Abstract
Bone marrow-derived cells are known to play important roles in repair/regeneration of injured tissues, but their roles in pathological fibrosis are less clear. Here, we report a critical role for the chemokine receptor CCR2 in the recruitment and activation of lung fibrocytes (CD45+, CD13+, collagen 1+, CD34−). Lung fibrocytes were isolated in significantly greater numbers from airspaces of fluorescein isothiocyanate-injured CCR2+/+ mice than from CCR2−/− mice. Transplant of CCR2+/+ bone marrow into CCR2−/− recipients restored recruitment of lung fibrocytes and susceptibility to fibrosis. Ex vivo PKH-26-labeled CCR2+/+ lung fibrocytes also migrated to injured airspaces of CCR2−/− recipients in vivo. Isolated lung fibrocytes expressed CCR2 and migrated to CCL2, and CCL2 stimulated collagen secretion by lung fibrocytes. Fibrocytes could transition into fibroblasts in vitro, and this transition was associated with loss of CCR2 expression and enhanced production of collagen 1. This is the first report describing expression of CCR2 on lung fibrocytes and demonstrating that CCR2 regulates both recruitment and activation of these cells after respiratory injury.
Pulmonary fibrosis is characterized by alveolar epithelial cell injury, hyperplasia, inflammatory cell accumulation, fibroblast proliferation, and deposition of extracellular matrix.1–6 In animals, the disease process can be modeled via the intratracheal administration of fluorescein isothiocyanate (FITC).7,8 Soluble monocyte chemoattractant protein-1 (CCL2) levels in the bronchoalveolar lavage (BAL) fluid peak by day 1 after FITC and remain elevated until day 7;8 matrix-bound CCL2 is bioactive and evident through day 21 after FITC. CC chemokine receptor 2 (CCR2) is the high-affinity receptor for CCL2.9,10 We previously demonstrated that mice that are deficient in CCR2 (CCR2−/− mice) are protected from the development of experimental pulmonary fibrosis induced either by FITC or bleomycin.8 However, recruitment of classical inflammatory cells (monocytes, macrophages, eosinophils, neutrophils, T, B, or NK cells) to the lung in response to FITC was not diminished when CCR2−/− mice were compared to CCR2+/+ mice.8 Because altered recruitment of classical inflammatory cells was not noted, we determined whether CCR2 played a role in mesenchymal cell recruitment to the lung in response to injury.
A circulating population of cells (termed fibrocytes) that share leukocyte (CD45, CD34, CD13) and mesenchymal markers [collagen 1 (col 1), fibronectin] has been described.11–13 Fibrocytes cultured from peripheral blood migrate to skin wound chambers13 and bronchial mucosa after antigen challenge.14 The findings that fibrocytes can differentiate into myofibroblasts,14,15 and the fact that fibrocytes are present in fibrosing conditions such as asthma,14 nephrogenic fibrosing dermopathy,16 and hypertrophic scarring15 suggest a potentially pathogenic role for this cell type. We hypothesized that CCR2 is a critical regulator of fibrocyte recruitment and activation and that fibrocytes are mediators of lung fibrosis.
Materials and Methods
Mice
C57BL/6 and B6/129F2 mice were from Jackson Laboratories (Bar Harbor, ME). CCR2−/− mice on the C57BL/6 or B6/129F2 background were bred in the University of Michigan Laboratory Animal Medicine facilities under SPF conditions and have been described previously.17 Mice were used at 6 to 8 weeks of age. The University Committee on the Use and Care of Animals approved these experiments.
FITC Inoculation
FITC inoculation was performed as previously described.8 Briefly, mice were anesthetized with sodium pentobarbital. The trachea was exposed and entered with a needle under direct visualization. FITC (21 mg, no. F-7250; Sigma, St. Louis, MO) was dissolved in 10 ml of sterile phosphate-buffered saline (PBS), vortexed extensively, and sonicated for 30 seconds. This slurry was transferred to multiuse vials and vortexed extensively before each 50-μl aliquot was removed for intratracheal injection using a 23-gauge needle.
Bronchoalveolar Lavage
Mice were euthanized by CO2 asphyxiation and the trachea was exposed in a sterile manner. The trachea was cannulated with polyethylene tubing (PE50, Intramedic; Clay Adams, Parsippany, NJ) attached to a 25-guage needle on a tuberculin syringe, and the lungs were lavaged three times with 0.75 ml of sterile 1× PBS. The lavage fluid from a single mouse was combined, spun at 500 × g, and the supernatant removed. The cell pellet was resuspended in complete media and cultured for 10 to 14 days to allow mesenchymal cells to expand before analysis.
Mesenchymal Cell Isolation from Whole Lungs
Murine lungs were perfused with 5 ml of normal saline and removed using aseptic conditions. Lungs were minced with scissors in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum. Lungs from a single animal were placed in 10 ml of media in 100-cm2 tissue culture plates. Mesenchymal cells were allowed to grow out of the minced tissue, and when cells reached 70% confluence they were passaged using trypsin digestion. Fibroblasts were grown for 10 to 14 days (two to three passages) before being used and were always used at or before passage 3.
Immunohistochemical (IHC) Staining
Cells fixed for 10 minutes with 10% neutral buffered formalin were analyzed by IHC using CD45-PE or CD13-PE (BD Pharmingen, San Diego, CA), and col 1 (rabbit anti-mouse antisera; Accurate Chemical, Westbury, NY) with PE- (BD Pharmingen) or AMCA-coupled secondary reagents (Vector, Burlingame, CA). For CCR2 IHC, fixed cells were first treated with 1% trypsin before anti-CCR2 Abs (Santa Cruz Biotechnology, Santa Cruz, CA) were detected with a Vector alkaline phosphatase kit. Images were visualized on a Nikon Eclipse E600 microscope (Melville, NY) equipped for epifluorescence with appropriate filters. Images were captured on a digital SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI).
