Abstract
Previous studies have demonstrated that peptidic and nonpeptidic δ-opioid receptor agonists have different effects depending on the measure. For example, nonpeptidic δ-opioid agonists, but not peptidic agonists, produce convulsions in rats, and in vitro studies suggested that peptidic and nonpeptidic δ-opioid agonists might have differential mechanisms of receptor down-regulation. The present study evaluated potential differences between peptidic and nonpeptidic δ-opioid agonists in their ability to activate G proteins using guanosine 5′-O-(3-[35S]thio)triphosphate ([35S]GTPγS) autoradiography experiments in rat brain slices. The peptidic agonist [D-Pen2,D-Pen5]-enkephalin and the nonpeptidic agonist (+)BW373U86 [(+)-4-[α(R)-α-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-hydroxyphenyl)methyl]-N,N-diethylbenzamide] demonstrated concentration-dependent increases in [35S]GTPγS binding that were attenuated by the δ-opioid antagonist naltrindole. (+)BW373U86 was more potent and efficacious than the peptidic agonist, and this difference remained consistent across brain regions where significant stimulation was observed. In addition, multiple δ-opioid compounds were evaluated for their agonist activity in this assay. These data suggested that differences between peptidic and nonpeptidic δ-opioid agonists in behavioral studies were most likely caused by differences in agonist efficacy. Finally, these data also revealed that [35S]GTPγS autoradiography could be used to compare efficacy differences among agonists across various brain regions in rat brain slices.
ABBREVIATIONS: [35S]GTPγS, 5′-O-(3-[35S]thio)triphosphate; DPDPE, [D-Pen2,D-Pen5]-enkephalin; SNC80, (+)-4-[α(R)-α-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide; OMI, oxymorphindole; SIOM, spiroindanyloxymorphone; (+)BW373U86, (+)-4-[α(R)-α-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-hydroxyphenyl)methyl]-N,N-diethylbenzamide; NSB, nonspecific binding; NTI, naltrindole; TAN67, 2-methyl-4aa-(3-hydroxyphenyl)-1,2,3,4,4a,5,12,12aα-octahydroquinolino[2,3,3-g]isoquinoline dihydrobro-mide (SB205607); FrCtx, frontal cortex; PCtx, prelimbic cortex; CCtx, cingulate cortex; NAcc nucleus accumbens; CP, caudate putamen; HPC, hippocampus; Amyg, amygdala; PirCtx, piriform cortex; Thal, thalamus; Hypothal, hypothalamus; SN, substantia nigra; PAG, periaqueductal gray; PN, pontine nuclei
The δ-opioid receptor is a member of the seven transmembrane G protein-coupled receptor superfamily and is coupled to inhibitory G proteins. Activation of this receptor induces many physiological and behavioral effects ranging from antinociception, potential changes in mood, modulation of the digestive system, and sensory perception. A number of peptidic and nonpeptidic δ-opioid receptor-selective compounds have been used to characterize the in vitro and in vivo effects of δ-opioid receptor activation; however, few studies have directly compared these compounds in terms of agonist efficacy (e.g., Chang et al., 2004).
δagonist efficacy can be determined at the level of receptor-G protein interaction by measuring agonist-stimulated binding of the nonhydrolysable GTP analog [35S]GTPγS. The peptidic δ-opioid agonists [D-Pen2, D-Pen5]enkephalin (DPDPE) and pCl-DPDPE stimulated similar levels of [35S]GTPγS binding as the nonpeptidic δ-opioid agonist SNC80 in Chinese hamster ovary cells expressing the human δ-opioid receptor, suggesting equivalent efficacies (Quock et al., 1997; Xu et al., 2001). In contrast, in C6 cells stably expressing the rat δ-opioid receptor, Clark et al. (1997) reported that DPDPE and pCl-DPDPE were less efficacious than SNC80. The peptidic δ-opioid agonist deltorphin II produced similar levels of stimulation as DPDPE, and nonpeptidic agonists oxymorphindole (OMI) and spiroindanyloxymorphone (SIOM) were very weak agonists (Clark et al., 1997). Likewise, in [35S]GTPγS binding assays in mouse brain homogenates (Hosohata et al., 2000) and guinea pig caudate membranes (Thomas et al., 1999), DPDPE was less efficacious than SNC80.
Behavioral experiments have shown differences and similarities between peptidic and nonpeptidic δ-opioid agonists depending on the test. For example, peptidic and nonpeptidic agonists have antinociceptive properties in hyperalgesia assays and allodynia models in mice (Wild et al., 1993; Hong et al., 1998; Broom et al., 2002c) and rats (Kovelowski et al., 1999; Fraser et al., 2000). In rhesus monkeys, nonpeptidic δ-opioid agonists demonstrated antinociceptive effects under conditions of chemically induced hypersensitivity (Butelmen et al., 1995; Brandt et al., 2001), although these data lack comparisons with peptidic δ-opioid agonists. In addition, in-tracerebroventricular (i.c.v) administration of SNC80 or deltorphin II produced similar profiles of locomotor activity in rats (Fraser et al., 2000a).
In contrast, nonpeptidic δ-opioid agonists produce overt convulsions in mice (Comer et al., 1993a; Hong et al., 1998), rats (Broom et al., 2002a,b), and monkeys (Dykstra et al., 1993; Negus et al., 1994; Pakarinen et al., 1995); however, peptidic δ-opioid agonists did not produce observable convulsions but produced epileptic discharges that were associated with myoclonic contractions, wet dog shakes, and loss of balance in rats (Haffmans and Dzoljic, 1983; DeSarro et al., 1992). Likewise, in pigeons trained to discriminate the nonpeptidic δ-opioid agonist BW373U86, DPDPE and [D-Ser2,Leu5]-enkephalin-Thr did not substitute completely for BW373U86 (Comer et al., 1993b), and in pigeons trained to discriminate i.c.v. injections of DPDPE, BW373U86 only partially generalized (Jewett et al., 1996).
