Abstract
OBJECTIVE
To review the evidence and provide an approach to management of low bone density among premenopausal women.
QUALITY OF EVIDENCE
MEDLINE was searched from January 1990 to November 2004 and articles graded by level of evidence (I to III). Diagnosis and management recommendations were based on evidence from randomized controlled trials and expert consensus.
MAIN MESSAGE
Bone mineral density (BMD) testing among premenopausal women should be completed only in the presence of approved indications. Current evidence does not support screening for osteoporosis among premenopausal women. Low BMD in premenopausal women is associated with a lower fracture risk than seen in postmenopausal women. In the absence of fragility fractures, low BMD might reflect low peak bone mass based on genetic predisposition, environment, and lifestyle factors. Clinical evaluation enables distinction between low peak bone mass and a systemic disorder resulting in low BMD and skeletal fragility. Common causes of low bone density among premenopausal women include ovulatory disturbances and low body weight.
CONCLUSION
Bone mineral density alone is insufficient for diagnosis of osteoporosis among premenopausal women in the absence of fragility fractures. Antiresorptive therapy has been evaluated and shown to benefit premenopausal women using glucocorticoid therapy or those with primary hyperparathyroidism.
Abstract
OBJECTIF
Vérifier les données probantes concernant la présence d’une densité osseuse basse chez les femmes en préménopause et proposer une approche pour le traitement de cette condition.
QUALITÉ DES PREUVES
On a consulté MEDLINE entre janvier 1990 et novembre 2004 et les articles retenus ont été classés selon leur niveau de preuve (I à III). Les recommandations de diagnostic et de traitement étaient fondées sur des preuves provenant d’essais cliniques randomisés et de consensus d’experts.
PRINCIPAL MESSAGE
Chez les femmes préménopausique, la densité minérale osseuse (DMO) ne devrait être déterminée qu’en présence d’indications reconnues. Les données actuelles indiquent que le dépistage de l’ostéoporose n’est pas indiqué pour ces femmes préménopausique. En présence d’une faible DMO, le risque de fracture est plus faible en préménopause qu’en postménopause. En absence de fracture de fragilité, une DMO basse pourrait refléter un pic de masse osseuse bas en lien avec le mode de vie, certains facteurs environnementaux ou une prédisposition génétique. L’évaluation clinique permet de distinguer un pic de masse osseuse bas d’une affection systémique causant une faible DMO et une fragilité squelettique. Les causes fréquentes de faible DMO chez la femme préménopausique incluent les troubles ovulatoires et un poids corporel bas.
CONCLUSION
Une faible densité minérale osseuse ne permet pas de poser un diagnostic d’ostéoporose chez la femme préménopausique quand il n’y a pas de fracture de fragilité. On a démontré que les agents anti-résorption osseuse sont avantageux pour les femmes préménopausiques traitées aux glucocorticoïdes ou souffrant d’hyperparathyroïdie primaire.
EDITOR’S KEY POINTS.
Bone density follows a bell curve, so that among premenopausal women, low bone density might reflect normal variation in BMD and achievement of a lower peak bone mass owing to genetic and environmental factors, such as poor calcium intake, lack of exercise, smoking, or excessive alcohol intake.
Osteoporosis should be diagnosed in premenopausal women only if fragility fractures are present and not on the basis of low bone density measurements alone.
Low bone density should be investigated as it might be secondary to medical conditions associated with bone loss.
In this population, bisphosphonates have been evaluated only for treating patients who have received glucocorticoid therapy; they have improved bone density. Other measures to increase bone mass, depending on the cause, include estrogen replacement and lifestyle changes.
POINTS DE REPÈRE DU RÉDACTEUR.
La densité osseuse suit une courbe en forme de cloche, de sorte qu’une faible densité osseuse en les femmes préménopause pourrait refléter une variation normale de ce paramètre et l’atteinte d’un pic de masse osseuse moins élevé en raison de facteurs génétiques ou environnementaux, tels qu’un apport insuffisant de calcium, un manque d’exercice, l’usage de tabac ou une consommation excessive d’alcool.
Un diagnostic d’ostéoporose chez les femmes préménopausique ne devrait être évoqué qu’en présence de fractures de fragilité osseuse, et non en raison d’une densitométrie osseuse trop basse seulement.