Magnetic Separation
Lung mesenchymal cells were grown from lung minces for a period of 10 to 14 days. The trypsinized cells were stained with anti-CD45 Abs coupled to magnetic beads (Miltenyi Biotech, Auburn, CA). Labeled cells were then sorted by binding the cell population to MS- or LS-positive selection columns using a SuperMacs apparatus (Miltenyi Biotech) according to the manufacturer’s instructions. Cells are then washed extensively. CD45+ cells are retained on the column and can be removed by flushing the column with buffer once it is removed from the magnetic field. CD45− cells are collected in the original flow through. For extra purity, CD45+ cells were sometimes reapplied to a second LS-positive selection column. The absolute number of lung fibrocytes is determined by counting the cells that were retained on the column by a hemocytometer. IHC staining or flow cytometry staining on this population confirmed that these cells were CD45+, CD13+, and col 1+.
Flow Cytometry Analysis
For Figure 2b, cells were incubated for 15 minutes on ice with Fc block (BD Pharmingen, San Diego, CA) before surface staining with CD45-PE (BD Pharmingen). Cells were then washed and fixed/permeabilized using the Cytofix/Cytoperm kit from BD Pharmingen and stained for col 1 (rabbit anti-mouse col 1, Accurate) followed by a donkey anti-rabbit Cy5-coupled secondary (Research Diagnostics, Inc., Flanders, NJ). For Figure 2d, CD45+ cells were first purified from lung mince cultures on a magnet as described above. Then CD45+ cells were stained with CD45-PE or CD34-PE (clone RAM34, BD Pharmingen) followed by fixation/permeabilization and staining with col 1 (rabbit anti-mouse, Accurate) followed by a goat anti-rabbit FITC-secondary (Pharmingen). Cells were analyzed on the flow cytometer (FACScan; BD Biosciences, Mountain View, CA).
Figure 2.
Lung mince cultures contain fibrocytes. a: Lung minces from unchallenged C57BL/6 mice were cultured for 14 days and stained for expression of col 1, CD45, or CD13. The negative control was a mixture of rat and rabbit irrelevant Ig. b: Flow cytometry analysis of lung mince cultures stained for surface expression of CD45 using a directly conjugated PE Ab and intracellular col I (rabbit anti-mouse) recognized with a Cy5-conjugated donkey anti-rabbit secondary Ab. c: RT-PCR analysis of extracellular matrix gene expression between fibrocytes and fibroblasts. CD45+ fibrocytes and CD45− fibroblasts were purified from lung mince cultures via magnetic separation. Total RNA was prepared from each group of cells and RT-PCR performed for col I, col III, and fibronectin gene expression. d: Flow cytometry analysis of magnetically sorted CD45+ fibrocytes from C57BL/6 lung mince cultures pooled from three mice. After a single round of magnetic purification, fibrocytes were stained with CD45-PE (B), col 1 and donkey anti-rabbit FITC secondary (C), or with Col 1-FITC and CD34PE (D). All panels are representative of at least three separate experiments.
Chemotaxis
Lung mince cultures were serum-starved for 24 hours before magnetic purification of CD45+ and CD45− cells. Chemotaxis on cells at 1 × 106/ml was performed in Boyden chambers to recombinant proteins (fibronectin at 100 μg/ml; Sigma) or CCL2 (50 ng/ml; R&D Systems, Minneapolis, MN) through gelatin-coated 5- to 8-μm filters. Checkerboard analysis proved the migration was directional.
Bone Marrow Transplantation (BMT)
The protocol for syngeneic BMT has been previously described.18,19 Recipient mice received 13 Gy of total body irradiation (137Cs source) delivered in two fractions separated by 3 hours.19 Five million bone marrow cells and 1 × 106 nylon wool-purified T cells were resuspended in Leibovitz’s L-15 medium (Life Technologies, Grand Island, NY) and transplanted intravenously (0.25 ml total volume). After transplantation, mice were housed in sterilized microisolator cages and were fed normal chow and autoclaved hyperchlorinated water for the first 2 weeks after BMT.
Lung Collagen Measurements
Total lung collagen levels were determined by harvesting lungs from mice on day 21 after FITC or saline administration. Animals were euthanized, and perfused with 3 ml of normal saline before all five lung lobes were removed and snap-frozen in liquid nitrogen. Before analysis, lungs were homogenized in 1 ml of PBS, and hydrolyzed by the addition of 1 ml of 12 N hydrochloric acid (HCl). Samples were then baked at 110°C for 12 hours. Aliquots (5 μl) were then assayed by adding chloramine T solution for 20 minutes followed by development with Erlich’s reagent at 65°C for 15 minutes as previously described.8 Absorbance was measured at 550 nm, and the amount of hydroxyproline was determined against a standard curve generated using known concentrations of hydroxyproline standard (Sigma).
Proliferation Assays
CCR2+/+ or CCR2−/− mice were injected with saline or FITC on day 0. On day 7, lungs were removed, minced, and cultured for 14 days. At this time, CD45+ fibrocytes and CD45− fibroblasts were isolated via magnetic sorting and total numbers of each cell type were enumerated. Five thousand cells of each cell type were plated in 96-well flat-bottomed tissue culture dishes in complete media containing 10% fetal calf serum and antibiotics. Cells were cultured for 24, 48, or 72 hours before the addition of 10 μCi of 3H-thymidine for an additional 16 hours. Cells were harvested onto glass fiber filters using an automated cell harvester and filters were counted using a β-scintillation counter.
