Abstract
Gastric acid production is important in intestinal iron absorption. The peptide hormone gastrin exists in both amidated and non-amidated forms, which stimulate and potentiate gastric acid secretion, respectively. Since non-amidated gastrins require ferric ions for biological activity in vitro, this study investigated the connection between iron status and gastrin by measurement of circulating gastrin concentrations in mice and humans with hemochromatosis. Gastrin concentrations are increased in the plasma and gastric mucosa of Hfe-/- mice, and in the sera of humans with HFE-related hemochromatosis. The discovery of a relationship between iron status and circulating gastrin concentrations opens a new perspective on the mechanisms of iron homeostasis.
Keywords: ferric, gastrin, hemochromatosis, iron
1. Introduction
The importance of gastric acid production in dietary iron absorption was first recognised nearly fifty years ago, when Baird and coworkers reported that iron-deficiency anemia is a long-term consequence of partial gastrectomy [1]. Although the peptide hormone gastrin has long been recognised as a major regulator of gastric acid secretion [2], the possibility of a connection between iron status and circulating gastrins has not been investigated previously.
All forms of gastrin, including amidated gastrin17 (Gamide) and glycine-extended gastrin17 (Ggly), are derived from an 80 amino acid precursor, progastrin [2]. C-terminal amidation is essential for the biological activities of Gamide as a stimulant of gastric acid secretion and a growth factor for the gastric mucosa [2]. However, non-amidated forms such as Ggly have been shown to potentiate gastric acid secretion [3], and to stimulate proliferation in the colorectal mucosa [4-6]. Amidated gastrins act via the cholecystokinin2 (CCK2 receptor), but the receptor that mediates the effects of non-amidated gastrins has not been defined [7]. We have previously reported that Ggly binds two ferric ions selectively and with high affinity via glutamates 7-9 [8,9]. Ferric ion binding is essential for Ggly’s biological activity since replacement of glutamate 7 by alanine [9], or addition of iron chelators [9] or bismuth ions [10], renders Ggly inactive. In contrast, although Gamide also binds two ferric ions [8], their removal has no effect on biological activity [11].
Although our in vitro observation that non-amidated gastrins require ferric ions for biological activity [9] suggests that a connection may exist between iron status and gastrins, this proposal has not been tested in vivo. We therefore measured concentrations of Gamide and Ggly in the plasma and in gastric mucosal extracts of mice with hemochromatosis as a result of deletion of the Hfe gene [12]. Serum Gamide and Ggly concentrations were also investigated in humans with HFE-related hemochromatosis.
2. Materials and methods
2.1. Patients.
Patients with hemochromatosis have been described previously [13]. Blood sample collection was approved by the Queensland Institute of Medical Research Human Ethics Committee and written informed consent was obtained from all individuals.
2.2. Mouse strains.
C57BL/6J Hfe-/-mice [12] were obtained from Dr. Robert Fleming (Department of Pediatrics, St Louis University School of Medicine, St Louis, MO). Equal numbers of male and female mice (9-11 weeks of age) were fed a commercial pelleted diet, given water ad libitum, and fasted overnight before being anesthetised with ethrane prior to euthanasia and collection of plasma and tissue. All experiments described in this study were approved by the Austin Health or Queensland Institute of Medical Research Animal Ethics Committees.
2.3. Peptide extraction and radioimmunoassay.
Peptides were extracted by boiling whole murine stomachs in 3 volumes of water followed by mechanical homogenisation (Polytron, Brinkman Instruments, Lucerne, Switzerland). After centrifugation (10000 rpm, 15 min, 4°C) supernatants were collected for radioimmunoassay. Murine plasma was collected from centrifuged blood obtained by cardiac puncture. For both human and murine samples amidated gastrin was measured as described previously by radioimmunoassay against an amidated gastrin17 standard curve with 125I-amidated gastrin17 as label with antiserum 1296, which recognises the amidated C-terminal pentapeptide, detects all fragments greater than the pentapeptide and does not cross react with cholecystokinin or Ggly [14]. Ggly was assayed as described previously by radioimmunoassay against a gastrin17gly standard curve with 125I-gastrin17gly as label with antiserum 7270, which does not cross-react with Gamide[14].
