Abstract
Mutations in myocilin result in ocular hypertension, likely due to decreased drainage of aqueous humor through the trabecular meshwork. Since less myocilin is found in the aqueous humor of those with disease-causing mutations, understanding myocilin’s role in the aqueous humor is of clinical importance. Recently, myocilin was shown to exit cultured trabecular meshwork cells in association with shed vesicles called exosomes. To examine relevance of this finding in a physiological setting, the present study examined three different types of ocular samples for the presence of myocilin-associated exosomes. Using differential centrifugation steps, we found that myocilin associated with exosomes isolated from effluent collected from human anterior segments in organ culture and aqueous humor obtained from human cadaveric eyes or from patients undergoing excisional surgery. Similar to results with cultured cells, myocilin appeared to predominately associate with exosomes in fresh samples, appeared mostly soluble at later times, and had biochemical properties (density of 1.13g/mL–1.19g/mL in linear sucrose gradient) similar to those characteristic of exosomes. These data indicate that exosomes are present and may facilitate the transport of myocilin into the extracellular space of human ocular cells.
Glaucoma is the second leading cause of irreversible blindness in the United States (Quigley 1996). The most common form, primary-open angle glaucoma (POAG), is a group of diseases characterized by the loss of retinal ganglion cells that is often coincident with elevated intraocular pressure (Epstein, Allingham and Schuman 1997). Because first-order relatives of individuals with POAG are 8 times more likely to develop POAG than the general population, some forms are thought to have a genetic component (Miller and Paterson 1962; Tielsch et al. 1994). In fact, recent genetic linkage studies have identified 3 loci containing genes that when mutated associate with POAG (Stone et al. 1997; Rezaie et al. 2002; Monemi et al. 2005). The first of these loci, GLC1A, contains a gene that encodes a protein called myocilin (MYOC) (Kubota et al. 1997; Ortego, Escribano and Coca-Prados 1997; Nguyen et al. 1998). Mutant MYOC is responsible for 3–4% of POAG in every population examined to date (Fingert et al. 2002).
Despite eight years of intensive research, the function of MYOC remains a mystery; likely because MYOC has a combination of several interesting properties. Myocilin is a secreted protein that is found both in the cytosol and associated with intracellular membranes, including the Golgi apparatus and intracellular vesicles (Lutjen-Drecoll et al. 1998; Nguyen et al. 1998; Stamer et al. 1998; Mertts et al. 1999; O’Brien, Ren and Wang 2000; Clark et al. 2001). These apparently conflicting subcellular and extracellular localizations were reconciled with a recent report showing that MYOC exits cultured trabecular meshwork cells in association with vesicles, called exosomes, that are released from cells when multivesicular bodies fuse with plasma membranes (Hardy et al. 2005).
Intracellularly, MYOC’s association with membranes appears to be mediated by its coiled-coil domain, possibly as a member of a vesicle coat complex (Stamer et al. 2006). In the extracellular compartment, MYOC’s association with released exosomes appears labile. Thus, when MYOC’s association with released exosomes was followed over time, the majority of extracellular MYOC was associated with exosomes soon after release into the extracellular space (Hardy et al. 2005). In contrast, MYOC was found soluble in the conditioned media at later time points.
The present study was conducted to determine whether MYOC’s association with extracellular membranes is physiologically relevant, or a product of the cell culture environment. We hypothesized that MYOC associates with exosomes in human samples isolated from anterior eye segments. To test our hypothesis we examined three different types of human ocular samples for the presence of MYOC-associated exosomes: effluent from perfused human anterior segments, aqueous humor from cadaveric human eyes and aqueous humor from patients undergoing incisional eye surgery. Effluent was collected from perfused human ocular anterior segments that had stable baseline facilities within a physiological range (Johnson 1996; Ethier et al. 2004). Collections were conducted at the end of 24 hr periods of perfusion. Aqueous humor was collected from human cadaveric eyes within 35 hours of death or from patients undergoing cataract or glaucoma filtering surgery by paracentesis as described elsewhere (Knepper et al. 2002). Human samples were compared to samples of conditioned media that were taken from stable, confluent monolayers of human trabecular meshwork cells in culture as described previously (Hardy et al. 2005). All samples were frozen at −80°C until time of analysis.
