Abstract
The glucan-binding protein-A (GbpA) of Streptococcus mutans has been shown to contribute to the architecture of glucan-dependent biofilms formed by this species and influence virulence in a rat model. Since S. mutans synthesizes multiple glucosyltransferases (GTF) and non-GTF glucan-binding proteins (GBPs), it’s possible that there is functional redundancy that overshadows the full extent of GbpA contributions to S. mutans biology. Glucan-associated properties such as adhesion, aggregation, and biofilm formation were examined independently of other S. mutans GBPs by cloning the gbpA gene into a heterologous host, Streptococcus gordonii, and derivatives with altered or diminished GTF activity. The presence of GbpA did not alter dextran-dependent aggregation nor the initial sucrose-dependent adhesion of S. gordonii. However, expression of GbpA altered the biofilm formed by wild-type S. gordonii as well as the biofilm formed by strain CH107 that produced primarily α-1,6-linked glucan. Expression of gbpA did not alter the biofilm formed by strain DS512 that produced significantly lower quantities of parental glucan. These data are consistent with a role for GbpA in facilitating the development of biofilms that harbor taller microcolonies via binding to α-1,6-linkages within glucan. The magnitude of the GbpA effect appears dependent on the quantity and linkage of available glucan.
Introduction
It is well established that glucan, synthesized from the glucose moiety of sucrose, plays a prominent role in the cariogenicity of the dental pathogen Streptococcus mutans. The loss of any of the three S. mutans glucosyltranferase (GTF) enzymes, which synthesize and bind glucans, results in decreased virulence under at least some conditions in animal models (Munro et al., 1991; Yamashita et al., 1993). The roles for non-GTF cell-surface or secreted proteins that bind glucan have also been the subject of investigation. Four of these glucan-binding proteins (GBPs) have been described in S. mutans: GbpA (Russell, 1979), GbpB (Smith et al., 1994), GbpC (Sato et al., 1997), and GbpD (Deepan et al., 2004). Comprising a functionally heterogenous group, they have been associated with altered biofilm formation (GbpA; Hazlett et al., 1999), cell wall stability and peptidoglycan hydrolase activity (GbpB; Chia et al., 2001), dextran-dependent aggregation (GbpC; Sato et al., 1997), and lipase activity (GbpD; Deepan et al., 2004). Contributions of GBPs to virulence in animal models have been variable (Hazlett et al., 1998; Matsumura et al., 2003).
It is uncertain whether the glucan binding affinity of any of the GBPs is influenced by the glucan products of specific GTFs, though GbpA has been shown to require α-1,6 linkages for binding (Russell, 1979; Haas & Banas, 2000). The α-1,6 linkages predominate in water-soluble glucan but are also represented in water-insoluble glucan that is primarily composed of α-1,3 linkages. The predominance of α-1,6 and α-1,3 linkages and their correlation with water solubility is well conserved among GTFs and glucan from numerous oral streptococcal species. However, individual GTFs and their glucan products may differ in precise linkage proportions, the length of the polymer and the degree of branching.
In an effort to further investigate GbpA, the gbpA gene was cloned and expressed in Streptococcus gordonii. S. gordonii is a primary plaque colonizer that possesses a single GTF that synthesizes glucans with both α-1,3 and α-1,6 linkages (Grahame & Mayer, 1985; Haisman & Jenkinson, 1991). Transcription of the structural gene, gtfG, is controlled by the positive regulator rgg (Sulavik et al., 1992). Using this heterologous host, the contribution of GbpA to adhesion and biofilm properties could be evaluated independent of other S. mutans GBPs. Here we report that the expression of plasmid-borne GbpA in S. gordonii did indeed affect the overall architecture of the sucrose-associated biofilms formed by this species. The changes were consistent with a role for GbpA in adding elevation to the biofilm by facilitating the formation of taller microcolonies. The magnitude of the change was the greatest when the proportion of water-soluble glucan was highest, consistent with an affinity of GbpA for α-1,6 glucan linkages (Russell, 1979; Haas & Banas, 2000).
Materials and methods
Strains and growth conditions
All cultures were stored at -80°C. Table 1 provides an overview and description of the strains used in this study. Briefly, the gbpA mutant strain of S. mutans was described previously (Hazlett et al., 1998). The S. gordonii strain Challis, CH1, was the parent for all strains with altered GTF expression. Strain DS512 (Sulavik et al., 1992) has an internal 12-bp deletion in the rgg positive regulatory gene and only ca. 3% of the parental GtfG gene product. Strain CH107 has a 585-bp deletion within the carboxyl terminal glucan-binding domain of gtfG. Glucans synthesized by the encoded 152 kDa GtfG of this strain show only α-1,6-linkages by NMR analysis (Vickerman et al., 1996). Strain CHΔgtfG was constructed by transformation of strain CH1 with a ca. 2.8 kb linear DNA fragment that contained DNA flanking gtfG and replaced the first ca. 4.7 kb of gtfG with a ca. 120-bp omega fragment (Frey & Krisch, 1985). Putative transformants resulting from allelic exchange were selected by their soft colony phenotype on 3% sucrose agar plates. Southern blots and nucleotide sequence analysis confirmed that strain CHΔgtfG has the rgg gene upstream intact up to the native stop codon, followed by an omega transcriptional and translational stop fragment and then 63 3′ nucleotides of gtfG. This strain had no detectable GTF activity. All strains were propagated in Todd-Hewitt (TH) medium (Difco) or Chemically-Defined Medium (CDM; JRH Biosciences, Lenexa, KS) supplemented with 0.25M sodium biocarbonate and, when appropriate, 1% sucrose. Media for plasmid-containing strains included erythromycin at 5μg/ml.