2.4. Immunohistochemistry.
The morphology of the gastric mucosa was assessed after staining with hematoxylin/eosin [15]. Gastric parietal cells were detected by immunofluorescent staining for the β-subunit of the gastric H+/K+-ATPase with the monoclonal antibody 2B6 as previously described [16].
2.5. Gastric pH determination.
The pH of the gastric contents was determined by the method of Koh and coworkers [17], except that 1 ml of saline was used for flushing.
2.6. Statistics.
Data are means ± S.E. Statistical significance relative to the control (*, p < 0.05; **, p < 0.01; #, p < 0.001) was assessed by one-way ANOVA, followed by Student’s t-test with Bonferroni correction.
3. Results and discussion
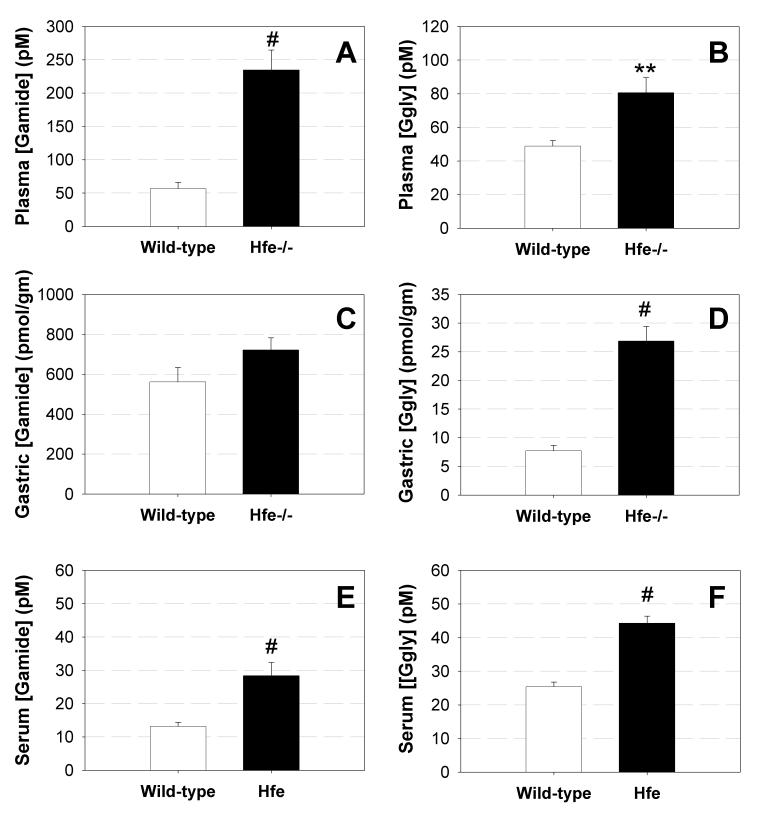
We have previously reported that gastrins bind two ferric ions with high affinity [8], and that ferric ion binding is essential for the biological activity of Ggly [9]. In order to determine whether there is a hitherto unsuspected connection between circulating gastrins and iron status, Gamide and Ggly concentrations were assayed in plasma and gastric mucosal extracts from Hfe-/- mice, which develop the iron loading disease hemochromatosis [12]. Both Gamide and Ggly concentrations were significantly higher in the plasma of Hfe-/- mice compared with wild-type controls (Figures 1A, 1B). The concentration of Ggly was also four-fold higher in the gastric mucosal extracts from Hfe-/- mice (Figure 1D). The failure to detect a significant increase in Gamide concentration in the gastric mucosal extracts from Hfe-/- mice (Figure 1C) may be a consequence of the far higher baseline concentration of Gamide relative to Ggly, since the same absolute increase in gastrin concentration will result in a much lower percentage increase in Gamide concentration. The changes in gastrin concentration are not an artefact caused by changes in the plasma iron concentration, since the measurement of Gamide concentration by radioimmunoassay is not affected by addition of ferric ions (112 ± 5 % of initial concentration), or by removal of any adventitious metal ions by chelation with EDTA (110 ± 3 %). The observed changes appear to be due to differences in translation of gastrin mRNA or in processing of progastrin since no significant differences in the concentration of gastrin mRNA between mouse strains were detected by real time PCR (data not shown).
