Abstract
Although hypoxia stimulates the expression of vascular endothelial growth factor (VEGF), little is known of the role or mechanism by which VEGF functions after ischemia and reperfusion (I/R) injury. In this report, we first evaluated the expression of VEGF in a mouse model of liver warm ischemia. We found that the expression of VEGF increased after ischemia but peaked between 2 and 6 hours after reperfusion. Mice were treated with a neutralizing anti-mouse VEGF antiserum (anti-VEGF) or control serum daily from day −1 (1 day before the initiation of ischemia). Treatment with anti-VEGF significantly reduced serum glutaminic pyruvic transaminase levels and reduced histological evidence of hepatocellular damage compared with controls. Anti-VEGF also markedly decreased T-cell, macrophage, and neutrophil accumulation within livers and reduced the frequency of intrahepatic apoptotic terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling-positive cells. Moreover, there was a reduction in the expression of pro-inflammatory cytokines (tumor necrosis factor-α and interferon-γ), chemokines (interferon-inducible protein-10 and monocyte chemoattractant protein-1) and adhesion molecules (E-selectin) in parallel with enhanced expression of anti-apoptotic genes (Bcl-2/Bcl-xl and heme oxygenase-1) in anti-VEGF-treated animals. In conclusion, hypoxia-inducible VEGF expression by hepatocytes modulates leukocyte trafficking and leukocyte-induced injury in a mouse liver model of warm I/R injury, demonstrating the importance of endogenous VEGF production in the pathophysiology of hepatic I/R injury.
Ischemia/reperfusion (I/R) injury, an antigen-independent inflammatory component of organ procurement, remains an important problem in clinical transplantation. In the case of the liver, I/R injury causes up to 10% of early transplant failures and can lead to a higher incidence of acute and chronic rejection.1 The mechanisms underlying hepatic I/R injury are complex but are known to involve leukocyte accumulation and activation (neutrophils, Kupffer cells, and monocytes/macrophages), pro-inflammatory cytokine and chemokine secretion, complement activation, and vascular cell adhesion molecule activation.2,3 However, the underlying mechanisms and mediators involved remain to be elucidated.
Vascular endothelial growth factor (VEGF), a well-established angiogenesis factor has been recently found to have potent pro-inflammatory properties in the early period after transplant. This effect of VEGF is mediated in part via its ability to facilitate intragraft mechanisms of leukocyte recruitment and to promote endothelial activation responses including adhesion molecule and chemokine production.4 VEGF expression is primarily regulated by hypoxia,5–7 and many different cell types have been found to express an increased amount of VEGF when subjected to hypoxia in vitro. Induced VEGF mRNA has been shown to be present in hypoxic zones of tumors,8 and it is also increased in expression after ischemic injury in vivo9 in the liver10,11 and in the lung.12 Furthermore, it is reported that hypoxia mediates VEGF expression via direct effects on the gene by both transcriptional and posttranscriptional mechanisms.13–16 These findings indicate that hypoxic induction of VEGF is likely a characteristic component of transplantation and that VEGF-induced biology might be a key component of reperfusion injury after transplant. Nevertheless, surprisingly little is reported on the role of VEGF in I/R or its function in inflammation after reperfusion.
In this study, we used a mouse model of hepatic warm ischemia followed by reperfusion to dissect the pathophysiological role of endogenous VEGF in the mechanism of I/R injury. Our findings suggest that induced VEGF expression after I/R mediates injury facilitates early leukocyte recruitment and leukocyte-induced hepatocellular damage. These findings indicate that targeting hypoxia-inducible genes such as VEGF at the time of reperfusion will have therapeutic effects to limit early innate immune inflammatory responses and subsequent leukocyte recruitment that predispose an organ to the development of rejection.
Materials and Methods
Animals
Male C57BL/6 mice (8 to 10 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were housed in the University of California, Los Angeles animal facility under specific pathogen-free conditions. All animals received humane care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institute of Health (NIH publication 86-23, revised 1985).
Generation of Anti-VEGF Antiserum
Rabbit anti-murine VEGF antiserum was prepared according to the methods of Tilton et al17 and as described previously.4 Briefly, New Zealand White rabbits were immunized with 500 μg of the N-terminal sequence of secreted VEGF (AAPTTEGEQKSHEVIKFMVYQRSY) coupled with keyhole limpet hemocyanin using the maleimidobenzyl-N-hydroxylsuccinimide ester crosslinker. Subsequently, rabbits received 250 μg of peptide every 3 weeks by subcutaneous injection. Every 6 weeks, the animals were bled, and anti-VEGF titers were tested using standard enzyme-linked immunosorbent assay (ELISA). ELISA was performed using 2 μg/ml VEGF protein antigen coated to bovine serum albumin. As illustrated in Figure 1A, the antisera used in these experiments contained high titers of anti-VEGF up to a dilution of 1:156,000. Neutralizing activity was assessed by evaluating the ability of the antiserum to inhibit murine VEGF-induced proliferation of human umbilical vein endothelial cells (HUVECs). Briefly, HUVECs were treated with murine VEGF (5 ng/ml) for 72 hours, and proliferation was assessed by 3[H]thymidine incorporation for the last 18 hours of co-culture. Addition of anti-VEGF antisera inhibited murine VEGF-induced proliferation of endothelial cells by approximately 30% at a dilution of 1:30 and by approximately 60% at a dilution of 1:10 (Figure 1B). In contrast, control serum from nonimmunized rabbits failed to inhibit VEGF-induced proliferation. Finally, we tested our antiserum for its neutralizing function in vivo using a CHO-VEGF angiogenesis assay, as described previously.4 The antisera used in these studies inhibited VEGF-induced angiogenesis by approximately 50 to 60% when given at a dose of 0.5 ml, and 80% inhibition was seen when a dose of 0.8 ml was given intraperitoneally daily. Thus, we used a dose of 0.8 ml for all studies.