Une densité osseuse trop basse doit être investiguée parce qu’elle pourrait être secondaire à une affection qui cause une lyse osseuse.
Dans cette population, les bisphosphonates ont été évalué seulement comme traitement des femmes qui avaient reçu des glucocorticoïdes; ils ont amélioré la densité osseuse. Les autres mesures susceptibles d’augmenter la masse osseuse incluent, selon la cause, l’oestrogénothérapie substitutive et la modification des habitudes de vie.
Osteoporosis is common among postmenopausal women. Younger premenopausal women who have diseases or conditions associated with progressive bone loss could be at increased risk of osteoporosis. Some asymptomatic women ask to have bone mineral density (BMD) tested. Premenopausal women can have low bone density without fragility fractures or serious risk factors for fracture. It is thus necessary for primary care physicians to know the indications for BMD testing among premenopausal women as well as the appropriate interpretation of BMD tests and management of low BMD among premenopausal women.
Low bone density (T-score of less than 1 standard deviation below the mean for young adults) affects approximately 15% of young healthy women between the ages of 30 and 40.1 Bone density follows a bell curve distribution, and approximately 0.5% of young healthy women between the ages of 30 and 40 have T-scores of –2.5 or less.2,3 Osteoporosis in premenopausal women is diagnosed when fragility fractures are present and diagnosis is not based solely on the results of a BMD test.1 Premenopausal women experiencing fragility fractures should be further evaluated to determine why bones fracture despite adequate estrogen levels. A few patients require a bone biopsy to evaluate the underlying pathology and to identify a possible cause for bone fragility before menopause.
This paper addresses managing low BMD among premenopausal women and reviews the underlying pathophysiology, diagnosis, and therapy.
Quality of evidence
MEDLINE was searched to identify all English-language abstracts evaluating low BMD among premenopausal women. The search was limited to articles published in peer-reviewed journals from January 1990 to November 2004. The term “low bone density” was cross-matched with “premenopausal women” and with the MeSH headings “management” and “pathophysiology.” All studies evaluating premenopausal women were included except for case reports.
Diagnosis
The World Health Organization defines osteoporosis as a progressive systemic disease characterized by low bone density and microarchitectural deterioration in bone that predisposes patients to increased bone fragility and fracture.1 The WHO criteria for diagnosis of osteoporosis by T-scores applies only to postmenopausal women. These criteria were not intended to apply to premenopausal women with low BMD.1
Low BMD among premenopausal women can be either physiologic or pathologic. Bone mineral density among young healthy women follows a bell curve distribution; approximately 15% of young healthy women have T-scores of less than –1, and 0.5% of young healthy women have T-scores of –2.5 or less.2 In the normal population, people at the upper or the lower end of the normal bell curve could represent the normal variation in BMD. Lower BMD might not reflect underlying disease and might not be associated with increased fracture risk before menopause.1 In premenopausal women without fragility fractures or height loss, low BMD could simply reflect an underlying low peak bone mass. Low peak bone mass is genetically determined and also affected by environmental factors, such as inadequate exercise and dietary calcium intake, as well as smoking and excess alcohol consumption during patients’ years as teenagers and young adults.1
Low BMD among young women is not associated with the same increased risk of fracture as low BMD among older women. Premenopausal women, being younger, have a substantially lower risk of fracture even with falls. Premenopausal women have relatively increased muscle mass. They are estrogen replete and have lower rates of bone turnover than postmenopausal women have. Therefore, the risk of fracture in young women with low BMD is much lower than that seen in postmenopausal women.4-6 Age is an independent risk factor for fracture,1 and low BMD among postmenopausal older women is associated with higher risk of fracture.1
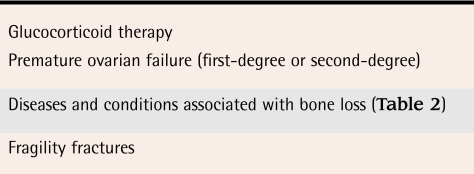
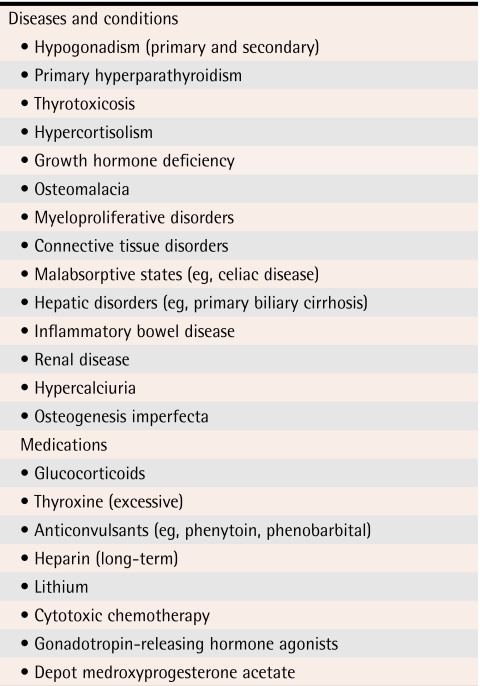
Bone density testing should be completed among women with identifiable causes of bone loss (Table 1).1 The decision to complete BMD testing is made on clinical grounds. Secondary causes of bone loss (Table 21) can be important indications for BMD testing.