One rationale for at least some of the observed differences between peptides and nonpeptides is that different agonists may have class-specific interactions with the δ-opioid receptor (Okura et al., 2003). For example, δ ligands may differentially activate δ-opioid receptor sites in different brain regions, thus producing different behavioral effects. One caveat to this hypothesis could be that peptidic and nonpeptidic δ-opioid agonists may differ in efficacy.
To address these issues, the present study investigated the effects of peptidic and nonpeptidic δ-opioid agonists on G protein activation in rat brain slices, as measured by the stimulation of [35S]GTPγS binding. These compounds were compared in terms of agonist efficacy as well as regional binding throughout the rat brain. Previous work with δ-opi-oid agonists in [35S]GTPγS autoradiography experiments used mainly DPDPE or pCl-DPDPE to stimulate G protein activation (Sim et al., 1996b; Park et al., 2000; Sim-Selley et al., 2002), and one study investigated the effects of SNC80 and related compounds on [35S]GTPγS binding in rat brain slices (Jutkiewicz et al., 2004). Comparing across studies, the nonpeptidic δ-opioid agonists seem to stimulate [35S]GTPγS binding to a greater extent than DPDPE. However, at present, no direct comparisons have been made between peptidic and nonpeptidic δ-opioid agonists in [35S]GTPγS autoradiography studies. Therefore, this study compared efficacy and regional differences between the peptidic δ-opioid agonist DPDPE and the active isomer of the nonpeptidic agonist (+)BW373U86 in their ability to stimulate [35S]GTPγS binding. Overall, (+)BW373U86 was more potent and efficacious than DPDPE in stimulating [35S]GTPγS binding. Consequently, DPDPE produced very low, if any, stimulation of G protein binding in brain regions with lower δ-opioid receptor expression. Finally, δ-opioid agonists can be rank-ordered by efficacy using [35S]GTPγS autoradiography.
Materials and Methods
Subjects
Male Sprague-Dawley rats (250–350 g) were obtained from Harlan (Indianapolis, IN) and housed in groups of three rats per cage. All animals were fed on a standard laboratory diet and maintained on a 12-h light/dark cycle, with lights on at 6:30 AM at an average temperature of 21°C. Studies were performed in accordance with the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health. The experimental protocols were approved by the University of Michigan University Committee on the Use and Care of Animals.
Agonist-Stimulated [35S]GTPγS Autoradiography
Agonist-stimulated [35S]GTPγS autoradiography was performed as previously described (Sim et al., 1995). Naive rats were decapitated, and brains were removed and fresh-frozen in 2-methylbutane (Sigma-Aldrich, St. Louis, MO) on dry ice. Coronal sections (20 μm) were cut in either level of the caudate putamen (+1.00 mm rostral to bregma) or throughout the whole brain on a cryostat maintained at −18°C, mounted on gelatin-subbed slides, and stored at −80°C for less than 4 weeks until use. Coronal sections were rinsed in TME buffer (50 mM Tris-HCl, 3 mM MgCl2, 0.2 mM EGTA, 100 mM NaCl, and 0.1% BSA, pH 7.4) at room temperature for 10 min. Following this first incubation, slices were incubated in TME buffer containing 2 mM GDP and 9.5 mU/ml adenosine deaminase for 15 min at room temperature. Then slices were incubated in TME buffer with 2 mM GDP, 9.5 mU/ml adenosine deaminase, 0.04 nM [35S]GTPγS with or without varying concentrations of an agonist [DPDPE or (+)BW373U86], or 10 μM of a selective δ-opioid agonist or 10 μM unlabeled GTPγS [to determine nonspecific binding (NSB)] for 2 h at room temperature. In some experiments, slices were incubated in TME buffer with GDP, adenosine deaminase, 0.04 nM [35S]GTPγS, and 10 nM of the selective δ-opioid antagonist naltrindole (NTI) or vehicle for 30 min before they were incubated with varying concentrations of agonist. After the 2-h incubation with agonist (or no agonist), slices were rinsed twice for 2 min in cold 50 mM Tris buffer (pH 7.4) and once in distilled water for 1 min. Slides were air-dried for a few hours and exposed to film for 48 h in film cassettes together with 14C standards (Amersham Biosciences Inc., Piscataway, NJ). Images were digitized, and densitometric analysis was conducted using NIH Image J software. Compounds were tested on duplicate slices at the level of the caudate putamen from six rats or one regional slice per rat from 7 to 8 rats. Brain structures were identified by reference to the stereotaxis atlas by Paxinos and Watson (1986).
Data Handling and Statistical Analysis
Density values were averaged for each drug condition depending on the brain region tested. Nonspecific binding for each brain region was subtracted from the basal and drug-treated values. Then drug-induced changes in [35S]GTPγS binding were calculated as percentage of stimulation over basal for each brain region and/or condition tested. DPDPE-induced binding (10 μM) was compared with (+)BW373U86-induced binding (1 nM and 10 μM) with a one-way analysis of variance, and significant differences were analyzed using Tukey’s post hoc test (GraphPad Prism Software, GraphPad Software Inc., San Diego, CA). For dose concentration curves, EC50 and maximum stimulation values for DPDPE and (+)BW373U86 were also compared with a Student’s t test (GraphPad Prism Software).