Figure 1.
Generation of anti-VEGF antiserum. A: Every 6 weeks, after immunization with VEGF peptide, New Zealand White rabbits were bled, and serum was collected. An ELISA is illustrated for the assessment of anti-VEGF in four postimmunization immune sera compared with pre-immune serum. ELISA was performed using 2 μg/ml VEGF peptide as the antigen and a goat anti-rabbit horseradish peroxidase-conjugated antibody as the secondary reagent. B: Human endothelial cells were treated with mouse VEGF (5 ng/ml) in triplicate cultures, and after 72 hours, proliferation was assessed by 3[H]thymidine incorporation. Anti-VEGF antiserum was added to cultures at increasing dilutions, as illustrated. The percent inhibition of mean VEGF-induced proliferation was calculated for each culture condition. No inhibition was found with control serum at identical concentrations (not shown). One representative experiment of 10 is shown.
Experimental Design
We used an established mouse model of partial warm hepatic I/R injury, as described previously.9,18 Briefly, mice were anesthetized with pentobarbital sodium (50 mg/kg intraperitoneally) and injected with heparin (100 U/kg), and an atraumatic clip was used to interrupt the artery and portal venous blood supply to the left and middle lobes of the liver. After 90 minutes of partial hepatic warm ischemia, the clamp was removed to initiate hepatic reperfusion. Mice were sacrificed at 0 to 6 hours of reperfusion, and liver/blood samples were collected. In the first set of experiments, VEGF gene/protein expression was examined serially in liver samples subjected to I/R injury (n = 4/time point). In the second set of experiments, the effects of VEGF blockade after treatment with neutralizing rabbit anti-mouse VEGF serum were assessed at 6 hours after reperfusion. The groups of mice were treated intraperitoneally with 0.8 ml of anti-VEGF serum (n = 8) or control serum (n = 9) twice: at day −1 and just before the ischemia insult. Sham mice underwent the same procedure but without vascular occlusion (n = 4).
Hepatocyte Function
Serum glutaminic-pyruvic transaminase (sGPT) levels, an indicator of hepatocellular injury, were measured in peripheral blood samples at 6 hours after reperfusion with an autoanalyzer (ANTECH Diagnostics, Los Angeles, CA).
Histology
Liver paraffin sections (5 μm thick) were stained with hematoxylin and eosin (H&E). The severity of I/R injury was blindly graded using modified Suzuki’s criteria.19 In this classification, sinusoidal congestion, hepatocyte necrosis, and ballooning degeneration are graded on a scale of 0 to 4. No necrosis, congestion, or centrilobular ballooning is given a score of 0, whereas severe congestion/ballooning and >60% lobular necrosis is given a value of 4.
Immunohistochemistry
Liver specimens were embedded in optimal cutting temperature compound (Tissue-Tec, Sakura Finetek, Inc., CA), snap frozen, and stored at −70°C. Cryostat sections (5 μm thick) were fixed in acetone, and then appropriate primary mouse antibody (Ab) against leukocytes (CD45), T cells (CD3) (1:50 dilution; BD Pharmingen, San Diego, CA), and macrophages (1:200 dilution; Biosource International, Camarillo, CA) was added. Specimens were incubated with a species-specific peroxidase-conjugated secondary Ab (Jackson Immunoresearch, Westgrove, PA). The specimens were counterstained in hematoxylin and were mounted in glycerol gelatin. The negative control was prepared by omitting primary Ab.
Neutrophil Infiltration
The activity of myeloperoxidase (MPO), an enzyme specific for polymorphonuclear neutrophils (PMNs), was used as an index of hepatic neutrophil accumulation.18 Briefly, the frozen tissue was thawed and placed in iced 0.5% hexadecyltrimethyl-ammonium bromide and 50 mmol of potassium phosphate buffer solution (pH 5.0). Each sample was homogenized and centrifuged at 15,000 rpm for 15 minutes at 4°C. Supernatants were then mixed with hydrogen peroxide-sodium acetate and tetramethyl-benzidine solutions. The change in absorbance was measured by spectrophotometry at 655 nm. One unit of MPO activity was defined as the quantity of enzyme degrading 1 μmol peroxide/min at 25°C/g tissue.
