Abstract
Systemically active, nonpeptidic delta opioid receptor agonists have been shown to produce antidepressant and anxiolytic effects in animal models in rodents. In addition, delta agonists have been shown to increase expression of brain-derived neurotrophic factor (BDNF) mRNA, an effect of some antidepressants, which may be important for the clinical efficacy of antidepressant drugs. The present study examined whether a variety of peptidic delta agonists, DPDPE, JOM-13, a systemically active derivative of DPDPE, deltorphin II, and H-Dmt-Tic-NH-CH2-Bid could produce convulsions and antidepressant-like effects in the forced swim test. In addition, some of these compounds were examined for their influence on BDNF mRNA expression. All four agonists dose-dependently decreased immobility in the forced swim test, indicating an antidepressant-like effect. Only JOM-13 produced convulsions at doses required for antidepressant-like effects. In addition, DPDPE increased BDNF mRNA expression, as measured by in situ hybridization, in the frontal cortex. The antidepressant-like effect of the agonists in the forced swim test and the increase in BDNF mRNA expression produced by DPDPE were blocked by the delta antagonist naltrindole. Therefore, activation of the delta receptor by centrally administered peptidic agonists and intravenously administered JOM-13 produces behavioral antidepressant-like effects without producing convulsions, and some peptidic agonists can increase BDNF mRNA expression, however, not as consistently as the systemically active nonpeptidic agonists.
Keywords: Delta opioid receptor, Antidepressant Forced, swim test BDNF, Peptide, In situ hybridization
1. Introduction
Delta opioid receptor agonists are known to produce many behavioral effects in rodents, including antinociception, increased locomotor activity, convulsions, and antidepressant-like effects (Fraser et al., 2000a,b; Broom et al., 2002a,b,c; Comer et al., 1993; Hong et al., 1998). In addition, the nonpeptidic delta agonist (+)BW373U86 has been shown to increase brain-derived neurotrophic factor (BDNF) mRNA expression in several brain regions in the rat (Torregrossa et al., 2004, 2005). Increased expression and activity of BDNF have been implicated in the mechanism of action of antidepressant drugs (Duman, 2004).
The potential use of delta agonists as analgesics and antidepressants has led to the development of many different compounds that activate the delta opioid receptor. The earliest selective delta agonists were peptides, including [Tyr-D-Pen-Gly-Phe-D-Pen] (DPDPE) and [Tyr-D-Ala-Phe-Glu-Val-Val-Gly-NH2] (deltorphin II). The poor bioavailability of peptidic compounds led to the development of systemically active, nonpeptidic delta agonists, including BW373U86 and SNC80. Most behavioral and physiological effects of delta agonists have been demonstrated to occur with both the peptidic and nonpeptidic agonists; however, there are a few exceptions. The nonpeptidic delta agonists produce convulsions in mice (Comer et al., 1993; Hong et al., 1998; Broom et al., 2002a), rats (Broom et al., 2002c), and nonhuman primates (Dykstra et al., 1993; Pakarinen et al., 1995; Negus et al., 1994). On the other hand, peptidic agonists have not been reported to produce convulsions in any species; however, they do produce wet dog shakes, unstable movement, and epileptic discharges as measured by electroencephalogram (EEG) (Haffmans and Dzoljic, 1983). These findings suggest that activation of the delta receptor by both peptidic and nonpeptidic agonists can lead to EEG changes, but only the nonpeptides produce overt convulsions.
Both peptidic and nonpeptidic agonists have been shown to produce antinociception (Fraser et al., 2000a) and increases in locomotor activity (Fraser et al., 2000b). In contrast, anti-depressant-like effects and increased BDNF mRNA expression have only been demonstrated with nonpeptidic agonists (Broom et al., 2002b; Saitoh et al., 2004; Torregrossa et al., 2004). However, there is evidence that peptidic agonists may produce antidepressant-like effects from studies showing that the enkephalinase inhibitor RB101 produces antidepressant-like effects in the learned helplessness and forced swim tests (Tejedor-Real et al., 1998; Baamonde et al., 1992; Jutkiewicz et al., 2005a,b). This effect can be blocked by the selective delta antagonist naltrindole, suggesting that endogenous opioid peptides such as met-enkephalin and leu-enkephalin can produce antidepressant-like effects by activating the delta opioid receptor. However, 32 mg/kg RB101 administered intravenously did not increase BDNF mRNA expression in the frontal cortex or hippocampus (Jutkiewicz et al., submitted for publication), indicating that there may be differences in the effects produced by the endogenous peptides and nonpeptidic agonists at the delta receptor.