Drugs
(+)BW373U86 and SNC80 were synthesized as previously described (Calderon et al., 1994). DPDPE was kindly donated by Dr. Henry Mosberg (College of Pharmacy, University of Michigan). TAN67 was obtained from Tocris Cookson Inc. (Ellisville, MO). NTI and deltorphin II were obtained from the National Institute on Drug Abuse. OMI and SIOM were synthesized as previously described (Portoghese et al., 1990, 1993). (+)BW383U86, DPDPE, OMI, SIOM, TAN67, and NTI were dissolved in sterile water. SNC80 was dissolved in 8% 1 M HCl.
Results
Concentration-effect curves were determined in caudate putamen slices of naive rats for the peptidic δ-opioid agonist DPDPE and the nonpeptide (+)BW373U86 (Fig. 1). Both compounds showed concentration-dependent increases in [35S]GTPγS binding. The EC50 values (±S.E.M.) for DPDPE and (+)BW373U86 with vehicle NTI pretreatment (sterile water) were 0.49 μM (±0.42) and 1.1 nM (±0.15), respectively. Maximum stimulation values (±S.E.M.) for DPDPE and (+)BW373U86 were 131.4% (±6.5) and 187.8% (±4.4) over basal, respectively.
Fig. 1.
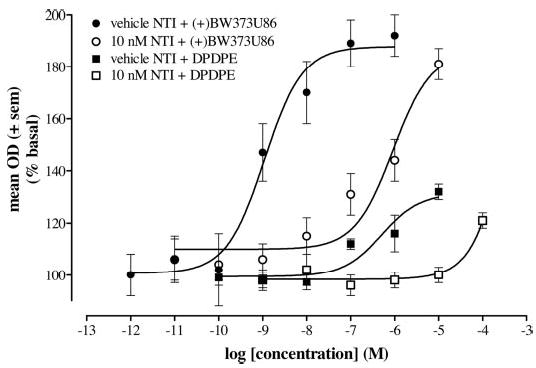
Percentage of [35S]GTPγS stimulation by increasing concentrations of (+)BW373U86 or DPDPE pretreated with either vehicle (sterile water) or 10 nM NTI compared with basal [35S]GTPγS binding in the caudate putamen of coronal brain slices from naive rats. EC50values were approximately 1.1 nM, 0.92 μM, 0.49 μM, and undetermined for vehicle NTI with (+)BW373U86 (•), 10 nM NTI with (+)BW373U86 (○), vehicle NTI with DPDPE (▪), and 10 nM NTI with DPDPE (□), respectively.
With a 30-min pretreatment of 10 nM NTI, the concentration-effect curves were shifted to the right for both compounds (Fig. 1). With (+)BW373U86, the curve was shifted approximately 1000-fold to the right with an EC50 (±S.E.M.) of 0.92 μM (±0.27). With DPDPE, treatment with the antagonist shifted the curve approximately 100-fold to the right; however, an EC50 could not be determined.
DPDPE- and (+)BW373U86-induced [35S]GTPγS binding was compared in different regions throughout the brain at nearly equieffective concentrations, as determined in the previous experiment (Fig. 2). DPDPE (10 μM) and (+)BW373U86 (1 nM) were compared in the frontal cortex (FrCtx), prelimbic cortex (PCtx), cingulate cortex (CCtx), nucleus accumbens (NAcc), caudate putamen (CP), hippocampus (HPC), amygdala (Amyg), piriform cortex (PirCtx), thalamus (Thal), hypothalamus (Hypothal), substantia nigra (SN), periaqueductal gray (PAG), and pontine nuclei (PN). These concentrations of DPDPE and (+)BW373U86 did not stimulate [35S]GTPγS binding above basal values in the hippocampus, thalamus, hypothalamus, and periaqueductal gray. The frontal cortex was the only region in which 1 nM (+)BW373U86 significantly stimulated (p < 0.05) [35S]GTPγS binding above levels produced by 10 μM DPDPE [F(2,19) = 6.85, p = 0.006]. Although the effects were not significant, (+)BW373U86 might produce a trend to stimulate more [35S]GTPγS binding than DPDPE in the cingulate cortex, caudate putamen, and pontine nuclei. DPDPE and (+)BW373U86 produce nearly equivalent levels of G protein stimulation in the prelimbic cortex, nucleus accumbens, amygdala, piriform cortex, and substantia nigra.
Fig. 2.
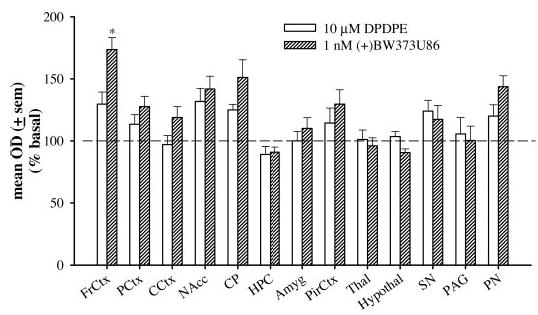
Percentage of [35S]GTPγS stimulation produced by 10 μM DPDPE or 1 nM (+)BW373U86 compared with basal [35S]GTPγS binding in coronal slices of different brain regions of Sprague-Dawley rats: FrCtx, PCtx, CCtx, NAcc, CP, HPC, Amyg, PirCtx, Thal, Hypothal, SN, PAG, and PN.
