Abstract
This study was designed to learn more about the changes in expression of rat anterior pituitary (AP) leptin during the estrous cycle. QRT-PCR assays of cycling rat AP leptin mRNA showed 2—fold increases from metestrus to diestrus followed by an 86% decrease on the morning of proestrus. Percentages of leptin cells increased in proestrus and pregnancy to 55–60% of AP cells. Dual labeling for leptin proteins and growth hormone (GH) or gonadotropins, showed that the rise in leptin protein-bearing cells from diestrus to proestrus was mainly in GH cells. Only 10–20% of leptin cells in male or cycling female rats co-express gonadotropins. In contrast, 50–73% of leptin cells from pregnant or lactating females co-express gonadotropins and only 19% co-express GH, indicating plasticity in the distribution of leptin. Leptin cells expressed GnRH receptors; and estrogen and GnRH together increased the co-expression of leptin mRNA and gonadotropins. GnRH increased cellular leptin proteins 3–4X and mRNA 9.8X in proestrous rats and stimulated leptin secretion in cultures from diestrous, proestrous and pregnant rats. These regulatory influences, and the high expression of AP leptin during proestrus and pregnancy, suggest a supportive role for leptin during key events involved with reproduction.
Keywords: Leptin, Anterior pituitary, gonadotropes, somatotropes, gonadotropin-releasing hormone, estrous cycle, pregnancy, lactation, males, rat, QRT-PCR, in situ hybridization, immunolabeling
Introduction
Leptin production by white fat cells signals the levels of fat depots in the body to the brain, and hence regulates food intake (Zhang et al, 1994; Vasselli, 2001; Rowland and Morien, 1996). It also makes critical nutrients, like glucose, available to cells (Schneider et al, 1999, 2000, 2002). As a regulator of appetite and metabolism, circulating leptin also plays a critical role in reproduction, because a threshold level of fat deposition is vital for normal puberty and fertility. The importance of leptin to the pituitary is further highlighted by the recognition of major problems in animals homozygous for a leptin receptor defect (Zucker rats) (Urbanski, 2001; Lloyd et al. 2001). These rats show losses in both the growth hormone (GH) and the reproductive system axes and are morbidly obese. Puberty is also delayed or absent, and fertility is severely impaired in these animals (Popovic et al. 2001, Urbanski, 2001). Giving exogenous leptin to leptin-deficient, obese animals will cure the infertility (Barash et al. 1996; Chehab et al. 1996). Urbanski (2001) and Mann and Plant (2002) indicate that leptin is one of many permissive factors that allow puberty to proceed. Mice that overexpress leptin go through accelerated puberty (Yura et al. 2000).
However, leptin has also recently been recognized as a multifunctional paracrine regulator in a number of organs, including the anterior pituitary (Jin et al. 1999; Morash et al. 1999; Popovic et al. 2001; Lloyd et al 2001. Jin et al. 2000; Vidal et al. 2000; Sone and Osamura, 2001; Sone et al. 2001; McDuffie et al, 2004). In humans, leptin proteins are found in subsets of corticotropes, somatotropes, gonadotropes or thyrotropes (Jin et al 1999; Vidal et al. 2000). Dual labeling at the electron microscopic level shows that leptin proteins are stored in secretory granules (Vidal et al, 2000). Recent studies in our laboratory detected the expression of leptin mRNA and proteins by somatotropes (McDuffie et al. 2004). However, the percentages of leptin-protein bearing cells varied with the reproductive state. Therefore, we hypothesized that gonadotropes might also be a source of leptin. We also postulated that regulators of gonadotropes might be involved in the production of anterior pituitary leptin.
One important candidate regulator proposed for testing was Gonadotropin releasing hormone (GnRH). This neuropeptide is produced by neurons scattered in the pre-optic and anterior regions of the hypothalamus stretching back to the arcuate nucleus (Clayton 1989; Conn et al. 1987; Conn 1994). GnRH is secreted in pulses. The GnRH pulse amplitude and frequency changes as the rats approach midcycle. Slower pulses of GnRH, seen earlier in the cycle, favor FSH secretion. Higher amplitude pulses during proestrus favor LH secretion over FSH secretion and eventually lead to the LH surge. (Belchetz et al. 1978; Levine and Ramirez 1982; Crowley et al. 1985; Wildt et al. 1981; Haisenleder et al. 1991; Bédécarrats and Kaiser 2003; Burger et al. 2002; Kaiser et al. 1997; Loumaye and Catt 1982; Savoy-Moore et al. 1980.) To enhance their response to GnRH, estrogens stimulate the production of GnRH receptors, which reach a peak late in diestrus or early in proestrus (Lloyd et al. 1988; Childs et al. 1994b). These known cyclic changes in GnRH receptors were used in the design of tests of GnRH effects on pituitary leptin.
In the present group of studies, we continued our analysis of changes in the expression of leptin with different reproductive states, adding tests of leptin expression by pituitaries from rats during the PM of proestrus as well as tests of pituitaries from pregnant or lactating rats. We tested the efficacy of GnRH in the regulation of AP leptin expression, comparing exposures of 1 h and 3 h in cells from rats in proestrus. The findings presented in this report show that the expression of leptin proteins and mRNA in normal male and cycling female rats predominates in somatotropes. However co-expression of leptin mRNA and proteins can also be found in subpopulations of gonadotropes, which predominate in pregnant or lactating females. The study will also present in vitro data demonstrating that leptin-bearing cells express GnRH receptors and that GnRH stimulates the expression of leptin mRNA, proteins, and secretion.
Materials and Methods
Animals
Male or Female Sprague-Dawley rats (Harlan, Sprague-Dawley, wt 200–250 g) were acclimated and housed 3–4/cage with a 12 h on, 12 h off light-dark cycle for 7–10 days before use. Vaginal smears were used to determine the stage of the cycle, as previously described (McDuffie et al. 2004). The Animal use protocol was approved annually by the Animal Care and Use Committee, with regular updates as specified in the guidelines.
To obtain pregnant females, proestrous females were placed individually in the same cage with a male for one night. The smear was checked for sperm the next morning. We then separated the female, if pregnant, and sacrificed her on the 4th day at 10 AM. We checked the uterus for embryos at the time of sacrifice and all 6 females were found to be pregnant. For the lactating females, we sacrificed the mothers (6 rats) on the third day of lactation at 10 AM.
Pituitaries from all animals were taken either at 10 AM (all stages of the cycle) or 2 PM (diestrus or proestrus) from animals that had completed two successful estrous cycles. Rats were sacrificed by decapitation under anesthesia as previously described (McDuffie et al. 2004).
Stimulation of dispersed pituitary cells
For secretion and cytochemical studies, freshly dispersed pituitary cells were used for several reasons. First, the use of whole cells for the detection of co-expression of two products prevented artifacts often seen in sections, which make it impossible to differentiate between a neighboring process filled with label and a patch of label at the periphery of a cell. Sections also may miss detection of dual label in areas in the cell that are above or below the plane of the section. Living, dispersed cells were also needed to detect GnRH receptors by biotinylated analogs in sectioned material (Childs et al. 1983a,b).
Tests of expression of leptin and other pituitary hormones show that there are no changes in percentages of labeled cells when the culture is extended for as long as 48 h. Percentages of LH and GH cells are similar to those freshly dispersed cultures as they are in sections, however the labeling is more intense in the whole cells and it is easier to detect labeled small cells. The enzymatic dispersion process and the subsequent 1–2 hrs of culture were not deleterious to the detection of product. In cases where we use a longer incubation in secretagogue, estrogen or vehicle, the percentages of leptin bearing cells remain comparable to those in freshly dispersed cultures.
After 1–2 h of plating, freshly dispersed pituitary cells from proestrous female or male rats were treated for 1 or 3 h with vehicle, or 10 pM-1000 pM GnRH. The vehicle was defined, serum-free media (McDuffie et al, 2004). The cells were fixed and then prepared for in situ hybridization or immunocytochemistry (McDuffie et al. 2004). Media were collected for leptin EIA.
Immunocytochemistry
Immunolabeling for leptin was done, as previously described (McDuffie et al, 2004), with a working dilution of anti-leptin of 1:37,500 (Sigma). Dual labeling for leptin and GH or LHβ was done by first detecting leptin with the streptavidin-biotin peroxidase protocol. Then, GH, FSHβ, or LHβ were detected by the ImmPRESS® protocol according to kit instructions (Vector Laboratories, Burlingame, Ca), as described previously (McDuffie et al, 2004; Childs et al. 2005). The dilution of anti-rat GH was 1:200,400 and the dilution of anti-rat LHβ or anti-human FSHβ were 1:30,000–1:40,000. Controls are illustrated in the previous reports for leptin antigens (McDuffie et al, 2004). The controls for the new protocols that detected GH antigens or mRNA are reported in Childs et al. 2005 or Iruthayanathan et al. 2005. Those for the anti-LHβ or anti-FSHβ antisera were described in Childs et al, 1994a.
Affinity cytochemistry for GnRH receptors
To detect GnRH receptors on leptin-bearing cells, pituitary cells from diestrous rats were stimulated for 10 min with 1 nM biotinylated GnRH, which was detected as previously described (Childs et al. 1983a, b, Lloyd and Childs 1988; Childs et al. 1994b). This was followed by immunolabeling for leptin antigens with streptavidin peroxidase and amber-orange DAB as previously described (Childs et al. 1983b; McDuffie et al. 2004). Controls included competition with unlabeled GnRH for receptor sites, which eliminated black labeling for GnRH and absorption of the anti-leptin with leptin, which eliminated the amber labeling for leptin.