Therefore, the aim of the present study was to determine if the peptidic agonists DPDPE, deltorphin II, Tyr-c[D-Cys-Phe-D-Pen]OH (JOM-13), and the pseudo-peptide H-Dmt-Tic-NH-CH2- Bid could produce antidepressant-like effects in a modified rat forced swim test, an assay used to predict the antidepressant potential of novel compounds. In addition, we determined the ability of some of the agonists to increase BDNF mRNA expression in the frontal cortex and hippocampus by in situ hybridization. We also determined whether these effects were mediated by the delta receptor by administering the selective delta antagonist naltrindole.
2. Results
2.1. Forced swim test studies
DPDPE dose-dependently decreased immobility in the forced swim test, indicating an antidepressant-like effect [F(3,22) = 4.964, P = 0.0088] that post-hoc analysis revealed was significant at the highest dose of 155 nmol (100 μg) (Fig. 1a). DPDPE did not significantly affect swimming [F(3,22) = 2.685, P = 0.715] or climbing behaviors [F(3,22) = 0.4217, P = 0.7393]. In addition, there was no effect of DPDPE in the forced swim test when it was given 1 h before the test (data not shown), indicating that the antidepressant effect of the peptide is short-lived.
Fig. 1.
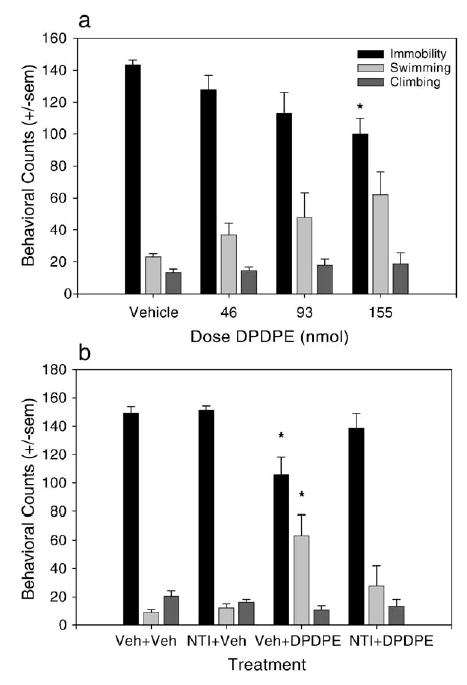
Antidepressant-like effects of DPDPE in the forced swim test. (a) Effect of increasing doses of DPDPE given i.c.v. on immobility, swimming, and climbing behaviors when given 30 min before the forced swim test. DPDPE dose-dependently and significantly decreased immobility indicating an antidepressant-like effect (n = 8 for vehicle and n = 6 for each dose of DPDPE). (b) Effect of 10 mg/kg naltrindole (NTI) given 30 min before 155 nmol DPDPE on behaviors in the forced swim test. NTI blocked the antidepressant-like effect of DPDPE (n = 5 per group, except Veh + DPDPE, n = 6). Data are expressed as the mean ± standard errors of the mean (SEM), and statistical differences are determined by comparison to the vehicle-treated control. *P < 0.05.
The reduction in immobility produced by 155 nmol of DPDPE was blocked by a 30 min pretreatment with 10 mg/ kg of the delta opioid receptor antagonist naltrindole (NTI), s.c. [F(3, 22) = 5.524, P = 0.055] (Fig. 1b), indicating that the antidepressant-like effect of DPDPE is mediated through the delta receptor. NTI had no effect on behavior in the forced swim test when given alone.