The mean optical density values (±S.E.M.) for 10 μM DP-DPE- and 1 nM (+)BW373U86-stimulated [35S]GTPγS binding together with basal- and nonspecific binding-stimulated [35S]GTPγS binding are displayed in Table 1. [35S]GTPγS binding for the basal condition is also expressed as nanocuries per gram (±S.E.M.) in Table 1. Basal stimulation was relatively similar in all brain regions except the amygdala and hypothalamus, which had slightly higher basal levels, and the pontine nucleus, which had lower basal levels. High basal stimulation in the amygdala and hypothalamus has been reported previously (Sim et al., 1996a). Nonspecific binding was low in all brain regions.
TABLE 1.
Values (±S.E.M.) determined from rat brain slices of different brain regions following incubation with no agonist (basal), 10 μM cold GTP-γS NSB, 10 μM DPDPE, or 1 nM (+)BW373U86 [35S]GTPγS binding is expressed as nCi/g (±S.E.M.) for basal stimulation and optical density values (±S.E.M.) for basal-, NSB-, DPDPE-, and (+)BW373U86-stimulated binding.
| FrCtx | PCtx | NAcc | CP | CCtx | HPC | Amyg | PirCtx | Thal | Hypothal | SN | PAG | PN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal (nCi/g) | 251.2 (23.8) | 392.1 (29.3) | 310.0 (13.2) | 299.1 (24.8) | 367.2 (38.1) | 260.7 (18.2) | 407.5 (40.6) | 312.4 (31.4) | 267.6 (27.0) | 443.8 (35.4) | 306.0 (36.1) | 323.1 (34.7) | 147.1 (16.9) |
| Basal (OD) | 76.3 (4.8) | 104.3 (5.4) | 89.7 (1.5) | 86.5 (5.2) | 99.1 (6.8) | 82.5 (4.6) | 106.9 (6.3) | 89.3 (5.8) | 79.7 (5.4) | 113.2 (5.1) | 86.6 (7.5) | 90.8 (7.1) | 49.7 (4.9) |
| NSB (OD) | 33.9 (2.4) | 43.8 (3.8) | 40.1 (4.1) | 32.1 (2.1) | 39.0 (3.3) | 28.8 (1.3) | 37.4 (2.1) | 37.2 (3.1) | 28.8 (1.5) | 32.7 (1.8) | 37.4 (8.9) | 27.6 (1.1) | 19.1 (2.5) |
| DPDPE (OD) | 86.0 (4.3) | 110.2 (3.8) | 103.7 (4.3) | 95.0 (5.0) | 95.7 (6.4) | 75.7 (3.2) | 101.8 (3.2) | 86.2 (4.6) | 79.1 (5.2) | 103.4 (4.1) | 93.1 (12.8) | 93.2 (3.8) | 52.2 (3.4) |
| (+)BW (OD) | 99.1 (3.8) | 123.2 (5.8) | 110.0 (3.3) | 101.1 (3.7) | 107.7 (5.3) | 76.7 (2.9) | 108.1 (3.1) | 92.0 (4.4) | 75.7 (3.7) | 104.4 (4.1) | 90.6 (14.6) | 90.4 (3.6) | 60.3 (4.8) |
OD, optical density; BW, (+)BW373U86.
Following the previous experiment, a supramaximal concentration of 10 μM (+)BW373U86 was used to stimulate [35S]GTPγS binding in the same regions of the rat brain (Fig. 3). This higher concentration of (+)BW373U86 generated higher levels of [35S]GTPγS binding in many brain regions compared with DPDPE. (+)BW373U86 (10 μM) produced more G protein stimulation than DPDPE in the frontal cortex (p < 0.05) [F(2,19) = 6.85, p < 0.006], prelimbic cortex (p < 0.01) [F(2,19) = 6.46, p < 0.007], caudate putamen (p < 0.01) [F(2,19) = 7.16, p < 0.005], cingulate cortex (p < 0.05) [F(2,19) = 5.65, p < 0.01], thalamus (p < 0.05) [F(2,19) = 6.73, p = 0.006], and pontine nuclei (p < 0.05) [F(2,19) = 4.79, p < 0.05]. In the hippocampus, the difference in [35S]GTPγS binding between 10 μM DPDPE, 1 nM (+)BW373U86, and 10 μM (+)BW373U86 was significant [F(2,19) = 3.78, p < 0.042]; however, this difference was not large enough to be identified by a post hoc test. Nevertheless, 10 μM (+)BW373U86 stimulated binding to 125% of basal compared with the lower concentration of (+)BW373U86 and DPDPE that did not stimulate binding in the hippocampus to any extent. In addition, the high concentration of (+)BW373U86 increased [35S]GTPγS binding in the thalamus compared with that produced by 1 nM (+)BW373U86 (p < 0.01) [F(2,19) = 6.73, p = 0.006]. In addition to the hippocampus and thalamus, differences in [35S]GTPγS binding were not statistically significant between 1 nM (+)BW373U86 and 10 μM (+)BW373U86 in any other brain region.
Fig. 3.
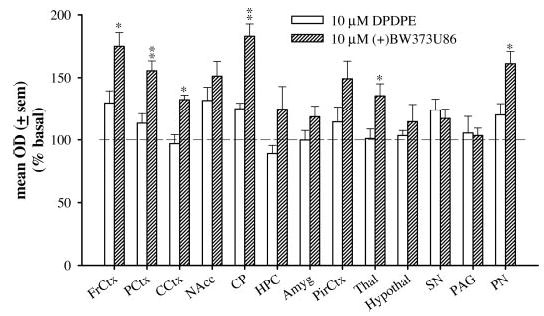
Percentage of [35S]GTPγS stimulation produced by 10 μM DPDPE or 10 μM (+)BW373U86 compared with basal [35S]GTPγS binding in coronal slices of different brain regions of Sprague-Dawley rats: FrCtx, PCtx, CCtx, NAcc, CP, HPC, Amyg, PirCtx, Thal, Hypothal, SN, PAG, and PN.