Figure 1.
Circulating gastrin is increased in hemochromatosis. The concentrations of Gamide (A, C, E) and Ggly (B, D, F) in plasma (A, B) and gastric mucosal extracts (C, D) of mice, and in the sera of patients with hemochromatosis (E, F), were measured by radioimmunoassay [14]. Wild-type C57BL/6J (n = 10) or Hfe-/- (n = 20) mice were fasted overnight before collection of plasma and tissue samples. The total numbers (n = 41 and 40, respectively) and the mean age of the control and HFE groups, and the numbers of male and female patients, were approximately equal. Data are means ± S.E. Statistical significance relative to the control (*, p < 0.05; **, p < 0.01; #, p < 0.001) was assessed by one-way ANOVA, followed by Student’s t-test with Bonferroni correction.
In order to investigate whether or not the connection between circulating gastrin concentrations and iron status observed in mice was also present in humans, gastrin concentrations were measured in sera from patients with HFE-related hemochromatosis. In parallel with the murine studies, circulating Gamide and Ggly (Figures 1E, 1F) concentrations were significantly greater in hemochromatotic patients compared with a group of normal controls with a similar mean age and sex ratio. Since infection with Helicobacter pylori (H. pylori) can increase circulating gastrin concentrations [2], the comparison was repeated in H. pylori-negative patients (n = 35). The fact that the difference was of similar magnitude and still significant (p < 0.001, data not shown) indicated that the observed increase in circulating gastrin concentrations in hemochromatotic patients was not due to infection with H. pylori.
Several alternative explanations were considered for the observed increases in circulating gastrin concentrations in mice and humans with HFE-related hemochromatosis. In the case of the murine samples the possibilities that the increases might have been due to structural changes or to differences in bacterial colonisation of the stomach [18] were eliminated by the failure to detect significant morphological differences between strains (Figure 2A,B). The possibility was also considered that the changes in gastrin concentrations might have been due to an increased luminal pH, since reduced acid secretion leads to a compensatory increase in circulating gastrin [2]. This explanation could be eliminated as there was no difference in the numbers of parietal cells (Figure 2C,D), and as the pH of the luminal contents was lower in the iron-overloaded mice than in controls (Figure 2E). The fact that animals were fasted overnight before collection of plasma and tissue also eliminates feeding differences as a cause of the observed changes in gastrin concentrations. Since the HFE protein is expressed in the gastric antrum [19] the observed increases in circulating gastrin concentrations may either be a direct effect of the HFE mutation, or alternatively may be the result of the altered iron homeostasis consequent on HFE mutation.
Figure 2.
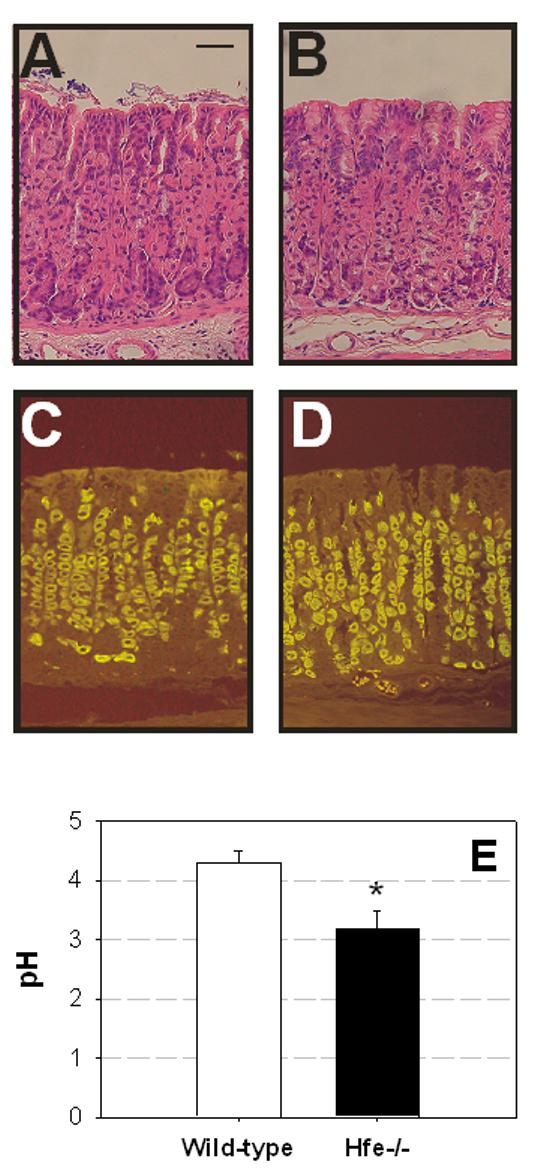
The observed changes in gastrin concentrations in mice with disordered iron metabolism are not due to structural changes in the gastric mucosa. The morphology (assessed by staining with hematoxylin/eosin (A, B)), and the numbers of gastric parietal cells (assessed by staining for the gastric H+/K+-ATPase (C, D)), were the same in wild-type (A, C) and Hfe-/- (B, D) mice. The scale bar in A represents 50 μm; panels A-D are at the same magnification. Similarly the changes in gastrin concentration were not due to an increased pH, as the pH of the luminal contents (E) was lower in the Hfe-/- mice than in controls.
