Abstract
Objectives:
Oral cancer has become an important healthcare problem in many countries. Since this disease develops slowly, early detection and intervention can greatly affect ultimate outcome. Celecoxib is a cyclooxygenase-2 inhibitor with significantly less toxicity. This study investigated the possibility of using it for chemoprevention of oral cancer at the early stages.
Study Design:
Randomized animal study.
Methods:
Dysplastic lesions were induced in the buccal pouches of 47 hamsters by a 5-week painting 9, 10-dimethl-1, 2-benzanthrancene (DMBA). Basal diets or diets containing 500 or 1,500 ppm of Celecoxib were orally given for 7 weeks. The T50 (50% incidence; i.e., the time to appearance of tumors in 50% of the hamsters) was observed, and volume of tumors was measured on day 1, 9, 19, 28, 35, and 48 with the Celecoxib treatment.
Results:
The T50 was 9, 19, and 28 days with the treatment in the control group, in the 500 ppm group, and in the 1,500 ppm group, respectively. It indicated that the Celecoxib treatment could delay progression of early lesion. The tumor measurement showed that this treatment was also effective in delaying tumor growth in both treatment groups. There was difference in the treatment efficacy between the 500 ppm and 1500 ppm of Celecoxib, to indicate a dose-dependent efficacy.
Conclusions:
Celecoxib is effective to delay onset of early lesions induced by DMBA and to slow down growth of the tumors, in hamster cheek pouches during the post-initiation stage. Its treatment efficacy appears to be dose-dependent.
Keywords: oral cancer, chemoprevention, hamster, Celecoxib, cyclooxygenase-2 inhibitor
INTRODUCTION
Oral cancer has become an important healthcare problem in clinic. 1 Since this disease develops slowly, often to take more than 20 years, there are a large number of individuals using tobacco and alcohol who are at risk for developing oral cancer. Therefore, an effective therapeutic and preventive strategy for the early stage of the oral carcinogenesis process would be important for management of patients. Cyclooxygenase (COX)-2 is known to be overexpressed in a variety of human premalignant and malignant lesions including oral ones. 2 Its overexpression enhances cell proliferation, inhibits apoptosis and increases metastatic potential, thereby contributing to carcinogenesis. 3 These findings provide the rationale for the use of selective inhibitors of COX-2 as a novel class of chemopreventive agents. Celecoxib is a highly selective inhibitor of COX-2, with less toxicity than traditional COX inhibitors. It may be used for reversing or stopping oral carcinogenesis at an early stage of disease.4
The previous studies involved topical or oral administration of Celecoxib on 9,10 - dimethyl -1,2-benzanthrancene (DMBA)-inducing hamster oral carcinogenesis or a nude mouse model inoculated with an oral squamous carcinoma cells. 2, 4-6 However, there were no studies to determine such effect of Celecoxib on those early oral lesions at the pre-cancer (post-initiation) stage. In this study, we topically painted the oral cheek pouch with 9, 10-dimethyl-1,2-benzanthrancene (DMBA), to create oral carcinogenesis in a hamster model. We would test our hypothesis that oral administration of Celecoxib can inhibit or delay the process of malignant transformation at the level of pre-cancerous lesions, with no significant risk of systemic toxicity. We also investigated the dose-response assay to determine the treatment effect of different levels of Celecoxib on oral carcinogenesis.
MATERIALS AND METHODS
This study was performed in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (A 3775-01). The animal-use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at Boston University School of Medicine, Boston, Massachusetts.
Celecoxib and an animal model
Celecoxib (Celebrex, GD, Searle, Skokie, IL) was supplied by the Boston University Pharmacy. Diets with Celecoxib at 1500 ppm and 500ppm were prepared by Harlan Teklad (Madison WI) by mixing the Celecoxib with Teklad Global 18% protein grounded rodent diet. Forty-seven (47) six-week old male golden Syrian hamsters (Mesocricetus auratus) were obtained from Charles River Laboratories, Inc (Wilmington MA). The hamsters were watched for 1 week before the study. Three hamsters were allocated to each cage and were fed standard laboratory chow and water ad libitum. Both buccal pouches of the hamsters were painted with 0.5% solution of DMBA in acetone (Sigma Chemicals, St. Louis MO) under general anesthesia of CO2 and O2 mixture. The painting was conducted by using a No. 4 sable brush, 3 times weekly for 5 weeks. The hamsters were then randomly divided into 3 groups: (1) control - 17 hamsters; (2) Celecoxib 1500ppm - 15 hamsters; and (3) Celecoxib 500 ppm - 15 hamsters. The weight of all hamsters was recorded prior to the medication and subsequently on the end of this study.