Table 1.
Indications for BMD testing in premenopausal women
Table 2.
Important secondary causes of bone loss
Data from Khan et al.1
Clinical evaluation
It is necessary to evaluate premenopausal women with low BMD to ensure that no secondary causes of bone loss have contributed to the low BMD. Clinical assessment includes taking a complete history, performing a physical examination of the patient, and requesting appropriate laboratory tests to exclude common conditions associated with bone loss.7 Patients with low BMD in the absence of fragility fractures need to be evaluated, as do those with a history of fracture and those who have a secondary cause of bone loss likely to have contributed to development of low BMD levels.
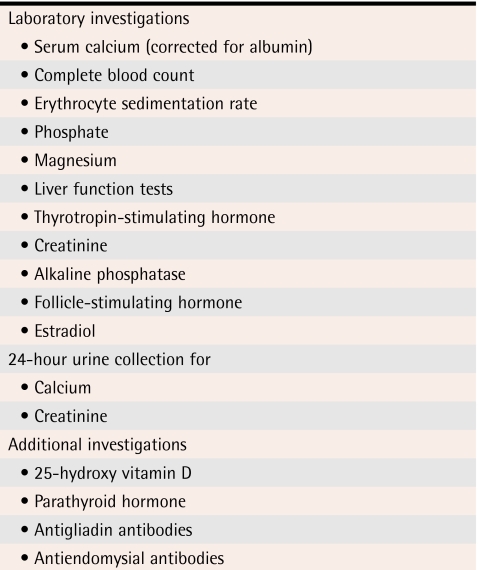
Evaluation should include assessment of thyroid, liver, and renal function (Table 3). Serum calcium (corrected for albumin) is elevated in hyperparathyroidism or malignancy. Low serum calcium levels are seen among people with vitamin D deficiency or malabsorption. Malabsorption of calcium and vitamin D can result in low BMD as well as osteomalacia. A low measurement for calcium in 24-hour urine assessment is an early indicator of inadequate calcium intake or malabsorption. If results from the 24-hour urine assessment for calcium do not return to normal when calcium supplementation is increased, the possibility of malabsorption or occult celiac disease should be considered. A celiac panel with assessment of antiendomysial and antigliadin antibodies can help to diagnose celiac disease. History taking and physical examination will guide physicians considering additional investigations. It is also important to ensure that young women truly are estrogen replete. Subclinical deficiency in estrogen has been associated with low BMD among young women.8-10
Table 3.
Workup for low bone mineral density in premenopausal women
Amenorrhea, as a result of estrogen deficiency, is associated with accelerated bone loss. Menstrual status is an important factor in achieving peak bone mass and in maintaining BMD among women before menopause.1,8-11 It is necessary to ensure that women with low bone mass are not experiencing estrogen deficiency either clinically or subclinically.1 Elevations in follicle-stimulating hormone of more than 20 mIU/L have been associated with increased bone turnover and progressive bone loss in the perimenopausal period.10,12 Therefore, a detailed assessment of menstrual status is necessary, as ovulatory disturbances are frequently observed among premenopausal women with low BMD.8-10
A premenopausal woman with fragility fractures or progressive bone loss should be referred to a metabolic bone clinic for further assessment. A bone biopsy can provide further information regarding the underlying pathology resulting in fractures and might be of value in the assessment. A bone biopsy is completed using a technique similar to that of a bone marrow biopsy; however, the bone biopsy needle removes a core of bone tissue with both cortical and cancellous bone intact. This removal is completed while the patient receives local anesthetic, and risks are essentially limited in experienced hands to local bleeding.