Like DPDPE, deltorphin II dose-dependently decreased immobility in the forced swim test [F(5,31) = 8.669, P < 0.0001], producing statistically significant reductions at doses of 0.03 and 0.1 nmol. Doses of 0.001 and 0.003 nmol and a high dose of 10 nmol deltorphin II did not produce an antidepressant-like effect (Fig. 2a). Deltorphin II also produced a significant increase in swimming at a dose of 0.1 nmol [F(5,31) = 6.294, P = 0.004] and a significant increase in climbing at a dose of 0.03 nmol [F(5,31) = 3.221, P = 0.0186]. The antidepressant-like effect of 0.1 nmol deltorphin II was blocked by 10 mg/kg NTI [F(3,18) = 8.004, P = 0.013] (Fig. 2b).
Fig. 2.
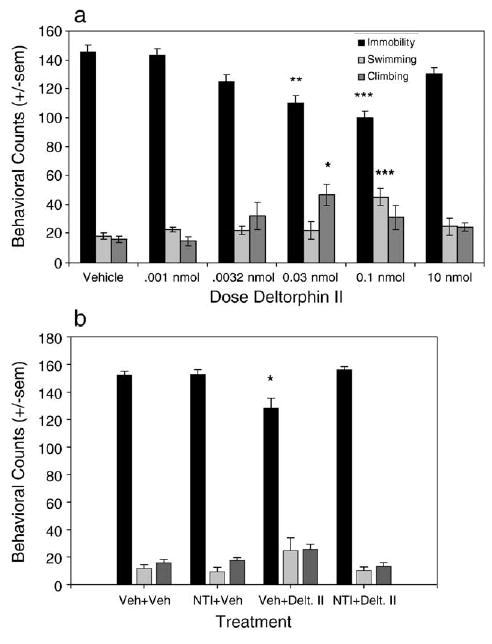
Antidepressant-like effects of deltorphin II in the forced swim test. (a) Effect of increasing doses of deltorphin II on behaviors in the forced swim test. Deltorphin II dose-dependently and significantly reduced immobility counts, indicating an antidepressant-like effect (Vehicle n = 9, 0.001 nmol n = 6, 0.0032 nmol n = 7, 0.03 nmol n = 4, 0.1 nmol n = 6, 10 nmol n = 5). (b) Effect of 10 mg/kg NTI given 30 min prior to 0.1 nmol deltorphin II on behaviors in the forced swim test. NTI blocked the antidepressant-like effect of deltorphin II (Vehicle n = 4, NTI + Veh and Veh + deltorphin II n = 6, NTI + deltorphin II n = 7). *P < 0.05, **P < 0.01, ***P < 0.001.
The pseudo-peptidic delta opioid receptor agonist H-Dmt-Tic-NH-CH2-Bid also dose-dependently decreased immobility in the forced swim test and reached statistical significance at a dose of 60 nmol [F(3,17) = 6.184, P = 0.049]. All three doses tested (30, 60, and 100 nmol) significantly increased swimming behaviors [F(3,17) = 10.06, P = 0.005] and had no effect on climbing behavior [F(3,17) = 1.9, P = 0.1679] (Fig. 3a). The reduction in immobility produced by 60 nmol of H-Dmt-Tic-NH-CH2-Bid was antagonized by 10 mg/kg NTI [F(3,18) = 7.824, P = 0.015] (Fig. 3b), indicating that H-Dmt-Tic-NH-CH2-Bid produces antidepressant-like effects through its action at the delta opioid receptor. JOM-13 produced significant reductions in immobility in the forced swim test at a dose of 32 mg/kg [F(2,15) = 5.431, P = 0.168], indicating that it also produces an antidepressant-like effect and that the compound can cross the blood–brain barrier when administered i.v. (Fig. 4a). The antidepressant-like effect of JOM-13 was blocked by 10 mg/ kg naltrindole [P = 0.01], indicating that the effect was mediated by the delta opioid receptor (Fig. 4b).
Fig. 3.