Chemoprevention and follow-up
After 5-week DMBA painting, all animals remained on the standard rodent diet for 1 additional week. Then we continued the control group on the same diet, but fed the second group with the diet supplemented with 1,500 ppm Celecoxib and the last group with the diet having 500 ppm Celecoxib. We conducted this treatment regime for 7 weeks. The time (T50) for tumor appearance in 50% of the hamsters was observed. We also measured the sizes of lesions on Days 1, 9, 19, 28, 35, and 48 from the beginning of the Celecoxib feeding. The tumor size in three (a, b, c) orthogonal directions was measured and the volume was calculated based on the following formula: V= ABC (∏/6). At the end of the study, the animals were euthanized with CO2; and mucosal biopsy specimens were taken for histological examination. The specimens were fixed in 10% formalin, processed in a standard manner, and stained with hematoxylin and eosin. Necropsy also was conducted under an operating microscope, including a gross examination of all internal organs including the stomach, duodenum, kidneys, and liver.
Data Analysis
Statistical analyses of the data were performed with the guidance of a statistician and the assistance of a computer program, Stat view (SAS Institute, Inc., Cary NC). Comparison of the tumor burdens was performed using a nonparametric Student t-test and the corresponding P-values were obtained. P-values less than 0.05 were considered significant.
RESULTS
This study had two findings relating to treatment effect of the Celecoxib on animals: (1) Delay onset of early lesion. The T50 was significantly different among the 3 groups: It was 9 days, 19 days, and 28 days in the control group, the 500 ppm group, and the 1,500 ppm group, respectively. This would indicate that the Celecoxib treatment was effective in delaying the onset of early lesions in both of the treatment groups; and (2) slow down the growth of oral tumor. There were no significant different on the tumor volume before 9 days of Celecoxib treatment, among all of these 3 groups. However, significant slowing on the tumor growth was found in both of Celecoxib treatment groups when their results were compared with the control group (P<0.05), starting from the measurement at the 19 days.
It should be indicated that such a significant treatment efficacy remained in the 1,500 ppm group during entire follow-up period until the end of this study at the 48 days, while the 500 ppm group diminished its treatment efficacy from 35 days. Moreover, there was significant difference to be found in the treatment efficacy between the 1500 ppm group and 500 ppm group (P<0.05 at day 19 and 28; P<0.001 at day 35 and 48, respectively, see Table I and Fig I.).
Table 1.
Statistical analysis (P-value) of tumor growth over time (day)
| Group/P value | Day1 | Day 9 | Day 19 | Day 28 | Day 35 | Day 48 |
|---|---|---|---|---|---|---|
| Cont. vs. Cele.1500 | .19 | .16 | .01 | .01 | .03 | .02 |
| Cont. vs. Cele.500 | .19 | .25 | .02 | .03 | .05 | .05 |
| Cele.1500 vs. Cele.500 | .84 | .28 | .04 | .03 | .001 | .001 |
Cont. = control; Cele.1500 = 1,500 ppm Celecoxib group; Cele.500 = 500 ppm Celecoxib group.
Figure I.