Once GnRH binding was detected on cells with leptin proteins, further tests of diestrous rats were conducted in which cells were treated overnight with vehicle or 100 pM estradiol (Childs et al, 2005) and then exposed to vehicle or 1 nM GnRH for 1 h or biotinylated GnRH for 10 min. These conditions had previously been shown to increase the numbers of target cells for GnRH (Lloyd et al, 1988). The cells were then fixed and prepared for the detection of biotinylated GnRH or leptin mRNA and LHβ or FSHβ.
In situ hybridization
In situ hybridization was carried out as described previously (McDuffie et al, 2004), with modifications (Childs et al. 2005). The leptin mRNA was hybridized with the 48 bp biotinylated oligonucleotide probe complementary to nucleotides 342–389 located within the coding sequence for rat leptin (accession number NM_013076). This probe and the control sequences were produced by GeneDetect.com (www.GeneDetect.com). The controls for this protocol were described in McDuffie et al, 2004, but never illustrated. They involved substituting biotinylated sense probe for the antisense probe (Figure 1). There is labeling in dense black patches in the field treated with biotinylated antisense probe. No labeling is evident in the field treated with the biotinylated sense probe. Dual labeling to detect the hormone content of cells that expressed leptin mRNA was done with the ImmPRESS® protocol, as described previously (Childs et al. 2005).
Figure 1.
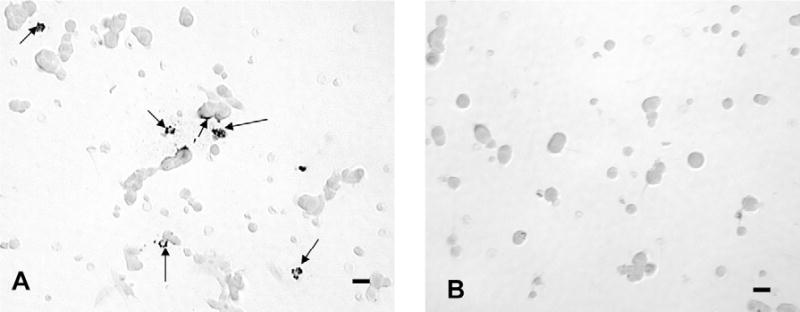
illustrates controls for the detection of leptin mRNA. Figure 1a is a field from a male rat pituitary culture showing label for leptin mRNA with 100 ng/ml biotinylated antisense oligonucleotide probe complementary to leptin mRNA. Label is in irregular dense black patches in the cells (arrows). Figure 1b shows a field treated with 100 ng/ml biotinylated sense oligonucleotide leptin mRNA probe. No labeling is evident in this field. Bar=15 μm.
Analysis of labeling and statistics
The cytochemical labeling was analyzed by either cell counts or the Bioquant Nova Image analysis equipment, with an algorithm that integrated label density and area and changes in both numbers of labeled cells as well as the amount of label per cell. This protocol is described in a recent report (Iruthayanathan et al, 2005). The approach for the statistical analysis, including the power analysis is also described in recent reports (McDuffie et al. 2004; Childs et al. 2005, Iruthayanathan et al. 2005).
Enzyme Immunoassays
The leptin mouse/rat EIA kit by American Laboratory Products Company (ALPCO Diagnostics, Salem, NH), was used to detect serum leptin proteins. The EIA was performed, following kit instructions. Interassay and intra-assay variation coefficients were < 4.7% and < 4.4%, respectively. The kit included the production of sample dilutions of leptin for assay, which was found to be linear over the standard range. The limit of sensitivity of the kit was 10–20 pg/ml.
RNA extraction, cDNA synthesis and QRT-PCR
Whole pituitaries from cycling female rats were used for these studies. We analyzed leptin mRNA expression in 3–5 rats/stage or time in the cycle. After sacrifice by decapitation, the whole pituitary was placed in RLT buffer (Qiagen) containing β mercaptoethanol (as per manufacturer's protocol). The ratio was 100:1- RLT: β mercaptoethanol. RNA was then extracted as described in previous studies, including the DNase steps that removed genomic DNA (Iruthayanathan et al, 2005).
The Biorad Iscript cDNA synthesis was used to reverse transcribe total RNA in an MJ PTC 150 Minicycler in 20uL reaction mixture containing 4 μl 5XiScript, 1 μl reverse transcriptase and 15 μL RNA as per manufacturer's protocol (25º C at 5 min, 50º C at 30 min and 85º C at 5 min). Tests of serial dilutions for the cDNA samples showed reproducible assays of leptin mRNA with dilutions spanning 1:10–1:100. Aliquots were frozen at −80º C and diluted 1:10 and 1:100 for the QRT-PCR assay for leptin mRNA.
Standards for each gene for QRT-PCR were prepared according to the method of Zhou et al (2003) and amplified as described in our previous study (Iruthayanathan et al 2004). A nested primer strategy was used to amplify leptin cDNA to provide a high enough concentration for the standards. This involved the use of two sets of primers, which increased the yield of amplicon. The pituitary leptin gene was cloned and used to produce cDNA for the template, which was then amplified by PCR in two rounds. The first round of PCR was done with forward and reverse primers F1 and R1, which amplified a 300 bp region from nucleotide 105 to 404 in NM_013076.1. The second round of PCR used primers F2 and R2, which amplified regions 179–249 nested in the first amplicon. The product was sent for sequencing and found to be identical to rat leptin. To make the standards, each cDNA fragment was diluted to 4.15 amol/μl and frozen. For the QRT-PCR assays, eight 10-fold serial dilutions were made for use as standards.
The QRT-PCR assays were run in a Roche Light Cycler 1.2 (Roche Applied Sciences, Indianapolis, IN). The QRT-PCR was carried out as in our previous study with the FAST-START DNA Master SYBR Green I enzyme mix (Roche, Indianapolis, IN). The housekeeping gene used to normalize the readings was hypoxanthine guanine phosphoribosyltransferase (HPRT) as described in our previous publication (Iruthayanathan et al, 2004). HPRT did not change with the stage of the reproductive cycle.
During the course of running the leptin assays, we experienced some difficulties with the SYBR green detection system, as it often resulted in product that included primer dimers, rendering the final product levels uninterpretable. Therefore, we switched to the Roche Applied Sciences “Universal Probe system”, which was run as in the kit instructions. The Universal probe designed for the detection of leptin was #13 (catalogue number 04685121001). Universal Probe #13 reacts with nucleotides 220–227, aggcagag in the leptin cDNA template. The forward primer designed by Roche for the amplification of leptin was ccaggatcaatgacatttcaca (nucleotides 179–200) and the reverse primer was aatgaagtccaaaccggtga (nucleotides 230–249) in NM_013076.1. The amplicon is a 71 bp sequence that includes one 1564 bp Intron spanning region at bp 203–204. We used the same standards that were produced for the SYBR Green protocol.
The Lightcycler Taqman Master kit was prepared as described on the kit instructions (Catalogue 04535286001) and then a master mix was prepared which included the following components (multiplied by a factor that varied with the numbers of tubes). For each tube, there was 10.4 μl of nuclease-free water, 0.2 μl of Universal probe 13 (10 μM stock), 0.2 μl each of forward and reverse primers (20 μM used to make final concentration of 200 nM) and 4 μl of the prepared TaqMan master. The master mix was added (15 μl) to each of the glass tubes and then 5 μl of standards (101–105), or 1:10 diluted sample cDNAs, or tris were added to individual tubes. All samples were run in duplicate. The tris or water control served as a negative control. After the tubes were set up, they were centrifuged for 10 seconds and then placed in the Light Cycler which was programmed as follows: Preincubation: 95º C, 10 min; 45 cycles: Denaturation- 95ºC, 10 sec, Annealing- 60ºC, 30 sec, Extension-72 ºC 1 sec; After 45-55 cycles- Cooling, 45 ºC, 1 minute. This protocol allowed us to successfully detect product in all samples. It avoids the formation of primer dimers and thus has the advantage of being more specific. With these primers, only leptin amplicons are detected by Universal probe 13.
Results
Changes in leptin expression with the reproductive state
We had previously reported changes in leptin proteins (McDuffie et al. 2004) with the stage of the cycle. The continuing analysis in this study added more data detecting both mRNA and proteins, including that from rats taken at 2 PM on the day of proestrus and a new graph has been constructed (Figure 2). All data in the following paragraphs are averages ± sem.
Figure 2.