Intervention
There are no data evaluating the usefulness of bisphosphonates among premenopausal women with low BMD in the absence of glucocorticoid therapy. Bisphosphonates have been evaluated only among premenopausal women who have received glucocorticoid therapy. Glucocorticoid therapy is associated with an increase in the rate of bone turnover in addition to decreasing intestinal calcium absorption, increasing urinary calcium losses, and impairing osteocyte lifespan and function. Bisphosphonates lower bone turnover and, therefore, are effective among premenopausal women who have been treated with glucocorticoid therapy.13-16 Women who have not received glucocorticoid therapy and who are estrogen replete could have normal bone turnover rates. Suppressing these rates with a bisphosphonate might not be safe or effective in improving bone density or reducing fracture risk. This patient population is thus best served by a specialized metabolic bone clinic before antiresorptive therapy is implemented.
Antiresorptive therapies.
Antiresorptive therapy benefits premenopausal women with secondary causes of bone loss, such as glucocorticoid use or primary hyperparathyroidism (level I evidence).17 Bisphosphonates have long-term skeletal retention, and these agents can be released from the skeleton several years later, potentially in a subsequent pregnancy. The effects of bisphosphonates on the developing fetal skeleton are unknown. Cyclic etidronate, a less potent bisphosphonate, has not been evaluated among premenopausal women with osteoporosis. Alendronate has been evaluated among premenopausal women with primary hyperparathyroidism and has been shown to be effective in improving BMD in this population.17 Alendronate and risedronate are effective in improving BMD in glucocorticoid-induced bone loss (level I evidence). Antiresorptive therapy has not been evaluated among premenopausal women with low BMD in the absence of secondary causes of osteoporosis.
Lifestyle modification.
Lifestyle modification should be encouraged among premenopausal women in order to improve BMD. This would include weight-bearing exercises, adequate dietary calcium intake, smoking cessation, limiting caffeine, and reducing excessive alcohol consumption (level II evidence).18
It is important to ensure adequate calcium and vitamin D intake. Maintenance of normal body weight, with a body mass index of 20 to 25, and a daily exercise program are of value in maintaining BMD (level II evidence).18
Estrogen supplementation.
Women who are estrogen deficient, either clinically or subclinically, benefit from estrogen supplements. Estrogen supplementation, in the form of either oral contraceptive pills or 17beta-estradiol in combination with a progestin, should be considered in order to prevent progressive bone loss (level III evidence). Estrogen supplementation is associated with improvements in BMD among estrogen-deficient women (level I evidence).19 Cyclic medroxyprogesterone in women with ovulatory disturbance has been shown in a randomized placebo-controlled prospective study over 1 year to result in gains in BMD (level I evidence).20 Prospective data are needed to evaluate further the effect of short luteal-phase cycles on BMD. Further evaluation of intervention with estrogen or progesterone supplements is also warranted. The role of estrogen supplementation for women who are not estrogen deficient is controversial (level III evidence).