How do our data fit with current models of iron homeostasis? The regulatory peptide hepcidin is known to play a central role in the regulation of iron transport [20,21]. A reduction in the circulating concentration of hepcidin, such as is observed in HFE-related hemachromatosis [22], results in reduced internalisation of the iron transporter ferroportin, and hence to increased iron export from intestinal enterocytes [23]. Our data suggest that a second unexpected correlate of reduced circulating hepcidin may be an increase in antral and/or circulating gastrins. The increase in circulating gastrins would stimulate gastric acid secretion, which would in turn lower luminal pH (Figure 2E), promote the release of ferric ions from food in the stomach, and hence increase the availability of iron for intestinal uptake. While other models are also feasible and require assessment, in this model hepcidin controls cellular iron export and gastrins, under the control of hepcidin, modulate the availability of dietary iron.
The model is consistent with the earlier observation that iron-deficiency anemia is a long-term consequence of partial gastrectomy [1]. The reduction in iron uptake could either be a direct consequence of the removal of the source of gastrins, or indirectly by reduction in the amount of gastric acid released in response to gastrins [1]. The observations that there was no reduction in ferrous ion absorption in humans after removal of the source of gastrin by antrectomy, but that absorption was lower in antrectomised patients with acid secretion reduced by vagotomy, suggested that gastric acid was the critical factor [24]. The importance of gastric acid was further demonstrated by the observation that administration of the histamine H2 receptor antagonist cimetidine to human volunteers resulted in reduced acid secretion and a 65% decrease in iron absorption [25]. However later reports indicated that long-term treatment of humans with the proton pump inhibitor omeprazole did not induce iron deficiency [26], and treatment of rats with omeprazole only decreased iron absorption if the animals were on an iron-deficient diet [27]. Interestingly iron deficiency in rats has itself been reported to reduce acid secretion [28]. Further studies using other means to modify iron status are clearly required to detail the relationships between iron status and gastrin. Such studies might include modification of dietary iron intake and chemical induction of anemia.
In conclusion, the experimental data presented in this paper provide convincing evidence for a previously unsuspected relationship between circulating gastrins and iron status. When taken together with our in vitro observations that non-amidated gastrins require ferric ions for biological activity [8,9], there is now strong evidence for relationships between gastrin expression, gastrin bioactivity and iron homeostasis, and tantalising glimpses of connections between gastrin and iron in colorectal cancer. For example the increased risk of colorectal cancer in patients with HFE mutations [29] may be related to their increased concentrations of circulating gastrins (Figure 1E, F), since circulating Gamide concentrations above normal are also associated with an increased risk of colorectal cancer [30]. Although the detailed implications of our findings remain to be explored, the connection between hemochromatosis and circulating gastrins opens new perspectives on the mechanisms of iron homeostasis and colorectal cancer development.
