Abstract
Rubisco activase is a nuclear-encoded chloroplast protein that is required for the light activation of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) in vivo. In most plants examined to date, there are two isoforms of Rubisco activase arising from alternative splicing that differ only at the carboxyl terminus. Here we demonstrate with recombinant proteins that in Arabidopsis the larger isoform has a unique role in the regulation of Rubisco activity. At physiological ratios of ADP/ATP, the 46-kDa isoform has minimal ATP hydrolysis and Rubisco activation activity in comparison with the 43-kDa isoform. Analysis of a series of carboxyl-terminal deletion and Ala substitution mutants of the 46-kDa isoform revealed that the presence of Cys residues at positions 411 and 392 were essential to preserve a low ATP hydrolysis and Rubisco activation activity in the presence of ADP. Consequently, incubation of the 46-kDa isoform with DTT and thioredoxin-f increased both activities, whereas incubations with DTT alone or with thioredoxin-m were ineffective. Thioredoxin-f and DTT had no effect on the 43-kDa isoform. However, premixing both isoforms before conducting a reduction and oxidation cycle demonstrated that the activity of both isoforms could be regulated. Reduction and oxidation also modulated the activity of native activase proteins isolated from either Arabidopsis or spinach, but not tobacco, which only has the smaller isoform. These findings suggest that in plants containing both isoforms, Rubisco activase regulates the activity of Rubisco in response to light-induced changes in both the ADP/ATP ratio and the redox potential via thioredoxin-f.
Rubisco activase regulates the activity of ribulose 1,5-bisphosphate (RuBP) carboxylase/oxygenase (Rubisco; EC 4.1.1.39), the enzyme that initiates photosynthetic carbon metabolism by combining atmospheric CO2 with RuBP to form 3-phosphoglyceric acid (1, 2). Rubisco activase varies the activity of Rubisco by removing the otherwise inhibitory sugar phosphates, RuBP, and in some plants, 2-carboxyarabinitol 1-phosphate. In addition to being a substrate, RuBP also binds to an inactive form of the enzyme and blocks the binding of the “activating” CO2 to form a carbamate on an active site Lys and the Mg2+ that are both necessary for catalysis (1, 2). In some plants 2-carboxyarabinitol 1-phosphate accumulates in darkness or low light and binds to the carbamylated form of the enzyme, blocking access by the RuBP substrate. The activity of Rubisco activase can be regulated by the ADP/ATP ratio in the stroma because ADP inhibits the ATP hydrolysis reaction of the activase, which is required to remove the inhibitors.
The activity of Rubisco varies with light intensity over the same range as photosynthesis via changes in the relative amounts of active and inactive forms of the enzyme (3, 4). The discovery of Rubisco activase and, subsequently, its ATP hydrolysis activity raised expectations that the elucidation of the mechanism for light regulation would be imminent. However, it soon became clear that the stromal ADP/ATP ratio under steady-state conditions did not vary greatly with light intensity (5, 6). Meanwhile, the presence of thylakoids and light was shown to have a stimulatory effect on the activation process mediated by Rubisco activase (7), but only limited biochemical characterization has been achieved (8–10).
Many other enzymes in the chloroplast, particularly several in the reductive pentose phosphate cycle, are also light/dark modulated (11). In these cases, a redox cascade generated by light plays a major role. In the light, electrons from photosystem I are shuttled through ferredoxin and ferredoxin–thioredoxin reductase to target enzymes via thioredoxins that reduce disulfide bonds in these proteins and thereby change their catalytic activities. Although both Rubisco and Rubisco activase contain numerous cysteines, no clear evidence that these proteins are redox regulated has been reported. Although often included for in vitro experiments and protection during storage, DTT is not necessary to observe the maximal activity of these proteins. Indeed, treatment of leaves with low concentrations of methyl viologen, which inhibits photosynthesis and inactivates redox-regulated enzymes in the reductive pentose phosphate cycle, results in a high activation of Rubisco at low light intensities (5, 12).
An intriguing feature of Rubisco activase has been its presence in two isoforms (13), differing only at the carboxyl terminus, which arise by alternative splicing of the gene transcript (14). Expression of the recombinant spinach isoforms was used to show that each isoform is capable of activating Rubisco in vitro, but with a slightly different maximal activity (15). More significantly, the larger isoform was more sensitive to inhibition by ADP, suggesting a possible regulatory role for this specific isoform (15). Recently, much clearer evidence for such a role in vivo and a possible direct link to light regulation was obtained. Transgenic Arabidopsis plants expressing only the smaller isoform were found to maintain Rubisco in a largely active form in the light regardless of the light intensity (R. Kallis and A.R.P., unpublished work). Conversely, Arabidopsis plants expressing only the larger isoform exhibit a light modulation of Rubisco activity more similar to the wild-type plants (N.Z., unpublished results).