Table 1.
Strain Descriptions
| Strain | Reference | Genotype | Relevant Phenotype/Use in This Study |
|---|---|---|---|
| S. mutans 3209 (GbpA ) | (Hazlett et al., 1998) | Chromosomal gbpA deleted. | Does not make GbpA. Used to supply exogenous glucan to S. gordonii strains making GbpA in transwell experiments. |
| S. gordonii CHR3 | (Vickerman et al., 1993) | recA-deficient via insertional inactivation. | Minimizes recombination. Used in the original cloning and propagation of gbpA in the plasmid pVA749. |
| S. gordonii CH1 | (Macrina et al., 1981) | Wild-type (WT) parental. | Used as the parental S. gordonii strain. |
| S. gordonii CH1(gbpA) | This study. | gbpA in plasmid pVA749 | Used to test the effect of GbpA when total glucan production is reduced. |
| S. gordonii DS512 | (Sulavik et al., 1992) | 12-bp deletion in rgg resulting in a premature translational stop. | Only about 3% of WT levels of GtfG produced. |
| S. gordonii DS512(gbpA) | This study. | gbpA in plasmid pVA749. | Used to test the effect of GbpA when total glucan production is reduced. |
| S. gordonii CH107 | (Vickerman et al., 1996) | 585-bp deletion within the carboxyl terminal Glucans synthesized by the altered gtfG. | Glucans synthesized by the altered GtfG show only α-1,6-linkages by NMR analysis. |
| S. gordonii CH107(gbpA) | This study. | gbpA in plasmid pVA749. | Used to test the effect of GbpA when the glucan contains solely α-1,6-linkages to which GbpA has highest affinity. |
| S. gordonii CHΔgtfG | This study. | gtfG replaced with an omega fragment. | No glucan synthesized. This strain has an intact rgg and therefore controls for the effects of interrupting rgg in strain DS512. |
| S. gordonii CHΔgtfG(gbpA) | This study. | gbpA in plasmid pVA749. | Used to test the effect of GbpA when glucan is not present. |
| S. gordonii CH1(pVA749) | (Macrina et al., 1981) | Parental strain containing the plasmid vector pVA749. | Used as a control strain for the effects of the vector harboring gbpA. |
Construction of plasmid-borne gbpA
The S. mutans gbpA gene and flanking region was amplified by PCR with S. mutans 3209 chromosomal template and primers 5′TAGATATCCGACAATTTGCAAGTAATAGAAGT3′ and 5′TAGATATCCGTTATCATACGACGACATACAA3′ using Elongase enzyme (Invitrogen, Carlsbad, CA) and an annealing temperature of 58°C. The resulting ca. 2.1-kb amplicon with engineered restriction sites contained the gbpA promoter and ribosomal binding site, the complete ORF and downstream transcriptional terminator region. The EcoRV-digested fragment was cloned into the compatible HaeIII site in the erythromycin-resistant streptococcal plasmid pVA749 (Macrina et al., 1981), transformed into the recA-deficient S. gordonii Challis strain CHR3 (Vickerman et al., 1993) to minimize the potential for recombination between chromosomal and cloned DNA, and the resulting plasmid, pVA749:gbpA, verified by nucleotide sequence analysis. The purified plasmid was then transformed into the S. gordonii strains made competent with serum as previously described (Vickerman et al., 1993). The corresponding strains harboring gbpA on the pVA749 plasmid were designated CH1(gbpA), DS512(gbpA), CH107(gbpA), and CHΔgtfG(gbpA). Vector control strains were transformed with pVA749.
Western immunoblotting
Strains of interest were grown overnight to stationary phase in 25 ml TH broth. The bacteria were pelleted by centrifugation and the pellet resuspended in 150μl of 2X cracking buffer (0.0375M Tris, 1% SDS, 2.5% 2-mercaptoethanol, 15% glycerol, and bromphenol blue) to extract surface protein. Although GbpA is a secreted protein there is a sufficient amount that remains associated with the bacterial cells. The cracking buffer solutions were left to incubate at room temperature for 2 hrs and then the bacteria were pelleted by centrifugation. Proteins were separated by SDS-polyacrylamide gel electrophoresis (10% gel) and blotted onto nitrocellulose. After blocking in 2% Tween 20 in PBS (pH 7.4) for 60 min at 37°C, anti-GbpA antibody was added at 1:5000 in PBS with 2% Tween 20 and left at 4°C overnight. After multiple washes with PBS-Tween 20 the gel was incubated at room temperature for 1 hr with protein-A conjugated to horse-radish peroxidase (Pierce, Rockford, IL). The GbpA bands were visualized by enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ).