Acknowledgements
This work was supported in part by grants from the National Health and Medical Research Council of Australia (GB, AS), the Austin Hospital Medical Research Foundation (GB, AS), and the National Institutes of Health (GA, GB, AS). We thank the Brisbane Hemochromatosis Group for provision of the sera from patients with hemochromatosis, and Dr. Adrienne Paterson and Mildred Yim for some of the gastrin radioimmunoassays.
Footnotes
- CCK2 receptor
- cholecystokinin2 receptor
- Gamide
- amidated gastrin17
- Ggly
- glycine-extended gastrin17
References
- [1].Baird IM, Blackburn EK, Wilson GM. The pathogenesis of anaemia after partial gastrectomy. I. Development of anaemia in relation to time after operation, blood loss, and diet. Q J Med. 1959;28:21–34. [PubMed] [Google Scholar]
- [2].Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–39. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- [3].Higashide S, Gomez G, Greeley GH, Jr., Townsend CM, Jr., Thompson JC. Glycine-extended gastrin potentiates gastrin-stimulated gastric acid secretion in rats. Am J Physiol. 1996;270:G220–4. doi: 10.1152/ajpgi.1996.270.1.G220. [DOI] [PubMed] [Google Scholar]
- [4].Koh TJ, Dockray GJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Wang TC. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103:1119–26. doi: 10.1172/JCI4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Koh TJ, Bulitta CJ, Fleming JV, Dockray GJ, Varro A, Wang TC. Gastrin is a target of the beta-catenin/TCF-4 growth-signaling pathway in a model of intestinal polyposis. J Clin Invest. 2000;106:533–9. doi: 10.1172/JCI9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aly A, Shulkes A, Baldwin GS. Short term infusion of glycine-extended gastrin(17) stimulates both proliferation and formation of aberrant crypt foci in rat colonic mucosa. Int J Cancer. 2001;94:307–13. doi: 10.1002/ijc.1483. [DOI] [PubMed] [Google Scholar]
- [7].Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochim Biophys Acta. 2004;1704:1–10. doi: 10.1016/j.bbcan.2004.01.004. [DOI] [PubMed] [Google Scholar]
- [8].Baldwin GS, Curtain CC, Sawyer WH. Selective, high-affinity binding of ferric ions by glycine-extended gastrin(17) Biochemistry. 2001;40:10741–6. doi: 10.1021/bi010016h. [DOI] [PubMed] [Google Scholar]
- [9].Pannequin J, Barnham KJ, Hollande F, Shulkes A, Norton RS, Baldwin GS. Ferric ions are essential for the biological activity of the hormone glycine-extended gastrin. J Biol Chem. 2002;277:48602–9. doi: 10.1074/jbc.M208440200. [DOI] [PubMed] [Google Scholar]
- [10].Pannequin J, Kovac S, Tantiongco JP, Norton RS, Shulkes A, Barnham KJ, Baldwin GS. A novel effect of bismuth ions: selective inhibition of the biological activity of glycine-extended gastrin. J Biol Chem. 2004;279:2453–60. doi: 10.1074/jbc.M309806200. [DOI] [PubMed] [Google Scholar]
- [11].Pannequin J, Tantiongco JP, Kovac S, Shulkes A, Baldwin GS. Divergent roles for ferric ions in the biological activity of amidated and non-amidated gastrins. J Endocrinol. 2004;181:315–25. doi: 10.1677/joe.0.1810315. [DOI] [PubMed] [Google Scholar]
- [12].Zhou XY, et al. HFE gene knockout produces mouse model of hereditary hemochromatosis. Proc Natl Acad Sci U S A. 1998;95:2492–7. doi: 10.1073/pnas.95.5.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Powell LW, et al. Screening for hemochromatosis in asymptomatic subjects with or without a family history. Arch Intern Med. 2006;166:294–301. doi: 10.1001/archinte.166.3.294. [DOI] [PubMed] [Google Scholar]