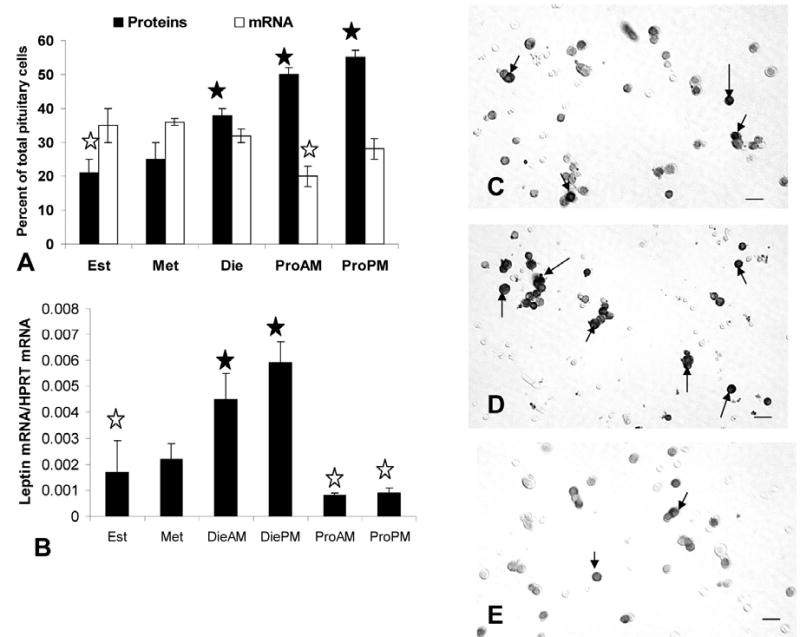
Expression of pituitary leptin proteins and mRNA in different stages of the cycle. Figure 2a is a graph showing the changes in percentage of AP cells with leptin proteins or mRNA with the stage of the cycle. Closed star=highest values in diestrus (die), or proestrus AM (ProAM) or PM (ProPM). Open stars=lowest values in estrus (est) or metestrus (met). Figure 2b shows a graph of the results from the QRT-PCR assays for leptin mRNA in extracts from pituitaries from cycling female rats. The rise in leptin mRNA from metestrus (met) to diestrus (die) is significant (p=0.014) as is the decline in expression from diestrus PM (diePM) to proestrus AM (p=0.002). Values remain low through estrus. Values for metestrous rat pituitaries are slightly higher than those for proestrous animals (p<0.022, proestrus AM and p<0.044, proestrus PM). Closed Star=highest levels; Open star=lowest levels. Figures 2c–e are light micrographs showing the increase in density of labeling from proestrous AM (Figure 2c) to proestrous PM (Figure 2d) and the loss of cellular leptin proteins on the AM of estrus (Figure 2e). Arrows=leptin bearing cell. Bar = 15 μm.
There is a gradual rise in percentages of AP cells with leptin proteins from a low of 21.0 ± 4.0% on the AM of estrus to a peak of 55.0 ± 3.0% of AP cells on the afternoon of proestrus. Figures 2c-e illustrate fields labeled for leptin proteins, comparing labeling on the morning and evening of proestrus and showing reduced labeling on the morning of estrus. The peak expression of leptin during proestrus is higher than that in all other groups (p<0.001), including male rats, which had 39.6 ± 1.0% of AP cells with leptin proteins.
Leptin mRNA is expressed in 32–37% of pituitary cells in male or in estrous, metestrous, or diestrous female rats (Figure 2). Thus, early in the cycle (estrus and metestrus), there are more cells with leptin mRNA than leptin proteins. There are no significant differences among these groups. However, there is a significant decline (p<0.002) in the percentages of cells with leptin mRNA to 20.0 ± 3.0% on the morning of proestrus. This reduction on the AM of proestrus was confirmed by the QRT-PCR assays (Figure 2b), which showed a significant 86% decline from diestrous PM to proestrous AM (p<0.007). The QRT-PCR assays show that leptin mRNA is maintained at relatively low levels through estrus. Then, levels rise during metestrus and diestrus. The 2—fold rise in diestrus AM and PM is to values higher than all other groups (p<0.015). The two diestrous values are not different from one another.
The increase in mRNA from metestrus to diestrus detected by the QRT-PCR assays, was not detected by a change in percentages of leptin-bearing cells. However, densitometric analysis showed that the total area of label for leptin mRNA increased from 844.0 ± 114.0 μm2 to 1246.0 ± 15.0 μm2 (p<0.01) during this period.
To learn if other reproductive states were associated with changes in pituitary leptin, cell populations from pregnant or lactating rats were studied. Figure 3 shows that populations from pregnant rats contained relatively the highest percentages of AP cells with leptin proteins (60.0 ± 2.0%) or mRNA (44.0 ± 2.0%), when compared with all other groups. The percentages of AP cells with leptin proteins are higher than those from males and all cycling groups except females taken on the afternoon of proestrus. The values for mRNA-bearing cells are higher than those from all other groups (p<0.001).
Figure 3.
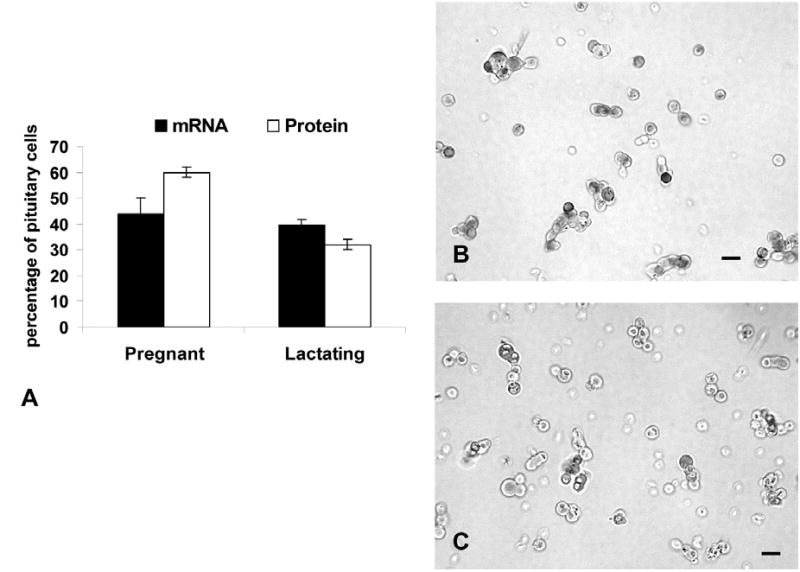
The graph shows the results of counts of leptin mRNA or protein-bearing cells in pregnant or lactating female rats. See text for statistics. The photographs depict immunolabeling for leptin in fields from pregnant (3b) or lactating (3c) rats. Bar=15 μm.
AP populations from females taken on the 3rd day of lactation had midrange levels of leptin proteins, when compared with other physiological states. There were 32.0 ± 3.0% cells with leptin proteins and 40.0 ± 2.0% cells with leptin mRNA (Figure 3). The percentages of AP cells with leptin proteins in lactating rats are higher than those from estrous rats (p<0.02) and lower than those from proestrous (p<0.047) or pregnant rats (p<0.001). The percentages of AP cells with leptin mRNA are higher than all groups, but pregnant rats. Photographs of fields from these rats are also illustrated in Figure 3.
Cell types that express co-leptin proteins
Our previous study had reported that most leptin-bearing cells were somatotropes. However, the timing and direction of changes in expression during the cycle (Figure 2) suggested that gonadotropes might be involved. Dual immunolabeling was therefore done on some of these experimental groups to test this hypothesis. The main question was focused on whether or not somatotropes or gonadotropes, or both, contributed to the rise in the percentage of leptin-bearing cells during proestrus and pregnancy.
When co-expression of leptin proteins and GH proteins was tested, there was a significant increase (p<0.001) in the percentages of dual-labeled AP cells from 25.0 ± 3.0% on the morning of diestrus to 37.0 ± 4.0% of the population on the morning of proestrus (Figure 4). This increment (12 percentage points) matches that seen when the total percentages of AP cells with leptin were calculated (Figure 2). The overall percentage of AP cells with GH proteins did not change significantly. It was 36.0 ± 6.0% on the AM of diestrus and 41.0 ± 4.0% on the morning of proestrus.
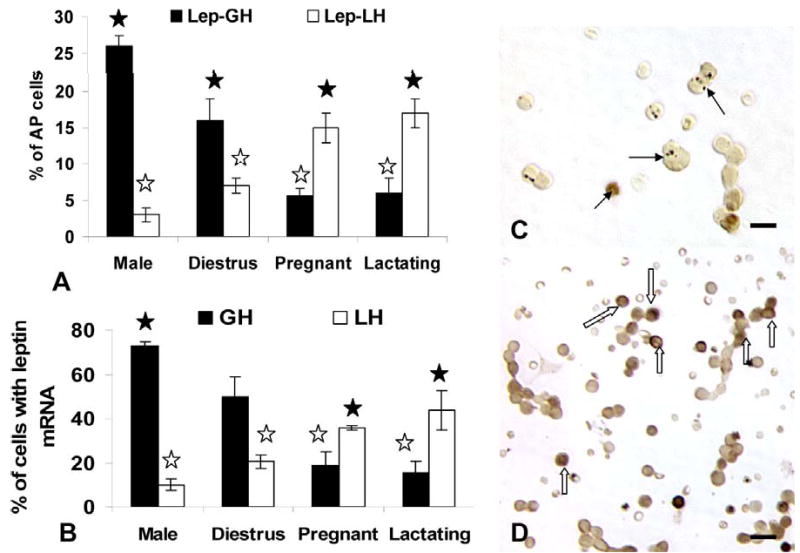
Figure 4.