RNA Extraction/Competitive Template Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
To study target gene expression patterns, we used competitive template RT-PCR, as described previously.20 Briefly, total RNA was extracted from frozen liver tissue samples using RNase Mini kit (Qiagen Inc., Chatsworth, CA), and RNA concentration was determined by a spectrophotometer. Five micrograms of RNA was reverse-transcribed using oligo (dT) primers and superscript reverse transcriptase (Invitrogen Life Technologies, CA). The resulting cDNA (1 μg) was used as a template for subsequent PCR. Primers used in PCR were as follows: tumor necrosis factor-α (TNF-α) sense (5′-GGC AGG TCT ACT TTG GAG TCA TTG C-3′) and TNF-α antisense (5′-ACA TTC GAG GCT CCA GTG AAT TCG G-3′); Interferon-γ (IFN-γ) sense (5′-AGC GGC TGA CTG AAC TCA GAT TGT AG-3′) and IFN-γ antisense (5′-GTC ACA GTT TTC AGC TGT ATA GGG-3′); E-selectin sense (5′-CTC TGA CAG AAG AAG CCA AG-3′) and E-selectin antisense (5′-ACT TGA GTC CAC TGA AGC CA-3′); IFN-inducible protein-10 (IP-10) sense (5′-TTA CCC AGT GGA TGG TGG CTA GTC CTA-3′) and IP-10 antisense (5′-CCC TTG GGA AGA TGG TGG TT-3′); and β-actin sense (5′-GTG GGC CGC TCT AGG CAC CA-3′) and β-actin antisense (5′-CGG TTG GCC TTA GGG TTC AGG GGG-3′). The RT2 PCR Primer Set (Superarray, Frederick, MD) was used for VEGF-A and monocyte chemoattractant protein (MCP)-1 (CCL2). PCR was performed by different cycle numbers at the annealing temperature optimized for each primer pair: 35 cycles, 55°C (VEGF-A); 35 cycles, 60°C (TNF-α); 37 cycles, 63°C (IFN-γ); 35 cycles, 60°C (E-selectin); 33 cycles, 53°C (IP-10); 35 cycles, 55°C (MCP-1); and 35 cycles, 60°C (β-actin), respectively. PCR products were analyzed in ethidium bromide-stained 2% agarose gel and scanned using Kodak Digital Science 1D Analysis software (version 2.0). To compare relative levels of each gene, all samples were normalized against the β-actin template cDNA ratio.
Western Blots
Protein was extracted from livers with ice-cold PBSTDS (1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) in PBS (pH 7.4)) buffer. Proteins (40 μg/sample) in sodium dodecyl sulfate-loading buffer were subjected to 10 to 20% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). The membrane was blocked with 5% dry milk and 0.1% Tween 20 (USB, Cleveland, OH). Polyclonal rabbit anti-mouse VEGF, Bcl-2, Bcl-xl, Bax (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), heme oxygenase-1 (HO-1) (StressGen Biotech, Victoria, BC, Canada), and monoclonal mouse anti-β-actin Abs (Abcam Inc., Cambridge, MA) were used. Relative quantities of protein were determined using a densitometer (Kodak Digital Science 1D Analysis Soft-ware, Rochester, NY) and presented in comparison with β-actin expression.
Apoptosis Assay
Apoptosis was detected using the terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) method. Cryostat sections (5 μm thick) of liver were investigated using the ApopTag Peroxydase kit (Chemicon International, Inc., Temecula, CA). The peroxidase activity was visualized with diaminobenzidine substrate, yielding a brown oxidation product; methyl green was used for counterstaining. The results were scored semiquantitatively by averaging the number of TUNEL+ cells/field at a magnification of ×200. Six fields/tissue sample were evaluated.
Statistical Analysis
All data are expressed as mean ± SD. Differences between experimental groups were analyzed using one-way analysis of variance or Student’s t-test for unpaired data. All differences were considered statistically significant at the P value of <0.05.
Results
Local VEGF Expression in Hepatic I/R Injury
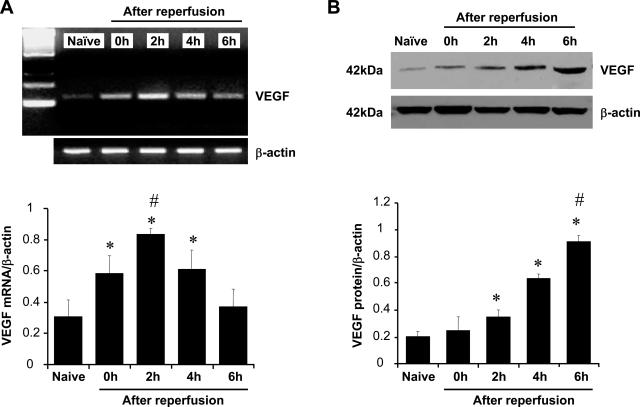
We used competitive template RT-PCR and Western blots to analyze the kinetics of endogenous VEGF production in a well-established mouse model of partial hepatic warm ischemia followed by reperfusion. As shown in Figure 2A, 90 minutes of ischemia alone (0 hours) triggered a significant increase of mRNA coding for VEGF in the liver compared with naïve controls (P < 0.05). Its expression increased further in the postischemic period, peaked at 2 hours of reperfusion (P < 0.05), and decreased thereafter. As shown in Figure 2B, VEGF protein levels increased progressively during ischemia and 6 hours of reperfusion period (P < 0.01 compared with naïve controls). Expression of VEGF was most notable locally on hepatocytes in periductal areas (data not shown)
Figure 2.