As reported before by others, we found MYOC in whole samples of aqueous humor (cadaveric), effluent from perfused human anterior segments and conditioned media from human trabecular meshwork cells in culture (figure 1, Pre-spin) (Nguyen et al. 1998; Rao, Allingham and Epstein 2000). After removal of cellular debris by centrifugation at 10,000g, exosomes were isolated from samples by collecting membrane pellets after two sequential centrifugation steps at 100,000g as described previously (Hardy et al. 2005). In all three samples, MYOC was found in the exosome fraction in different amounts. In contrast, a control protein, a soluble fragment of CD44 (sCD44 or CD44 ectodomain), was detected in all fractions except exosomes in preparations from aqueous humor and effluent. Soluble CD44 is present in body fluids including blood and aqueous humor as a result of cleavage from full-length CD44 protein from cell surfaces (Knepper et al. 2002). Interestingly, we were able to detect full-length CD44 in trabecular meshwork cells (not shown), but not sCD44 in any fraction prepared from conditioned media of human trabecular meshwork cells; possibly due to lack of a stimulus to initiate liberation from full-length CD44.
Figure 1. Analysis of human aqueous humor, effluent from perfused human anterior segments and conditioned media from human trabecular meshwork cells for the presence of MYOC-associated exosomes.
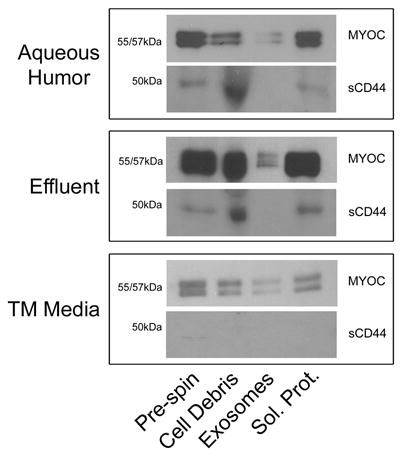
A. Whole aqueous humor, effluent and conditioned media (Pre-spin) were subjected to differential centrifugation steps, to first pellet cellular debris and then to pellet extracellular membranes (Exosomes). The resulting supernatant was considered to contain only soluble proteins (Sol. Prot.). Fractions were analyzed by SDS-PAGE/western blotting using affinity purified antibodies that recognize myocilin (Stamer et al. 1998) or sCD44 (BU52). Shown is one representative experiment of 4 total experiments for cadaveric aqueous humor and effluent and of 12 total experiments for conditioned media.
When comparing MYOC content in the exosome fraction to MYOC that remained soluble in the supernatant (Sol. prot.) after centrifugation at 100,000g, MYOC in human samples and conditioned media shown in figure 1 was found predominantly soluble in supernatant, similar to results reported previously (Hardy et al. 2005). To test the hypothesis that MYOC’s association with exosomes is labile over time in physiologically relevant samples, we analyzed aqueous humor obtained fresh from patients undergoing cataract (normal) or filtering surgery (glaucoma). Characteristics of aqueous humor donors are shown in supplemental table 1. Significantly, figure 2 shows that the majority of MYOC associates with exosomes in both normal and glaucomatous samples compared to the soluble fractions; indicating that the majority of MYOC in aqueous humor from live humans is associated with exosomes and not free in solution. Notice that for volume equivalent samples between normal and glaucoma patients (at the same exposure time to film), more MYOC signal was observed in glaucoma samples, likely due to protein concentration that occurs in glaucoma patients on aqueous suppressants. Since only 3–4% of glaucoma patients have mutations in MYOC and pooled patient samples were tested in the present study, it was not surprising that the relative amount of MYOC between fractions was not different in aqueous humor from normal and glaucomatous eyes. Thus, there was a small probability (1/1000) that one of the three samples in each pool tested was from a patient having a mutation in MYOC. Unfortunately, none of the glaucoma patients were screened for MYOC mutations. As with cadaveric aqueous humor samples, we tested all aqueous humor samples obtained from living patients for the presence of sCD44. Due to the dilutions necessary for the fractionation procedure and the small amount of starting material (i.e.:≤500ng/lane), we were unable to detect sCD44 in any of the patient samples (not shown).