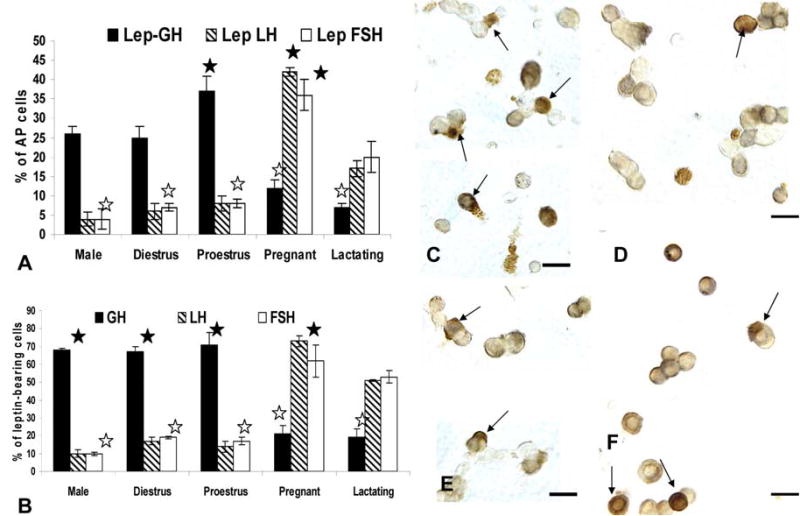
The top graph (Figure 4a) shows the counts of cells following dual immunolabeling for leptin proteins and LHβ, FSHβ or GH proteins and differences based on gender and reproductive state. The Y axis expresses the counts as percentages of total pituitary cells counted (% AP cells). See text for statistical details. Figure 4b shows the same counts expressed as percentages of leptin-bearing cells in these same experimental groups. See text for statistical differences. Filled stars=highest values and open stars=lowest values. Figure 4c and d illustrate fields from proestrous (Figure 4c) and pregnant (Figure 4c) dual labeled for leptin (black) and GH (orange). Numerous dual labeled cells (arrows) are evident only in the field from proestrous rats. In the pregnant rat, most of the gray, leptin-bearing cells do not contain GH. In contrast, Figures 4e and f are fields labeled for leptin (black) and LHβ (orange) from proestrous (Figure 4e) or pregnant (Figure 4f) animals. Only the field from the pregnant rat shows numerous dual labeled cells (arrows). In the proestrous rat, most of the gray, leptin bearing cells do not contain LHβ.
In contrast, the percentage of AP cells that co-expressed leptin and gonadotropins did not change from diestrus to proestrus. Diestrous populations had 6.5 ± 1.0% or 7.0 ± 1.0% cells with leptin and LH or FSH, respectively and proestrous populations had 8.0 ± 2.0% AP cells with leptin and LH or FSH (Figure 4). Previous studies have shown that bihormonal (cells with both LH and FSH) gonadotropes predominate in the population of diestrous and proestrous rats, representing over 70% of the gonadotrope population (Childs et al, 1987, 1994a), which suggests that leptin is being expressed by cells that are mostly bihormonal.
Cultures from male rats exhibited a profile similar to that of diestrous rats with 26.0 ± 2.0% of AP cells co-expressing leptin and GH. Only 4.0% of AP cells co-expressed leptin and LH or FSH in the male, values which were also not different from diestrous rats. Those for AP cells co-expressing leptin and LH were significantly lower than percentages seen on the AM of proestrus (p=0.045).
Significant plasticity in expression was seen in populations from pregnant or lactating rats (Figure 4a) as leptin-bearing cells shifted from being predominantly somatotropes to one that is predominantly gonadotropes. There were no significant changes in the percentages of cells with GH proteins in pregnant or lactating rats. There was a significant reduction (p<0.001) in the percentages of AP cells that co-expressed GH and leptin proteins to 12.0 ± 2.0% in the populations from pregnant females and 6.6 ± 1.0% in those from lactating females. In pregnant rat cells, there was a significant rise in percentage of AP cells that co-expressed LH or FSH and leptin to 42.0 ± 1.0% or 36.0 ± 4.0%, respectively. Cells from lactating females also had significantly more AP cells with LH and leptin (18.7 ± 0.4%) or FSH and leptin (17.0 ± 1.0%) than all other groups, but the pregnant rats.
The analysis also focused on the proportion of leptin-bearing cells that expressed each of the pituitary hormones tested. These values were also used to predict if other cells contributed to leptin-bearing cells and to validate the data in Figure 4a. The validation was done by manually multiplying the percentages of leptin cells that contained each of the hormones by the overall percentages of AP cells that contained leptin, reported in Figures 2 and 3. These calculated percentages were within 0.5–1.0 percentage points of those derived from the cell counts in Figure 4a.
GH stores are found in 67–71% of the leptin protein-bearing cells in males, diestrous or proestrous females, values that are not significantly different from one another (Figure 4b). In contrast, only 19–20% of leptin cells co-express GH proteins in pregnant or lactating animals, which is significantly lower than values from the other groups. (p<0.001).
LH is found in 14–17% and FSH is found in 17–19% of leptin cells in diestrous and proestrous rat populations, values which are not different from one another. As stated above, it is likely that leptin may be expressed in gonadotropes, at least half of which are bihormonal (store both LH and FSH). In populations from pregnant rats, most leptin-bearing cells express LH (73.0 ± 2.0% of leptin cells) and/or FSH (62.0 ± 6.0% of leptin cells). These data show clear overlap in the percentages of leptin bearing cells with gonadotropins, which supports the hypothesis that leptin is expressed in part by bihormonal gonadotropes.
In male rats, only 10% of leptin cells co-express LHβ or FSHβ proteins. The LH values are significantly lower (p<0.006) than all female groups, except those from proestrus AM. The percentage of leptin cells with FSH in the male are lower than all female groups (Student’s T test).
Figure 4 also depicts the dual labeling for leptin proteins and GH (Fig 4c and d) or LHβ proteins (Fig 4e and f) in proestrous (Fig 4c and e) or pregnant (Fig 4d and f) rats. The fields dual-labeled for leptin and GH show the contrast between the numerous dual labeled cells in the proestrous female rat (Fig 4c) with only one in the field from the pregnant rat (Fig 4d). Similarly, few LH cells co-express leptin in the field from the proestrous rat (Fig 4e) and there are numerous dual labeled LH-leptin cells in the field from the pregnant rat (Fig 4f).
Cell types that co-express leptin mRNA
Dual labeling for leptin mRNA and LH or GH proteins was also done on some of these animals, focusing on diestrus because it was a peak time of expression of mRNA. Figure 2 had shown that, during most stages of the cycle, but diestrus, leptin mRNA was found in 32–36% of pituitary cells. In diestrous cell populations, 19.0 ± 2.0% of AP cells co-expressed leptin mRNA and GH, which is 51% of leptin mRNA bearing cells (Figure 5). Among LHβ gonadotropes, the percentages were similar to those seen when leptin protein-bearing cells were counted (7.25 ± 1.0% of AP with leptin mRNA and LHβ). Similar results were seen for FSHβ labeling (data not shown).
Figure 5.
The top graph (Figure 5a) shows the counts of cells following dual labeling for leptin mRNA (with in situ hybridization) and LHβ or GH proteins and differences based on gender or reproductive state. The Y axis shows the counts as percentages of anterior pituitary cells. The bottom graph (Figure 5b) expresses these same counts as percentages of leptin-bearing cells. See text for statistical details. The colored photographs in Figure 5c depicts a dual labeled field from a pregnant rat showing a number of cells leptin mRNA (black patches, arrows) and GH (orange), but no dual-labeled cells. Bar=15 μm Figure 5d depicts a field from a pregnant rat dual labeled for leptin mRNA (black) and LHβ proteins (orange). Many dual labeled cells are shown in this field (hollow arrows) Bar=20 μm.
The loss in cells co-expressing GH and leptin mRNA was again evident in pregnant and lactating females; values are significantly lower than values in diestrous animals (p<0.001) (Figure 5a). In contrast, more AP cells co-expressed leptin mRNA and LHβ proteins (16–17%), values which were significantly higher than those from diestrous rats (p<0.001).
In cells from male rats, 26.0 ± 1.0% of AP cells express leptin mRNA and GH proteins, which is comparable to the levels seen in the dual immunolabeled fields and greater than values in the diestrous female or those from pregnant or lactating animals (p<0.001) (Figure 5a). When LH gonadotropes were analyzed, the percentages of AP cells with leptin mRNA and LHβ in the male were comparable to those seen after dual immunolabeling.
The percentages of leptin mRNA bearing cells that contain GH are higher in the male then all of the female groups (73.0 ± 2.0%; p<0.001) (Figure 5b). Percentages of leptin-mRNA bearing cells that contain LHβ are only 10.0 ± 2.0% in cells from male rats and 20.0 ± 2.0% in those from diestrous females, which is significantly higher than values in the male (p<0.01, Student’s T test).
As in the case of the dual immunolabeling, pregnant and lactating rats had more leptin-bearing cells with LHβ proteins (36.0 ± 1.0 or 44.0 ± 9.0% of leptin cells in pregnant or lactating groups, respectively). These values were not different from one another, but they were significantly higher than those from all other groups. Figures 5c and 5d also illustrates the dual labeling for leptin mRNA and GH or LHβ in pregnant rats, showing the low expression of leptin mRNA in GH cells (Fig 5c) and the high expression in cells with LHβ antigens (Fig 5d).
GnRH and estrogen effects on leptin expression
The timing of the rise in leptin protein expression during the cycle from diestrus to proestrus coincided with the rise in estrogen, which stimulates the production of GnRH receptors by gonadotropes (Lloyd et al, 1988) and somatotropes (Childs et al, 1994a). We hypothesized that leptin expression might be regulated in vivo by GnRH pulses. To determine if GnRH could bind directly to leptin-bearing cells, we exposed freshly dispersed pituitary cells from 3 groups of diestrous rats (3 rats/group) to 1 nM biotinylated GnRH for 10 min. After biotinylated GnRH was detected by avidin-biotin complexes (McDuffie et al, 2004), dual labeling was used to identify leptin in these cells. Figure 6a and b illustrate dual labeling for Bio-GnRH and leptin proteins. The biotinylated neuropeptide is seen as dark patches on or near the surface of the cell and the leptin labeling is seen inside the cells. In diestrous rats, cells with GnRH receptors and leptin proteins are 11.5 ± 2.0%. This represented 30.0 ± 3.0% of leptin-bearing cells and 73.0 ± 3.0% of GnRH target cells.