PKH-26 Labeling of Fibrocytes
Fibrocytes were purified from lung mince cultures by magnetic separation and labeled ex vivo with PKH-26 (Sigma) according to the manufacturer’s instructions. Cells were washed extensively and reinfused (5 × 105) via tail vein into mice on day 4 after FITC. PKH-26-labeled cells were visualized using fluorescent light and appropriate filters in frozen sections 24 hours after infusion.
Gene Array Analysis for Chemokine Receptors
Cellular mRNA pooled from cells from three mice was prepared using Trizol reagent (Invitrogen, Rockville, MD) according to the manufacturer’s instructions. Total mRNA was converted to labeled cDNA using the SuperArray AmpoLabeling-LPR kit and hybridized to GEArray Q series mouse chemokines and receptors gene array according to the manufacturer’s instructions (SuperArray Biosciences, Frederick, MD). Blots shown represent data from two to three experiments per cell type.
mRNA Analysis
Cellular mRNA was prepared using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. Gene expression for col 1, col 3, and fibronectin was determined by reverse transcriptase (RT)-polymerase chain reaction (PCR) using the Promega Access RT-PCR kit (Promega, Madison, WI) according to manufacturer’s instructions. The conditions for PCR were 95°C for 1 minute, 55°C for 1 minute, and 68°C for 1 minute. Extracellular matrix genes were amplified for 35 cycles. β-Actin was amplified for 25 cycles. Primer sequences were as follows: col 1 sense, TGGTGCCAAGGGTCTCACTGGC; col 1 anti-sense, GGACCTTGTACACCACGTTCACC; col 3 sense, GCAGTCCAACGTAGATGAATTGG; col 3 anti-sense, GAAGGCCTGGTGGACCAGCTGG; fibronectin sense, AAGTGTGATCCCCATGAAGCAACG; fibronectin anti-sense, CTCTCGAGAATCGTCTCTGTCAGC; β-actin sense, GTGGGGCTCCCCAGGCACCA; β-actin anti-sense, GCTCGGCCGTGGTGGTGAAGC.
Western Blotting
Cells were seeded (4 × 105/well) and serum-starved in 35-mm dishes. Cultures were exposed to treatments [interleukin (IL)-13 at 10 ng/ml or transforming growth factor (TGF)-β1 at 2 ng/ml or CCL2 at 10 ng/ml] all from R&D Systems for 24 hours and then were washed with ice-cold PBS and lysates were prepared, adjusted for protein concentration, and analyzed for col 1 expression by Western blot analysis as previously described.20 Blots were stripped and reprobed for β-tubulin. Digital photographs of blots were taken and the density of the bands was calculated using the Image J 1.32 program available for free download from http://rsb.info.nih.gov/ij/. The density of the collagen signal for each lane was normalized to the density of β-tubulin in each lane. The values for the serum-free conditions were normalized to 1 in each experiment and the relative density for each condition was calculated relative to serum-free media for each experiment. Four experiments were averaged together to obtain statistical significance.
Statistics
In all graphs where error bars are shown, data represent mean ± SEM. Statistical significance was analyzed using the InStat v. 3 program (Graphpad Software) for Windows on a Dell GX260 computer. Student’s t-tests were run to determine P values when comparing two groups. For three or more groups, analysis of variance was performed with a posthoc Bonferroni test. A value of P < 0.05 was considered significant.
Results
Lung Fibrocytes Can Be Isolated from the Alveolar Space of FITC-Injured Mice
Cells obtained by BAL from wild-type C57BL/6 mice on days 0, 1, 3, 5, 7, 10, 14, 17, and 21 after FITC were cultured and analyzed for the expression of the fibrocyte markers, col 1, CD45, and CD13 (Figure 1a) by IHC. Virtually all cells that could be isolated from lavages of FITC-treated mice at all time points were positive for each of these markers. The dual expression of CD45 and col 1 was confirmed in BAL fibrocytes isolated after FITC (Figure 1b). The number of fibrocytes that can be cultured from BAL increases after FITC exposure (Figure 1c) and correlates with the kinetics of CCL2 expression within the BAL after FITC injury.8
Figure 1.
Cells with both mesenchymal and leukocyte markers accumulate in the airspaces of FITC-injured mice. a: C57BL/6 mice were injected with FITC on day 0 and BAL was performed on day 3 after FITC. Lavage cells were cultured for 14 days and stained for expression of col 1, CD45, or CD13. The negative control is a mixture of rabbit and rat irrelevant Ig. Positive cells were identified with alkaline phosphatase activity (red color). Staining pattern was similar for cells isolated on all other days after FITC. b: Dual-IHC staining demonstrated the presence of both mesenchymal (col 1, stained with ACMA; blue color) and leukocyte (CD45, stained with PE; red color) markers on the same cells cultured from day 5 BAL of FITC-treated mice. Data in a and b are representative of three experiments per time point in two different genetic backgrounds. c: Absolute numbers of CD45+, col 1+ cells isolated from BAL cultures on various days after FITC (n = 5 per time point, representative of three similar experiments).
Lung Fibrocytes Can Be Isolated from Lung Mince Cultures
Approximately 15 to 25% of the mesenchymal cells that are present in 10- to 14-day cultures of lung mince cells express the fibrocyte markers CD45 and CD13 (Figure 2a). Flow cytometry analysis of lung mince cultures demonstrates the dual expression of surface CD45 and intracellular col 1 (Figure 2b) on the fibrocyte population. The col I-positive, CD45-negative population of cells in the lung mince cultures represents effector fibroblasts. The level of extracellular matrix proteins being produced by fibrocytes relative to fibroblasts is appreciably lower when analyzed either by flow cytometry for intracellular col 1 expression (Figure 2b) or by RT-PCR for the expression of col I, col III, or fibronectin genes (Figure 2c). Fibrocytes isolated from alveolar spaces or lung mince cultures are 2% positive for CD34 (Figure 2d). Fibrocytes were purified from lung mince cultures via magnetic separation on CD45 beads and stained by flow cytometry for the expression of CD45 (Figure 2d, B), col 1 (Figure 2d, C), or dual expression of col 1 and CD34 (Figure 2d, D). It should be noted that under the culture conditions used in this study, both fibrocytes and fibroblasts express α-smooth muscle actin.