Figure 2. Comparison of myocilin-associated exosomes in aqueous humor samples from normal and glaucoma patients.
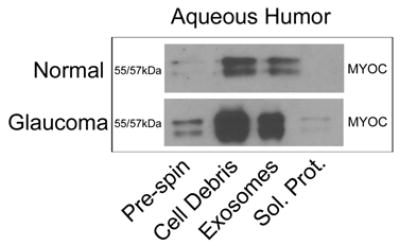
Due to the limited quantity of aqueous humor per sample (~70μL), three samples were combined for a total of ~200μL (Pre-spin) and subjected to differential centrifugation steps; to first pellet cellular debris and then to pellet extracellular membranes (Exosomes). The resulting supernatant was considered to contain only soluble proteins (Sol. Prot.). Fractions were analyzed by SDS-PAGE/western blotting using affinity purified antibodies that recognize myocilin. Shown is one representative experiment of four total independent experiments. Aqueous samples were obtained from normal or glaucoma patients who were undergoing cataract or filtration surgery, respectively as previously described (Knepper et al. 2002). Patients provided informed consent in accordance with the tenets of the Declaration of Helsinki and procurement protocols were approved by the Duke University’s Institutional Review Board.
To determine whether MYOC-associated extracellular membranes, isolated from human ocular samples, have biochemical properties characteristic of exosomes, we analyzed the density of exosome preparations in sucrose gradients as described previously (Hardy et al. 2005). Washed exosome pellets were placed at the bottom of tubes and floated upward into sucrose gradients (2M–0.25M) under a centripetal force of 100,000×g for 18 hrs. Fractions were collected, proteins in fractions were separated by SDS-PAGE and MYOC was identified by western blot using affinity purified antibodies (Stamer et al. 1998). Results in figure 3 show that MYOC-associated exosomes in all samples equilibrate at a density characteristic of exosomes (1.13–1.19 g/ml) (Raposo et al. 1996; Escola et al. 1998; Wubbolts et al. 2003). Shown in panel A are MYOC blots for individual gradients. Panel B shows a comparative summary of blots shown in panel A that accounts for differences in density between fractions of individual experiments. Mean densities for MYOC-associated exosomes in aqueous humor samples was 1.13 g/ml (n=2), effluent was 1.17 g/ml (n=3) and conditioned medium was 1.14 g/ml (n=3). Thus, buoyant properties of exosomes from three different human ocular sources were remarkably similar and within characteristic range.
Figure 3. Myocilin-associated extracellular membranes prepared from cadaveric aqueous humor, effluent and media from trabecular meshwork cells equilibrate at a density characteristic of exosomes.
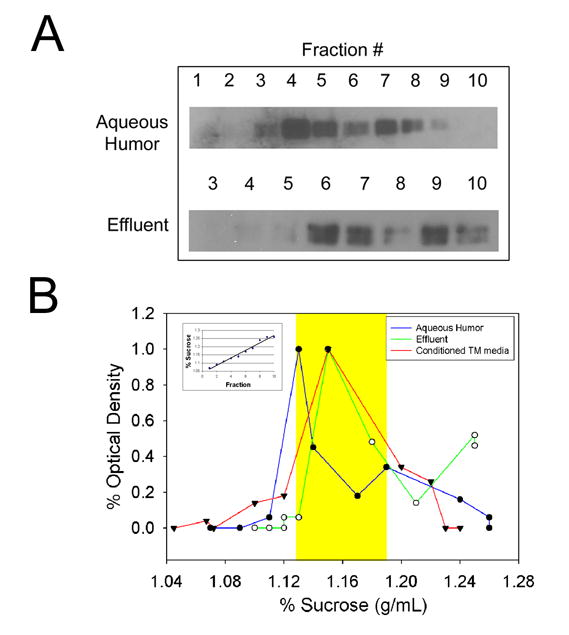
A. Extracellular membranes were isolated from human samples by differential centrifugation and floated upward into linear sucrose gradients. Fractions were collected, analyzed by SDS-PAGE and probed with anti-myocilin IgGs. B. Analysis of myocilin content (triangle=effluent; square=aqueous humor; circle=conditioned media) in gradient fractions by densitometric analysis of western blots and linearity of gradients by refractometry (example shown in inset). Shown are representative experiments of two total experiments for aqueous humor and three for effluent and conditioned media. Yellow shaded area indicates the reported density range of exosomes (1.13g/mL–1.19g/mL).