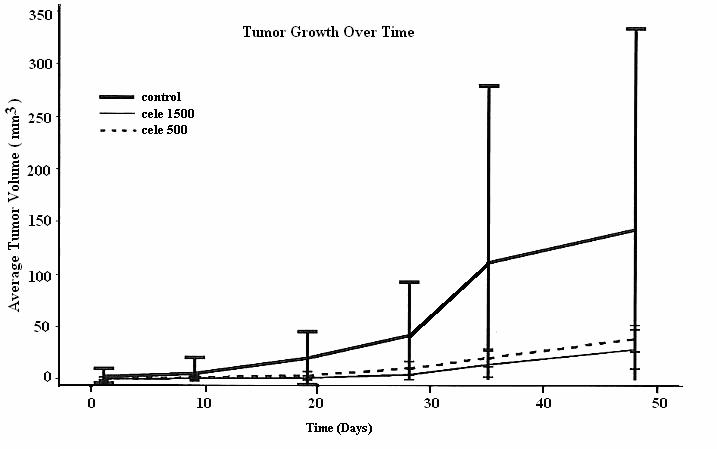
Measurement (mm3) of tumor growth
In this study, there were no detectable side effects or adverse reactions observed during the entire follow-up period. Statistically, no significant difference (P>0.5) was found among the 3 groups (P> 0.5), in the body weight to be measured between pre-treatment and the end of study. No any abnormal finding was identified at our microscopic examination and necropsy.
DISCUSSION
As in human oral cancer, although the carcinogen is applied to the entire surface of the cheek pouch, the process of cancerization is not uniform. The severity of the lesions increases with time. However, at the end of the process, tumors coexist with preneoplastic lesions and areas of apparently normal mucosa. 7 In each of hamsters treated with DMBA, more than one grade of angiogenesis may exist in the buccal pouch simultaneously. For example, one hamster with a lesion of squamous cell carcinoma (SCC) probably also had dysplasia or hyperkeratosis on the mucosa around the SCC lesion. Therefore, in contrast to our previous cancer studies by inoculation of oral SCC cells on mouse skin, the DMBA-induced hamster cheek pouch model of the oral cancer is ideal. It would help us to understand the oral carcinogenesis process because it closely mimics the development of premalignant and malignant lesions in the human oral cavity. The generally accepted carcinogenesis protocol involves topical application of 0.5% DMBA on cheek pouches 3 per week over a period of not less than 14 weeks, preferably greater than 4 months. 8 Typically, there is a significant increase of karyotypes demonstrating tetraploidy or near-tetraploidy at the beginning of the second week of DMBA treatment. 9 Therefore, this 5-week DMBA treatment may be regarded as the precancerous stage of the oral carcinogenesis process. To the best of our knowledge, this is the first study to report oral administration of Celecoxib during the pre-cancer (post-initiation) stage and to find it can significantly delays progression of oral carcinogenesis and in an animal model.
Clinically, physicians usually use daily 200 mg or 400 mg Celecoxib for treatment of rheumatoid arthritis or other diseases, and both doses have been approved by FDA. There is no specific guidance and dose for its application in animal model. However, currently 1,500 p.p.m probably is the most common dose to be used in rodent models. 10 Kawamori reported their findings to establish a therapeutic blood level of Celecoxib for chemoprevention in such models. The study identified daily administration of 1,500 p.p.m as an ideal dose to significantly suppress the colonic aberrant crypt foci formation. 11 These findings served as the basis for our dose selected for this study. We also added a 500 p.pm dose in this study, because there is evidence to indicate that the efficacy of Celecoxib appears to be dose-dependent. 12 Additionally, it is recommended that this medication should be prescribed at the lowest effective dose with individual treatment goals, to reduce potential side-effect. This additional dose will help us to identify any potential relationship of the dose to efficacy and toxicity, and to find the optimal dose for future use.
This result clearly shows that Celecoxib can effectively delay the onset and slow down the growth of DMBA-induced oral tumor in hamster cheek pouches during the post-initiation stage. It was found that the T50 in both treatment groups were significantly later than that in the control group. From day 19 with Celecoxib administration, a treatment effect on the tumor growth was found in both of the treatment groups, suggesting that this medication was effective, even at a relative low dose. It is noteworthy that there was significant difference in the treatment efficacy to delay the tumor growth between the lower (500 ppm) and higher (1500 ppm) doses of Celecoxib, found from day 19. This implies that the chemopreventive efficacy of Celecoxib appeared to be dose-dependent during the post-initiation periods. In this study with a 48-days follow up, there were no detectable side effects observed. Further study is warranted to identify the optimal dose of Celecoxib and to facilitate the application of this agent in a clinical setting.