Here we demonstrate that the presence of two Cys residues, found only in the larger isoform, is the basis for the different activities exhibited by the two isoforms by analyzing the activities of carboxyl-terminal deletion and Ala substitution mutants. We then directly examined the effects of thioredoxins on the activities of Rubisco activase in the presence of DTT and oxidized glutathione.
MATERIALS AND METHODS
Reagents.
Recombinant spinach thioredoxins-f and -m were gifts from P. Schürmann (Université de Neuchâtel, Switzerland).
Cloning and Purification of Recombinant Rubisco Activase.
Three oligonucleotides were constructed on the basis of cDNA sequences of Arabidopsis Rubisco activase (16). The upstream 30-mer oligonucleotide (primer A), corresponding to the amino acid sequence V-K-E-D-K-Q-T and which is the N terminus of the mature polypeptide, contained an NcoI restriction site (underlined hexamer) at the 5′ end and had the following sequence: 5′-CACACCATGGTG AAA GAA GAC AAA CAA ACC-3′. Two downstream primers, corresponding to the carboxyl-terminal amino acid sequences of the 43-kDa and 46-kDa isoforms, respectively, were 5′-CCCCGGATCCTTA CTT GCT GGG CTC CTT TTC-3′ and 5′-CCCCGGATCCTCA AAA GTT GTA GAC ACA GGT-3′ (primer B) and contained a BamHI restriction site (underlined hexamer). A cDNA of each isoform (14) was used as the template for the PCR reactions. Double-stranded PCR products were digested with NcoI and BamHI restriction enzymes and introduced into a pET-28a expression vector (Novagen). The resulting constructs, named Pet43 and Pet46, were transformed into Escherichia coli strain BL21(DE3) (Novagen). Expression and purification of these two recombinant Rubisco activase isoforms were performed as previously described (17).
Purification of Native Rubisco Activase and Rubisco from Leaves.
All purifications were performed as previously described, except that after 35% (NH4)2SO4 precipitation, the precipitated proteins were dissolved in buffer A, desalted (with Sephadex G-25), and resolved by chromatography on a 30-ml Q-Sepharose column (18).
Cloning and Purification of the Recombinant 46-kDa Isoform Rubisco Activase Deletion Mutants.
Five mutants were constructed by inverse PCR on the basis of the cDNA sequence of 46-kDa isoform with deletions of −7, −14, −21, −28, and −35 amino acids from the carboxyl terminus. The 31-mer upstream oligonucleotide sequence, corresponding to the cDNA sequence of the 3′ untranslated region, was 5′-CACAGGATCCCGAATATTATCCTGCTTATTA-3′ and contained a BamHI restriction site (underlined hexamer). The five different downstream oligonucleotide sequences were as follows: −7del, 5′-CCCCGGATCCTCA ATC GTC ACT TCT AGC CGT TGG-3′; −14del, 5′-CCCCGGATCCTCA ATC AAA GTT TTC AGC CAC AGG-3′; −21del, 5′-CCCCGGATCCTCA ATC AGT ACA CCC TTC AGG AAC-3′; −28del, 5′-CCCCGGATCCTCA TGG CAG GTT TAC TTG CTG GGC-3′; −35del, 5′-CCCCGGATCCTCA TCC TTT TCC GTA GAA AGT TCC-3′. Each contained a BamHI restriction site (underlined hexamer) and a stop codon (italicized trimer). The inverse PCR reactions were performed (19) by using a Petnk46 plasmid as template. Petnk46 is similar to the Pet46 plasmid, but also contains about 200 bp nucleotides of the 3′-untranslated region of the 46-kDa cDNA sequence. The final PCR products were digested with BamHI, self-ligated, and transformed into BL21(DE3). Expression and purification of these mutant proteins were performed as previously described (17).
Cloning and Purification of Five Site-Directed Mutants of the 46-kDa Isoform of Rubisco Activase.
The upstream oligonucleotide was primer A (see above). The downstream oligonucleotide sequences were as follows: for Y413A, 5′-CCCCGGATCCTCA AAA GTT GGC GAC ACA GGT TCC-3′; for V412A, 5′-CCCCGGATCCTCA AAA GTT GTA GGC ACA GGT TCC-3′; for C411A, 5′-CCCCGGATCCTCA AAA GTT GTA GAC AGC GGT TCC ATC GTC-3′; for T410A, 5′-CCCCGGATCCTCA AAA GTT GTA GAC ACA GGC TCC ATC GTC ACT-3′. Each oligonucleotide contained a BamHI restriction site (underlined hexamer) and substituted nucleotides (italicized and underlined) necessary to change the proper codon. A PCR-based site-directed mutagenesis method (20) was performed to obtain the C392A mutant. Primer B and a primer (5′-GTT CCT GAA GGG GCT ACT GAT CCT-3′) containing the desired mutation (underlined) were used for the first-stage PCR reaction. Primer A and the first-stage PCR product were used for the second-stage PCR reaction. Pet46 was used as the template for all PCR reactions. The final PCR product was digested by NcoI and BamHI and cloned into the pET-28a vector. All mutations were confirmed by DNA sequencing. Expression and purification of the recombinant mutant Rubisco activase isoforms were performed as previously described (17).