Lung Fibrocytes Increase in the Interstitium in Response to FITC
Cultures of minced lungs from naïve mice contain 2.76 ± 0.8 × 106 lung fibrocytes whereas cultures from day 7 FITC-treated mice contain 4.64 ± 1.0 × 106 lung fibrocytes after the same length of culture (n = 3, P < 0.05). Thus, lung fibrocytes increase in number in the lung interstitium in response to FITC challenge.
Lung Fibrocytes Express CCR2
Interstitial lung mesenchymal cells are a mixture of lung fibrocytes and fibroblasts. Fibroblasts are col 1+, CD45−, CD13−, and CD34−. These two cell populations were purified from B6/129F2 mice by magnetic sorting for CD45 and were analyzed for chemokine receptor expression via hybridization to SuperArray gene arrays for mouse chemokines and receptors. Lung fibrocytes from this genetic background express modest levels of CXCR4 and CCR1, and high levels of CCR2, CCR5, and CCR7 (Figure 3a). CXCR4, CCR5, and CCR7 have previously been reported on human peripheral blood fibrocytes,13 however CCR2 expression has not previously been evaluated. The fibroblast population expressed only CXCR4 (not shown).
Figure 3.
Lung fibrocytes express functional CCR2 receptors and migrate to CCL2 in vitro. a: Superarray analysis of chemokine receptor gene expression in fibrocytes from B6129/F2 mice. b: CCR2 IHC on cytospins of fibrocytes (CD45+) and fibroblasts (CD45−) purified from n = 3 pooled lung minces. Red alkaline phosphatase staining represents positive staining. Only the CD45+ population expressed CCR2. Data are representative of three experiments. c: CD45+ fibrocytes and CD45− fibroblasts were purified from lung mince cultures and in vitro chemotaxis assays were performed. Fibronectin was used as a positive control for mesenchymal cell migration (n = 6 per condition and represents three similar experiments).
Lung Fibrocytes Express CCR2 and Migrate in Response to CCL2
Lung fibrocytes from B6/129F2 mice were separated from fibroblasts in lung mince cultures via two rounds of positive selection on anti-CD45-coupled magnetic beads. The postsort lung fibrocyte population was 99.5% positive for CD45, CD13, and col 1. In contrast, the fibroblast population was 99.5% positive for col 1 but negative for both CD45 and CD13 (not shown). IHC staining confirmed the presence of CCR2 protein on the lung fibrocytes, but not fibroblasts (Figure 3b). Purified lung fibrocytes migrated in response to CCL2 in vitro whereas the fibroblast population did not (Figure 3c). Both lung fibrocytes and fibroblasts were able to migrate in response to the potent mesenchymal cell chemoattractant fibronectin.
CCR2−/− Mice Are Protected from FITC-Induced Fibrosis on Two Genetic Backgrounds
CCR2−/− mice are protected from FITC- and bleomycin-induced pulmonary fibrosis on the B6/129F2 background.8 CCR2−/− mice are also protected on the C57BL/6 background. C57BL/6 mice accumulate 7.3 ± 0.5 μg/ml hydroxyproline (collagen surrogate) per g of body weight in their lungs after FITC challenge compared to 4.2 ± 0.77 μg/ml hydroxyproline per g of body weight in the B6/CCR2−/− mice (n = 8, P < 0.05). Interestingly, the overall magnitude of the fibrotic response to FITC in the C57BL/6 background is higher than previously noted in the B6/129F2 background.8
Fibrocyte Chemokine Receptor Expression Profiles Do Not Change in Response to FITC
To compare the chemokine receptor profile on fibrocytes purified from both genetic backgrounds in which CCR2−/− mice are protected, we analyzed the chemokine receptor profile of fibrocytes purified via magnetic selection from lung mince cultures of saline- or FITC-treated C57BL/6 mice. Figure 4 demonstrates that fibrocytes from C57BL/6 mice express CCR1, CCR2, CCR5, and CCR7 as did fibrocytes from B6/129F2 mice (Figure 3a). There were some notable differences however. Fibrocytes from C57BL/6 mice also expressed low levels of CCR3 and had reduced expression of both CXCR4 and CCR7 compared to the fibrocytes from the B6/129F2 mice. Of note, the chemokine receptor profile of fibrocytes purified from FITC-treated mice was not different from the profile seen on fibrocytes from saline-treated mice on either genetic background. Thus, fibrocytes from two different genetic backgrounds express CCR2 and the chemokine receptor profile does not change in response to FITC.
Figure 4.
Fibrocyte chemokine receptor profiles do not change in response to FITC. Fibrocytes were purified via magnetic sorting from day 14 lung mince cultures from saline- or FITC-treated C57BL/6 mice. Receptor profiles did not change after FITC administration. Blots shown represent RNA pooled from three mice and were repeated two times in this background strain. Similarly, the profile of chemokine receptors seen on fibrocytes purified from B6129/F2 mice were not different from the profile seen in Figure 3a (not shown). RPL13A is ribosomal protein 13A.
Lung Fibrocytes Are Dependent on CCR2 Expression for Migration to Injured Airspaces in Vivo
Wild-type and CCR2−/− mice on both the C57BL/6 or B6/129F2 backgrounds were injected with FITC on day 0. On day 4 after FITC, BAL cell pellets were cultured and analyzed for the dual expression of col 1 and CD45. The absolute number of lung fibrocytes present in cultures from injured airspaces of wild-type mice were significantly greater than noted in cultures from CCR2−/− mice (Figure 5a). The absolute number of lung fibrocytes in C57BL/6 mice was higher than in B6/129F2 mice correlating with differences observed in the magnitude of the fibrotic response to FITC between these strains. Accumulation of lung fibrocytes in injured airspaces is dependent on expression of the CCL2 receptor, CCR2.