Glucan activity assay
Protein preparations and separation by SDS-PAGE were as above for western immunoblotting. Relative amounts of GTF activity for each strain were measured via glucan production in acrylamide gels, as previously described (Vickerman et al., 1996). Briefly, strains to be compared were grown to the same mid-to late-log stage (OD600 of ca. 1.6) and equal volumes of 1% (w/v) SDS-extracted cells and cell-free supernatants were run on 8.75% acrylamide SDS-PAGE. After electrophoresis, gels were incubated overnight in 3% sucrose, 0.5% Triton X-100 in 10mM sodium phosphate, pH 6.8, at 37°C, and the resulting glucan products were stained with paraosanaline. Band intensities reflect the relative amount of GTF activity and the water solubility of the glucan products (Vickerman et al., 1996).
Adhesion assay
Strains CHR3 and CHR3(gbpA) were grown overnight to late log phase in CDM and diluted to OD600=0.05 in fresh CDM. One milliliter aliquots were added to vials containing equivalent amounts of saliva-coated hydroxyapatite beads (BDH Chemicals Ltd., Poole, England) and set in an anaerobic chamber at 37°C on a rotator (10 rpm). At 0, 2, 3.5, and 5 hrs post-inoculation the beads were softly pelleted and the supernatant sampled for the enumeration of colony forming units (CFU). Following washing the pellets were similarly enumerated for CFU by sonication plating on Todd-Hewitt agar.
Dextran-dependent aggregation
The procedure for measuring dextran-dependent aggregation was based on that used to measure the activity of the S. sobrinus glucan-binding lectin (GBL) (Ma et al., 1996). Briefly, the panel of S. gordonii strains was grown overnight in TH broth to late log phase, pelleted by centrifugation, washed in phosphate-buffered saline (PBS), and resuspended in PBS at approximately one-tenth the volume. Dextran T2000 (Fisher Scientific, Fair Lawn, NJ) was added to a concentration of 100μg/ml. The suspensions were incubated at 37°C for up to 2 hrs and the absorbance read at wavelength 600.
Generation of biofilms
Biofilms for confocal microscopy were generated by inoculating wells of a two-well Lab-Tek Borosilicate Coverglass System (Nalge Nunc International, Rochester, NY) with 75μl of an overnight culture normalized to an equivalent optical density (OD). Each well held 1.5 ml of CDM with 5% sucrose. The biofilms were allowed to develop over two days at 37°C while rotating at 10 rpm. The medium was changed at 24 hrs.
Early biofilm deposition of strains CH1 and CH1(gbpA) was tested in the presence of a S. mutans GbpA-negative strain in a transwell system that presumably allowed the cell-free flow of GTFs and glucan to diffuse through the filter. An equivalent OD of each S. gordonii strain was inoculated into wells of a Falcon 24-well tissue culture plate (Becton Dickinson, Franklin Lakes, NJ) in CDM plus 5% sucrose. A circular, glass coverslip was added to each well. A 0.4 μm Falcon transwell cell culture insert (Becton Dickinson) was placed into the inoculated wells and inoculated with an equivalent OD of S. mutans (GbpA-) in CDM plus 5% sucrose. The biofilms were allowed to develop for six hours at 37°C with constant rotation at 10rpm. The glass coverslips were then collected, stained with crystal violet, and photographs of five independent, random fields were imaged. A grid was overlaid onto the photographic images to estimate coverage. When less than 40% of the boxes in the grid were empty the biofilm was designated as having “heavy” coverage. If greater than 40% of the grid boxes were empty the biofilm coverage was designated as “light.” A total of 115 images each for strains CH1 and CH1(gbpA) were analyzed by this semi-quantitative protocol.
Confocal Microscopy
For confocal microscopy the planktonic phase was aspirated from the biofilm wells, the biofilm washed once with PBS, and then stained with SYTO 9 (Molecular Probes, Eugene, OR) for 15 min. The stain was aspirated, the biofilm washed in PBS, and then 1.5 ml of PBS added to retain hydration. The stained biofilms were imaged directly using a Zeiss 510 META NLO-confocal microscope system on an Axiomat 200 M inverted microscope equipped with a 25 x 0.8 NA multi immersion DIC lens (Zeiss, USA, Thornwood, NY). The SYTO 9 fluorescent dye was excited using two-photon excitation with the 800 nm line from a Coherent Chameleon Ti:Sa pulsed laser and fluorescence emission detected using a 500-550 IR band pass filter. Images were collected at 1024 x 1024 pixel resolution with scaling of 0.36 μm (X) x 0.36 μm (Y) x 1.0 μm (Z). Images were processed using the native Zeiss LSM software and Adobe Photoshop.