CONCLUSION
In summary, this study demonstrates for the first time that oral administration of Celecoxib during the pre-cancer (post-initiation) stage significantly delay progression of local disease to oral cancer. This finding provides preliminary evidence to support the use of Celecoxib as a chemopreventive agent for premaglinant oral lesions and high-risk individuals. The development of preventive strategies on the basis of experimental studies will offer a practical approach to designing chemoprevention trials in humans.
ACKNOWLEDGMENT
This work was supported by grants from the National Institutes of Health (R21DE16021). The authors thank Ms. Nina Leech, Department of Otolaryngology-head Neck Surgery, Boston University School of Medicine, for her great help and editorial assistant.
Footnotes
This work was supported by grants from the National Institutes of Health (R21DE16021).
This study was conducted in accordance with the PHS Policy on Human Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq)
REFERENCES
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–19. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura N, Urade M, Hashitani S, Noguchi K, Manno Y, Takaoka K, Sakurai K. Increased expression of cyclooxygenase (COX)-2 in DMBA-induced hamster cheek pouch carcinogenesis and chemopreventive effect of a selective COX-2 inhibitor celecoxib. J Oral Pathol Med. 2004;33:614–621. doi: 10.1111/j.1600-0714.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z. The role of COX-2 in oral cancer development, and chemoprevention/treatment of oral cancer by selective COX-2 inhibitors. Currrent Pharmaceutical Design. 2005;11:1771–1777. doi: 10.2174/1381612053764887. [DOI] [PubMed] [Google Scholar]
- 4.Ning L, Sandeep S, Su W, Mingzhu F, Peng W, Zheng S, Chung SY, Xiaoxin Ch. Overexpression of 5-Lipoxygenase and Cyclooxygenase 2 in Hamster and Human Oral Cancer and Chemopreventive Effects of Zileuton and Celecoxib. Clinical Cancer. 2005 March 1;11:2089–2096. doi: 10.1158/1078-0432.CCR-04-1684. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Polavaram R, Shapshay SM. Topical inhibition of oral carcinoma cell with polymer delivered celecoxib. Cancer Lett. 2003 Jul 30;198(1):53–58. doi: 10.1016/s0304-3835(03)00272-6. [DOI] [PubMed] [Google Scholar]
- 6.Makitie AA, Chau M, Lim S, Viani MA, Gilbert R, Lim MS, Jordan RC. Selective inhibition of cyclooxygenase 2 induces p27kip1 and skp2 in oral squamous cell carcinoma. J otolaryngol. 2003 Aug;32(4):226–229. doi: 10.2310/7070.2003.41701. [DOI] [PubMed] [Google Scholar]
- 7.Raimondi A, Cabrini R, Itoiz ME. Ploidy analysis of field cancerization and cancer development in the hamster cheek pouch carcinogenesis model. J Oral Pathol Med. 2005;34(4):227–231. doi: 10.1111/j.1600-0714.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- 8.Harada K, Yura Y, Tsujimoto H, Kusaka J, Yoshida H, Sato M. Effect of local administration of epidermal growth factor on 9,10-dimethyl-1,2-benzanthracene-induced tumor formation in hamster cheek pouch. Eur J Cancer B Oral Oncol. 1995 Jan;31B(1):27–31. doi: 10.1016/0964-1955(94)00035-3. [DOI] [PubMed] [Google Scholar]
- 9.Lin MH, Hsieh SC, Li SY, Shih HC, Chiang T, McBride J, Todd R, Chou LS, Chou MY, Wong DT. Sequential cytogenetic alterations in hamster oral keratinocytes during DMBA-induced oral carcinogenesis. Eur J Cancer B Oral Oncol. 1994 Jul;30B(4):252–64. doi: 10.1016/0964-1955(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 10.Pendland A, Schoggins SJ, Scott GA, Khan KN, Han R. Reduction of NU-induced skin tumors in Hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–44. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 11.Kawamori T, Rao CV, Seihbert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–12. [PubMed] [Google Scholar]
- 12.Reddy BS, Rao CV, Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res. 1996;56:4566–9. [PubMed] [Google Scholar]