Kinetics of VEGF expression in mouse liver after 90 minutes of warm ischemia followed by 6 hours of reperfusion. A: Competitive-template RT-PCR analysis. The expression of VEGF mRNA was significantly up-regulated after 90 minutes of ischemia (0 hours) and peaked at 2 hours of reperfusion compared with naïve control liver. It decreased at 6 hours after reperfusion (mean ± SD; n = 4/time-point). *P < 0.05 versus naïve, #P < 0.05 versus 0 or 4 hours. B: Western blot analysis. The expression of VEGF protein was significantly up-regulated beginning at 2 hours of reperfusion with peak up-regulation taking place at 6 hours of reperfusion (mean ± SD; n = 4). *P < 0.01 versus naïve, #P < 0.01 versus 0, 2, and 4 hours.
VEGF Antagonist Ameliorates Hepatic I/R Injury
We next assessed whether VEGF blockade protected mouse livers against I/R injury. We found that 90 minutes of hepatic warm ischemia and 6 hours of reperfusion increased sGPT levels compared with sham controls (Figure 3; 3334 ± 549 and 48 ± 16 IU/L, respectively; P < 0.01). Treatment with anti-VEGF markedly decreased sGPT levels (500 ± 106 IU/L; P < 0.01). Livers were also analyzed by histology, and hepatocellular damage was graded using Suzuki’s criteria.19 The ischemic lobes in the control group showed moderate to severe hepatocyte necrosis and sinusoidal congestion (Figure 4, C and D; score, 7.96 ± 1.08). In contrast, the anti-VEGF group revealed minimal necrosis/sinusoidal congestion and almost complete preservation of lobular architecture (Figure 4, E and F; score, 1.00 ± 0.87; P < 0.01).
Figure 3.
Serum GPT levels (IU/L). The sGPT levels were significantly lower in anti-VEGF serum-treated mice compared with control mice (mean ± SD; n = 4–9/group). *P < 0.01 versus control group.
Figure 4.
Photomicrograms of representative mouse livers. A and B: Sham group. C and D: Control group with severe sinusoidal congestion and hepatocyte necrosis (Suzuki’s score = 7.97 ± 1.08). E and F: Anti-VEGF group with good preservation of lobular architecture without edema, congestion, or absence of centrilobular necrosis (Suzuki’s score = 1.00 ± 0.87). H&E stain; representative of four to nine per group.
Evaluation of VEGF Gene and Protein Expression after VEGF Antagonist Therapy
Competitive template RT-PCR and Western blot analysis were performed at 6 hours of reperfusion to measure VEGF gene and protein expression, respectively. Treatment with anti-VEGF serum did not affect VEGF mRNA expression compared with sham and control groups (Figure 5A). As shown in Figure 5B, VEGF protein expression significantly decreased in anti-VEGF serum-treated animals compared with controls (P < 0.01).
Figure 5.
VEGF mRNA/protein expression after anti-VEGF serum treatment (at 6 hours of reperfusion). A: Competitive-template RT-PCR analysis. No significant differences in mRNA coding for VEGF were observed among sham, control, and treatment groups (mean ± SD; n = 4–6/group). B: Western blot analysis. The expression of VEGF protein was significantly increased in the control and anti-VEGF serum treatment groups compared with sham group. Anti-VEGF decreased VEGF protein synthesis compared with controls. Results are representative of four different experiments. *P < 0.01 versus sham. #P < 0.01.
VEGF Antagonist Prevents Leukocyte Infiltration
To determine how anti-VEGF treatment affected local leukocyte infiltration, we assessed PMN infiltration using an MPO assay and mononuclear cell infiltration by immunohistology. As shown in Figure 6, we found that MPO activity was significantly reduced in anti-VEGF-treated animals after 6 hours of reperfusion (3.10 ± 0.56 versus 6.82 ± 1.34 U/g; P < 0.05) compared with control group. We also found marked immunohistochemical staining for T cells and macrophages in animals treated with control Ab (Figure 7, B, E, and H) compared with those pretreated with anti-VEGF serum (Figure 7, C, F, and I).
Figure 6.
Intrahepatic neutrophil accumulation. MPO enzyme activity levels (U/g) were significantly lower in anti-VEGF serum treatment group compared with control group. These data represent the mean ± SD of four to six experiments. *P < 0.05.
Figure 7.
Immunohistochemical staining for intrahepatic leukocytes (CD45), T cells (CD3), and macrophages (Mac). Control serum-treated mice with increased intrahepatic leukocyte (B), T-cell (E), and macrophage (H) infiltration. In contrast, mice treated with anti-VEGF Ab revealed minimal leukocyte (C), T-cell (F), and macrophage (I) infiltration. Representative of four animals per group. Original magnification, ×400.