Figure 6.
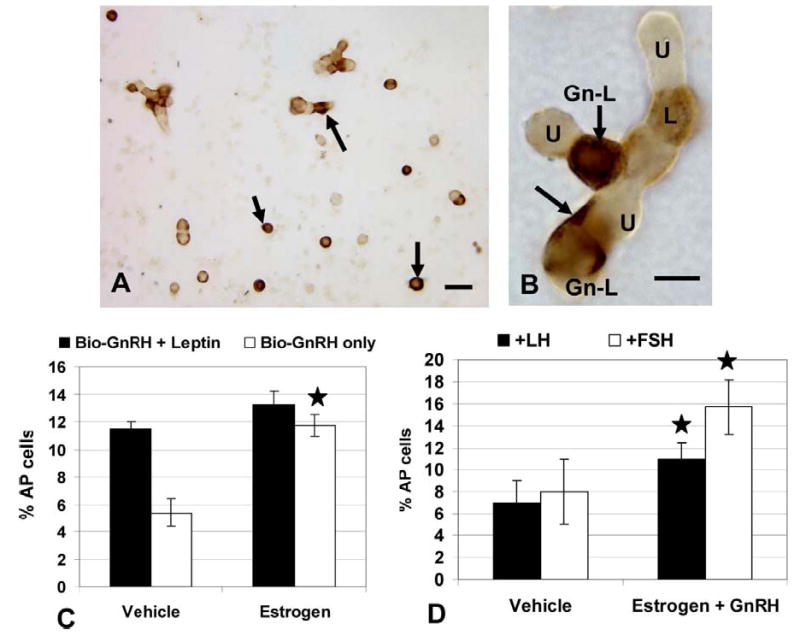
The top photograph depicts dual labeling for biotinylated analog of GnRH (black) followed by immunolabeling for leptin proteins (orange). Arrows in Figure 6a indicated leptin-bearing cells with GnRH receptors. Bar=20 μm. Figure 6b shows a cluster of cells that includes a leptin containing GnRH target cell (Gn-L, arrows show Biotinylated GnRH binding) and a leptin-bearing cell with no GnRH receptors (L) and unlabeled cells (U). Figure 6c shows the effect of overnight estrogen on the expression of biotinylated GnRH-labeled cells with and without leptin proteins. Star=significantly different from vehicle control. Figure 8d shows the effect of estrogen and GnRH overnight on the co-expression of leptin mRNA and LHβ or FSHβ proteins. The Y axis is the percentage of pituitary cells. Star=significantly different from vehicle control.
Estrogen is a well established modulator of GnRH receptors and our previous studies have shown that 100 pM increases the percentage of GnRH-target cells, when given overnight to diestrous rats (Lloyd and Childs 1988). These studies used this experimental approach on an additional 3 groups of diestrous rats to learn if estrogen increased the number of GnRH receptors on leptin-bearing cells. Figure 6c shows that, whereas estrogen does increase the overall percentage of GnRH-target cells as in our previous studies (Lloyd et al. 1988), it does not significantly increase the number of leptin-bearing cells that bind GnRH, which remain at 13.25 ± 2.0% of AP cells.
The next study was designed to learn if estrogen and GnRH could increase leptin expression by gonadotropes. Three groups of cells pooled from 3 diestrous rats/group were treated with and without 100 pM estradiol overnight and then given vehicle or 1 nM GnRH for 1 h the next morning. They were then fixed and labeled for leptin mRNA followed by immunolabeling for LHβ or FSHβ. Neither estrogen nor GnRH alone stimulated more gonadotropes to express leptin mRNA (data not shown). However, Figure 6d shows that when added together, estrogen and GnRH stimulated a significant increase in AP cells that co-express LHβ and leptin mRNA from 7.0 ± 2.0% to 11.0 ± 3.0% (p<0.03) of AP cells. Similarly, estrogen and GnRH together stimulated an even greater increase in the percentages of cells with leptin mRNA and FSHβ from 8.0 ± 3.0% to 15.0 ± 6.0% (p<0.02).
To test if the overnight incubation in estrogen may have caused losses in expression of leptin mRNA, these data were compared with those from freshly dispersed cultures (7.0 ± 1.0% of AP cells from diestrous rats express leptin mRNA and LHβ, as shown in Figure 5a). Similarly, 7.25 ± 2.8% of AP cells co-express leptin mRNA and FSHβ in these same cultures. Both sets of findings point to no losses in leptin mRNA during the overnight incubation in estrogen.
GnRH stimulation of cellular and secreted leptin
Because GnRH receptors are at a peak late in diestrus, extending to the morning of proestrus (Lloyd and Childs 1988; Childs et al. 1994b) cells from proestrous AM female rats were studied to learn more about the specific effects of GnRH on leptin mRNA and protein expression.
Figure 7a illustrates the study of freshly dispersed cells from rats taken on the AM of proestrus. There is a significant increase in the average IOD of labeling after 1 h in 100 pM GnRH (p<0.001), which plateaus at 500 pM. However, there is a significant decrease in IOD of labeling for leptin proteins after 1 nM GnRH (p<0.03), when compared with that following 500 pM. The value for 1 nM GnRH is still higher than the IOD for the vehicle treated group (p=0.009). Similar studies were done of cells treated for 3 h with GnRH and there were no differences in expression of leptin proteins or mRNA.
Figure 7.
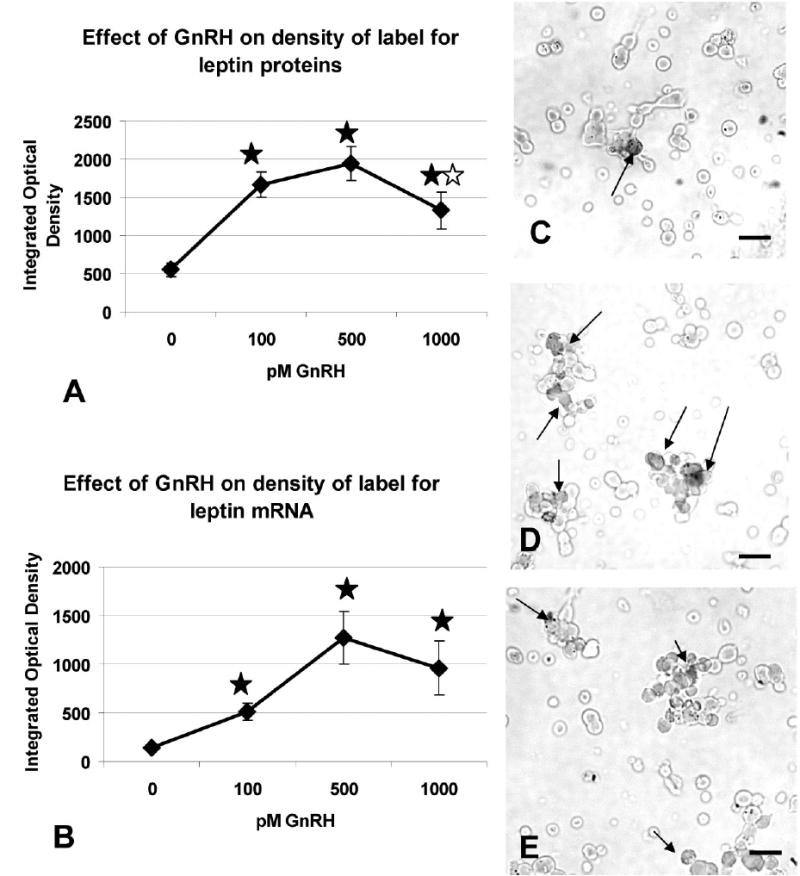
Cellular leptin proteins (Figure 7a) and mRNA (Figure 7b) were analyzed by automated image analysis and the Integrated Optical Density was calculated for each field. The Y axis is the average integrated optical density calculated over 20–25 randomly selected fields ± sem. Closed star= significantly different from vehicle control (see text for statistical details). Open star=significantly different from IOD with 500 pM. Photographs depict the labeling for leptin mRNA in the vehicle (Figure 7c), 100 pM GnRH treated (Figure 7d) or 1 nM GnRH treated (Figure 7e) cultures. Arrows show labeling for leptin mRNA. Bar=15 μm.
Figure 7b shows a similar response to GnRH when IOD of label for mRNA was detected. Note that the average IOD in the vehicle control is about 5—fold lower than that for the proteins (Figure 7a). This reflects the lower expression of mRNA on the AM of proestrus seen in Figure 2. The IOD for leptin mRNA label is significantly increased in all three concentrations of GnRH; the increase with 100 pM is significant by Student’s T test (p<0.001) and there is a further increase to reach a peak with 500 pM (p<0.008). The IOD following 1 nM is not different from that with 500 pM.
GnRH also stimulated secretion of leptin from cultures of pituitary cells taken from diestrus, proestrus or pregnant females. Figure 8 compares basal and GnRH stimulated secretion in these groups, Basal secretion is significantly higher when one compares media from pregnant rat AP cells with that from diestrous rats (p=0.012). GnRH- stimulated secretion is increased over basal in each of the groups (diestrous p=0.016; proestrus p<0.015 and pregnant p<0.01). The cells from pregnant rats show the highest responses to GnRH, when compared with all others.
Figure 8.