Figure 5.
Lung fibrocytes migrate to injured airspaces of CCR2+/+ mice in greater numbers than in CCR2−/− mice, and these differences do not reflect proliferative changes in culture. a: C57BL/6, B6/129F2, or CCR2−/− mice on either background were injected with FITC on day 0. On day 4 after FITC, BAL was performed and cell pellets were cultured and analyzed for dual expression of CD45 and col 1. Data represent n = 4 mice per group and are representative of three separate experiments. b: Fibrocytes and fibroblasts were purified from pooled lung mince cultures of day 7 FITC-treated CCR2+/+ or CCR2−/− mice. Equal numbers of cells were plated and cultured for 48 hours before the addition of 3H-thymidine for a final 16 hours. Although fibrocytes are less proliferative than fibroblasts, no differences were seen in the proliferative capacity of these cells from CCR2+/+ or CCR2−/− mice at any time point tested (24 to 72 hours, n = 6). Representative of three separate experiments.
Fibrocytes from CCR2+/+ and CCR2−/− Mice Proliferate at Similar Rates in Vitro
To be certain that differences in fibrocyte numbers noted in BAL cell cultures were not because of proliferative differences between CCR2+/+ and CCR2−/− cells, we purified fibrocytes via CD45 magnetic selection and fibroblasts from lung mince cultures of CCR2+/+ or CCR2−/− mice treated with saline or FITC. Purified fibrocytes and fibroblasts were cultured for 24 to 72 hours and proliferation was assessed. Figure 5b shows that the proliferation of CCR2+/+ and CCR2−/− fibrocytes and fibroblasts were similar after 48 hours of culture. No differences were noted in the proliferation of the CCR2+/+ or CCR2−/− cells at any time point tested from either FITC- or saline-treated animals. Furthermore, the addition of CCL2 to cells in culture did not alter proliferation (not shown). Thus the differences in fibrocyte accumulation in the BAL of CCR2+/+ and CCR2−/− mice treated with FITC likely reflect differences in recruitment, and not expansion in culture.
CCR2+/+ BMT into CCR2−/− Mice Restores Lung Fibrocyte Recruitment to Injured Airspaces
Bone marrow-derived fibroblasts can be incorporated into fibrotic lung lesions.21,22 CCR2−/− mice were lethally irradiated and reconstituted with a BMT from CCR2+/+ mice. Mice were allowed to recover from the BMT for at least 7 weeks. At 7 weeks, macrophages purified from the peripheral blood, spleen, and BAL were donor-derived (CCR2-positive, not shown). CCR2+/+ mice, CCR2−/− mice, or CCR2−/− mice that had received a CCR2+/+ BMT (CCR2+/+ BMT) were injected with FITC and BAL was performed on day 4 after FITC. BAL cell pellets were cultured and cells that dually expressed CD45 and col 1 were enumerated (Figure 6a). Recruitment of lung fibrocytes in response to FITC was restored in the CCR2+/+ BMT mice. Lung fibrocytes cultured from the CCR2+/+ BMT mice were positive for CCR2 (not shown) indicating donor origin.
Figure 6.
CCR2+/+ BMT into CCR2−/− mice restores lung fibrocyte recruitment and fibrotic susceptibility. a: Wild-type B6/129F2 CCR2+/+, CCR2−/−, or CCR2−/− mice that had received a BMT with CCR2+/+ bone marrow were injected with FITC. BAL was harvested on day 4 after FITC for culture and enumeration of CD45+, col 1+ cells. The lung fibrocytes isolated from CCR2+/+ BMT mice were CCR2-positive by IHC; RT-PCR confirms that they were of the CCR2+/+ donor origin. Data represent n = 5 per group and were repeated twice. b: CCR2−/− mice were given BMT from CCR2−/− or CCR2+/+ donors and rested for 7 weeks. Mice were then injected with saline or FITC. Lung hydroxyproline content was determined 21 days later. Data represent five mice per group in the saline injections and eight mice per group in the FITC injections. All experiments were repeated two times. c: PKH-26-labeled CCR2+/+ fibrocytes were visualized in FITC-treated lungs within 24 hours of tail vein infusion.
CCR2+/+ BMT into CCR2−/− Mice Restores Fibrotic Susceptibility to FITC
CCR2−/− mice were given BMT from either CCR2−/− or CCR2+/+ donors. Mice were rested at least 7 weeks after BMT and each group was injected intratracheally with either saline or FITC. Lungs were harvested 21 days later and hydroxyproline assays were performed (Figure 6b). CCR2−/− mice that received either a CCR2+/+ BMT or a CCR2−/− BMT showed an equivalent level of hydroxyproline in response to saline injection (compare open bar to stippled bar). CCR2−/− mice that received a CCR2−/− BMT were protected from FITC-induced fibrosis as expected (hatched bar). However, CCR2−/− mice receiving CCR2+/+ BMT were now susceptible to FITC-induced fibrosis (black bar, n = 8; P < 0.03) generating a fibrotic response to FITC similar in magnitude to that previously reported in CCR2+/+ mice.8 Thus, fibrotic susceptibility and lung fibrocyte recruitment are both restored to CCR2−/− mice via CCR2+/+ BMT.