Biofilm Analysis
Biomass, percent substratum coverage, average thickness, roughness coefficient and surface to volume ratio were determined using COMSTAT image-processing software (Heydorn et al., 2000) written as a script in Matlab 5.1 (The MathWorks) equipped with the Image Processing Toolbox. These scripts also functioned perfectly using our current version of Matlab 7.0.1. Z-stacks were collected from five randomly selected regions from each independent biofilm in a single well and were processed and analyzed by COMSTAT. The five image stacks covered a total area of ∼7.41×10-5 μm2, an area that more than satisfies the recommended minimum surface area that should be investigated in order to obtain representative data (Korber et al., 1993).
Results
The panel of S. gordonii strains represented an opportunity to examine the influence of GbpA on adhesion, aggregation, and biofilm formation by CH1 strains with quantitative and qualitative differences in glucan synthesis. These glucan variations also served as implicit controls in these analyses since it was expected that GbpA should not influence the properties of S. gordonii unless glucan was present.
In order to confirm that S. gordonii strains carrying plasmid-borne gbpA secreted the protein in a manner analogous to its native S. mutans, western immunoblots were performed using rabbit sera directed against the glucan-binding domain of GbpA. As seen in Figure 1A, intense bands representing GbpA were evident in extracts from each of the S. gordonii plasmid-bearing strains. The band migration distances for the recombinant S. gordonii strains were the same as from S. mutans indicating that signal peptide cleavage of GbpA was occurring at or near the native cleavage site. The blot also confirmed the lack of degradation of GbpA in the recombinant strains.
Fig 1.

(A) Western immunoblot using anti-GbpA sera. Proteins were separated by SDS-PAGE. GbpA was detected migrating just below the 84 kDa molecular weight band. GbpA routinely runs at a higher molecular weight than that calculated from its amino acid sequence (Russell, 1979; Banas et al., 1990). The band from S. mutans was run as a control; the single band represented the mature form of GbpA in which the signal peptide has been cleaved. CH1, DS512, and CH107 are strains of S. gordonii as described in the Materials and Methods, and strains CH1(gbpA), DS512(gbpA), and CH107(gbpA) are the corresponding derivatives that express GbpA. (B) Activity gel depicting glucan bands from the panel of S. gordonii strains. Proteins from S. gordonii were separated by SDS-PAGE, washed, incubated in sucrose to allow in situ synthesis of glucan, and stained with Schiff’s reagent. CH1, DS512, and CH107 are the parental S. gordonii strains and CH1(gbpA), DS512(gbpA), and CH107(gbpA) are the corresponding derivatives that express GbpA. The faster migrating bands for strains CH107 and CH107(gbpA) are due to an internal deletion within the glucosyltransferase enzyme that results in a smaller molecular weight protein that synthesizes water-soluble glucan. Relative levels of activity between the strains were similar for supernatant and cell activities (data not shown).
It was also important to confirm that the glucan synthesizing capacity of the strains was maintained in the presence of GbpA. An activity gel demonstrating similar glucan synthesis, for each strain pair (with and without GbpA), corresponding to the glucosyltransferase bands is shown in Figure 1B. The DS512 strains have an interrupted rgg gene resulting in reduced transcription of the downstream gtfG gene (Sulavik et al., 1992). The expected >90% decrease in glucan synthesis in the DS512 strains relative to the CH1 strains was evident. The CH107 strains harbor a 585-bp internal deletion within the glucan binding domain of gtfG that encodes a GtfG that synthesizes α-1,6-linked water-soluble glucans and has reduced enzymatic activity (Vickerman et al., 1996). The faster migrating 152 kDa GTF band is noticeable on the gel as is the relative reduction in the intensity of the glucan band. However, the intensity of the CH107 glucan bands was likely also diminished by the water solubility of the glucans formed making it appear that the strain synthesized less glucan than it does. The amounts of glucan associated with CH107 biofilms were more similar to CH1 biofilms than DS512 biofilms. In all the S. gordonii strains the GTF activity was not affected by the presence of pVA749:gbpA.
In the absence of glucan S. gordonii is capable of efficient adhesion to hydroxyapatite that has been coated with salivary proteins. The presence of GbpA did not enhance or inhibit adhesion to saliva-coated hydroxyapatite beads (data not shown).
Since some glucan-binding proteins, notably GbpC of S. mutans and the GBL of Streptococcus sobrinus, facilitate dextran-dependent aggregation the GbpA-producing strains of S. gordonii were compared to the parental strains for this property. Neither the plasmid-free strains nor those carrying plasmid-borne GbpA demonstrated significant dextran-dependent aggregation.