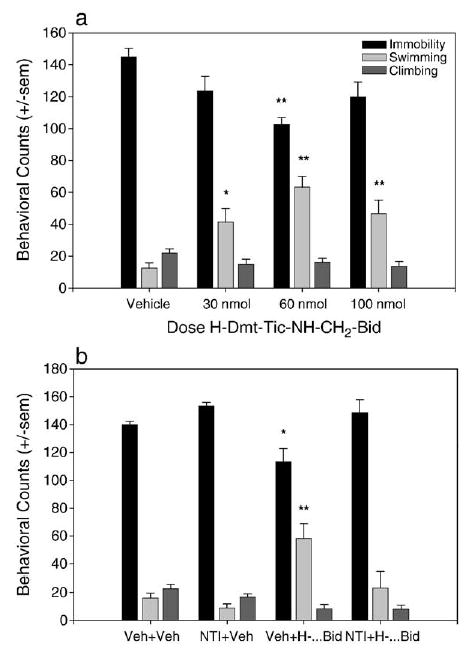
Antidepressant-like effects of H-Dmt-Tic-NH-CH2-Bid in the forced swim test. (a) 60 nmol of H-Dmt-Tic-NH-CH2-Bid produced a significant decrease in immobility in the forced swim test, indicating an antidepressant-like effect. All doses tested (30, 60, and 100 nmol) produced significant increases in swimming behavior (Vehicle n = 5, all doses agonist n = 6). (b) 10 mg/kg NTI given 30 min prior to 60 nmol H-Dmt-Tic- NH-CH2-Bid blocked the antidepressant-like effect (Veh + Veh and NTI + Veh n = 6, Veh and NTI + agonist n = 5). *P < 0.05, **P < 0.01.
Fig. 4.

Antidepressant-like effect of JOM-13 in the forced swim test. (a) 32 mg/kg JOM-13, i.v., produced a significant decrease in immobility in the forced swim test, indicating an antidepressant-like effect, and significantly increased swimming behavior (n = 6 per group). (b) 10 mg/kg NTI given 30 min prior to 32 mg/kg JOM-13 blocked the antidepressant-like effect (n = 5 per group). (c) 32 mg/kg produced convulsions in 2 out of 6 rats (33.33%). (d) When 10 mg/kg NTI was given as a pretreatment, no convulsions were observed with administration of 32 mg/kg JOM-13. *P < 0.05, **P < 0.01.
2.2. Convulsion observations
JOM-13 produced convulsions in 20–30% of rats at a dose of 32 mg/kg (Fig. 4c). On average, the convulsion occurred 2 min after the i.v. injection. This effect appeared to be blocked by 10 mg/kg naltrindole (Fig. 4d); however, because JOM-13 produces convulsions in such a small percentage of animals, it is difficult to determine if naltrindole was truly blocking the convulsive effect. JOM-13 was the only compound to produce convulsions at the dose required to produce an antidepressant-like effect. Overt convulsions were not observed after DPDPE, deltorphin II, or H-Dmt-Tic-NH-CH2-Bid administration at any dose that produced antidepressant-like effects (data not shown).
2.3. BDNF mRNA expression studies
DPDPE dose-dependently increased BDNF mRNA expression in the frontal cortex, reaching significance at a dose of 155 nmol [F(2,8) = 12.62, P = 0.034] (Fig. 5). DPDPE also appeared to increase BDNF mRNA expression in the hippocampal CA1 region; however, the effect was not significant [F (2,9) = 1.508, P = 0.3866], and DPDPE did not alter BDNF expression in the CA3 or dentate gyrus regions of hippocampus. The increase in BDNF mRNA expression in frontal cortex produced by 155 nmol DPDPE was antagonized by 1 mg/kg NTI [F(3,11) = 10.43, P = 0.015] (Fig. 6), while 0.1 mg/kg naltrexone had no effect on DPDPE's tendency to increase BDNF in the frontal cortex [F(3,15) = 1.061, P = 0.3948] (Fig. 6). Overall, there was a great amount of variability in the amount of BDNF mRNA expressed across the animals, particularly in the DPDPE-treated groups. DPDPE may be distributed differently in the brain across animals with the i.c.v. route of administration, resulting in both high variability and less statistically significant results.