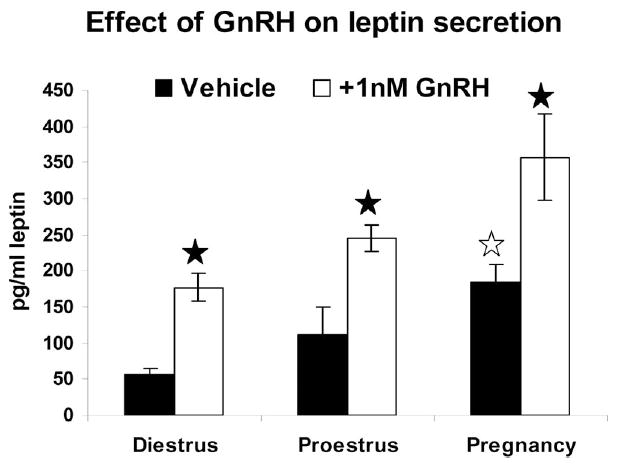
Cells from diestrous, proestrous AM and pregnant females were treated for 1 h with 1 nM GnRH. Media were assayed by EIA for leptin. Basal secretion from pregnant rat cultures is higher than that from diestrous rat cultures (open star). GnRH treatment stimulated higher media levels in all experimental groups (closed star). GnRH treated cultures from pregnant rats have higher media levels than all other groups. See text for statistics.
Discussion
This study was designed to learn more about changes in pituitary leptin with different reproductive states. Our earlier study had shown leptin mRNA and protein expression in somatotropes and changes in protein expression with the estrous cycle (McDuffie et al. 2004). In this study, we added tests of rats in proestrous PM and showed that this period was distinguished by continued high expression of AP leptin proteins comparable to that seen in the AM. Cell counts did not detect major overall changes during the estrous cycle that would account for the changes in the percentages of leptin-bearing cells (Childs et al, 1992a,b). We also added tests of pregnant and lactating rats showing increased expression of leptin in both groups. At this point, we cannot rule out the possibility of mitotic activity contributing to these changes.
As in the previous study, over 2/3rds of leptin-bearing cells in cycling females and normal males co-express growth hormone. This study adds data showing that less than 10% of leptin-bearing cells are gonadotropes in male rats, but 14–19% are gonadotropes in female rats in diestrus or proestrus. A more striking shift is seen in AP populations from pregnant females in which 62–73% of leptin-bearing cells express FSH or LH proteins and only 19% express GH. The reason for this plasticity in expression is unclear at this point, however some of the studies of regulation of leptin to be discussed below may provide clues.
Leptin regulation during the estrous cycle
Leptin appears to be differentially regulated in cycling females so that percentages of AP cells that express leptin proteins reach a peak just before the LH surge on the afternoon of proestrus. Then, the leptin-bearing cells lose stores of leptin by the morning of estrus and become invisible to immunolabeling. This suggests that the stores may have been secreted, although we cannot rule out degradation as a cause of the reduction. The leptin-bearing cells could still be detected, however, by their content of mRNA. Thus, the cells themselves did not disappear.
The rise in leptin protein-bearing cells seen just before the LH surge indicates that it might play an important role with respect to ovulation. A more specific paracrine role is suggested by the fact that leptin is known to be a secretagogue for LH, both in vivo and in vitro (Gonzales et al. 1999, 2000; DiBasi et al. 2001; Yu et al. 1997a,b). Whereas it is tempting to speculate that the rise and fall in pituitary leptin from proestrus to estrus helps support the LH surge, at this point the evidence must be considered circumstantial. Future studies would be needed to prove this paracrine function for pituitary leptin.
Leptin mRNA expression was in 32–37% of the cells early in the cycle (from estrus to diestrus). Quantification by QRT-PCR showed a dramatic rise in transcripts on diestrus AM and PM, 12 h before peak expression of leptin proteins is detected. This rise during diestrus thus supports the increased proteins needed for proestrous activity. As we studied the changes in percentages of cells with leptin mRNA, we noted a significant decline on the morning of proestrus, which was confirmed by the QRT-PCR assays. This rapid decline is intriguing and suggests that leptin transcripts are tightly regulated during the cycle, perhaps by rapid degradation. This pattern of expression indicates that the timing of this periovulatory expression of pituitary leptin may be important. Leptin is an anorexigenic hormone, which has potent inhibitory effects on neurons that stimulate appetite (Zhang et al, 1994; Vasselli, 2001; Rowland and Morien, 1996). In our previous studies, we suggested that pituitary leptin might be regulated to ensure adequate nutrition for pregnancy or other reproductive activities (McDuffie et al 2004). Of greater importance in this regard may be its role in facilitating the utilization of nutrients, like glucose (Schneider et al, 1999, 2000, 2002). Thus, because of its other functions, pituitary leptin may be tightly regulated to allow it to perform supportive roles for the reproductive system, which may include both glucose utilization and LH stimulation. However, its expression may be carefully timed to prevent anorexigenic effects that might compromise a pregnancy.
The changes in leptin expression during the cycle and after pregnancy suggested that reproductive hormones like estrogen or GnRH might be involved in its regulation. Our previous studies (McDuffie et al, 2004) found that estrogen alone did not stimulate leptin in cells from metestrous rats. However, an overnight treatment with estrogen followed by exposure to 2 nM growth hormone releasing hormone (GHRH) resulted in an increase in percentages of leptin-bearing cells, in vitro. In a recent publication (Childs et al, 2005), we reported an increase in GHRH receptors on GH cells following exposure to the same concentrations and times in estradiol. Thus, the stimulation of leptin seen in our first study may have been mediated by estrogen’s stimulatory effects on GHRH receptors (McDuffie et al, 2004).
In the present study, the same paradigm was used because of evidence that estrogen also stimulates GnRH receptors in metestrous or diestrous rats (Lloyd et al, 1988). In addition, we have reported that there is an increased expression of GnRH receptors by gonadotropes and somatotropes from diestrous to proestrous AM (Childs et al, 1994). The present findings showed that cells with leptin did bind biotinylated analogs of GnRH. Furthermore, estrogen exposure to cells from diestrous females increased cells with GnRH receptors. However, estrogen did not increase the percentage of AP cells that bound GnRH and contained leptin.
GnRH regulation of pituitary leptin
The biotinylated analog of GnRH detected GnRH receptors on leptin-bearing cells, which suggests that this hormone may regulate leptin directly. The counts of cells with leptin and GnRH-receptors appears to have detected 2X more “gonadotropes” (defined by their GnRH binding) with leptin than were detected with dual immunolabeling for LH or FSH, which showed only 6 ± 1% of diestrous AP cells with leptin and LHβ, and 6.6 ± 0.6% with leptin and FSHβ. This is partially explained by the appearance, in diestrus and proestrus, of somatotropes which express GnRH receptors (Childs et al, 1994b) and LH and FSH mRNA (Childs et al 1994a). These “somatogonadotropes” represent 11–16% of the AP population during proestrus. Thus, the predominance of somatotropes in the leptin-bearing cell population suggests that the GnRH might be affecting leptin from this cell type or its somatogonadotrope subtype.
To learn if estrogen and GnRH affected the gonadotropin content of leptin mRNA bearing cells, diestrous rats were stimulated for 24 h with estrogen and then treated for 1 h with GnRH. In groups treated with both hormones, there were significant increases in the percentages of AP cells with leptin mRNA and LHβ or FSHβ, which shows the potential of these reproductive hormones. Neither hormone was effective by itself. At this point, we can not rule out the possibility that the increase could have been by mitotic activity, as GnRH is mitogenic for gonadotropes (Childs et al, 2001).
GnRH effects on expression of cellular leptin mRNA and proteins was studied in proestrous rats, which express maximal numbers of GnRH receptors (Lloyd et al, 1988). The average integrated optical density array was used to integrate information from changes in density and area of label in the leptin-bearing pituitary cells. There was an increase in IOD of label for leptin proteins and mRNA in the cell populations was seen only in groups treated for 1 h with physiological concentrations of GnRH (less than 1 nM) indicating a sensitive, relatively short term response. Groups treated for 3 h did not respond, which suggests again that responses to GnRH may be timed, in vivo, to match its pulses. We also compared the IOD from the immunolabeling with that from the in situ hybridization and confirmed the significant reduction in expression of leptin mRNA seen on the AM of proestrus by the QRT-PCR assay. It is worthwhile to note, however, that the cells were still able to respond to GnRH by the production of more transcripts.
The final set of studies compared basal and GnRH-mediated secretion in cells from diestrus, proestrus, and pregnant female rats. If one compares the percentages of leptin-bearing cells in different physiological states with their secretory activity, there was also good correlation between the abundance of leptin protein bearing cells in the population and their basal and GnRH-stimulated responses.
The possibility that secretion of leptin is regulated by a neuroendocrine route suggests that this protein may have a secretory pathway distinct from that of adipocyte leptin. Insulin-mediated leptin secretion from adipocytes is considered to be via constitutive pathways, although a subpopulation of leptin vesicles may be secreted by a regulated pathway (Bradley et al. 2001). Vidal et al. (2000) detected leptin co-expression with pituitary hormones at the electron microscopic level and their photographs depict labeling for leptin in the same granules that store LH or GH. This suggests that the leptin secretory cycle may be similar to that of gonadotropins or GH. Collectively, our experiments with GnRH and the morphological data from Vidal et al (2000) support regulatory pathways for leptin that are similar to those that regulate pituitary hormones.
Plasticity in the site(s) of production of pituitary leptin
The studies of co-expression of leptin and gonadotropins or GH suggest that more leptin bearing cells are GH cells in cycling female rats and in the male. However, in pregnancy, most leptin-bearing cells are gonadotropes. Future studies would be needed to follow the progression in leptin expression by gonadotropes with early and later pregnancy. In addition, future dual labeling studies are needed to know if other cells contribute to the leptin-bearing population during these reproductive states.