Biochemical Assays of Rubisco Activase Activity.
ATP hydrolysis activity was determined by measuring the rate of Pi formation from ATP. The reaction buffer contained 50 mM Tricine⋅KOH (pH 8.0), 10 mM MgCl2, 20 mM KCl, ATP and ADP totaling 4 mM, other additions as indicated, and 20 μg Rubisco activase in a total volume of 200 μl. The reactions were terminated after 4 min by adding 200 μl 10% (wt/vol) SDS. Pi in the reaction buffer was measured as described (21). The Rubisco activation activity of Rubisco activase was assayed by measuring the increase in Rubisco activity with time. The assay mixture (500 μl) contained 50 mM Tricine⋅KOH (pH 8.0), 20 mM MgCl2, 0.1 mM EDTA, 4 mM RuBP, ATP and ADP totaling 4 mM, 10 mM [14C]NaHCO3 (10 μCi/ml). Rubisco activase (20 μg) was added immediately followed by Rubisco (75 μg) in an inactive noncarbamylated form (previously desalted in a buffer without Mg2+ or CO2 and preincubated with RuBP). Aliquots of 50 μl were quenched with 100 μl of 4 N formic acid/1 N HCl every 30 sec. The samples were dried, and the incorporation of 14CO2 into acid stable products was determined by liquid scintillation counting. Rubisco activity was calculated from the difference in fixed CO2 at successive time points. Rubisco activase activity (Figs. 2–5) corresponds to the increase in Rubisco activity with time. The effects of pretreatment of Rubisco activase were determined by incubation of Rubisco activase (100 μg) with either thioredoxin-f or -m (7.5 μg) in 100 μl containing 100 mM Tris⋅Cl (pH 7.9) and 5 mM DTT at room temperature for 10 min. In some experiments, subsequent additions were made as indicated.
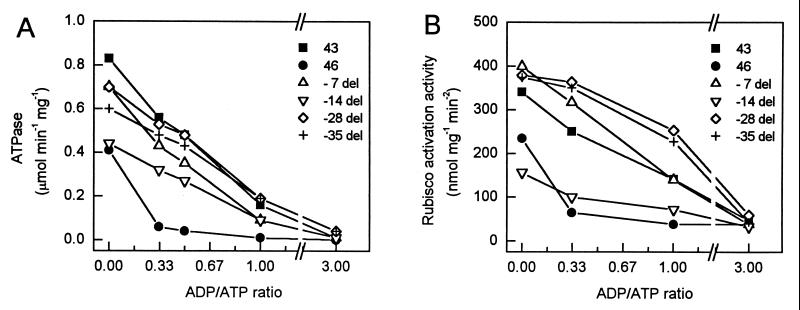
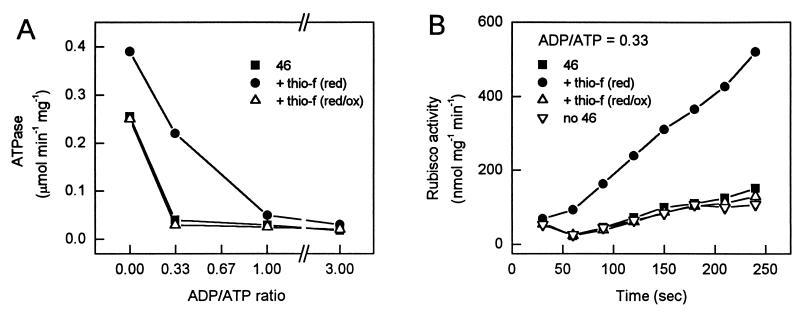
Figure 2.
Comparison of the ATPase (A) and Rubisco activation (B) activities for the 43-kDa, 46-kDa, and the 46-kDa carboxyl-terminal deletion mutant proteins at different ADP/ATP ratios.