Fig. 5.
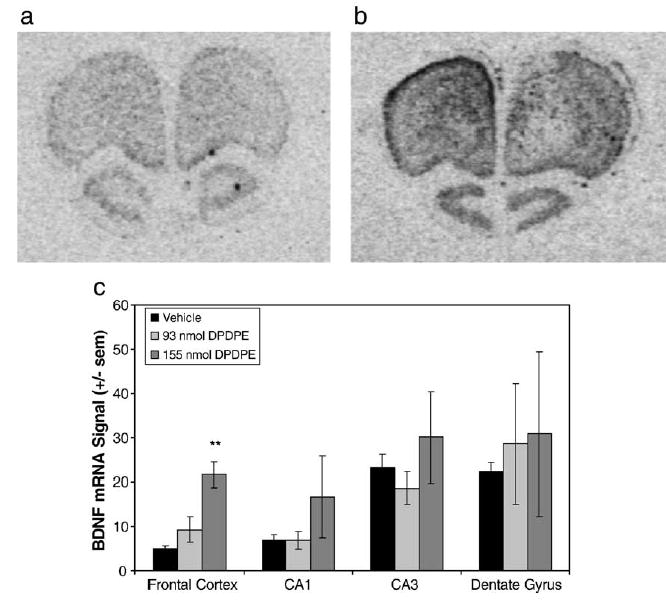
DPDPE increased BDNF mRNA expression in the frontal cortex. (a, b) Representative photomicrographs from X-ray films exposed for 14 days after in situ hybridization with the antisense cRNA probe to rat BDNF mRNA. Photos show sections through the frontal cortex. (a) Section from a vehicle-treated animal. (b) Section from an animal treated with 155 nmol (100 μg) DPDPE. (c) Quantification of BDNF mRNA signal in frontal cortex (FC), CA1, CA3, and dentate gyrus (DG) of hippocampus. 155 nmol of DPDPE significantly increased BDNF mRNA expression in the frontal cortex (n = 4 per group, except 155 nmol DPDPE where n = 3).** P < 0.01.
Fig. 6.
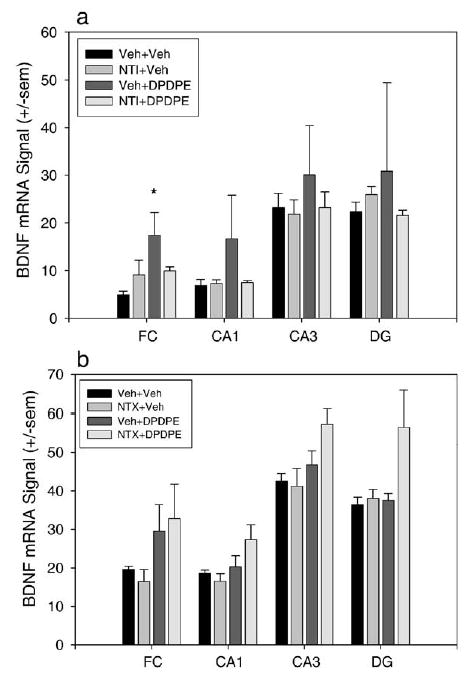
NTI, not NTX, blocks increases in BDNF mRNA expression produced by DPDPE. Quantification of BDNF mRNA signal in the FC, CA1, CA3, and DG of hippocampus. (a) 1 mg/kg NTI given 30 min before 155 nmol DPDPE prevents the DPDPE-induced increase in BDNF mRNA expression in frontal cortex and prevents any animals from showing increased BDNF signal in the hippocampus (Veh + Veh and Veh + DPDPE n = 4, NTI + Veh and NTI + DPDPE n = 3). *P < 0.05. (b) 0.1 mg/kg NTX administered 15 min before 155 nmol DPDPE did not block the trend towards DPDPE-induced increases in BDNF mRNA expression in frontal cortex (Veh + Veh n = 3, NTI + Veh n = 4, Veh and NTX + DPDPE n = 6).