VEGF Antagonist Decreases Pro-Inflammatory Gene Expression Patterns
We next used competitive-template RT-PCR to analyze cytokine (TNF-α and IFN-γ), chemokine (IP-10 and MCP-1), and adhesion molecule (E-selectin) gene expression in the ischemic liver lobes. As shown in Figure 8, the control group revealed significantly increased levels of TNF-α (0.73 ± 0.14 versus 0.13 ± 0.02; P < 0.01), IFN-γ (0.63 ± 0.04 versus 0.09 ± 0.02; P < 0.01), IP-10 (0.81 ± 0.13 versus 0.18 ± 0.08; P < 0.01), MCP-1 (0.69 ± 0.16 versus 0.24 ± 0.10; P < 0.01), and E-selectin (0.53 ± 0.08 versus 0.07 ± 0.02; P < 0.01) compared with sham controls. Treatment with anti-VEGF serum decreased hepatic expression of mRNA coding for TNF-α (0.29 ± 0.08), IFN-γ (0.15 ± 0.06), IP-10 (0.52 ± 0.20), MCP-1 (0.41 ± 0.12), and E-selectin (0.19 ± 0.08) compared with controls (P < 0.05).
Figure 8.
Competitive template RT-PCR-assisted expression of mRNA coding for TNF-α, IFN-γ, IP-10, MCP-1, and E-selectin. The control group shows significant up-regulation of pro-inflammatory cytokines (TNF-α and IFN-γ), chemokines (IP-10 and MCP-1), and E-selectin expression compared with those treated with anti-VEGF Ab. These data represent the mean ± SD of four to six experiments. *P < 0.05.
VEGF Antagonist Prevents Apoptosis
Apoptosis of hepatocytes was evaluated by TUNEL staining of livers after ischemia and 6 hours reperfusion. We found a large number of apoptotic cells (20.1 ± 7.3 TUNEL+ cells/field; Figure 9, B and D) in control serum-treated animals. In contrast, anti-VEGF serum profoundly decreased the frequency of apoptotic cells (4.3 ± 1.8 TUNEL+ cells/field, P < 0.01; Figure 9, C and D). Using Western blot, we also analyzed the expression of anti-apoptotic (Bcl-2/Bcl-xl), pro-apoptotic (Bax), and antioxidant (HO-1) gene products. The relative expression levels were determined by densitometry and expressed as ratios to β-actin as a housekeeping gene. As shown in Figure 10, A and B, the expression of Bcl-2/Bcl-xl and HO-1 was strongly enhanced in anti-VEGF treatment group (0.38 ± 0.11, 0.45 ± 0.11, and 0.61 ± 0.07, respectively; P < 0.01) compared with controls (0.13 ± 0.02, 0.14 ± 0.04, and 0.21 ± 0.10, respectively; P < 0.01). In contrast, the anti-VEGF treatment group suppressed Bax expression (0.23 ± 0.06 versus 1.11 ± 0.08; P < 0.01) compared with control group.
Figure 9.
TUNEL-assisted detection of apoptosis. A: Sham group. B: Control group with large numbers of TUNEL+ cells (dark brown spots). C: Anti-VEGF group with markedly decreased frequency of TUNEL+ cells. D: The results were scored semiquantitatively by averaging the number of TUNEL+ cells (mean ± SD) per microscopic field at magnification ×400. Minimum of 10 fields were evaluated per sample in four different experiments. *P < 0.01.
Figure 10.
Western blot-assisted analysis of anti-apoptotic (Bcl-2/Bcl-xl), pro-apoptotic (Bax), and antioxidant (HO-1) proteins. A: Bcl-2, Bcl-xl, Bax, and HO-1 proteins were detected by polyclonal rabbit anti-mouse Abs. Antibody against β-actin was used as internal control. B: Each bar graph shows the ratio of protein and β-actin expression. Bcl-2/Bcl-xl and HO-1 expression was increased in anti-VEGF group compared with controls. The expression of Bax was suppressed by VEGF antagonist. Results are representative of four different experiments. *P < 0.01.
Discussion
This study provides new insights into the emerging role of VEGF in the pathophysiology of liver I/R injury. Our findings indicate that VEGF is increased during the reperfusion period of I/R injury and that its expression is functional in leukocyte trafficking and the liver injury response. Moreover, our findings are suggestive that blockade of VEGF with an anti-VEGF antiserum can ameliorate the associated liver injury. In addition, our studies demonstrate that VEGF may function via the expression of pro-inflammatory cytokines (TNF-α/IFN-γ), chemokines (IP-10/MCP-1), and endothelial activation responses (E-selectin) and that blockade of VEGF has biological effects to limit intrahepatic apoptosis and promote anti-apoptotic (Bcl-2/Bcl-xl)/antioxidant (HO-1) protective genes.
Leukocyte-endothelial cell interactions are well established to play a role in the pathophysiology of hepatic I/R injury.1,2 Initial leukocyte tethering in sinusoidal venules requires expression of selectins on endothelial cells. In venular endothelial cells, P-selectin/E-selectin and intracellular adhesion molecule-1 (ICAM-1) become up-regulated by I/R. These molecules participate in the rolling of leukocytes onto endothelial cells during an inflammatory reaction. Leukocyte infiltration is augmented by the release of cytokines and chemokines derived in part from infiltrating monocytes/macrophages and T cells, which can increase the expression of P-selectin/E-selectin and ICAM-1. VEGF may activate this inflammatory cascade and thus promote the development of I/R injury. It is known that VEGF induces the expression of adhesion molecules (E-selectin, ICAM-1, and VCAM-1) and chemokines (monocyte chemoattractant protein 1 [MCP-1], interleukin [IL]-8, and IFN-inducible protein-10 [IP-10]) both in vitro and in vivo.4 In our analyses, anti-VEGF treatment markedly reduced intrahepatic expression of adhesion molecule and chemokine expression. Furthermore, intrahepatic infiltration of leukocytes including PMNs (MPO assay), macrophages (Mac), and T cells (CD3) was markedly suppressed in anti-VEGF-treated mice compared with control mice. Thus, we propose that a major mechanism underlying the pro-inflammatory action of VEGF in hepatic I/R injury involves leukocyte trafficking and local activation.