A concentration-effect curve for (+)BW373U86 was also obtained for the frontal cortex. In the frontal cortex, the (+)BW373U86 was shifted 3-fold to the left of the (+)BW373U86 curve in the caudate putamen (Fig. 4) with an EC50 value (±S.E.M.) of 0.33 nM (±0.06). Concentration-effect curves were obtained for the frontal cortex and caudate putamen only because large increases in [35S]GTPγS binding could be determined; however, most brain regions lacked these large stimulation levels needed to determine reliable concentration curves.
Fig. 4.
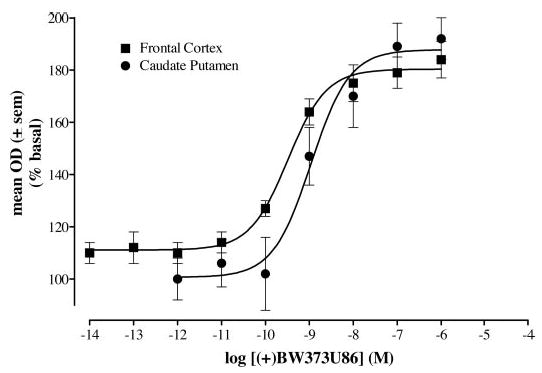
Percentage of [35S]GTPγS stimulation by increasing concentrations of (+)BW373U86 compared with basal [35S]GTPγS binding in the frontal cortex or caudate putamen of coronal brain slices from naive rats. EC50 values were 0.33 nM for (+)BW373U86 in the frontal cortex (▪) and 1 nM for (+)BW373U86 in the caudate putamen (•).
The levels of GTPγS binding as a percentage of basal (±S.E.M.) were compared in the caudate putamen for a number of δ-opioid agonists at a concentration of 10 μM: (+)BW373U86, SNC80, the putative δ-2 agonist deltorphin II, the putative δ-1 agonists DPDPE, TAN67, OMI, or SIOM (Fig. 5). (+)BW373U86 produced the greatest levels of [35S GTPγS binding at 205% (±5), followed by SNC80 at 177% (±4), deltorphin II at 152% (±8), DPDPE at 147% (±4), TAN67 at 141% (±6), OMI at 123% (±4), and SIOM at 104% (±5). In addition, a 30-min pretreatment with 10 μM OMI or SIOM blocked (+)BW3737U86-induced increases in [35S]GTPγS binding, lowering stimulation levels to 117 (±3.8) and 110 (±2.3), respectively (Fig. 6).
Fig. 5.
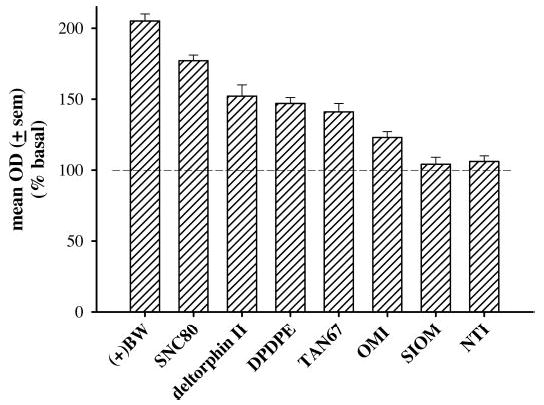
Percentage of [35S]GTPγS stimulation 10 μM (+)BW373U86, SNC80, deltorphin II, DPDPE, TAN67, OMI, SIOM, and NTI compared with basal [35S]GTPγS binding in the caudate putamen of coronal brain slices from naive rats. Compound names are listed under Materials and Methods and defined in Abbreviations.
Fig. 6.
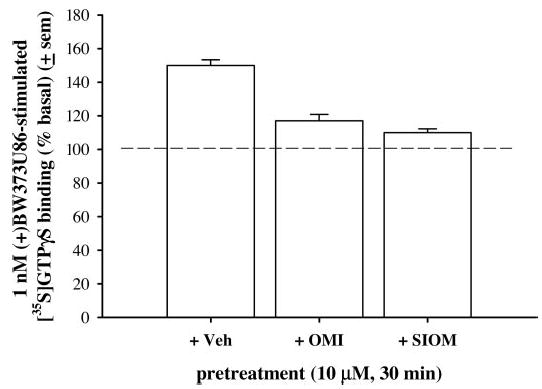
Percentage of [35S]GTPγS stimulation by 1 nM (+)BW373U86 pretreated with either vehicle (sterile water), 10 μM OMI, or 10 μM SIOM compared with basal [35S]GTPγS binding in the caudate putamen of coronal brain slices from naive rats.
Discussion
The present study investigated the effects of peptidic and nonpeptidic δ-opioid agonists on [35S]GTPγS binding in rat brain slices. The peptidic agonist DPDPE and the nonpeptidic agonist (+)BW373U86 stimulated [35S]GTPγS binding in a concentration-dependent manner in brain slices from the caudate putamen. This stimulation was mediated through the δ-opioid receptor as the concentration effect curves were shifted to the right following treatment with the selective δ-opioid receptor antagonist NTI. The nonpeptidic agonist (+)BW373U86 was more potent and efficacious than the peptidic agonist DPDPE. Previous research has also found potency and efficacy differences in [35S]GTPγS binding in striatal slices of rat brains with some nonpeptidic δ-opioid agonists (Jutkiewicz et al., 2004) as well as with the δ-opioid agonists morphine and [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (Sim et al., 1996a).