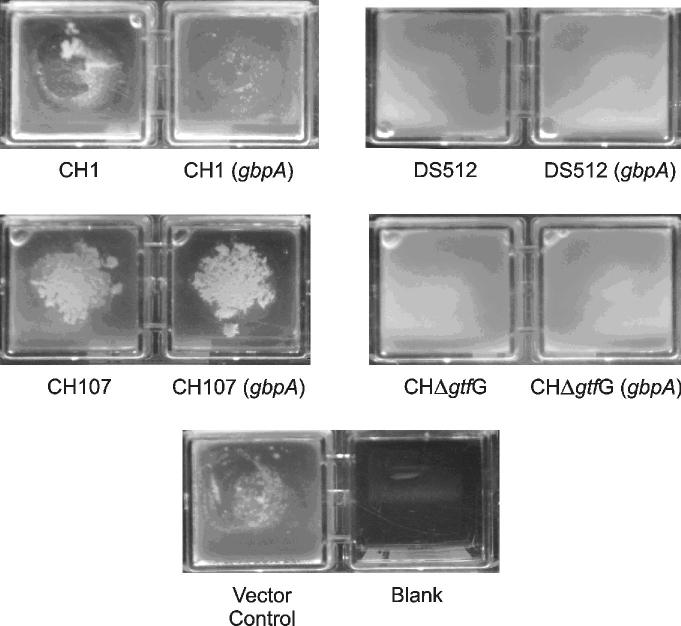
The lack of an effect by GbpA on sucrose-independent adhesion and dextran-dependent aggregation was not unexpected. In S. mutans, GbpA appears to make its most significant contribution to biofilm architecture when grown in the presence of sucrose. Therefore, this was tested next by comparing two-day biofilms formed by the S. gordonii parental strains and the cognate strains secreting GbpA. Unlike S. mutans, where an obvious macroscopic difference in the biofilm was visible between wild-type and a GbpA- strain (Hazlett et al., 1999), there were no macroscopic differences in the biofilms between S. gordonii strains with or without GbpA as evident in Figure 2. However, Figure 2 also shows that there were profound differences between the various parental strains that paralleled the differences in glucan synthesis noted above. Both the CH1 and CH107 strains formed rougher, more elevated biofilms compared to DS512. The planktonic bacteria in the CH107 wells formed large aggregates while the DS512 strain appeared colloidal in the planktonic phase. A strain with a deleted gtfG gene was also investigated as a control for pleiotropic effects on biofilm formation that might accompany the inactivation of rgg in strain DS512. However, the biofilms formed by the DS512 and CHΔgtfG strains appeared macroscopically identical. The vector control biofilm was similar to the CH1 wild-type as expected. The blank control ensured the absence of contamination in the media used to propagate the bacteria.
Fig 2.
Macroscopic views of the microtiter dish wells containing planktonic phase bacteria and biofilms formed by the panel of S. gordonii strains. The wells display two days growth in CDM with 1% sucrose. The medium was changed after 24 hrs. CH1, DS512, CH107 and CHΔgtfG were the parental S. gordonii strains and CH1(gbpA), DS512(gbpA), CH107(gbpA), and CHΔgtfG(gbpA) were the corresponding derivatives that expressed GbpA. The vector control was CH1 with the plasmid pVA749 without the gbpA. The blank well was a negative control to monitor potential contamination of the medium.
In order to determine if GbpA contributed differences to the biofilms at the microscopic level the biofilms were imaged by confocal scanning laser microscopy and analyzed using the biofilm analysis program COMSTAT (Heydorn et al, 2000). The results, shown in Table 2 and Figure 3, indicate that GbpA does make a difference and it depends on the quality and quantity of glucan available. The strains capable of synthesizing significant quantities of glucan were CH1 and CH107, though CH107 produces less than CH1. When GbpA was expressed in those strains there was an increase in biomass that was statistically significant for CH1(gbpA) and at the threshold of significance for CH107(gbpA). For CH107(gbpA) this increase was likely due to the formation of significantly taller microcolonies. The surface to volume ratio decreased significantly in the biofilm formed by the CH1(gbpA) strain. There was a similar trend for CH107(gbpA) but it did not reach statistical significance. The biofilm formed by the CH107(gbpA) strain had a significantly higher roughness coefficient. GbpA did not affect the biofilm formed by DS512. When examined microscopically the CHΔgtfG biofilm did not remain completely still. Perhaps the total absence of glucan resulted in enhanced Brownian motion that blurred the images obtained by confocal microscopy which in turn negated the ability to perform COMSTAT analysis.
Table 2.