Ex Vivo-Labeled CCR2+/+ Fibrocytes Migrate to FITC-Injured Airspaces
Fibrocytes purified from C57BL/6 mice were labeled ex vivo with PKH-26 red dye and 5 × 105 cells were injected intravenously into CCR2−/− mice on day 4 after FITC. On day 5 after FITC, frozen sections were prepared and analyzed. PKH-26-labeled CCR2+/+ fibrocytes were readily visible in FITC-injured airspaces (Figure 6c).
CCL2 and TGF-β1 Induce Collagen Secretion in Lung Fibrocytes
The effect of the profibrotic mediators CCL2, TGF-β1, and IL-13 on collagen production by lung fibrocytes (Figure 7, a and b) and fibroblasts (Figure 7, c and d) was determined by Western blot. Shown are two representative Western blots for each cell type. Blots were stripped and reprobed with β-tubulin to normalize for loading. Densitometry analysis was performed on four separate experiments to normalize the col I signal to β-tubulin. The value obtained for the serum-free media control in each experiment was set at 1. CCL2 stimulation was able to up-regulate collagen synthesis in purified lung fibrocytes in a manner similar to TGF-β1. However, IL-13 had no effect in fibrocytes. IL-13 and TGF-β1 both stimulate the production of col I by fibroblasts, however, CCL2 had no effect on this population. The functional ability of CCL2 to augment col I expression in fibrocytes, but not fibroblasts correlates well with the CCR2 receptor expression differences noted in these two populations (Figure 3b). Thus, CCL2 stimulates col I production in lung fibrocytes as well as functions as a chemotaxin to recruit fibrocytes to injured airspaces.
Figure 7.
CCL2 and TGF-β1 stimulate collagen production in lung fibrocytes. Sorted populations of lung fibrocytes (a and b) and fibroblasts (c and d) were serum-starved for 24 hours before the addition of fresh serum-free media or serum-free media + IL-13 (10 ng/ml), CCL2 (10 ng/ml), or TGF-β1 (2 ng/ml) for an additional 24 hours. Col 1 protein was analyzed by Western blot. Two blots, representative of four total blots are shown for each cell type (a and c). Blots were stripped and reprobed with β-tubulin. Densitometry was performed to normalize col I expression to β-tubulin. In each experiment, the value for serum-free media was normalized to 1 and statistical analyses were performed (b and d). CCL2 and TGF-β1 both significantly stimulated the production of col I in fibrocytes. IL-13 and TGF-β1 both stimulated the production of col I in fibroblasts.
Fibrocytes Transition into Fibroblasts in Vitro
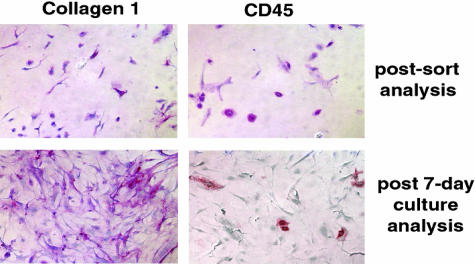
Fibrocytes might transition to fibroblasts in vitro. Lung fibrocytes were purified via two rounds of magnetic selection of CD45 beads from lung mince cultures of CCR2+/+ mice. The fibrocyte population isolated after this double immunoselection was 99.5% dual-positive for CD45 and col I as assessed by IHC (Figure 8, top). After 7 days of in vitro culture, the cell population was 94% CD45-negative, but remained col I-positive (Figure 8, bottom). Given the purity of the starting population, these results suggest that lung fibrocytes can transition into fibroblasts during in vitro culture.
Figure 8.
Fibrocytes transition into fibroblasts in vitro. Fibrocytes were purified via two rounds of magnetic selection and the final purified population was greater than 99.5% positive for col I and CD45 after sorting. Purified fibrocytes were cultured for 7 days in complete media and reanalyzed for expression of col I and CD45 via IHC using alkaline phosphatase-coupled secondary reagents. After 1 week of culture, the purified fibrocytes retained col I expression, but lost CD45 expression suggesting a transition from the fibrocyte to the fibroblast phenotype. Similar results were obtained in three separate experiments.
Discussion
Lung fibrocytes are recruited to the alveolar space in response to fibrotic injury. Lung fibrocytes share both mesenchymal and leukocyte markers, express functional CCR2 receptors, and migrate to CCL2 in vitro and in vivo. Recruitment of lung fibrocytes to injured airspaces is diminished in CCR2−/− mice, correlating with their protection from experimental pulmonary fibrosis. CCR2+/+ BMT into CCR2−/− mice restores both lung fibrocyte recruitment as well as susceptibility to FITC-induced lung matrix deposition. Both CCL2 and TGF-β1 augment col I secretion from lung fibrocytes. Fibrocytes can transition into fibroblasts in vitro, and this transition is accompanied by a loss of CCR2 and an increase in extracellular matrix production.
A population of cells that express both leukocyte and mesenchymal cell markers is present in greater numbers in the airspaces of injured versus uninjured animals. These cells express CD45, the common leukocyte antigen and CD13, a marker of myeloid lineage cells while expressing and producing col I, a mesenchymal marker. In contrast to peripheral blood fibrocytes that uniformly express CD34,12,13,23 only 2% of lung fibrocytes expressed the stem cell marker CD34. These data are consistent with a previous report demonstrating that CD34 is lost on peripheral blood fibrocytes that migrate to lung airways in response to Ag challenge.14 In aggregate, these data suggest that expression of CD34 is compartmentalized. Thus, our work extends the finding that lung fibrocytes are CD34-negative to the lower respiratory tract of the lung. Furthermore, these data demonstrate that CCR2 is a critical mediator for the recruitment of lung fibrocytes to areas of injury.