Deltorphin II alone increased BDNF expression in the dentate gyrus at a dose of 0.1 nmol (P = 0.143) and in the CA3 region of hippocampus at a dose of 1 nmol (P = 0.366), and H-Dmt-Tic-NH-CH2-Bid alone increased BDNF mRNA expression in the frontal cortex (P = 0.001). However, when the delta antagonist NTI or vehicle was given before deltorphin II or H-Dmt-Tic-NH-CH2-Bid, there was no significant increase in BDNF mRNA expression in any brain region [P < 0.05]. In addition, there was no effect of either peptide with or without a pretreatment of naltrexone [P < 0.05] (Fig. 7).
Fig. 7.
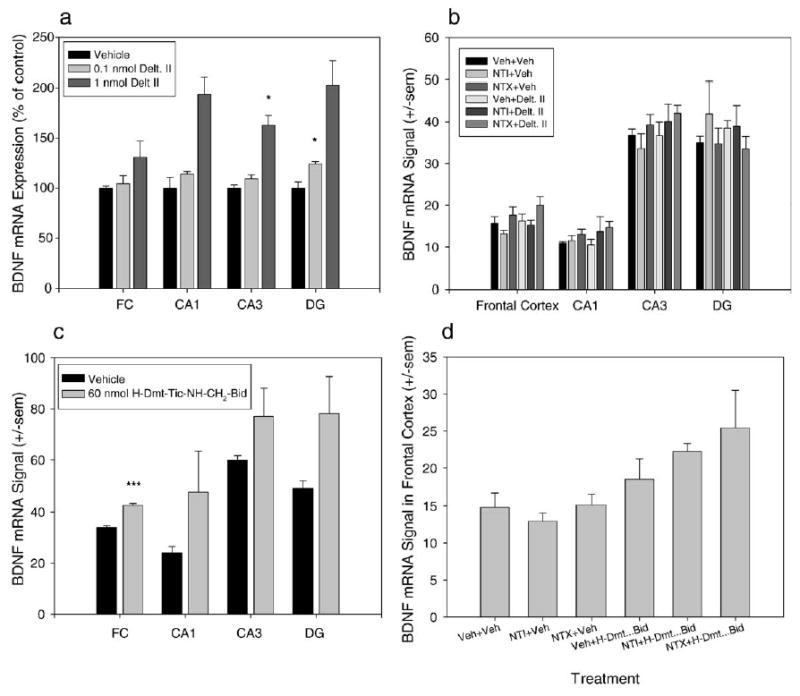
Deltorphin II and H-Dmt-Tic-NH-CH2-Bid do not reliably increase BDNF mRNA expression. (a–c) Quantification of BDNF mRNA signal in the FC, CA1, CA3, and DG of hippocampus. (d) Quantification of BDNF mRNA signal in the frontal cortex. (a) Effect of 0.1 and 1 nmol deltorphin II on BDNF mRNA expression. Both doses tended to increase BDNF expression in the hippocampus. 0.1 nmol significantly increased BDNF expression in the DG, and 1 nmol significantly increased BDNF expression in the CA3 region of hippocampus. (b) When vehicle, NTI, or NTX was given prior to deltorphin II, no treatment had any significant effect on BDNF mRNA expression. (c) 60 nmol H-Dmt-Tic-NH-CH2-Bid significantly increased BDNF mRNA expression in the frontal cortex. (d) H-Dmt-Tic-NH-CH2-Bid had no effect on BDNF mRNA expression in the FC when vehicle, NTI, or NTX was given as a pretreatment. *P < 0.05, ***P < 0.001.