COMSTAT Analysis of Biofilms Formed by Streptococcus gordonii With or Without GbpA*
| Strain | Biomass | %Coverage at Substratum | Average Thickness | Roughness Coefficient# | Surface/Volume |
|---|---|---|---|---|---|
| CH1 | 6.7 +/- 0.4 | 56.4 +/- 2.6 | 8.8 +/- 0.6 | 0.75 +/- 0.03 | 2.2 +/- 0.1 |
| CH1(gbpA) | 8.7 +/- 0.9 | 67.3 +/- 5.8 | 10.5 +/- 1.0 | 0.64 +/- 0.09 | 1.8 +/- 0.1 |
| Student t-test result | p<0.05 | NS | NS | NS | p<0.05 |
| CH107 | 11.1 +/- 0.9 | 86.9 +/- 2.0 | 11.5 +/- 0.9 | 0.40 +/- 0.02 | 1.3 +/- 0.1 |
| CH107(gbpA) | 13.7 +/- 0.9 | 85.6 +/- 1.9 | 14.3 +/- 0.9 | 0.49 +/- 0.03 | 1.0 +/- 0.1 |
| Student t-test result | p=0.05 | NS | p<0.05 | p<0.05 | NS |
| DS512 | 1.2 +/- 0.2 | 20.7 +/- 1.8 | 1.5 +/- 0.4 | 1.31 +/- 0.06 | 3.3 +/- 0.3 |
| DS512(gbpA) | 1.1 +/- 0.4 | 21.9 +/- 5.8 | 1.1 +/- 0.4 | 1.43 +/- 0.13 | 3.3 +/- 0.2 |
| Student t-test result | NS | NS | NS | NS | NS |
The values in each column represent the mean plus or minus the standard error of the mean.
The roughness coefficient is a measure of the variation in biofilm thickness (Heydorn et al., 2000).
NS = Not significant
Fig 3.
Representative confocal microscopic views of the respective biofilms at 1μm above the substratum. The bacteria have been stained with the fluorescent dye SYTO9 and appear green. The lines through the images represent the planes for the x-z and y-z cross-sectional views that appear at the top and right hand margins of the images respectively. CH1, CH107, and DS512 are the parental S. gordonii strains and CH1(gbpA), CH107(gbpA), and DS512(gbpA) are the corresponding derivatives that express GbpA. The scale bar is 100 μm.
The rationale behind using a variety of parental strains was supported by the fact that for biomass, percent coverage at the substratum, average thickness, and roughness coefficient, the strains differed significantly from one another (except for CH1 vs. CH107 in average thickness and CH1(gbpA) vs. CH107(gbpA) in roughness coefficient).
An attempt was made to ascertain the effect that exogenously supplied glucan from S. mutans would have on the initial stages of biofilm formation by S. gordonii CH1 or CH1(gbpA). Strains CH1 or CH1(gbpA) were grown in microtiter dish wells in which was placed a circular glass coverslip. A transwell chamber containing a GbpA- strain of S. mutans was also placed into the well presumably allowing diffusion of S. mutans glucosyltransferases. Controls using S. mutans in the transwell and sterile medium in the microtiter dish confirmed the lack of diffusion of the bacterial cells. Analysis of the coverslips after six hours indicated that the CH1(gbpA) strain was almost twice as likely (66% of the time) to be heavily colonized as the parental CH1 strain (38% of the time). This effect was not evident if the transwell contained S. gordonii or sterile medium.
Discussion
The GBPs of S. mutans display heterogeneous functions (Banas & Vickerman, 2003), but it is also possible that they make overlapping contributions, particularly in the realm of sucrose-dependent biofilm formation. If overlapping, the full phenotypic significance of a particular GBP might not be evident upon inactivation of its corresponding gene within its native host. The rationale for expressing gbpA within S. gordonii was to isolate it from the other S. mutans GBPs. Though it is conceivable thatS. gordonii harbors endogenous GBPs none have been described in the literature.
Using this strategy it was hypothesized that the addition of GbpA would alter biofilms formed by S. gordonii to an extent that was at least comparable to that seen when comparing biofilms formed by wild-type and GbpA- strains of S. mutans. However, this was not the case. The loss of GbpA in S. mutans led to both macroscopic and microscopic changes in biofilm architecture (Hazlett et al., 1999). The addition of GbpA to S. gordonii resulted in more subtle differences that were evident only at the microscopic level. This difference in the magnitude of the biofilm change may indicate that GbpA has another as yet unknown function. But a simpler, more likely explanation is that the overall quantity of glucan is as important for visualizing the effects of GbpA as isolating it from other GBPs.
The results also underscore the importance of the glucan profile produced by the host organism. The addition of GbpA increased the biomass of biofilms formed by either CH1(gbpA) or CH107(gbpA). In CH107(gbpA) the increase could be explained by a significant increase in the average thickness of the biofilms. For CH1(gbpA) there were trends toward increases in both substratum coverage and biofilm thickness but neither attained statistical significance. In its native host, the loss of GbpA increases substratum coverage but reduces thickness (Hazlett et al., 1999). Since in its native host the mass of the biofilm is maintained, with or without GbpA, it follows that the loss of ability to elevate the biofilm will lead to an increase in substratum coverage. When gbpA is expressed in S. gordonii it appears that it aids in elevating the biofilm but with a concomitant increase in biomass that may include modification of substratum coverage depending on the proportion of water-soluble and -insoluble glucan. GbpA has the highest affinity for α-1,6-linked glucose that predominates in water-soluble glucan (Russell, 1979; Haas & Banas, 2000). Since sticking to smooth surfaces is primarily attributed to water-insoluble glucan, the binding of GbpA to α-1,6-linkages within the mostly α-1,3-linked water-insoluble glucan molecules may promote the accumulation of CH1(gbpA) biomass via proportional increases in biofilm height and coverage. For CH107(gbpA), which synthesized only α-1,6-linked glucan, the binding of GbpA appeared to preferentially promote biofilm elevation. The ability of GbpA to contribute to biofilm elevation has now been supported by two studies; the addition of GbpA to S. gordonii led to an increase in elevation (this study) and its loss in S. mutans led to a reduction in biofilm height (Hazlett et al., 1999).