Lung fibrocytes express functional CCR2 receptors. These experiments are the first to identify expression of CCR2 on fibrocytes. Expression of CCR3, CCR5, CCR7, and CXCR4 mRNA by human fibrocytes isolated from peripheral blood in healthy volunteers has previously been reported.14 CCR2 expression was not evaluated. Murine peripheral blood fibrocytes have been reported to express CCR7 and CXCR4.13 However, accumulation of prelabeled fibrocytes to skin in vivo only occurred to the CCR7 ligand, CCL21, and not in response to CXCL12 suggesting that CXCR4 was not functional in vivo.14 CCR2 receptors on lung fibrocytes are functional both in vitro as assessed by chemotaxis (Figure 3c) and in vivo (Figure 6). Accumulation of lung fibrocytes in the alveolar spaces of FITC-injured mice was significantly reduced in CCR2−/− mice (Figure 5a). The enhanced appearance of lung fibrocytes in the injured BAL by day 3 after FITC correlates well with the kinetics of CCL2 expression in the BAL after FITC. CCL2 is induced in the airspaces within 24 hours of FITC inoculation.8 Thus this work is the first to document an important role for CCR2 in mesenchymal cell precursor recruitment after tissue injury both in vitro and in vivo.
The magnitude of the fibrotic response correlates with the absolute numbers of fibrocytes that are recruited to the alveolar space in response to FITC. Wild-type mice readily accumulate fibrocytes and develop fibrosis after FITC-induced lung injury. In contrast, CCR2−/− mice do not recruit fibrocytes to the alveolar space and are protected. Reconstitution of CCR2+/+ bone marrow cells restores both fibrocyte recruitment and fibrotic susceptibility. These data provide compelling evidence for an important role for CCR2-expressing fibrocytes in the fibroproliferative responses after acute lung injury.
The origin of murine fibrocytes is controversial. A bone marrow origin was suggested because of CD34 expression. Early studies that evaluated male BMTs into female recipients after 800 rads of total body irradiation failed to identify significant numbers of male bone marrow-derived peripheral blood fibrocytes.12 However, studies using BMTs from green fluorescent protein (GFP)-expressing mice into wild-type mice have suggested bone marrow-derived fibroblasts are incorporated into fibrotic lesions after bleomycin injury or irradiation.21,22 Neither of these studies characterized the GFP+ fibroblasts for fibrocyte markers. Our work is the first to demonstrate that bone marrow precursors give rise to CD45+, CD13+, col 1+, CCR2+ lung fibrocytes. Furthermore, the uniform staining of CCR2 on lung fibrocytes and the fact that CCR2 is expressed at high levels (gene array analysis of two different genotypes of mice) suggests that CCR2 is an important receptor for the recruitment and activation of these cells. Furthermore, our transplantation and adoptive transfer experiments demonstrate that CCR2+/+ lung fibrocytes home to FITC-injured airspaces within the lung.
CCR2+/+ bone marrow-derived cells are required for fibroproliferative responses. These studies are the first to demonstrate a role for bone marrow-derived CD45+, col 1+, CCR2+ cells in excess collagen accumulation after injury. Reconstitution of mice with a specific genetic defect in CCR2 expression with CCR2+/+ bone marrow enhanced the amount of collagen deposited in the FITC-injured lung and restored the fibrotic response (Figure 6b). Lung fibrocytes respond to CCL2 by increasing col 1 synthesis (Figure 7, a and b) demonstrating that the CCR2 receptor plays a role in activation as well as recruitment of lung fibrocytes. Extracellular matrix production by mesenchymal cells is known to be regulated by soluble factors such as TGF-β1 and IL-13.20,24–29 CCL2, TGF-β1, and IL-13 have been reported to be produced in response to bleomycin or FITC.8,25,29–31 CCL2 and TGF-β1 increased col 1 production in lung fibrocytes, but IL-13 did not. In contrast, TGF-β1 and IL-13 both stimulated the production of col I by fibroblasts.
In sum, these data define a new mechanism whereby CCL2/CCR2 participate in tissue remodeling after injury. Alveolar injury leads to the generation of CCL2 within the alveolar space. Bone marrow-derived CCR2+/+ fibrocytes are recruited to the lung after lower respiratory tract injury in response to secreted CCL2. Under the influence of profibrotic factors (TGF-β1 and CCL2), lung fibrocytes are activated to secrete extracellular matrix in sites of tissue injury.
Fibrocytes may contribute to fibrogenesis in several ways. First, the fibrocytes may directly contribute to fibrosis by secreting collagen. The amount of collagen secreted by CCR2+/+ fibrocytes exposed to CCL2 would be greater than from CCR2−/− fibrocytes (Figure 7, a and b). Second, the fibrocytes secrete TGF-β111 and thus, the CCR2-mediated recruitment of fibrocytes to the lung may serve to activate resident fibroblasts via the secretion of TGF-β1. Third, fibrocytes may differentiate into effector fibroblasts. The data in Figure 8 support this concept and demonstrate that fibrocytes can differentiate into fibroblasts in culture. The transition of fibrocytes into fibroblasts is accompanied by the loss of CCR2. The loss of CCR2 may serve to trap the effector fibroblasts within the lung and prevent their migration out of the injured lung. Additionally, the transition of fibrocytes into fibroblasts is associated with a significant up-regulation of extracellular matrix synthetic capacity and increased chemotactic responses to fibronectin. Thus, transition of CCR2-recruited fibrocytes into fibroblasts in vivo would change the functional behavior of these cells and significantly promote fibrogenesis. These results suggest that strategies aimed at blocking CCL2/CCR2 interactions may prevent the early recruitment of fibrocytes and their eventual differentiation into fibroblasts in the setting of fibrosis after acute lung injury.
Footnotes
Address reprint requests to Bethany B. Moore, Ph.D., 6301 MSRB III, 1150 W. Medical Center Dr., Ann Arbor, MI 48109-0642. E-mail: bmoore@umich.edu.