The changes in the expression of GH proteins by AP cells does not vary significantly with the stage of the cycle (Childs et al, 2000). In this study, the counts showed 36% GH cells in diestrus and 41% in proestrus. These data agree with those from previous studies (Childs et al, 2000). Thus, the 12 percentage point increase in leptin expression from diestrus to proestrus could be accounted for by the increase in the subset of AP cells that express GH and leptin proteins. LH or FSH-protein bearing cells also do not change after diestrus (Childs et al, 1987, 1992a, Childs et al, b) and their expression of leptin also remains at 6–8% of the population.
In male rats or cycling female rats, adding the percentages of leptin cells that contain each hormone brings the values to between 87–103% of leptin-bearing cells. One must recognize however, that in cycling female rats, 50–70% of gonadotropes are bihormonal (Childs et al, 1987; Childs et al, 1994a). Furthermore, in both males and cycling females 30–60% of GH cells co-express one of the gonadotropins (Childs et al, 1994a). Thus, leptin could be produced by cells that also produce both gonadotropins and/or GH.
Overlap in storage may also apply to the populations from lactating or pregnant rats in which adding the percentages brings the total to 130% or 156% of leptin-bearing cells. Overlap in storage of gonadotropins with or without GH has not yet been studied or described for these experimental groups, however, other “stimulated states”, like castration show an increase in the proportion of bihormonal gonadotropes to nearly 100% of gonadotropes (Childs, 1994; 2006).
Leptin production by somatogonadotropes might help explain the plasticity evident during pregnancy, especially if the shift reflects a reduction in GH expression by this subset. Future studies of GH expression after pregnancy would be needed to learn more about this phenomenon. The average percentages of GH cells in pregnant rat cultures are not lower than those normally seen in diestrous female rats.
In light of the probable overlap in storage of gonadotropins and GH in leptin-bearing cells, future studies will be needed to learn if other pituitary cell types change expression of leptin with the reproductive cycle. In our previous studies, (McDuffie et al, 2004), we reviewed the literature in which dual labeling for leptin and other hormones was reported. We are the only group to report expression of leptin mRNA. However, previous workers have reported leptin protein distribution in rodents to vary from just thyroid stimulating hormone (TSH) cells (Jin et al, 2000), to TSH and gonadotropes, but not somatotropes (Sone et al, 2001). The overall percentages of leptin bearing cells were lower than those reported in our studies. Studies in humans found leptin in most cell types, including 70% of corticotropes, 21% of GH cells, 33% of FSH cells, 29% of LH cells, and 32% of TSH cells. Leptin was also found in 64% of folliculostellate cells, but less than 3% of prolactin cells. Considering the plasticity of leptin and other hormone expression in the pituitary, one would need to know more about the percentages of each of these cell types in the human and the physiological state of the donors at death to fully compare their work with the present studies. However, the broad distribution suggests that other cell types have the potential to produce leptin and they could contribute to the changes in leptin-bearing cells.
To conclude, this study has demonstrated gender and cyclic differences in leptin expression, which could be regulated by GnRH supported by estrogen feedback. In females, the highest basal and GnRH-mediated leptin secretory activity was found in pituitary cells from proestrous or pregnant rats. This proves that pituitary leptin can be secreted and strengthens evidence for a role for pituitary leptin during these physiological states. Collectively, this and our previous study (McDuffie et al, 2004) suggest that leptin expression, including secretion, may be regulated by the same neuroendocrine hormones that regulate somatotropes and gonadotropes.
Acknowledgments
The authors acknowledge with thanks, the help and advice of Dr. Mary Iruthayanathan and Dr. Yi-hong Zhou during the development of the QRT-PCR assays for pituitary leptin. They also appreciate the help of Dr. Alex Pierson, Roche Life Sciences, with the Universal Probe system for leptin mRNA. The authors thank AF Parlow and the Hormone Distribution Office, NIDDK, NIH for the antisera to rat growth hormone and Dr. JG Pierce for the anti-bovine LHβ.
This paper was presented, in part, in a poster at the 2004 meetings of the Endocrine society, New Orleans, La, and in a platform session on July 31 at the 2006 meetings for the Society for the Study of Reproduction, Omaha, NE.
This publication was made possible by funding from NSF IBN 0240907, NIH R03 HD 44875, and 1 P20 RR020146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal in the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- Bédécarrats GY, Kaiser UB. Differential Regulation of Gonadotropin Subunit Gene Promoter Activity by Pulsatile Gonadotropin-Releasing Hormone (GnRH) in perifused LBT2. Cells: Role of GnRH Endocrinology. 2003;144:1802–1811. doi: 10.1210/en.2002-221140. [DOI] [PubMed] [Google Scholar]
- Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- Bradley RL, Cleveland KA, Cheatham B. The Adipocyte as a Secretory Organ: Mechanisms of Vesicle Transport and Secretory Pathways Recent Progress in Hormone. Research. 2001;56:329–358. doi: 10.1210/rp.56.1.329. [DOI] [PubMed] [Google Scholar]
- Burger LL, Dalkin AC, Aylor KW, Haisenleder DJ, Marshall JC. GnRH pulse frequency modulation of gonadotropin subunit gene transcription in normal gonadotropes-assessment by primary transcript assay provides evidence for roles of GnRH and follistatin. Endocrinology. 2002;143:3243–3249. doi: 10.1210/en.2002-220216. [DOI] [PubMed] [Google Scholar]
- Chehab PF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nature Genetics. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- Childs GV, Naor Z, Hazum E, Tibolt R, Westlund KN, Hancock MB. Cytochemical characterization of pituitary target cells for biotinylated gonadotropin releasing hormone. Peptides. 1983a;4(4):549–555. doi: 10.1016/0196-9781(83)90061-x. [DOI] [PubMed] [Google Scholar]
- Childs GV, Naor Z, Hazum E, Tibolt R, Westlund KM, Hancock MB. Localization of biotinylated gonadotropin releasing hormone on pituitary monolayer cells with avidin-biotin peroxidase complexes. J Histochem Cytochem. 1983b;31:1422–1425. doi: 10.1177/31.12.6195217. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Tibolt R, Lloyd JM. Cytological factors that support non-parallel secretion of LH and FSH during the estrous cycle. Endocrinology. 1987;121:1801–1813. doi: 10.1210/endo-121-5-1801. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Lloyd J. Recruitment and maturation of small subsets of luteinizing hormone (LH) gonadotropes during the estrous cycle. Endocrinology. 1992a;130:335–345. doi: 10.1210/endo.130.1.1727707. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Lloyd JM. Maturation of FSH gonadotropes during the rat estrous cycle. Endocrinology. 1992b;131(1):29–36. doi: 10.1210/endo.131.1.1612007. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Rougeau D. Cells that Express Luteinizing Hormone (LH) and Follicle Stimulating Hormone (FSH) Beta (∃) Subunit mRNAs during the Estrous Cycle: The major contributors contain LH∃, FSH∃ and/or Growth Hormone. Endocrinology. 1994a;134:990–997. doi: 10.1210/endo.134.2.8299592. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Miller BT. Cytochemical detection of GnRH binding sites on rat pituitary cells with LH, FSH and GH antigens during diestrous upregulation. Endocrinology. 1994b;134:1943–1951. doi: 10.1210/endo.134.4.8137763. [DOI] [PubMed] [Google Scholar]
- Childs GV. Division of Labor among Gonadotropes. Vitamins and Hormones. 1994;1994;50:217–283. doi: 10.1016/s0083-6729(08)60657-3. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Wu P. Differential expression of growth hormone messenger ribonucleic acid by somatotropes and gonadotropes in male and cycling female rats. Endocrinology. 2000;141:1560–1570. doi: 10.1210/endo.141.4.7429. [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G. Epidermal growth factor and gonadotropin releasing hormone stimulate proliferation of enriched populations of pituitary gonadotropes. Endocrinology. 2001;142:847–854. doi: 10.1210/endo.142.2.7953. [DOI] [PubMed] [Google Scholar]
- Childs GV, Iruthayanathan M, Akhter N, Unabia G, Whitehead-Johnson B. Bipotential Effects of Estrogen on Growth Hormone Synthesis and Storage in Vitro. Endocrinology. 2005 Apr;146:1780–1788. doi: 10.1210/en.2004-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs GV. Gonadotropes and Lactotropes. In: Neill J, Knobil E, editors. Physiology of Reproduction. Elsevier Press; N.Y: 2006. pp. 1483–1579. Chapter 29. [Google Scholar]
- Clayton RN. Gonadotrophin releasing hormone: its actions and receptors. J Endocrinol. 1989;120:11–19. doi: 10.1677/joe.0.1200011. [DOI] [PubMed] [Google Scholar]