In addition to leukocyte recruitment/activation, apoptosis mediated by TNF-α, Fas ligand, cytokines, chemokines, and the generation of reactive oxygen species represents another key mechanism of I/R injury.21 Consistent with the pro-inflammatory function of VEGF to stimulate apoptosis during hepatic warm I/R injury, blockade of VEGF in this study markedly diminished the apoptotic pathway. One possible anti-apoptotic mechanism of anti-VEGF treatment might be associated with suppression of infiltration and leukocyte-induced apoptosis mechanisms. TNF-α, interleukins, and reactive oxygen species, established participants in the development of apoptosis, are all delivered into the local site by monocyte/macrophages and are released by activated Kupffer cells. The VEGF antagonist in our study also triggered an increased expression of anti-apoptotic (Bcl-2/Bcl-xl) and antioxidant (HO-1) proteins and diminished the expression of pro-apoptotic Bax protein. This is consistent with a role for VEGF in the regulation of the balance between pro- and anti-apoptotic mediators in hepatic I/R injury. Gene therapy-induced anti-apoptotic Bcl-2 or Bag-1 induction can also prevent hepatic I/R injury,22,23 and the down-regulation of pro-apoptotic Bax has been found to decrease apoptosis after cardiac I/R injury.24 Moreover, our previous studies have shown that pharmacological or gene therapy-induced HO-1 expression prevents hepatic, cardiac, and renal I/R injury via the anti-apoptotic pathway.25 In addition, other agents known to limit hepatic I/R such as CD154-CD40 blockade also increase anti-apoptotic Bcl2/Bcl-xl and antioxidant HO-1 expression, suggesting that a common mechanism by which all of these agents protect against hepatic damage may involve prevention of apoptosis in the course of I/R injury.20,26
VEGF is a potent mediator of physiological and pathological angiogenesis.27,28 It was originally cloned and identified as a vascular permeability factor because of its ability to induce vascular leak. Based on its profound angiogenic properties, it is a powerful agent to reverse critical limb ischemia, myocardial ischemia, and nonhealing skin ulcers.29 However, some recent studies have raised concerns about the harmful in vivo effects of VEGF that may in part relate to its pro-inflammatory functions.30–33 Interestingly, a number of cytokines (such as TNF-α, IL-1β, and IL-6), hormones, and cell surface molecules (eg, CD154 and CD40) known to be expressed and functional in I/R are known also to regulate VEGF expression.27,34 For instance, stimulation of CD40 is a major and potent signal for VEGF expression,35,36 and we have recently reported the importance of CD154-CD40 signaling in the mechanism of hepatic I/R injury.9,20,26 Consistent with this possibility, disruption of CD154-CD40 signaling after gene therapy, pharmacological blockade, or genetic engineering prevented hepatic I/R injury and decreased VEGF levels.9 Thus, it is possible that CD154-CD40 signaling functions to increase VEGF expression and thus to promote VEGF-dependent hepatic warm I/R injury and severe hepatic damage. Together, the findings identified in this study and those reported by others using rat10 and mouse9,11 models support the concept that the inducible expression of VEGF is an important underlying mechanism in I/R injury.
The time points used for analysis in the present study revealed that VEGF gene expression increased after 90 minutes of warm ischemia and peaked 2 hours after reperfusion. This is suggestive that ischemia alone is a weak inducer of VEGF and that most of its functional effects occur at later times in association with reperfusion. Given these results, we believe that it is most likely that VEGF is induced by several of the factors known to be of functional importance in I/R and that it acts to augment and further to amplify the response. Our results suggest that antiserum-mediated suppression of VEGF occurred via down-regulation of inflammatory response of VEGF in hepatic I/R. Together, these findings indicate that VEGF may be a major and central mediator of injury response.
The mechanism by which VEGF functions in I/R injury remains controversial. Boros et al10 reported increased local VEGF expression in a cold liver I/R model and that anti-VEGF treatment decreased the hepatic damage. Also, in agreement with our present data, Tsurui et al11 reported that hepatic warm I/R injury up-regulated local VEGF. However, they found that systemic exogenous infusion of VEGF exerted cytoprotective effects, as it did earlier in a model of myocardial I/R injury.37 This unexpected effect may have resulted, at least in part, from VEGF-dependent NO synthesis by vascular endothelial cells.38 Another possibility (as suggested11) is that systemically administered VEGF likely binds to VEGF receptors expressed on circulating leukocytes and inhibits their ability to respond to local VEGF expressed in the inflammatory site. In this scenario, the systemic administration of VEGF may function as an inhibitor of local VEGF-mediated leukocyte trafficking. Endogenous VEGF expression is mediated by hypoxia-inducible factor-1 (HIF-1). Redaelli et al39 have reported that VEGF gene transfer decreased HIF-1 and provided functional hepatic recovery after partial hepatectomy. The involvement of HIF-1 in the activation of VEGF has been reported.40 In addition, signal transducer and activation of transcription-3 might also modulate HIF-1-mediated VEGF expression.41 Collectively, all of these findings suggest that local endogenous VEGF is an important factor initiating and pro-moting hepatic I/R injury, and its blockade results in cytoprotection.