3. Discussion
The present study examined the ability of peptidic and pseudo-peptidic delta opioid receptor agonists to produce convulsions and antidepressant-like effects in the forced swim test and to increase BDNF mRNA expression as has been reported to occur with nonpeptidic delta opioid receptor agonists (Broom et al., 2002b; Torregrossa et al., 2004). The peptides DPDPE, deltorphin II, JOM-13, and the pseudo-peptide H-Dmt-Tic-NH-CH2-Bid all produced antidepressant-like effects in the forced swim test, similar to the nonpeptidic agonists (+)BW373U86 and SNC80 (Broom et al., 2002b). In addition, the antidepressant-like effects of these compounds were mediated by the delta opioid receptor as the selective delta antagonist naltrindole blocked the effect. These observations extend the findings from previous studies that a wide variety of agonists at the delta opioid receptor produce antidepressant-like behavioral effects (Broom et al., 2002b,c; Saitoh et al., 2004; Zhang et al., 2005).
However, DPDPE, deltorphin II, and JOM-13 did not produce as great a decrease in immobility as compared to previous studies with the systemic delivery of the nonpeptidic delta agonists. In addition, there was much more variability among animals in response to centrally administered peptidic agonists. The lower magnitude of effect produced by the peptidic agonists is likely due to the fact that these compounds appear to be partial agonists at the delta receptor when compared to the nonpeptidic agonists. Indeed, Jutkiewicz and colleagues found that DPDPE and deltorphin II produced less G-protein stimulation in most brain regions in the rat when compared to the nonpeptidic agonists (2005). In addition, the increased variability is probably a property of the route of administration. While cannula placement was always verified to be in the right lateral ventricle, it is hard to control how uniformly the compounds distribute in the brain across animals.
Nevertheless, these compounds did produce significant antidepressant-like effects in the forced swim test, indicating that at least some lower efficacy agonists at the delta receptor retain antidepressant-like effects while not producing convulsions, unlike the higher efficacy nonpeptidic compounds, which produce convulsions at high doses (Broom et al., 2002c). Therefore, it may be possible to develop systemically active delta partial agonists that produce antidepressant-like effects without producing the unwanted side effect of a convulsion. In addition, Jutkiewicz et al. demonstrated that slowing the i.v. infusion of nonpeptidic delta agonists eliminates the convulsions without affecting the antidepressant-like behavioral effect, suggesting that the convulsions can be avoided by several means while retaining the beneficial effects of delta agonists (2005).
The ability of JOM-13 to produce convulsions in some animals by the i.v. route of administration (fast infusion) suggests that this compound may be more efficacious than the other peptides. Alternatively, it may be that the increased stability of JOM-13 over the other peptides allows for increased delta receptor occupancy and a higher probability of producing a convulsion. It would be interesting to determine the antidepressant-like and convulsive effects of centrally administered JOM-13, however, we did not have sufficient access to this compound to complete those studies.
DPDPE, deltorphin II, and H-Dmt-Tic-NH-CH2-Bid all in-creased BDNF mRNA expression in either the frontal cortex or the hippocampus; however, this effect was low in magnitude and was not consistent across experiments. However, the tendency to increase BDNF mRNA expression in the frontal cortex and hippocampus may provide further evidence for the antidepressant potential of delta opioid receptor agonists. Increased BDNF mRNA expression has been implicated in the mechanism of action of antidepressant drugs (Duman, 2004). Nibuya et al. (1995, 1996) reported that chronic antidepressant administration increased BDNF mRNA expression in the frontal cortex and hippocampus. In addition, BDNF injected into the midbrain (Siuciak et al., 1997) and into the hippocampus (Shirayama et al., 2002) produces antidepressant-like behavioral effects in animal models, including the forced swim test. Additionally, human depressed subjects have reduced serum levels of BDNF, however, when these individuals are treated with antidepressants, BDNF levels normalize (Aydemir et al., 2005), and post-mortem studies have shown that people diagnosed with major depression had lower BDNF immunoreactivity than controls (Chen et al., 2001). Therefore, reductions in growth factors are likely to play some role in the pathogenesis of depression, and it may be necessary for antidepressant treatments to normalize the growth factor abnormalities before therapeutic effects are observed.