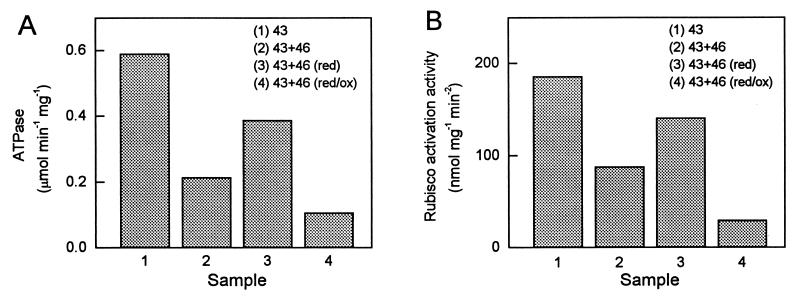
Figure 5.
Effects of combining the 43- and 46-kDa isoforms and reduction or reduction followed by oxidation via thioredoxin-f. The ATPase (A) and Rubisco activation (B) activities of the samples after treatment were measured at an ADP/ATP ratio of 0.33 at the same total activase concentration. Activity observed in the absence of activase was subtracted from the Rubisco activation activities. Samples were treated as follows: (1) 43-kDa only; (2) equal amounts of 43- and 46-kDa isoforms mixed before assay; (3) equal amounts of 43- and 46-kDa isoforms mixed and then reduced with DTT and thioredoxin-f before assay; (4) as in (3) but then oxidized with glutathione (GSSG) before assay. Similar results were observed in two other experiments and the calculated SE was less than 10% of the values shown for each sample.
Miscellaneous Procedures.
DNA cloning and PCR techniques followed standard procedures (22). The Biotechnology Center at the University of Illinois performed all DNA sequencing.
RESULTS
Activity Comparisons with Five Deletion Mutants of the 46-kDa Isoform.
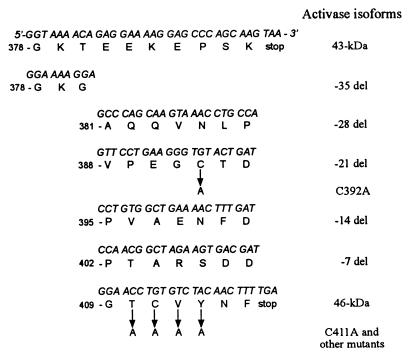
The 43-kDa and 46-kDa isoforms of Arabidopsis Rubisco activase differ only at the carboxyl terminus (Fig. 1). The 46-kDa isoform has 28 additional amino acids (residues 388–415), and the eight residues at positions 380 to 387 also differ. To determine whether only part of this carboxyl-terminal extension is responsible for the greater ADP sensitivity of the larger isoform (15), five deletion mutants were made, which removed seven amino acids at a time as shown in Fig. 1. After purifying the recombinant proteins from an E. coli expression system, the ATPase and Rubisco activation activities were examined at several ADP/ATP ratios (Fig. 2). We examined both activities because numerous types of experiments have shown that the two activities are not strictly coupled (2), although ATP hydrolysis is essential for Rubisco activation. The −21-aa deletion resulted in a protein with very low ATPase and Rubisco activation activity (not shown). The response of the other four deletion mutants to the ADP/ATP ratio was similar to the 43-kDa isoform. The mutants and the 43-kDa isoform retained more than half of their maximum ATPase (Fig. 2A) and Rubisco activation (Fig. 2B) activities at an ADP/ATP ratio of 0.33 in contrast to the 46-kDa isoform, which exhibited little activity at this ratio. An ADP/ATP ratio of 0.33 is typical of the stromal values observed in the light (23). The results suggested that the greater ADP sensitivity of the 46-kDa isoform is caused by the presence of the last seven amino acids.
Figure 1.
Nucleotide and amino acid sequences of the carboxyl-terminal domains of the various Rubisco activase isoforms used in this study.
Activity Comparisions with Five Site-Directed Mutants of the 46-kDa Isoform.
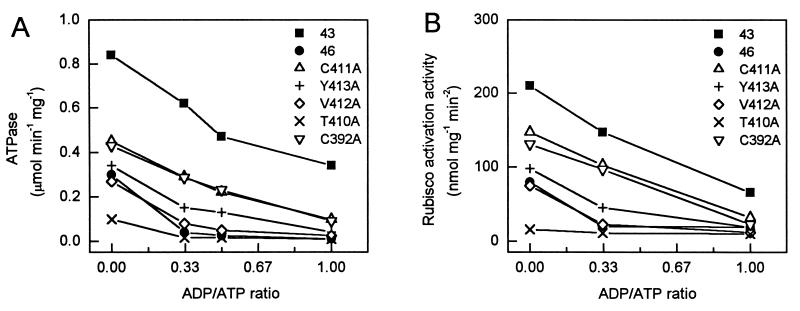
To determine which amino acid(s) might be critical for the greater ADP sensitivity of the 46-kDa isoform, initially four mutants (T410A, C411A, V412A, and Y413A) were made by a site-directed mutagenesis method (Fig. 1). The T410A mutant had very low activity under all conditions, while in the absence of ADP the other three mutants had ATPase activities that were similar to the 46-kDa isoform (Fig. 3A). At an ADP/ATP ratio of 0.33, only the C411A mutant retained more than half its maximum activity. Similar responses to the ADP/ATP ratio were obtained when the Rubisco activation activities of the mutants were compared (Fig. 3B). Only the C411A mutant displayed a high Rubisco activation activity at an ADP/ATP ratio of 0.33. Because Cys residues are capable of forming disulfide bonds, we also mutated the other Cys at position 392 in the carboxyl-terminal extension unique to the 46-kDa isoform. Mutation of this Cys to Ala was also sufficient to markedly lessen the ADP sensitivity and yielded a protein whose response resembled that of the 43-kDa isoform. These results indicate that both Cys-411 and Cys-392 are essential for the greater ADP sensitivity of the 46-kDa isoform.