Taken together, data of the present study indicate that MYOC-exosomes are present in human ocular samples. MYOC was found associated with exosomes in effluent from perfused anterior segments and in aqueous humor from human cadaveric and living eyes. These findings were consistent with our discovery of MYOC-associated exosomes in conditioned medium from cultured human trabecular meshwork cells. In fact, similar to results found in cultured cells, MYOC in human ocular samples appears to disassociate from exosomes over time. For example, the majority of MYOC was found in the exosomal fraction of aqueous humor from living patients, while the minority of MYOC was found in exosome fraction in aqueous humor isolated from cadaveric eyes (25–35 hours post mortem) or in effluent collected after 24 hours of perfusion. These data indicate that MYOC’s appearance extracellularly may be facilitated by exosome release. Interestingly, when these three sources of MYOC-associated exosomes were compared, ten times more conditioned media and 3 times more effluent was required to detect MYOC-associated exosomes on a volume and cell equivalent basis (supplementary figure 1). MYOC-associated exosomes appear more prominently in human samples and thus are likely physiologically relevant, and not an artifact of the cell culture environment.
It is intriguing to speculate how exosomes function in the conventional drainage pathway of humans; and how MYOC may contribute to and how mutations in MYOC may inhibit exosome function. Exosomes are known to have two main roles, antigen presentation and cell-cell communication (Stoorvogel et al. 2002; Fevrier and Raposo 2004). Because of their lipid nature, exosomes travel short distances in a paracrine fashion to deliver ligands or antigens to cell neighbors. Since disease-causing mutations disrupt MYOC transport out of cells (Jacobson et al. 2001), it is possible that ocular hypertension in MYOC-related POAG may result from a disruption in cell to cell communication in the conventional drainage pathway. These are interesting ideas that need to be tested in future studies.
Supplementary Material
Supplemental Figure 1: Relative content comparison of myocilin-associated exosomes between human ocular samples. The exosome fraction for each of the human samples was run side by side for comparison. Equivalent chemiluminescent signals were obtained when approximately 10 times the amount of conditioned media and 3 times the amount of effluent was loaded onto gel compared to cadaveric aqueous humor. Samples were compared on a volume/cell equivalent basis.
Acknowledgments
The authors thank Zhou Wan and Mike Fautsch for effluent and cadaveric aqueous humor samples, and Cecilia Santiago for assistance with aqueous humor samples from living patients.
References
- Clark A, Steely H, Dickerson J, English-Wright S, Stropki K, McCartney M, Jacobson N, Shepard A, Clark J, Matsushima H, Peskind E, Leverenz J, Wilkinson C, Swiderski R, FIngert J, Sheffield V, Stone E. Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2001;42:1769–1780. [PubMed] [Google Scholar]
- Epstein D, Allingham R, Schuman J, editors. Chandler and Grant’s, Glaucoma. Williams and Wilkins; Baltimore: 1997. [Google Scholar]
- Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. Journal of Biological Chemistry. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- Ethier C, Wada S, Chan D, Stamer W. Experimental and numerical studies of adenoviral delivery to outflow tissues of perfused human anterior segments. Invest Ophthalmol Vis Sci. 2004;45:1863–1870. doi: 10.1167/iovs.03-1133. [DOI] [PubMed] [Google Scholar]
- Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Current Opinion in Cell Biology. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Fingert J, Stone E, Sheffield V, Alward W. Myocilin Glaucoma. Surv Ophthalmol. 2002;47:547–561. doi: 10.1016/s0039-6257(02)00353-3. [DOI] [PubMed] [Google Scholar]
- Hardy K, Hoffman E, McKay B, Gonzalez P, Stamer W. Extracellular trafficking of myocilin in human trabecular meshwork cells. J Biol Chem. 2005;280:28917–28926. doi: 10.1074/jbc.M504803200. [DOI] [PubMed] [Google Scholar]