Data regarding oral contraceptives have been conflicting; some studies demonstrate positive effects on BMD in premenopausal women and other studies demonstrate negative effects on BMD (level II evidence).21,22 Data from the Canadian Multicentre Osteoporosis Study indicated that oral contraceptive users had lower BMD at the trochanter and spine than non-users.23 Evaluation of this patient population indicated, however, that these women also had higher rates of smoking and alcohol use and had a higher prevalence of menstrual irregularity before initiating oral contraceptive use than non-users had (level II evidence).23 Thus, prospective data are required to assess the effects of oral contraceptive use on BMD among premenopausal women. Depot medroxyprogesterone acetate, an injectable contraceptive, inhibits release of luteinizing hormone and follicle-stimulating hormone and results in suppression of ovarian synthesis of estradiol and progesterone (level II evidence).24,25 Depot medroxyprogesterone use has been associated with decreased BMD at the lumbar spine (level II evidence).26,27 Discontinuation of depot medroxyprogesterone is associated with rapid improvement in BMD and reversal of bone loss, as demonstrated in a prospective cohort study.28
Conclusion
Low BMD among premenopausal women should be further evaluated. Low BMD can be due to genetically predetermined low peak bone mass. Environmental factors, such as inadequate calcium intake, alcohol and tobacco excess, low body weight, and estrogen deficiency, can contribute to development of lower peak bone mass or to bone loss in the premenopausal years. Osteoporosis among premenopausal women is diagnosed in the presence of fragility fractures and diagnosis is not based solely on the results of a BMD test. Secondary causes of bone loss should be excluded, and any underlying condition contributing to low BMD should be corrected. Referral to a metabolic bone clinic is appropriate for patients with fragility fractures or progressive bone loss. Antiresorptive therapy has been evaluated only for premenopausal women receiving glucocorticoid therapy or those with primary hyperparathyroidism. Only in these conditions has antiresorptive therapy been shown to improve BMD measurements. Screening for osteoporosis among premenopausal women is not justified based on available evidence.
Levels of evidence.
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
Acknowledgments
This article was originally published as “Management of low bone mineral density in premenopausal women.” J Obstet Gynaecol Can 2005;27(4):345-9 and is reprinted courtesy of the Society of Obstetrics and Gynaecologists of Canada.
Biography
Dr Khan is Clinical Professor of Medicine in the Divisions of Endocrinology and Geriatrics at McMaster University in Hamilton, Ont.
Footnotes
Competing interests: None declared
References
- 1.Khan AA, Bachrach L, Brown JP, Hanley DA, Josse RG, Kendler DL, et al. Standards and guidelines for performing central dual-energy x-ray absorptiometry in premenopausal women, men, and children. J Clin Densitom. 2004;7:51–64. doi: 10.1385/jcd:7:1:51. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, Melton LJ, III, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9:1137–1141. doi: 10.1002/jbmr.5650090802. [DOI] [PubMed] [Google Scholar]
- 3.Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int. 1997;7:407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 4.Hui SL, Slemenda CW, Johnston CC. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest. 1988;81:1804–1809. doi: 10.1172/JCI113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sowers M, Kshirsagar A, Crutchfield M, Updike S. Body composition, age and femoral bone mass of young adult women. Ann Epidemiol. 1991;1:245–254. doi: 10.1016/1047-2797(91)90003-u. [DOI] [PubMed] [Google Scholar]
- 6.Hosmer WD, Genant HK, Browner WS. Fractures before menopause: a red flag for physicians. Osteoporos Int. 2002;13:337–341. doi: 10.1007/s001980200035. [DOI] [PubMed] [Google Scholar]
- 7.Khan AA, Syed Z. Bone densitometry in premenopausal women. J Clin Densitom. 2004;7:85–92. doi: 10.1385/jcd:7:1:85. [DOI] [PubMed] [Google Scholar]
- 8.Prior JC, Vigna YM, Schechter MT, Burgess AE. Spinal bone loss and ovulatory disturbances. N Engl J Med. 1990;323:1221–1227. doi: 10.1056/NEJM199011013231801. [DOI] [PubMed] [Google Scholar]