Figure 3.
Comparion of the ATPase (A) and Rubisco activation (B) activities for the 43-kDa, 46-kDa, and 46-kDa carboxyl-terminal Ala substitution mutant proteins at different ADP/ATP ratios.
Thioredoxin Regulation of 46-kDa Isoform.
The effect of mutating the carboxyl-terminal cysteine residues in the 46-kDa isoform suggested that redox regulation of Rubisco activase via thioredoxin should be investigated. Previously an increase in Rubisco activation in vivo by methyl viologen treatment had been interpreted to indicate that Rubisco was not regulated by redox changes in the stroma (12). Thus, the effects of the strong reductant, DTT, and thioredoxin-f or -m (from spinach) were examined. The 46-kDa isoform, 100 μg (22 μM), was preincubated with 5 mM DTT and 7.5 μg (5.4 μM) of thioredoxin-f for 10 min and the ATPase activity was measured at different ADP/ATP ratios. In a parallel preincubation, 10 mM oxidized glutathione was subsequently added and incubated for another 10 min. Other experiments (not shown) indicated that with 5.4 μM thioredoxin, incubations of 10 min were more than sufficient to achieve maximal activation or inactivation. As shown in Fig. 4A, when the 46-kDa isoform was incubated with DTT and thioredoxin-f, its ATPase activity increased 50% in the absence of ADP, and at an ADP/ATP ratio of 0.33, the activity increased nearly 10-fold relative to the untreated protein. Addition of 10 mM oxidized glutathione completely reversed the stimulation. As shown in Fig. 4B, incubation with thioredoxin-f and DTT also increased the Rubisco activation activity of the 46-kDa isoform when assayed at an ADP/ATP ratio of 0.33. The subsequent addition of oxidized glutathione completely reversed the stimulation. In other experiments (not shown), by using a preincubation time of 5 min, only 0.3 μM spinach thioredoxin-f was required for 50% of the maximal increase in the ATPase activity of the 46-kDa isoform when measured at an ADP/ATP ratio of 0.33. Preincubations of the 46-kDa isoform with DTT and spinach thioredoxin-m for 10 min or DTT alone for up to 15 h at 5°C had no effect on its activity. Furthermore, spinach thioredoxin-f and DTT or oxidized glutathione had no effect on the activity of the 43-kDa isoform or the 46-kDa C411A and C392A mutants. These combined results show that the 46-kDa isoform is reversibly activated by reduction specific to thioredoxin-f.
Figure 4.
Effects of reduction and oxidation of the 46-kDa isoform via thioredoxin-f. The 46-kDa isoform was either untreated (■), reduced with DTT and thioredoxin-f (●), or reduced with DTT and thioredoxin-f and then oxidized with glutathione (GSSG) (▵), as described in the text. The ATPase activity at the indicated ADP/ATP ratios (A) or the ability of the protein to activate Rubisco at an ADP/ATP ratio of 0.33 (B) was then determined. In B, activation of Rubisco with time in the absence of activase (▿) is also shown.
Thioredoxin Effects on the Combined 43-kDa and 46-kDa Isoforms.