Supported by the National Institutes of Health (grants P50HL56402 to B.B.M., C.H., G.B.T., HL071586 to B.B.M., P50HL074024 to V.J.T., and HL51083 to G.B.T.).
References
- Thannickal VJ, Toews GB, White E, Lynch JI, Martinez F. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–471. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- Lynch JI, Toews G: Idiopathic pulmonary fibrosis. Fishman A, Pulmonary Diseases and Disorders. Edited by Fishman A. Philadelphia PA, 1997, pp 1069-1084 [Google Scholar]
- Kuhn C. Pathology. Phan S, Thrall R, editors. New York: Marcek Dekker, Inc.,; Pulmonary Fibrosis. 1995:pp 59–83. [Google Scholar]
- King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164:1025–1032. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- Coultas D, Zumwalt R, Black W, Sobonya R. The epidemiology of interstitial lung disease. Am J Respir Crit Care Med. 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society ERS Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- Christensen P, Goodman R, Pastoriza L, Moore B, Toews G. Induction of lung fibrosis in the mouse by intratracheal instillation of fluorescein isothiocyanate is not T-cell dependent. Am J Pathol. 1999;155:1773–1779. doi: 10.1016/S0002-9440(10)65493-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Paine R, Christensen P, Moore T, Sitterding S, Ngan R, Wilke C, Kuziel W, Toews G. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;167:4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- Kurihara T, Bravo R. Cloning and functional expression of mCCR2, a murine receptor for the CC-chemokines JE and FIC. J Biol Chem. 1996;271:11603–11607. doi: 10.1074/jbc.271.20.11603. [DOI] [PubMed] [Google Scholar]
- Yamagami S, Tokuda Y, Ishii K, Tanaka H, Endo N. cDNA cloning and functional expression of a human monocyte chemoattractant protein 1 receptor. Biochem Biophys Res Commun. 1994;202:1156–1162. doi: 10.1006/bbrc.1994.2049. [DOI] [PubMed] [Google Scholar]
- Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Sun G, Stacey M, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;170:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- Aiba S, Tagami H. Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J Cutan Pathol. 1997;24:65–69. doi: 10.1111/j.1600-0560.1997.tb01098.x. [DOI] [PubMed] [Google Scholar]
- Cowper SE, Bucala R. Nephrogenic fibrosing dermopathy: suspect identified, motive unclear. Am J Dermatopathol. 2003;25:358. doi: 10.1097/00000372-200308000-00017. [DOI] [PubMed] [Google Scholar]
- Kuziel W, Morgan S, Dawson T, Griffin S, Smithies O. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke KR, Krenger W, Hill G, Martin TR, Kobzik L, Brewer J, Simmons R, Crawford JM, van den Brink MR, Ferrara JL. Host reactive donor T cells are associated with lung injury after experimental allogeneic bone marrow transplantation. Blood. 1998;92:2571–2580. [PubMed] [Google Scholar]
- Down JD, Mauch P, Warhol M, Neben S, Ferrara JL. The effect of donor T lymphocytes and total-body irradiation on hemopoietic engraftment and pulmonary toxicity following experimental allogeneic bone marrow transplantation. Transplantation. 1992;54:802–808. doi: 10.1097/00007890-199211000-00007. [DOI] [PubMed] [Google Scholar]
- Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29:537–544. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- Epperly M, Guo H, Gretton J, Greenberger J. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Resp Crit Care Med. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue S, Phan S. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesney J, Bucala R. Peripheral blood fibrocytes: mesenchymal precursor cells and the pathogenesis of fibrosis. Curr Rheumatol Rep. 2000;2:501–505. doi: 10.1007/s11926-000-0027-5. [DOI] [PubMed] [Google Scholar]
- Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, Essayan DM. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther. 2000;292:988–994. [PubMed] [Google Scholar]
- Kolodsick J, Toews G, Jakubzick C, Hogaboam C, Moore T, McKenzie A, Wilke C, Chrisman C, Moore B. Protection from FITC-induced fibrosis in IL-13 deficient, but not IL-4 deficient mice results from impaired collagen synthesis by fibroblasts. J Immunol. 2004;172:4068–4076. doi: 10.4049/jimmunol.172.7.4068. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Gon Y, Takeshita I, Maruoka S, Horie T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J Allergy Clin Immunol. 2001;107:1001–1008. doi: 10.1067/mai.2001.114702. [DOI] [PubMed] [Google Scholar]
- Xu YD, Hua J, Mui A, O’Connor R, Grotendorst G, Khalil N. Release of biologically active TGF-beta1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol. 2003;285:L527–L539. doi: 10.1152/ajplung.00298.2002. [DOI] [PubMed] [Google Scholar]
- Kenyon NJ, Ward RW, McGrew G, Last JA. TGF-beta1 causes airway fibrosis and increased collagen I and III mRNA in mice. Thorax. 2003;58:772–777. doi: 10.1136/thorax.58.9.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker RK, Laurent GJ, Shahzeidi S, Lympany PA, du Bois RM, Jeffery PK, McAnulty RJ. Transforming growth factors-beta 1, -beta 2, and -beta 3 stimulate fibroblast procollagen production in vitro but are differentially expressed during bleomycin-induced lung fibrosis. Am J Pathol. 1997;150:981–991. [PMC free article] [PubMed] [Google Scholar]
- Belperio JA, Dy M, Burdick MD, Xue YY, Li K, Elias JA, Keane MP. Interaction of IL-13 and C10 in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;27:419–427. doi: 10.1165/rcmb.2002-0009OC. [DOI] [PubMed] [Google Scholar]
- Zhang K, Gharaee-Kermani M, Jones ML, Warren JS, Phan SH. Lung monocyte chemoattractant protein-1 gene expression in bleomycin-induced pulmonary fibrosis. J Immunol. 1994;153:4733–4741. [PubMed] [Google Scholar]