- Jacobson N, Andrews M, Shepard A, Nishimura D, Searby C, Fingert J, Hageman G, Mullins R, Davidson B, Kwon Y, Alward W, Stone E, Sheffield V. Non-secretion of mutant proteins of the glaucoma gene myocilin in cultured trabecular meshwork cells and in aqueous humor. Hum Mol Genetics. 2001;10:117–125. doi: 10.1093/hmg/10.2.117. [DOI] [PubMed] [Google Scholar]
- Johnson D. Human Trabecular Meshwork Cell Survival Is Dependent on Perfusion Rate. Invest Ophthal and Vis Science. 1996;37:1204–1207. [PubMed] [Google Scholar]
- Knepper PA, Mayanil CS, Goossens W, Wertz RD, Holgren C, Ritch R, Allingham RR. Aqueous humor in primary open-angle glaucoma contains an increased level of CD44S. Investigative Ophthalmology & Visual Science. 2002;43:133–139. [PubMed] [Google Scholar]
- Kubota R, Noda S, Wang Y, Minoshima S, Asakawa S, Kudoh J, Mashima Y, Oguchi Y, Shimizu N. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: Molecular cloning, tissue expression, and chromosomal mapping. Genomics. 1997;41:360–369. doi: 10.1006/geno.1997.4682. [DOI] [PubMed] [Google Scholar]
- Lutjen-Drecoll E, May C, Polansky J, Johnson D, Bloemendal H, Nguyen T. Localization of the stress proteins alpha-B crystallin and trabecular meshwork inducible glucocorticoid response protein in normal and glaucomatous trabecular meshwork. Invest Ophthalmol Vis Sci. 1998;39:517–525. [PubMed] [Google Scholar]
- Mertts M, Garfield S, Tanermoto K, Tomarev S. Identification of the region in the N-terminal domain responsible for the cytoplasmic localization of MYOC/TIGR and its association with microtubules. Lab Invest. 1999;79:1237–1245. [PubMed] [Google Scholar]
- Miller S, Paterson G. Studies on glaucoma relatives. Br J Ophthalmol. 1962;46:513–522. doi: 10.1136/bjo.46.9.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Heon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Human Molecular Genetics. 2005;14:725–733. doi: 10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Chen P, Huang W, Chen H, Johnson D, Polansky J. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–6350. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- O’Brien E, Ren X, Wang Y. Localization of myocilin to the golgi apparatus in Schlemm’s canal cells. Invest Ophthalmol Vis Sci. 2000;41:3842–3849. [PubMed] [Google Scholar]
- Ortego J, Escribano J, Coca-Prados M. Cloning and characterization of subtracted cDNAs from a human ciliary body library encoding TIGR, a protein involved in juvenile open angle glaucoma with homology to myosin and olfactomedin. FEBS Letters. 1997;413:349–353. doi: 10.1016/s0014-5793(97)00934-4. [DOI] [PubMed] [Google Scholar]
- Quigley H. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao P, Allingham R, Epstein D. TIGR/Myocilin in human aqueous humor. Exp Eye Res. 2000;71:637–641. doi: 10.1006/exer.2000.0920. [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. Journal of Experimental Medicine. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. [see comment] Science. 2002;295:1077–1079. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- Stamer W, Perkumas K, Hoffman E, Roberts B, Epstein D, McKay BS. Coiled-coil targeting of myocilin to intracellular membranes. Exp Eye Res. 2006 doi: 10.1016/j.exer.2006.07.018. in press. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Roberts BC, Howell DN, Epstein DL. Isolation, culture and characterization of endothelial cells from Schlemm’s canal. Invest Ophthalmol Vis Sci. 1998;39:1804–1812. [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WLM, Nguyen TD, Polansky JR, Sunden SLF, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Shefield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- Tielsch J, Katz J, Sommer A, Quigley H, Javitt J. Family history and risk of primary open-angle glaucoma. Arch Ophthalmol. 1994;112:69–73. doi: 10.1001/archopht.1994.01090130079022. [DOI] [PubMed] [Google Scholar]
- Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. Journal of Biological Chemistry. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Relative content comparison of myocilin-associated exosomes between human ocular samples. The exosome fraction for each of the human samples was run side by side for comparison. Equivalent chemiluminescent signals were obtained when approximately 10 times the amount of conditioned media and 3 times the amount of effluent was loaded onto gel compared to cadaveric aqueous humor. Samples were compared on a volume/cell equivalent basis.