In Arabidopsis, the two Rubisco activase isoforms are present in the chloroplast stroma in nearly equal amounts (24), and the protein appears to function in the form of a high molecular mass aggregate (25, 26). Previous experiments indicate that mixing inactive mutant forms of the protein with active forms reduces the net activity cooperatively (27, 28). Therefore, we examined the ability of redox modulation of the 46-kDa isoform to regulate the activity of both isoforms when they are mixed together at a 1:1 ratio. ATPase and Rubisco activation activity were examined at an ADP/ATP ratio of 0.33 after the mixture of 43-kDa and 46-kDa isoforms was incubated in various ways (Fig. 5). At this ADP/ATP ratio, the 46-kDa isoform has very low ATPase and Rubisco activation activity in comparison with the 43-kDa isoform (e.g., Figs. 2 and 3). Therefore, assays with a 1:1 mixture at the same total activase concentration should result in about half the activity obtained with the 43-kDa isoform alone. However, the resulting ATPase activity was somewhat less than predicted when the two forms were mixed together immediately before assay and without pretreatment (Fig. 5A, Sample 2 vs. 1). DTT and thioredoxin-f pretreatment of the mixture increased the ATPase activity of the mixture because of the increase in the activity of 46-kDa isoform (Fig. 5A, Sample 3 vs. 2). Most importantly, subsequent treatment of the mixture with 10 mM oxidized glutathione reduced the activity to a very low level as seen by comparison with the activity of the mixture before pretreatment (Fig. 5A, Sample 4 vs. 2). Reduction and oxidation of the mixture after mixing the isoforms had similar effects on the Rubisco activation activity (Fig. 5B). These experiments demonstrate that redox changes in the 46-kDa isoform via thioredoxin-f can effectively regulate the activities of both isoforms.
Thioredoxin Effects on Native Rubisco Activase.
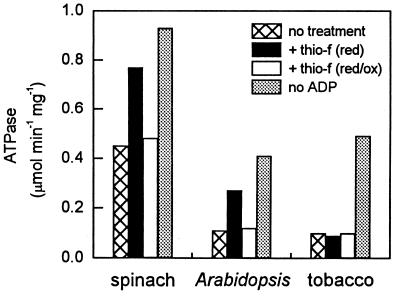
Mixing experiments with the recombinant isoforms may not duplicate exactly the effects of redox regulation by thioredoxin-f because of unknown factors. Therefore, we examined the effects of redox changes on the ATPase activities of the native proteins isolated from Arabidopsis, spinach, and tobacco. Tobacco was of particular interest because the protein apparently consists of only the smaller isoform (13, 29), and evidence for alternative splicing in this species is lacking. Fig. 6 shows that preincubation with DTT and spinach thioredoxin-f increased the ATPase activity (assayed at an ADP/ATP ratio of 0.33) of both native spinach and Arabidopsis Rubisco activase but had no effect on tobacco Rubisco activase. The stimulation was reversed by oxidation with glutathione. A similar redox modulation of the Rubisco activation activities of native Arabidopsis and spinach Rubisco activase has been observed (data not shown). The results of this experiment further suggest that the larger isoform allows for the redox regulation of Rubisco activase in vivo in species that contain both isoforms.
Figure 6.
Effects of reduction and oxidation of native Rubisco activase proteins isolated from spinach, Arabidopsis, and tobacco on their ATPase activity. ATPase activity of each protein was assayed at an ADP/ATP ratio of 0.33 after either: “no treatment;” reduction with DTT and thioredoxin-f, “+ thio-f (red);” reduction followed by oxidation with glutathione (GSSG), “+ thio-f (red/ox);” or assayed in the absence of ADP and without treatment, “no ADP.”
DISCUSSION
In the results reported here, we show that the larger isoform of Arabidopsis Rubisco activase plays a unique role in the regulation of Rubisco in vitro. Earlier studies of the recombinant spinach isoforms revealed that the larger isoform was more easily inhibited by ADP than the smaller isoform, suggesting a possible regulatory function (15). The only difference in the amino acid sequence of these proteins is in the carboxyl-terminal domains. Using carboxyl-terminal deletion mutants, we demonstrate that the removal of the last seven residues from the Arabidopsis 46-kDa isoform is sufficient to decrease the inhibition by ADP to resemble that of the 43-kDa isoform. We then used Ala substitution mutagenesis in the carboxyl-terminal region to demonstrate that Cys-411 and Cys-392 were required to maintain the greater ADP sensitivity of the 46-kDa isoform.
Light/dark regulation of key enzymes other than Rubisco in the reductive pentose phosphate cycle in chloroplasts by reduction of disulfide bonds in these enzymes via thioredoxin is well established (11). We demonstrate that the low activity of the 46-kDa isoform in the presence of ADP can be greatly increased by reduction with DTT via thioredoxin-f. Although the activity of the 43-kDa isoform alone is not altered by reduction/oxidation, we show that alteration of the activity of the larger isoform is sufficient to regulate the activity of both isoforms when they are present together. This is consistent with previous evidence that Rubisco activase operates in the form of a large complex (25, 26). The Ala substitution results indicate that Cys-392 and Cys-411 probably form the critical disulfide bond in the larger isoform that is reduced by thioredoxin-f. However, our results do not eliminate the possibility that other cysteines or intermolecular disulfides may form between the isoforms and also play a role in redox regulation.