Equiefficacious concentrations of 1 nM (+)BW373U86 and 10 μM DPDPE, determined from the initial experiments in caudate putamen slices, were compared across various brain regions. (+)BW373U86 at a concentration of 1 nM produced statistically significant increases in [35S]GTPγS binding compared with 10 μM DPDPE in the frontal cortex only. Based on these data, it seemed that (+)BW373U86 might be more potent in the frontal cortex than in the caudate putamen; indeed, the concentration curve was shifted 3-fold to the left of that determined in the caudate putamen. Although many regions demonstrated a trend to see higher G protein activation with (+)BW373U86 than DPDPE, a number of regions had little or no [35S]GTPγS activation following treatment with these concentrations of agonists. Therefore, [35S]GTPγS stimulation was measured following incubation with a supramaximal concentration, 10 μM (+)BW373U86. These results demonstrated that the nonpeptidic δ-opioid agonist (+)BW373U86 was more efficacious than DPDPE in many brain regions, including the frontal cortex, prelimbic cortex, caudate putamen, cingulate cortex, thalamus, and pontine nuclei. In addition, 10 μM (+)BW373U86 stimulated [35S]GTPγS binding in the hippocampus, whereas the lower concentration of (+)BW373U86 and 10 μM DPDPE did not. Overall, the degree of [35S]GTPγS binding was well correlated with δ-opioid receptor expression levels in different brain regions (Mansour et al., 1995).
The differences between the peptidic agonist DPDPE and nonpeptidic agonist (+)BW373U86 remained relatively consistent throughout the brain, such that DPDPE never caused more G protein activation than (+)BW373U86 in any region of the brain. These findings suggest that behavioral disparities between peptidic and nonpeptidic compounds may be attributable to efficacy differences between the compounds or classes of compounds and may not be caused by other functional differences among the compounds in various brain regions. For example, the hippocampus is thought to be the initiation site for δ-opioid agonist-induced convulsions. Although peptidic δ agonists were demonstrated to produce changes in electrocorticographic recording, these compounds do not produce apparent convulsions (Haffmans and Dzoljic, 1983; Tortella et al., 1984; Stutzmann et al., 1986; DeSarro et al., 1992); however, nonpeptidic δ-opioid agonists produce both electrocorticographic changes (seizures) and resultant convulsions (Marrosu et al., 1997; Broom et al., 2002a). In the current studies, (+)BW373U86, but not DPDPE, stimulated [35S]GTPγS binding in the hippocampus. These data suggest that high-efficacy agonist activity may be required for the manifestation of convulsions; therefore, only the more efficacious nonpeptidic δ-opioid agonists would induce convulsions.
Peptidic and nonpeptidic δ-opioid agonists produced similar antinociceptive effects in rats when administered i.c.v. (Fraser et al., 2000b). However, central administration of some peptidic and nonpeptidic δ-opioid agonists was shown to produce antinociceptive effects that were mediated through non-δ-opioid receptor-mediated mechanisms (Wild et al., 1993; Fraser et al., 2000b). Therefore, it may be difficult to compare δ-opioid agonist efficacy by this route of administration. In addition, the type of assay used to measure antinociception can alter the effectiveness of δ-opioid agonists. For example, in monkeys, nonpeptidic δ-opioid agonists demonstrated little or no antinociceptive effects in tail withdrawal assays but were effective antinociceptive ligands in chemically induced hypersensitivity experiments (Butelman et al., 1995; Brandt et al., 2001). These data suggest that δ-opioid agonists acting at δ-opioid receptors in regions involved in analgesia, such as the thalamus and PAG, might only produce antinociceptive effects in assays with low efficacy requirements. Likewise, in the current data, DPDPE and (+)BW373U86 produced low, if any, [35S]GTPγS stimulation in the thalamus and PAG, regions thought to be involved in antinociceptive responses.
Finally, in the present study, the level of [35S]GTPγS binding was determined for a number of peptidic (DPDPE and deltorphin II) and nonpeptidic [(+)BW373U86, SNC80, TAN67, OMI, and SIOM] δ-opioid agonists in rat brain slices from the caudate putamen following incubation with a high concentration of agonist (10 μM). These compounds demonstrated a rank order of efficacy: (+)BW373U86 > SNC80 > deltorphin II > DPDPE > TAN67 > OMI > SIOM. This rank order of efficacy corresponded with the efficacy determinations in cell cultures performed by Clark et al. (1997). As expected, high concentrations of the lower efficacy agonists (OMI and SIOM) antagonized [35S]GTPγS binding stimulated by the high-efficacy agonist (+)BW373U86, further supporting δ-opioid receptor mediation of the measured [35S]GTPγS stimulation.
Generally, the nonpeptidic agonists displayed a larger range of stimulation, and (+)BW373U86 and SNC80 were the most efficacious agonists tested. The peptidic agonists deltorphin II and DPDPE seemed equally efficacious but were less efficacious than (+)BW373U86 and SNC80. Likewise, (+)BW373U86 was more efficacious than DPDPE in most areas of the brain tested, except in regions with low or no [35S]GTPγS binding, such as the hypothalamus, periaqueductal gray, and substantia nigra. These data suggested that differences between peptidic and nonpeptidic δ-opioid agonists in behavioral studies are not due to differential binding in various brain regions but rather are caused by agonist efficacy differences such that nonpeptidic δ-opioid agonists are able to activate more G protein binding. These data revealed that [35S]GTPγS autoradiography can be used to compare efficacy differences among agonists across various brain regions. In addition, the present data suggested that agonist efficacy should be considered when using agonist-induced [35S]GTPγS stimulation experiments in brain slices.