It can also be speculated that water-soluble or -insoluble glucan synthesized by different GTFs may not function equivalently when interacting with glucan-binding proteins due to differences in minor linkage configurations, length of the polymer, or distribution of branch points. In this study glucan synthesized by native host enzymes more profoundly enhanced the initial adhesion and accumulation of S. gordonii in the presence of GbpA than in its absence. It is possible that this effect was mediated by increased glucan quantity, the ratio of water-soluble and -insoluble glucan, or subtle differences that conferred unique specificity to the S. mutans glucans.
The surface to volume ratio was significantly reduced in the CH1(gbpA) strain. The trend when comparing all the strains was that increased glucan, particularly water-soluble glucan, correlated with the lowest surface to volume ratios. The lower ratios are consistent with increased elevation of microcolonies within the biofilm. The lower ratios are also associated with nutrient-rich environments that are likely to exist when sucrose is present.
Strains DS512 and CHΔgtfG, which synthesize little or no glucan respectively, were unaffected by the presence of GbpA. These results were consistent with the expectation that any effect due to GbpA would require the presence of a glucan ligand. The total lack of glucan in strain CHΔgtfG appeared to affect biofilm architecture making it susceptible to slight movement that interfered with the ability to obtain precise confocal microscopic images. The remaining GTF activity in DS512, approximately 3% of the parental CH1 level, seemed to be sufficient to overcome this difficulty. This observation may highlight the stabilizing effect that glucan has on a streptococcal biofilm. Nevertheless, since rgg regulators have been implicated as playing global roles in Streptococcus pyogenes gene expression (Chaussee et al., 2002), and microarray analysis suggests that gtfG-independent genes are differentially expressed in strains DS512 and CHΔgtfG (Gill et al., 2005), we cannot rule out the possibility that the biofilm integrity seen in strain DS512 is due to an effect of rgg on a gene other than gtfG.
In summary, the expression of GbpA in a heterologous host, S. gordonii, led to changes in biofilm synthesis that mimicked those found in its native host, S. mutans. The magnitudes of the changes were less in S. gordonii suggesting that the maximal contribution of GbpA requires larger amounts of exogenous glucan or host-specific glucan properties. It is recognized that these are monospecies biofilms and caution must be exercised in drawing conclusions regarding the role of GbpA in dental plaque. However, it is conceivable that localized aggregates of predominantly S. mutans exist within carious lesions and rely on GBPs to optimally shape the biofilm architecture.
Acknowledgements
This work was supported by grants DE10058 (J.A.B.) and DE11090 (M.M.V.) from the National Institute of Dental and Craniofacial Sciences and RR017926 (J.E.M.). We thank Dr. Lisa Petti (Albany Medical College) for assistance with ECL.