In summary, anti-VEGF treatment inhibited hepatic damage and reduced inflammatory responses and apoptosis in a stringent mouse liver model of warm I/R injury. These beneficial effects involve several mechanisms including the up-regulation of antioxidant/anti-apoptotic cytoprotective genes and simultaneous inhibition of intrahepatic leukocyte accumulation and their activation. Thus, by modulating leukocyte trafficking, VEGF plays a paramount role in the pathophysiology of hepatic I/R injury. These results provide the rationale for improved therapeutic approaches to prevent hepatic I/R injury and thus increase the potential liver transplant donor pool.
Footnotes
Address reprint requests to Jerzy W. Kupiec-Weglinski, M.D., Ph.D., The Dumont-UCLA Transplant Center, 7–120 CHS, Box 957054, 10833 Le Conte Ave., Los Angeles, CA 90095-7054. E-mail: jkupiec@mednet.ucla.edu.
Supported by National Institutes of Health grants RO1-DK-062357, AI23847, and AI42223 (to J.W.K.-W.) and R01-HL74436 (to D.M.B.) and by The Dumont Research Foundation.
D.M.B. and J.W.K.-W. contributed equally to this work.
References
- Farmer DG, Amersi F, Busuttil RW, Kupiec-Weglinski JW. Current concepts in ischemia and reperfusion injury in the liver. Transplant Rev. 2000;14:106–126. [Google Scholar]
- Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury: a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol. 2003;18:891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- Reinders ME, Sho M, Izawa A, Wang P, Mukhopadhyay D, Koss KE, Geehan CS, Luster AD, Sayegh MH, Briscoe DM. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J Clin Invest. 2003;112:1655–1665. doi: 10.1172/JCI17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270:13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Shweiki D, Neeman M, Itin A, Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis, Proc Natl Acad Sci USA. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Ke B, Shen XD, Gao F, Tsuchihashi S, Farmer D, Briscoe DM, Busuttil RW, Kupiec-Weglinski JW. The CD154-CD40 T cell costimulation pathway in liver ischemia and reperfusion inflammatory responses. Transplantation. 2005;79:1110–1115. doi: 10.1097/01.tp.0000161248.43481.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros P, Tarcsafalvi A, Wang L, Megyesi J, Liu J, Miller CM. Intrahepatic expression and release of vascular endothelial growth factor following orthotopic liver transplantation in the rat. Transplantation. 2001;72:805–811. doi: 10.1097/00007890-200109150-00011. [DOI] [PubMed] [Google Scholar]
- Tsurui Y, Sho M, Kuzumoto Y, Hamada K, Akashi S, Kashizuka H, Ikeda N, Nomi T, Mizuno T, Kanehiro H, Nakajima Y. Dual role of vascular endothelial growth factor in hepatic ischemia-reperfusion injury. Transplantation. 2005;79:1110–1115. doi: 10.1097/01.tp.0000161627.84481.5e. [DOI] [PubMed] [Google Scholar]
- Abraham D, Taghavi S, Riml P, Paulus P, Hofmann C, Baumann C, Kocher A, Klepetko W, Aharinejad S. VEGF-A and -C but not -B mediate increased vascular permeability in preserved lung grafts. Transplantation. 2002;73:1703–1706. doi: 10.1097/00007890-200206150-00003. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay D, Knebelmann B, Cohen HT, Ananth S, Sukhatme VP. The von Hippel-Lindau tumor suppressor gene product interacts with Sp1 to repress vascular endothelial growth factor promoter activity. Mol Cell Biol. 1997;17:5629–5639. doi: 10.1128/mcb.17.9.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995;375:577–581. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- Pal S, Claffey K, Dvorak HF, Mukhopadhyay D. The von Hippel-Lindau gene product inhibits vascular permeability factor/vascular endothelial growth factor expression in renal cell carcinoma by blocking protein kinase C pathways. J Biol Chem. 1997;272:27509–27512. doi: 10.1074/jbc.272.44.27509. [DOI] [PubMed] [Google Scholar]
- Zarember KA, Malech HL. HIF-1alpha: a master regulator of innate host defenses? J Clin Invest. 2005;115:1702–1704. doi: 10.1172/JCI25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton RG, Kawamura T, Chang KC, Ido Y, Bjercke RJ, Stephan CC, Brock TA, Williamson JR. Vascular dysfunction induced by elevated glucose levels in rats is mediated by vascular endothelial growth factor. J Clin Invest. 1997;99:2192–2202. doi: 10.1172/JCI119392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, Busuttil RW, Kupiec-Weglinski JW. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury: modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- Ke B, Shen XD, Gao F, Busuttil RW, Lowenstein PR, Castro MG, Kupiec-Weglinski JW. Gene therapy for liver transplantation using adenoviral vectors: CD40-CD154 blockade by gene transfer of CD40Ig protects rat livers from cold ischemia and reperfusion injury. Mol Ther. 2003;9:38–45. doi: 10.1016/j.ymthe.2003.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudiger HA, Graf R, Clavien PA. Liver ischemia: apoptosis as a central mechanism of injury. J Invest Surg. 2003;16:149–159. [PubMed] [Google Scholar]