Acknowledgments
We thank Sarah Kaminsky for technical assistance on this project.
Footnotes
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
This work was supported by U.S. Public Health Service Grants DA00254, T32 GM07767, and T32 DA07267.
References
- Brandt MR, Furness MS, Mello NK, Rice KC, Negus SS. Antinociceptive effects of delta-opioid agonists in rhesus monkeys: effects on chemically induced thermal hypersensitivity. J Pharmacol Exp Ther. 2001;296:939–946. [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a nonpeptidic delta-opioid receptor agonist is not required for antidepressant-like effects. Psychopharmacology. 2002a;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002b;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002c;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Gatch MB, Chang KJ, Woods JH. BW373U86, a delta-opioid receptor agonist, reverses bradykinin-induced thermal allodynia in rhesus monkeys. Eur J Pharmacol. 1995;277:285–287. doi: 10.1016/0014-2999(95)00134-7. [DOI] [PubMed] [Google Scholar]
- Calderon SN, Rothman RB, Porreca F, Flippen-Anderson JL, McNutt RW, Xu H, Smith LE, Bilsky EJ, Davis P, Rice KC. Probes for narcotic receptor mediated phenomena. 19. Synthesis of (+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5 dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC80): a highly selective, nonpeptide δ opioid receptor agonist. J Med Chem. 1994;37:2125–2128. doi: 10.1021/jm00040a002. [DOI] [PubMed] [Google Scholar]
- Chang K-J, Porreca F, Woods JH. The Delta Receptor. Marcel Dekker; New York: 2004. [Google Scholar]
- Clark MJ, Emmerson PJ, Mansour A, Akil H, Woods JH, Portoghese PS, Remmers AE, Medzihradsky F. Opioid efficacy in a C6 glioma cell line stably expressing the delta opioid receptor. J Pharmacol Exp Ther. 1997;283:501–510. [PubMed] [Google Scholar]
- Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang K-J, De Costa BR, Mosberg HI, Woods JH. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993a;267:888 – 895. [PubMed] [Google Scholar]
- Comer SD, McNutt RW, Chang K-J, De Costa BR, Mosberg HI, Woods JH. Discriminative stimulus effects of BW373U86: a nonpeptide ligand with selectivity for delta opioid receptors. J Pharmacol Exp Ther. 1993b;267:866–874. [PubMed] [Google Scholar]
- DeSarro GB, Marra R, Spagnolo C, Nisticò G. Delta opioid receptors mediate seizures produced by intrahippocampal injection of ala-deltorphin in rats. Funct Neuro. 1992;7:235–238. [PubMed] [Google Scholar]
- Dykstra LA, Schoenbaum GM, Yarbrough J, McNutt R, Chang K-J. A novel delta-opioid agonist, BW373U86, in squirrel monkeys responding under a schedule of shock titration. J Pharmacol Exp Ther. 1993;267:875–882. [PubMed] [Google Scholar]
- Fraser GL, Parenteau H, Tu T-M, Ducharme J, Perkins MN, Clarke PBS. The effects of delta agonists on locomotor activity in habituated and non-habituated rats. Life Sci. 2000a;67:913–922. doi: 10.1016/s0024-3205(00)00690-1. [DOI] [PubMed] [Google Scholar]
- Fraser GL, Pradhan AA, Clarke PBS, Wahlestedt C. Supraspinal an-tinociceptive response to [D-Pen2,5]-enkephalin (DPDPE) is pharmacologically distinct from that to other delta-agonists in the rat. J Pharmacol Exp Ther. 2000b;295:1135–1141. [PubMed] [Google Scholar]
- Haffmans J, Dzoljic MR. Differential epileptogenic potentials of selective μ and δ opiate receptor agonists. J Neural Transmission. 1983;57:1–11. doi: 10.1007/BF01250043. [DOI] [PubMed] [Google Scholar]
- Hong EJ, Rice KC, Calderon S, Woods JH, Traynor JR. Convulsive behavior of nonpeptide delta-opioid ligands: comparison of SNC80 and BW373U86 in mice. Analgesia. 1998;3:269–276. [Google Scholar]
- Hosohata Y, Vanderah TW, Burkey TH, Ossipov MH, Kovelowski CJ, Sora I, Uhl GR, Zhang X, Rice KC, Roeske WR, et al. δ-Opioid receptor agonists produce antinociception and [35S]GTPγS binding in μ receptor knockout mice. Eur J Pharmacol. 2000;388:241–248. doi: 10.1016/s0014-2999(99)00897-3. [DOI] [PubMed] [Google Scholar]
- Jewett DC, Mosberg HI, Woods JH. Discriminative stimulus effects of a centrally administered delta-opioid peptide (D-Pen2-D-Pen5-enkephalin) in pigeons. Psychopharmacology. 1996;127:225–230. [PubMed] [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH. Delta-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague-Dawley rats. J Pharmacol Exp Ther. 2004;309:173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- Kovelowski CJ, Bian D, Hruby VJ, Lai J, Ossipov MH, Porreca F. Selective opioid delta agonists elicit antinociceptive supraspinal/spinal synergy in the rat. Brain Res. 1999;843:12–17. doi: 10.1016/s0006-8993(99)01803-x. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trend Pharmacol Sci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Cozzolino A, Puligheddu M, Giagheddu M, Di Chiara G. Hippocampal θ activity after systemic administration of a non-peptide δ-opioid agonist in freely-moving rats: relationship to D1 dopamine receptors. Brain Res. 1997;776:24–29. doi: 10.1016/s0006-8993(97)00969-4. [DOI] [PubMed] [Google Scholar]