References
- Banas JA, Vickerman MM. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 2003;14:89–99. doi: 10.1177/154411130301400203. [DOI] [PubMed] [Google Scholar]
- Banas JA, Russell RRB, Ferretti JJ. Sequence analysis of the gene for the glucan-binding protein of Streptococcus mutans Ingbritt. Infect. Immun. 1990;58:667–673. doi: 10.1128/iai.58.3.667-673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussee MS, Sylva GL, Sturdevant DE, Smoot LM, Graham MR, Watson RO, Musser JM. Rgg influences the expression of multiple regulatory loci to coregulate virulence factor expression in Streptococcus pyogenes. Infect. Immun. 2002;70:762–770. doi: 10.1128/iai.70.2.762-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia J-S, Chang LY, Shun C-T, Chang Y-Y, Chen J-Y. A 60-kilodalton immunodominant glycoprotein is essential for cell wall integrity and the maintenance of cell shape in Streptococcus mutans. Infect. Immun. 2001;69:6987–6998. doi: 10.1128/IAI.69.11.6987-6998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepan S, Shah H, Russell RRB. A novel glucan-binding protein with lipase activity from the oral pathogen Streptococcus mutans. Microbiology. 2004;150:1947–1956. doi: 10.1099/mic.0.26955-0. [DOI] [PubMed] [Google Scholar]
- Frey J, Krisch HM. ω mutagenesis in gram-negative bacteria: a selectable interposon which is strongly polar in a wide range of bacterial species. Gene. 1985;36:143–150. doi: 10.1016/0378-1119(85)90078-2. [DOI] [PubMed] [Google Scholar]
- Gill SR, Iobst S, Remortel B, Vickerman MM. Transciptome analysis of Streptococcus gordonii rgg and gtfG mutants. J. Dent. Res. 2005;84(Spec Iss B) Abstract #325. [Google Scholar]
- Grahame DA, Mayer RM. Purification and comparison of two forms of dextransucrase from Streptococcus sanguis. Carbohydr. Res. 1985;142:285–298. doi: 10.1016/0008-6215(85)85030-8. [DOI] [PubMed] [Google Scholar]
- Haas W, Banas JA. Ligand-binding properties of the carboxyl terminal repeat domain of the Streptococcus mutans glucan-binding protein-A. J. Bacteriol. 2000;182:728–733. doi: 10.1128/jb.182.3.728-733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haisman RJ, Jenkinson HF. Mutants of Streptococcus gordonii Challis overproducing glucosyltransferase. J. Gen. Microbiol. 1991;137:483–489. doi: 10.1099/00221287-137-3-483. [DOI] [PubMed] [Google Scholar]
- Hazlett KRO, Michalek SM, Banas JA. Inactivation of the gbpA gene of Streptococcus mutans increases virulence and promotes in vivo accumulation of recombinations between the glucosyltransferase B and C genes. Infect. Immun. 1998;66:2180–2185. doi: 10.1128/iai.66.5.2180-2185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett KRO, Mazurkiewicz JE, Banas JA. Inactivation of the gbpA gene of Streptococcus mutans alters structural and functional aspects of plaque biofilm which are compensated by recombination of the gtfB and gtfC genes. Infect. Immun. 1999;67:3909–3914. doi: 10.1128/iai.67.8.3909-3914.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Korber DR, Lawrence JR, Hendry MJ, Caldwell DE. Analysis of spatial variability within Mot+ and Mot-Pseudomonas fluorescens bioflms using representative elements. Biofouling. 1993;7:339–358. [Google Scholar]
- Ma Y, Lassiter MO, Banas JA, Galperin MY, Taylor KG, Doyle RJ. Multiple glucan-binding proteins of Streptococcus sobrinus. J. Bacteriol. 1996;178:1572–1577. doi: 10.1128/jb.178.6.1572-1577.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina FL, Tobian JA, Jones KR, Evans RP. Molecular cloning in streptococci. In: Hollaender A, DeMoss R, Kaplan S, Konisky J, Savage D, Wolfe R, editors. Genetic engineering of microorganisms for chemicals. Plenum Publishing Corp.; New York, NY: 1981. pp. 195–210. [Google Scholar]
- Matsumura M, Izumi T, Matsumoto M, Tsuji M, Fujiwara T, Ooshima T. The role of glucan-binding proteins in the cariogenicity of Streptococcus mutans. Microbiol. Immunol. 2003;47:213–215. doi: 10.1111/j.1348-0421.2003.tb03389.x. [DOI] [PubMed] [Google Scholar]
- Munro C, Michalek SM, Macrina FL. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect. Immun. 1991;59:2316–2323. doi: 10.1128/iai.59.7.2316-2323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RRB. Glucan-binding proteins of Streptococcus mutans serotype c. J. Gen. Microbiol. 1979;112:197–201. doi: 10.1099/00221287-112-1-197. [DOI] [PubMed] [Google Scholar]
- Sato Y, Yamamoto Y, Kizaki H. Cloning and sequence analysis of the gbpC gene encoding a novel glucan-binding protein of Streptococcus mutans. Infect. Immun. 1997;65:668–675. doi: 10.1128/iai.65.2.668-675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Akita H, King WF, Taubman MA. Purification and antigenicity of a novel glucan-binding protein of Streptococcus mutans. Infect. Immun. 1994;62:245–2552. doi: 10.1128/iai.62.6.2545-2552.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulavik MC, Tardiff G, Clewell DB. Identification of a gene, rgg, which regulates expression of glucosyltransferase and influences the Spp phenotype of Streptococcus gordonii Challis. J. Bacteriol. 1992;174:3577–3586. doi: 10.1128/jb.174.11.3577-3586.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman MM, Heath DG, Clewell DB. Construction of recombination-deficient strains of Streptococcus gordonii by disruption of the recA gene. J. Bacteriol. 1993;175:6354–6357. doi: 10.1128/jb.175.19.6354-6357.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman MM, Sulavik MC, Minick PE, Clewell DB. Changes in the carboxyl-terminal repeat region affect extracellular activity and glucan products of Streptococcus gordonii glucosyltransferase. Infect. Immun. 1996;64:5117–5128. doi: 10.1128/iai.64.12.5117-5128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect. Immun. 1993;61:3811–3817. doi: 10.1128/iai.61.9.3811-3817.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]