- 9.Sowers M, Randolph JF, Jr, Crutchfield M, Jannausch ML, Shapiro B, Zhang B, et al. Urinary ovarian and gonadotropin hormone levels in premenopausal women with low bone mass. J Bone Miner Res. 1998;13:1191–1202. doi: 10.1359/jbmr.1998.13.7.1191. [DOI] [PubMed] [Google Scholar]
- 10.Sowers M, Crutchfield M, Bandekar R, Randolph JF, Shapiro B, Schork MA, et al. Bone mineral density and its change in pre- and perimenopausal white women: the Michigan Bone Health Study. J Bone Miner Res. 1998;13:1134–1140. doi: 10.1359/jbmr.1998.13.7.1134. [DOI] [PubMed] [Google Scholar]
- 11.Sowers MR, Clark MK, Hollis B, Wallace RB, Jannausch M. Radial bone mineral density in pre- and perimenopausal women: a prospective study of rates and risk factors of loss. J Bone Miner Res. 1992;7:647–657. doi: 10.1002/jbmr.5650070609. [DOI] [PubMed] [Google Scholar]
- 12.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–112. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 13.for the Glucorticoid-Induced Osteoporosis Intervention Study Group. Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339:292–299. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 14.Adachi JD, Saag KG, Delmas PD, Liberman UA, Emkey RD, Seeman E, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. doi: 10.1002/1529-0131(200101)44:1<202::AID-ANR27>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 15.Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15:1006–1013. doi: 10.1359/jbmr.2000.15.6.1006. [DOI] [PubMed] [Google Scholar]
- 16.Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. 2000;67:277–285. doi: 10.1007/s002230001146. [DOI] [PubMed] [Google Scholar]
- 17.Khan AA, Bilezikian JP, Kung AW, Ahmed MM, Dubois SJ, Ho AY, et al. Alendronate in primary hyperparathyroidism: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:3319–3325. doi: 10.1210/jc.2003-030908. [DOI] [PubMed] [Google Scholar]
- 18.Josse RG; Scientific Advisory Council of the Osteoporosis Society of Canada. Brown JP. Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. CMAJ. 2002;167(10 Suppl):1–34. [PMC free article] [PubMed] [Google Scholar]
- 19.Kuohung W, Borgatta L, Stubblefield P. Low dose oral contraceptives and bone mineral density: an evidence based analysis. Contraception. 2000;61(2):77–82. doi: 10.1016/s0010-7824(00)00086-x. [DOI] [PubMed] [Google Scholar]
- 20.Prior JC, Vigna YM, Barr SI, Rexworthy C, Lentle BC. Cyclic medroxyprogesterone treatment increases bone density: a controlled trial in active women with menstrual cycle disturbances. Am J Med. 1994;96:521–530. doi: 10.1016/0002-9343(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 21.DeCherney A. Bone-sparing properties of oral contraceptives. Am J Obstet Gynecol. 1996;174:15–20. doi: 10.1016/s0002-9378(96)70366-6. [DOI] [PubMed] [Google Scholar]
- 22.Ott SM, Scholes D, LaCroix AZ, Ichikawa LE, Yoshida CK, Barlow WE. Effects of contraceptive use on bone biochemical markers in young women. J Clin Endocrinol Metab. 2001;86:179–185. doi: 10.1210/jcem.86.1.7118. [DOI] [PubMed] [Google Scholar]
- 23.Prior JC, Kirkland SA, Joseph L, Kreiger N, Murray TM, Hanley DA, et al. Oral contraceptive use and bone mineral density in premenopausal women: cross-sectional, population-based data from the Canadian Multicentre Osteoporosis Study. CMAJ. 2001;165(8):1023–1029. [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser IS, Weisberg E. A comprehensive review of injectable contraception with special emphasis on depot medroxyprogesterone acetate. Med J Aust. 1981;24(Suppl 1):3–19. doi: 10.5694/j.1326-5377.1981.tb135992.x. [DOI] [PubMed] [Google Scholar]
- 25.Jeppsson S, Johansson EDB. Medroxyprogesterone acetate, estradiol, FSH, and LH in peripheral blood after intramuscular administration of Depo-Provera® to women. Contraception. 1976;14:461–469. doi: 10.1016/s0010-7824(76)80060-1. [DOI] [PubMed] [Google Scholar]
- 26.Paiva LC, Pinto-Neto AM, Faundes A. Bone density among long-term users of medroxyprogesterone acetate as a contraceptive. Contraception. 1998;58:351–355. doi: 10.1016/s0010-7824(98)00125-5. [DOI] [PubMed] [Google Scholar]
- 27.Wanichsetakul P, Kamudhamas A, Watanaruangkovit P, Siripakarn Y, Visutakul P. Bone mineral density at various anatomic bone sites in women receiving oral contraceptives and depot-medroxyprogesterone acetate for contraception. Contraception. 2002;65:407–510. doi: 10.1016/s0010-7824(02)00308-6. [DOI] [PubMed] [Google Scholar]
- 28.Scholes D, LaCroix AZ, Ichikawa LE, Barlow WE, Ott SM. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159(2):139–144. doi: 10.1001/archpedi.159.2.139. [DOI] [PubMed] [Google Scholar]