- Bilbao G, Contreras JL, Eckhoff DE, Mikheeva G, Krasnykh V, Douglas JT, Thomas FT, Thomas JM, Curiel DT. Reduction of ischemia-reperfusion injury of the liver by in vivo adenovirus-mediated gene transfer of the antiapoptotic Bcl-2 gene. Ann Surg. 1999;230:185–193. doi: 10.1097/00000658-199908000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawitzki B, Amersi F, Ritter T, Fisser M, Shen XD, Ke B, Busuttil RW, Volk HD, Kupiec-Weglinski JW. Upregulation of Bag-1 by ex vivo gene transfer protects rat livers from ischemia/reperfusion injury. Hum Gene Ther. 2002;13:1495–1504. doi: 10.1089/10430340260185120. [DOI] [PubMed] [Google Scholar]
- Wang N, Minatoguchi S, Chen X, Uno Y, Arai M, Lu C, Takemura G, Fujiwara T, Fujiwara H. Antidiabetic drug miglitol inhibits myocardial apoptosis involving decreased hydroxyl radical production and Bax expression in an ischaemia/reperfusion rabbit heart. Br J Pharmacol. 2004;142:983–990. doi: 10.1038/sj.bjp.0705863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katori M, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 system in organ transplantation. Transplantation. 2002;74:905–912. doi: 10.1097/00007890-200210150-00001. [DOI] [PubMed] [Google Scholar]
- Shen XD, Ke B, Zhai Y, Amersi F, Gao F, Anselmo DM, Busuttil RW, Kupiec-Weglinski JW. CD154-CD40 T-cell costimulation pathway is required in the mechanism of hepatic ischemia/reperfusion injury, and its blockade facilitates and depends on heme oxygenase-1 mediated cytoprotection. Transplantation. 2002;74:315–319. doi: 10.1097/00007890-200208150-00005. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Isner J, Pieczek A, Schainfeld R, Blair R, Haley L, Asahara T, Rosenfield K, Razvi S, Walsh K, Symes J. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet. 1996;348:370–374. doi: 10.1016/s0140-6736(96)03361-2. [DOI] [PubMed] [Google Scholar]
- Celletti F, Waugh J, Amabile P, Brendolan A, Hilfiker P, Dake M. Vascular endothelial growth factor enhances atherosclerotic plaque progression. Nat Med. 2001;7:425–429. doi: 10.1038/86490. [DOI] [PubMed] [Google Scholar]
- Detmar M, Brown LF, S MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Epstein S, Kornowski R, Fuchs S, Dvorak H. Angiogenesis therapy: amidst the hype, the neglected potential for serious side effects. Circulation. 2001;104:115–119. doi: 10.1161/01.cir.104.1.115. [DOI] [PubMed] [Google Scholar]
- Thurston G, Suri C, Smith K, McClain J, Sato T, Yancopoulos G, McDonald D. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- Uchida J, Kanai K, Asano K, Watanabe S, Hisamitsu T, Suzaki H. Influence of fluticasone propionate on the production of vascular endothelial growth factor and basic fibroblast growth factor from nasal fibroblasts in vitro. In Vivo. 2004;18:767–770. [PubMed] [Google Scholar]
- Melter M, Reinders ME, Sho M, Pal S, Geehan C, Denton MD, Mukhopadhyay D, Briscoe DM. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood. 2000;96:3801–3808. [PubMed] [Google Scholar]
- Reinders ME, Sho M, Robertson SW, Geehan CS, Briscoe DM. Proangiogenic function of CD40 ligand-CD40 interactions. J Immunol. 2003;171:1534–1541. doi: 10.4049/jimmunol.171.3.1534. [DOI] [PubMed] [Google Scholar]
- Luo Z, Diaco M, Murohara T, Ferrara N, Isner J, Symes J. Vascular endothelial growth factor attenuates myocardial ischemia-reperfusion injury. Ann Thorac Surg. 1997;64:993–998. doi: 10.1016/s0003-4975(97)00715-7. [DOI] [PubMed] [Google Scholar]
- van der Zee R, Murohara T, Luo Z, Zollmann F, Passeri J, Lekutat C, Isner JM. Vascular endothelial growth factor/vascular permeability factor augments nitric oxide release from quiescent rabbit and human vascular endothelium. Circulation. 1997;95:1030–1037. doi: 10.1161/01.cir.95.4.1030. [DOI] [PubMed] [Google Scholar]
- Redaelli CA, Semela D, Carrick FE, Ledermann M, Candinas D, Sauter B, Dufour JF. Effect of vascular endothelial growth factor on functional recovery after hepatectomy in lean and obese mice. J Hepatol. 2004;40:305–312. doi: 10.1016/j.jhep.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–633. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK, Chung MH. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]