- [14].Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology. 1997;113:1576–88. doi: 10.1053/gast.1997.v113.pm9352860. [DOI] [PubMed] [Google Scholar]
- [15].Zavros Y, Rieder G, Ferguson A, Samuelson LC, Merchant JL. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterology. 2002;122:119–33. doi: 10.1053/gast.2002.30298. [DOI] [PubMed] [Google Scholar]
- [16].Toh BH, et al. The 60- to 90-kDa parietal cell autoantigen associated with autoimmune gastritis is a beta subunit of the gastric H+/K(+)-ATPase (proton pump) Proc Natl Acad Sci U S A. 1990;87:6418–22. doi: 10.1073/pnas.87.16.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koh TJ, Goldenring JR, Ito S, Mashimo H, Kopin AS, Varro A, Dockray GJ, Wang TC. Gastrin deficiency results in altered gastric differentiation and decreased colonic proliferation in mice. Gastroenterology. 1997;113:1015–25. doi: 10.1016/s0016-5085(97)70199-9. [DOI] [PubMed] [Google Scholar]
- [18].Rathinavelu S, Zavros Y, Merchant JL. Acinetobacter lwoffii infection and gastritis. Microbes Infect. 2003;5:651–7. doi: 10.1016/s1286-4579(03)00099-6. [DOI] [PubMed] [Google Scholar]
- [19].Parkkila S, et al. Cell surface expression of HFE protein in epithelial cells, macrophages, and monocytes. Haematologica. 2000;85:340–5. [PubMed] [Google Scholar]
- [20].Frazer DM, Anderson GJ. The orchestration of body iron intake: how and where do enterocytes receive their cues? Blood Cells Mol Dis. 2003;30:288–97. doi: 10.1016/s1079-9796(03)00039-1. [DOI] [PubMed] [Google Scholar]
- [21].Ganz T, Nemeth E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol. 2006;290:G199–203. doi: 10.1152/ajpgi.00412.2005. [DOI] [PubMed] [Google Scholar]
- [22].Bridle KR, et al. Disrupted hepcidin regulation in HFE-associated haemochromatosis and the liver as a regulator of body iron homoeostasis. Lancet. 2003;361:669–73. doi: 10.1016/S0140-6736(03)12602-5. [DOI] [PubMed] [Google Scholar]
- [23].Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- [24].Magnusson B, Solvell L, Rehnberg O. Iron absorption from ferrous ascorbate in normal male subjects and in patients 3-6 years after antrectomy with gastroduodenostomy. Scand J Haematol Suppl. 1976;26:53–67. doi: 10.1111/j.1600-0609.1976.tb01455.x. [DOI] [PubMed] [Google Scholar]
- [25].Skikne BS, Lynch SR, Cook JD. Role of gastric acid in food iron absorption. Gastroenterology. 1981;81:1068–71. [PubMed] [Google Scholar]
- [26].Stewart CA, Termanini B, Sutliff VE, Serrano J, Yu F, Gibril F, Jensen RT. Iron absorption in patients with Zollinger-Ellison syndrome treated with long-term gastric acid antisecretory therapy. Aliment Pharmacol Ther. 1998;12:83–98. doi: 10.1046/j.1365-2036.1998.00274.x. [DOI] [PubMed] [Google Scholar]
- [27].Golubov J, Flanagan P, Adams P. Inhibition of iron absorption by omeprazole in rat model. Dig Dis Sci. 1991;36:405–8. doi: 10.1007/BF01298866. [DOI] [PubMed] [Google Scholar]
- [28].Murray MJ, Stein N. The effect of iron stores on gastric acid secretion by rats. Br J Haematol. 1968;15:401–7. doi: 10.1111/j.1365-2141.1968.tb01556.x. [DOI] [PubMed] [Google Scholar]
- [29].Shaheen NJ, Silverman LM, Keku T, Lawrence LB, Rohlfs EM, Martin CF, Galanko J, Sandler RS. Association between hemochromatosis (HFE) gene mutation carrier status and the risk of colon cancer. J Natl Cancer Inst. 2003;95:154–9. doi: 10.1093/jnci/95.2.154. [DOI] [PubMed] [Google Scholar]
- [30].Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275–80. doi: 10.1016/s0016-5085(98)70193-3. [DOI] [PubMed] [Google Scholar]