Because Rubisco activase regulates the activity of Rubisco, our in vitro results suggest that it may join the other chloroplast enzymes that are known to be light/dark regulated by redox changes in the stroma. We believe that it is quite significant that redox regulation of Rubisco activase is most dramatic at physiological ratios of ADP/ATP (≅0.33) typical of values observed in the light (23). At an ADP/ATP ratio of 1, which is typical of the dark, both isoforms have much lower activity, and this would conserve ATP when photosynthesis is not occurring. These aspects of the regulation by Rubisco activase may allow Rubisco to respond to and integrate both indicators of the stromal energy status, but with the ADP/ATP ratio having an overriding control. Hence, redox regulation of Rubisco was not detected in previous experiments by using methyl viologen-treated leaves (12). Even though the stroma became oxidized, and other redox-regulated enzymes lost activity (12), our experiments here indicate that the increase in Rubisco activase activity by the observed decrease in the ADP/ATP ratio after methyl viologen treatment (5) would have been sufficient to overcome the effect of oxidation of the 46-kDa isoform.
The most noticeable environmental factor controlling Rubisco activity is the diurnal change in light intensity (1, 3). The mechanism of light regulation has been enigmatic because the one factor known to alter Rubisco activase activity, the ADP/ATP ratio, was found to not vary much with light intensity (5, 6). The redox regulation of Rubisco activase that we have shown here provides a straightforward mechanism for the light modulation if the stromal redox status varies to an analogous extent with variations in light intensity. Previous studies examining the variation of fructose 1,6-bisphosphatase (30, 31) and malate dehydrogenase (31, 32) activation with light intensity indicate that such modulation is possible, and a close correlation between fructose 1,6-bisphosphatase and Rubisco activation under steady-state conditions has been noted (30). An alternative is that redox regulation via thioredoxin primarily serves as a coarse light/dark signal, which can be modulated at the individual target enzymes by substrate levels or other factors like stromal pH and [Mg2+] (33). Factors besides the ADP/ATP ratio that could modulate the redox regulation of Rubisco activase uncovered here are unknown. Ultimately, direct measurements of the reduction status of thioredoxin and the target enzymes will be required to distinguish between these possibilities. Redox-induced changes in Rubisco activase activity may also account for the reported effects of light and thylakoids on Rubisco activation in chloroplast extracts (7, 8) and reconstituted systems (9, 10). Additional studies with these systems will be necessary to examine this possibility.
Redox regulation of Rubisco via Rubisco activase may not occur in all species. A few species examined to date, like tobacco, maize, and Chlamydomonas, do not appear to have both isoforms (13). The results of our experiment with the native tobacco protein were consistent with the absence of a larger isoform in the isolated protein. The extent to which Rubisco activity in tobacco is modulated in the light is not clear, and it is complicated by the presence of the Rubisco inhibitor, 2-carboxyarabinatol 1-phosphate (34). However, for maize, the observation that light intensity does not modulate Rubisco activity (35) is consistent with the reported absence of the larger isoform. More extensive species surveys of the presence and relative amounts of the two isoforms are needed but are problematic because of the known proteolytic vulnerability of the larger isoform (13, 36).
Rubisco activase was one of the earliest examples of alternative splicing in plants (14), and some differences in the developmental and diurnal expression of the activase mRNAs have been observed in barley (37). More generally, alternative splicing has been associated with developmental patterns, tissue specificity, and cellular targeting, but relatively few examples of alternative splicing have been identified in plants, and in most cases, its biological significance remains unclear (38). In the results presented here, we show that the two isoforms of Rubisco activase have substantially different functions in the regulation of Rubisco. Although both are capable of activating Rubisco, only the larger isoform is redox regulated. Redox regulation of the larger isoform appears to be sufficient to alter the activity of both isoforms in response to redox changes. The biochemical significance of alternative splicing of Rubisco activase seems to be clear, and it represents a new rationale for the occurrence of this process.
Acknowledgments
We thank P. Schürmann for spinach thioredoxins-f and -m. This work was supported in part by a grant from the United States Department of Energy (DE-AI02–97ER20268).