- Conn PM. The molecular mechanism of gonadotropin-releasing hormone action in the pituitary. In: Knobil E Neill., editor. The Physiology of Reproduction. 2nd Ed. New York: Raven Press; 1994. pp. 1815–1832. [Google Scholar]
- Conn PM, Huckle WR, Andrews WV, McArdle CA. The molecular mechanism of action of gonadotropin releasing hormone (GnRH) in the pituitary. Rec Prog Hrm Res. 1987;43:29–69. doi: 10.1016/b978-0-12-571143-2.50007-1. [DOI] [PubMed] [Google Scholar]
- Crowley WF, Jr, Filicori M, Spratt DL, Santoro NF. The physiology of Gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog Horm Res. 1985;41:473–531. doi: 10.1016/b978-0-12-571141-8.50015-9. [DOI] [PubMed] [Google Scholar]
- Di Biasi SN, Apfelbaum LI, Apfelbaum ME. In vitro effect of leptin on LH Release by anterior pituitary glands from female rats at the time of spontaneous and steroid-induced LH surge. Eur J Endo. 2001;145:659–665. doi: 10.1530/eje.0.1450659. [DOI] [PubMed] [Google Scholar]
- Finn PD, Cunningham MJ, Pau K-YF, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139:4652–4662. doi: 10.1210/endo.139.11.6297. [DOI] [PubMed] [Google Scholar]
- Gonzalez LC, Pinilla L, Tena-Sempere M, Aguilar E. Leptin (116–130) stimulates prolactin and luteinizing hormone secretion in fasted adult male rats. Neuroendocrinology. 1999;70:213–220. doi: 10.1159/000054479. [DOI] [PubMed] [Google Scholar]
- Gonzalez LC, Pinilla L, Tena-Sempere M, Dieguez C, Casanueva FF, Aguilar Effect of acute immunoneutralization of endogenous leptin on prolactin and LH secretion during the afternoon of pro-oestrus or in steroid-treated Ovariectomized female rats. J Reprod Fertil. 2000;118(1):39–45. doi: 10.1530/jrf.0.1180039. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Dalkin AC, Ortolano GA, Marshall JC, Shupnik MA. A pulsatile gonadotropin-releasing hormone stimulus is required to increase transcription of the gonadotropin subunit genes: evidence for differential regulation of transcription by pulse frequency in vivo. Endocrinology. 1991;128:509–517. doi: 10.1210/endo-128-1-509. [DOI] [PubMed] [Google Scholar]
- Iruthayanathan M, Zhou Y-H, Childs GV. DHEA Restoration of GH Gene Expression in Aging Female Rats, in vivo and in vitro - Evidence for Actions Via Estrogen Receptors. Endocrinology. 2005 2005 September 8;146:5176–5187. doi: 10.1210/en.2005-0811. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin I, Zhang S, Burguera BG, Couce ME, Osamura RY, Kulig E, Lloyd RV. Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology. 2000;141:333–339. doi: 10.1210/endo.141.1.7260. [DOI] [PubMed] [Google Scholar]
- Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, Kulig E, Lloyd RV. Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab. 1999;94:2903–2911. doi: 10.1210/jcem.84.8.5908. [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Jakubowiak A, Steinberger A, Chin WW. Differential effects of gonadotropin-releasing hormone (GnRH) pulse frequency on gonadotropin subunit and GnRH receptor messenger ribonucleic acid levels in vitro. Endocrinology. 1997;138:1224–1231. doi: 10.1210/endo.138.3.4968. [DOI] [PubMed] [Google Scholar]
- Levine JE, Ramirez VD. LHRH release during the rat estrous cycle and after ovariectomy, as estimated with push-pull cannulae. Endocrinology. 1982;111:1439–1448. doi: 10.1210/endo-111-5-1439. [DOI] [PubMed] [Google Scholar]
- Lloyd RV, Tsumanuma I, Vidal S, Kovaks K, Horvath E, Scheithauer BW, Couce ME, Burguera B. Leptin and leptin receptor expression in anterior pituitary function. Pituitary 2001. 2001;4:33–47. doi: 10.1023/a:1012982626401. [DOI] [PubMed] [Google Scholar]
- Lloyd JM, Childs GV. Changes in the number of GnRH-receptive cells during the rat estrous cycle: biphasic effects of estradiol. Neuroendocrinology. 1988;48:138–146. doi: 10.1159/000125001. [DOI] [PubMed] [Google Scholar]
- Loumaye E, Catt KJ. Homologous regulation of gonadotropin-releasing hormone receptors in cultured pituitary cells. Science. 1982;215:983–985. doi: 10.1126/science.6296998. [DOI] [PubMed] [Google Scholar]
- Mann R, Plant TM. Leptin and pubertal development Seminars in Reproductive. Medicine. 2002;20:93–102. doi: 10.1055/s-2002-32500. [DOI] [PubMed] [Google Scholar]
- McDuffie I, Akhter N, Childs GV. Regulation of leptin expression in anterior pituitary somatotropes. J Histochem Cytochem. 2004;52:263–273. doi: 10.1177/002215540405200214. [DOI] [PubMed] [Google Scholar]
- Morash B, Li A, Murphy PR, Wilkinson M, Ur E. Leptin gene expression in the brain and pituitary gland. Endocrinology. 1999;140:5995–9558. doi: 10.1210/endo.140.12.7288. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Morton GJ, Niswender KD, Gelling RW, Schwartz MW. Leptin inhibits hypothalamic Npy and Agrp gene expression via a mechanism that requires phosphatidylinositol 3-OH-kinase signaling. Am J Physiol Endocrinol Metab. 2005;2005;289:E1051–E1057. doi: 10.1152/ajpendo.00094.2005. [DOI] [PubMed] [Google Scholar]
- Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology. 1998;67:370–376. doi: 10.1159/000054335. [DOI] [PubMed] [Google Scholar]
- Popovic V, Damjanovic S, Dieguez C, Casaneuva FF. Leptin and the pituitary. Pituitary. 2001;2001;4:7–14. doi: 10.1023/a:1012938308654. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Morien A, Li B. The physiology and brain mechanism of feeding. Nutrition. 1996;12:626–693. doi: 10.1016/s0899-9007(96)00227-4. [DOI] [PubMed] [Google Scholar]
- Savoy-Moore RT, Schwartz NB, Duncan JA, Marshall JC. Pituitary gonadotropin-releasing hormone receptors during the rat estrous cycle. Science. 1980;209:942–944. doi: 10.1126/science.6250218. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Zhou D. Interactive effects of central leptin and peripheral fuel oxidation on estrous cyclicity. Am J Physiol. 1999;277:R1020–R1024. doi: 10.1152/ajpregu.1999.277.4.R1020. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Blum RM, Wade GN. Metabolic control of food intake and estrous cycles in Syrian hamsters. 1. Plasma insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2000;278(2):R476–485. doi: 10.1152/ajpregu.2000.278.2.R476. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Buckley CA, Blum RM, Zhou D, Szymanski L, Day DE, Bartness TJ. Metabolic signals, hormones and neuropeptides involved in control of energy balance and reproductive success in hamsters. Eur J Neurosci. 2002;16:377–379. doi: 10.1046/j.1460-9568.2002.02118.x. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone M, Nagata H, Takekochi S, Osamura RY. Expression and localization of leptin receptor in the normal rat pituitary gland. Cell Tissue Res. 2001;305:351–356. doi: 10.1007/s004410100407. [DOI] [PubMed] [Google Scholar]
- Sone M, Osamura RY. Leptin and the pituitary. Pituitary. 2001;4:15–23. doi: 10.1023/a:1012978525492. [DOI] [PubMed] [Google Scholar]
- Tena-Sempere M, Pinilla L, Gonzalez LC, Dieguez C, Casanueva FF, Aguilar E. Leptin inhibits testosterone secretion from adult rat testis in vitro. J Endocrinol. 1999;161(2):211–8. doi: 10.1677/joe.0.1610211. [DOI] [PubMed] [Google Scholar]
- Urbanski HF. Leptin and puberty. Trends in Endocrinology and Metabolism. 2001;12:428–429. doi: 10.1016/s1043-2760(01)00505-7. [DOI] [PubMed] [Google Scholar]
- Vasselli JR. Behavioral and biological determinants of leptin resistance. Appetite. 2001;37:115–117. doi: 10.1006/appe.2001.0418. [DOI] [PubMed] [Google Scholar]
- Vidal S, Cohen SM, Horvath E, Kovacs K, Scheithauer BW, Burguera BG, Lloyd RV. Subcellular localization of leptin in non-tumorous and adenomatous human pituitaries: an immuno-ultrastructural study. J Histochem Cytochem. 2000;48:1147–1152. doi: 10.1177/002215540004800811. [DOI] [PubMed] [Google Scholar]
- Walczewsk A, Yu WH, Karanth S, McCann SM. Estrogen and leptin have differential effects on FSH and LH release in female rats. Proc Soc Exp Biol Med. 1999;222:171–177. doi: 10.1046/j.1525-1373.1999.d01-128.x. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Takebe K. Evidence that neuropeptide Y secretion in the median eminence increases prior to the luteinizing hormone sure in ovariectomized steroid-primed rats: estimation by push-pull perfusion. Neurosci Lett. 1992;146:57–59. doi: 10.1016/0304-3940(92)90171-3. [DOI] [PubMed] [Google Scholar]
- Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, Knobil E. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology. 1981;109:376–385. doi: 10.1210/endo-109-2-376. [DOI] [PubMed] [Google Scholar]
- Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci USA. 1997;94:1023–1028. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology. 1997;128:5055–5058. doi: 10.1210/endo.138.11.5649. [DOI] [PubMed] [Google Scholar]
- Yura S, Ogawa Y, Sagawa N, Masuzaki H, Itoh H, Ebihara K, Aizawa-Abe M, Fuji S, Nakao K. Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J Clin Invest. 2000;105:749–755. doi: 10.1172/JCI8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proence R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;72:425–431. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zhou YH, Tan F, Hess KR, Yung WK. The expression of PAX6, PTEN, vascular endothelial growth factor and epidermal growth factor receptor in gliomas: relationship to tumor grade and survival. Clin Cancer Res. 2003;9:3369–3375. [PubMed] [Google Scholar]


