubMed] [Google Scholar]
- 15.Sato N, Rosty C, Jansen M, et al. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 2001;159:2017–22. doi: 10.1016/S0002-9440(10)63053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahin F, Maitra A, Argani P, et al. Loss of Stk11/Lkb1 expression in pancreatic and biliary neoplasms. Mod Pathol. 2003;16:686–91. doi: 10.1097/01.MP.0000075645.97329.86. [DOI] [PubMed] [Google Scholar]
- 17.Fujii H, Inagaki M, Kasai S, et al. Genetic progression and heterogeneity in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 1997;151:1447–54. [PMC free article] [PubMed] [Google Scholar]
- 18.Sato N, Ueki T, Fukushima N, et al. Aberrant methylation of CpG islands in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2002;123:365–72. doi: 10.1053/gast.2002.34160. [DOI] [PubMed] [Google Scholar]
- 19.House MG, Guo M, Iacobuzio-Donahue C, Herman JG. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis. 2003;24:193–8. doi: 10.1093/carcin/24.2.193. [DOI] [PubMed] [Google Scholar]
- 20.Terris B, Blaveri E, Crnogorac-Jurcevic T, et al. Characterization of gene expression profiles in intraductal papillary-mucinous tumors of the pancreas. Am J Pathol. 2002;160:1745–54. doi: 10.1016/S0002-9440(10)61121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato N, Fukushima N, Maitra A, et al. Gene expression profiling identifies genes associated with invasive intraductal papillary mucinous neoplasms of the pancreas. Am J Pathol. 2004;164:903–14. doi: 10.1016/S0002-9440(10)63178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–75. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 23.Domin J, Waterfield MD. Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett. 1997;410:91–5. doi: 10.1016/s0014-5793(97)00617-0. [DOI] [PubMed] [Google Scholar]
- 24.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 25.Carpenter CL, Duckworth BC, Auger KR, Cohen B, Schaffhausen BS, Cantley LC. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990;265:19704–11. [PubMed] [Google Scholar]
- 26.Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998;1436:127–50. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- 27.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–54. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 28.Kodaki T, Woscholski R, Hallberg B, Rodriguez-Viciana P, Downward J, Parker PJ. The activation of phosphatidylinositol 3-kinase by Ras. Curr Biol. 1994;4:798–806. doi: 10.1016/s0960-9822(00)00177-9. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. Embo J. 1996;15:2442–51. [PMC free article] [PubMed] [Google Scholar]
- 30.Hiles ID, Otsu M, Volinia S, et al. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992;70:419–29. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- 31.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644–6. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 32.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J. 2000;346(Pt 3):561–76. [PMC free article] [PubMed] [Google Scholar]
- 33.Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 34.Campbell IG, Russell SE, Choong DY, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–81. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 35.Lee JW, Soung YH, Kim SY, et al. PIK3CA gene is frequently mutated in breast carcinomas and hepatocellular carcinomas. Oncogene. 2005;24:1477–80. doi: 10.1038/sj.onc.1208304. [DOI] [PubMed] [Google Scholar]
- 36.Broderick DK, Di C, Parrett TJ, et al. Mutations of PIK3CA in anaplastic oligodendrogliomas, high-grade astrocytomas, and medulloblastomas. Cancer Res. 2004;64:5048–50. doi: 10.1158/0008-5472.CAN-04-1170. [DOI] [PubMed] [Google Scholar]
- 37.Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–5. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 38.Qiu W, Schönleben F, Li X, et al. PIK3CA mutations in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:1441–6. doi: 10.1158/1078-0432.CCR-05-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li VS, Wong CW, Chan TL, et al. Mutations of PIK3CA in gastric adenocarcinoma. BMC Cancer. 2005;5:29. doi: 10.1186/1471-2407-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci U S A. 2005;102:802–7. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121:271–80. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Helland A, Holm R, Kristensen GB, Borresen-Dale AL. PIK3CA mutations in advanced ovarian carcinomas. Hum Mutat. 2005;25:322. doi: 10.1002/humu.9316. [DOI] [PubMed] [Google Scholar]
- 43.Gallmeier E, Calhoun E, Kern SE. No mutations in PIK3CA identified in pancreatic carcinoma. NOGO. 2004;8:2. [Google Scholar]
- 44.Sasaki S, Yamamoto H, Kaneto H, et al. Differential roles of alterations of p53, p16, and SMAD4 expression in the progression of intraductal papillary-mucinous tumors of the pancreas. Oncol Rep. 2003;10:21–5. [PubMed] [Google Scholar]
- 45.Biankin AV, Biankin SA, Kench JG, et al. Aberrant p16(INK4A) and DPC4/Smad4 expression in intraductal papillary mucinous tumours of the pancreas is associated with invasive ductal adenocarcinoma. Gut. 2002;50:861–8. doi: 10.1136/gut.50.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fukushima N, Sato N, Sahin F, Su GH, Hruban RH, Goggins M. Aberrant methylation of suppressor of cytokine signalling-1 (SOCS-1) gene in pancreatic ductal neoplasms. Br J Cancer. 2003;89:338–43. doi: 10.1038/sj.bjc.6601039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsubayashi H, Sato N, Fukushima N, et al. Methylation of cyclin D2 is observed frequently in pancreatic cancer but is also an age-related phenomenon in gastrointestinal tissues. Clin Cancer Res. 2003;9:1446–52. [PubMed] [Google Scholar]
- 48.Su GH, Hruban RH, Bansal RK, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835–40. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Couzin J. Cancer drugs. Smart weapons prove tough to design. Science. 2002;298:522–5. doi: 10.1126/science.298.5593.522. [DOI] [PubMed] [Google Scholar]