ABBREVIATION
- RuBP
ribulose 1,5-bisphosphate
References
- 1.Portis A R., Jr Annu Rev Plant Physiol Plant Mol Biol. 1992;43:415–437. [Google Scholar]
- 2.Salvucci M E, Ogren W L. Photosynthesis Res. 1996;47:1–11. doi: 10.1007/BF00017748. [DOI] [PubMed] [Google Scholar]
- 3.Perchorowicz J T, Raynes D A, Jensen R G. Proc Natl Acad Sci USA. 1981;78:2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooks A, Portis A R., Jr Plant Physiol. 1988;87:244–249. doi: 10.1104/pp.87.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks A, Portis A R, Jr, Sharkey T D. Plant Physiol. 1988;88:850–853. doi: 10.1104/pp.88.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietz K J, Heber U. Biochim Biophys Acta. 1986;848:392–401. [Google Scholar]
- 7.Campbell W J, Ogren W L. Plant Physiol. 1990;92:110–115. doi: 10.1104/pp.92.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell W J, Ogren W L. Plant Physiol. 1990;94:479–484. doi: 10.1104/pp.94.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell W J, Ogren W L. Plant Cell Physiol. 1992;33:751–756. [Google Scholar]
- 10.Byrd G T, Ort D R, Ogren W L. Plant Physiol. 1995;107:585–591. doi: 10.1104/pp.107.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchanan B B. Arch Biochem Biophys. 1991;288:1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- 12.Salvucci M E, Portis A R, Jr, Ogren W L. Plant Physiol. 1986;80:655–659. doi: 10.1104/pp.80.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvucci M E, Werneke J M, Ogren W L, Portis A R., Jr Plant Physiol. 1987;84:930–936. doi: 10.1104/pp.84.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werneke J M, Chatfield J M, Ogren W L. Plant Cell. 1989;1:815–825. doi: 10.1105/tpc.1.8.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen J B, Orozco E M, Ogren W L. J Biol Chem. 1991;266:8963–8968. [PubMed] [Google Scholar]
- 16.Orozco B M, McClung C R, Werneke J M, Ogren W L. Plant Physiol. 1993;102:227–232. doi: 10.1104/pp.102.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van de Loo F J, Salvucci M E. Biochemistry. 1996;35:8143–8148. doi: 10.1021/bi9604901. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z Y, Synder G W, Esau B D, Portis A R, Jr, Ogren W L. Plant Physiol. 1992;100:1858–1862. doi: 10.1104/pp.100.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gasch A, Aoyama T, Foster R, Chua N-H. In: Methods in Arabidopsis Research. Koncz C, Chua N-H, Schell J, editors. Teaneck, NJ: World Scientific; 1992. pp. 352–355. [Google Scholar]
- 20.Chen B W, Przybyla A E. BioTechniques. 1994;17:657–659. [PubMed] [Google Scholar]
- 21.Chifflet S, Torriglia A, Chiesa R, Tolosa S. Anal Biochem. 1988;168:1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 23.Stitt M, Lilley R M, Heldt H W. Plant Physiol. 1982;70:971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckardt N A, Snyder G W, Portis A R, Jr, Ogren W L. Plant Physiol. 1997;113:575–586. doi: 10.1104/pp.113.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z Y, Ramage R T, Portis A R., Jr Biochim Biophys Acta. 1993;1202:47–55. doi: 10.1016/0167-4838(93)90061-u. [DOI] [PubMed] [Google Scholar]
- 26.Lilley R M, Portis A R., Jr Plant Physiol. 1997;114:605–613. doi: 10.1104/pp.114.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvucci M E, Klein R R. Arch Biochem Biophys. 1994;314:178–185. doi: 10.1006/abbi.1994.1427. [DOI] [PubMed] [Google Scholar]
- 28.van de Loo F J, Salvucci M E. Biochemistry. 1998;37:4621–4625. doi: 10.1021/bi972566e. [DOI] [PubMed] [Google Scholar]
- 29.Mate C J, Hudson G S, von Caemmerer S, Evans J R, Andrews T J. Plant Physiol. 1993;102:1119–1128. doi: 10.1104/pp.102.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sassenrathcole G F, Pearcy R W, Steinmaus S. Photosynthesis Res. 1994;41:295–302. doi: 10.1007/BF00019407. [DOI] [PubMed] [Google Scholar]
- 31.Harbinson J, Genty B, Foyer C H. Plant Physiol. 1990;94:545–553. doi: 10.1104/pp.94.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheibe R, Stitt M. Plant Physiol Biochem. 1988;26:473–481. [Google Scholar]
- 33.Leegood R C, Kobayashi Y, Neimanis S, Walker D A, Heber U. Biochim Biophys Acta. 1982;682:168–178. [Google Scholar]
- 34.Hammond E T, Andrews T J, Woodrow I E. Plant Physiol. 1998;118:1463–1471. doi: 10.1104/pp.118.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sage R F, Seemann J R. Plant Physiol. 1993;102:21–28. doi: 10.1104/pp.102.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zielinski R E, Werneke J E, Jenkins M E. Plant Physiol. 1989;90:516–521. doi: 10.1104/pp.90.2.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rundle S J, Zielinski R E. J Biol Chem. 1991;266:14802–14807. [PubMed] [Google Scholar]
- 38.Simpson G G, Filipowicz W. Plant Mol Biol. 1996;32:1–41. doi: 10.1007/BF00039375. [DOI] [PubMed] [Google Scholar]