- Negus SS, Butelman ER, Chang KJ, De Costa BR, Winger G, Woods JH. Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. J Pharmacol Exp Ther. 1994;270:1025–1034. [PubMed] [Google Scholar]
- Okura T, Varga EV, Hosohata Y, Navratilova E, Cowell SM, Rice KC, Nagase H, Hruby VJ, Roeske WR, Yamamura HI. Agonist-specific down-regulation of the human delta-opioid receptor. Eur J Pharmacol. 2003;459:9–16. doi: 10.1016/s0014-2999(02)02823-6. [DOI] [PubMed] [Google Scholar]
- Pakarinen ED, Woods JH, Moerschbaecher JM. Repeated acquisition of behavioral chains in squirrel monkeys: comparisons of a mu, kappa, and delta opioid agonist. J Pharmacol Exp Ther. 1995;272:552–559. [PubMed] [Google Scholar]
- Park Y, Ma T, Tanaka S, Jang C-G, Loh HH, Ko KH, Ho IK. Comparison of G-protein activation in the brain by μ-, δ- and κ-opioid receptor agonists in μ-opioid receptor knockout mice. Brain Res Bull. 2000;52:297–302. doi: 10.1016/s0361-9230(00)00265-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando, FL: 1986. [Google Scholar]
- Portoghese PS, Moe ST, Takemori AE. A selective δ1 opioid receptor agonist derived from oxymorphone. Evidence for separate recognition sites for δ1 opioid receptor agonists and antagonists. J Med Chem. 1993;36:2572–2574. doi: 10.1021/jm00069a017. [DOI] [PubMed] [Google Scholar]
- Portoghese PS, Sultana M, Takemori AE. Design of peptidomimetic δ opioid receptor antagonists using the message-address concept. J Med Chem. 1990;33:1714–1720. doi: 10.1021/jm00168a028. [DOI] [PubMed] [Google Scholar]
- Quock RM, Hosohata Y, Knapp RJ, Burkey TH, Hosohata K, Zhang X, Rice KC, Nagase H, Hruby VJ, Porreca F, et al. Relative efficacies of δ-opioid receptor agonists at the cloned human δ-opioid receptor. Eur J Pharmacol. 1997;326:101–104. doi: 10.1016/s0014-2999(97)83488-7. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Childers SR. In vitro autoradiography of receptor-activated G-proteins in rat brain by agonist-stimulated guanylyl 5′-[γ-[35S]thio]-triphosphate binding. Proc Natl Acad Sci USA. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Dworkin SI, Childers SR. Effects of chronic morphine administration on μ opioid receptor-stimulated [35S]GTPγS autoradiography in rat brain. J Neurosci. 1996a;16:2684–2692. doi: 10.1523/JNEUROSCI.16-08-02684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim LJ, Selley DE, Xiao R, Childers SR. Differences in G protein activation by μ- and δ-opioid and cannabinoid receptor in rat striatum. Eur J Pharmacol. 1996b;307:97–105. doi: 10.1016/0014-2999(96)00211-7. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Sharpe AL, Vogt LJ, Brunk LK, Selley DE, Samson HH. Effect of ethanol self-administration on mu- and delta-opioid receptor-mediated G-protein activity. Alcoholism Clin Exp Res. 2002;26:688–694. [PubMed] [Google Scholar]
- Stutzmann J-M, Bohme GA, Roques BP, Blanchard J-C. Differential electrographic patterns for specific μ- and δ-opioid peptides in rats. Eur J Pharmacol. 1986;123:53–59. doi: 10.1016/0014-2999(86)90686-2. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Herault XM, Rothman RB, Burgess JP, Mascarella SW, Xu H, Horel RB, Dersch CM, Carroll FI. (+)-4-[(N-Allyl-cis-3-methyl-4-piperidinyl)phenylamino]-N,N-diethylbenzamide displays selective binding for the delta opioid receptor. Bioorg Med Chem Lett. 1999;9:3053–3056. doi: 10.1016/s0960-894x(99)00525-9. [DOI] [PubMed] [Google Scholar]
- Tortella FC, Robles L, Mosberg HI, Holaday JW. Electrocephalographic assessment of the role of δ receptors in opioid peptide-induced seizures. Neuropeptides. 1984;5:213–216. doi: 10.1016/0143-4179(84)90065-9. [DOI] [PubMed] [Google Scholar]
- Wild KD, McCormick J, Bilsky EJ, Vanderah T, McNutt RW, Chang K-J, Porreca F. Antinociceptive action of BW373U86 in the mouse. J Pharmacol Exp Ther. 1993;267:858–865. [PubMed] [Google Scholar]
- Xu H, Lu Y-F, Thomas JB, Carroll FI, Rice KC, Rothman RB. Opioid peptide receptor studies. 15. Relative efficacy of 4-[(N-allyl-3-methyl-4-piperindinyl)phenylamino]-N,N-diethylbenzamide and related compounds at the cloned human δ-opioid receptor. Synapse. 2001;40:269–274. doi: 10.1002/syn.1049. [DOI] [PubMed] [Google Scholar]