The ability of both nonpeptidic and peptidic delta agonists to increase BDNF mRNA expression provides further evidence that the delta opioid receptor is a promising target for the development of antidepressants. However, it should be noted that there have been several reports that chronic administration of certain antidepressants has no effect on BDNF mRNA expression, indicating that an increase in BDNF expression may not be required to obtain antidepressant effects (Miro et al., 2002; Coppell et al., 2003; DeFoubert et al., 2004; Torregrossa et al., 2005).
While the peptidic and pseudopeptidic agonists did increase BDNF mRNA expression, they did not increase BDNF in the same brain regions to the same extent, and they did not always produce significant increases in expression across experiments. Both DPDPE and H-Dmt-Tic-NH-CH2-Bid increased BDNF mRNA expression in the frontal cortex and tended to increase expression in the hippocampus, while deltorphin II increased BDNF expression in the CA3 and dentate gyrus regions of hippocampus and had no effect in the frontal cortex. However, the amount of expression was highly variable among animals in all brain regions and with all treatments. The variability is likely due to differences in drug distribution among animals and possibly due to variations in the extent of damage done to the brain by cannula insertion. The insertion of a cannula into the brain will cause some amount of cell death, and BDNF is a growth factor whose expression will increase in response to brain damage (Kobori et al., 2002). Therefore, the amount of BDNF expressed due to the surgery may affect the subsequent ability of various treatments to affect BDNF expression. This idea is purely hypothetical, but animals that had observable damage through sections of the hippocampus also had the highest BDNF mRNA expression in the hippocampus (particularly in the dentate gyrus), regardless of the treatment they received. While these animals were excluded from analysis, variation in brain damage among animals may explain some of the within-groups expression differences that were observed. Again, at least DPDPE did significantly increase BDNF expression in the frontal cortex, despite the variability, suggesting that activation of the delta receptor by partial agonists may be sufficient to affect BDNF expression. We may have seen less variable and more reliable effects in the frontal cortex because this region was sufficiently far from the site of injection that there was little brain damage induced BDNF expression.
In conclusion, there is mounting evidence demonstrating antidepressant-like behavioral effects of several types of delta opioid receptor agonists, in several animal models, suggesting that activation of the delta receptor consistently produces effects similar to clinically prescribed antidepressants (Broom et al., 2002a; Saitoh et al., 2004; Jutkiewicz et al., 2004; present study). In addition, the ability of delta opioid receptor agonists to increase BDNF mRNA expression suggests that these compounds could be important in treating depression, but also in treating patients suffering stroke, head trauma, or possibly other neurodegenerative disorders. Therefore, the delta opioid receptor is a promising target for the development of novel treatments for several disorders, including depression.
4. Experimental procedure
4.1. Animals
All experiments were conducted using male Sprague–Dawley rats (Harlan Sprague–Dawley, Indianapolis, IN) delivered weighing 250–300 g. The rats were housed 3 per cage and were allowed ad libitum access to food and water. Animal rooms were kept on a 12-h light/dark cycle with lights on at 06:30 h and a temperature of 21 °C. Experiments were carried out in accordance with the Declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The study protocols were approved by the University of Michigan University Committee on the Use and Care of Animals.
4.2. Drugs
DPDPE [Tyr-D-Pen-Gly-Phe-D-Pen], deltorphin II [Tyr-D-Ala-Phe-Glu-Val-Val-Gly-NH2] trifluoroacetate, and naltrindole were obtained from the National Institute on Drug Abuse. H-Dmt-Tic-NH-CH2-Bid was synthesized according to published protocols (Balboni et al., 2002). Tyr-c[D-Cys-Phe-D-Pen]OH (JOM-13) was synthesized according to published protocols (Heyl et al., 1991). DPDPE was dissolved in sterile saline. Deltorphin II, H-Dmt-Tic-NH-CH2-Bid, JOM-13, and naltrindole were dissolved in sterile water. In addition, ketamine hydrochloride (Vedco Inc., St. Joseph, MO) and xylazine hydrochloride (Fermenta Animal Health Co., Kansas City, MO) were dissolved in sterile water.
4.3. Intracerebroventricular cannulation surgery
Rats were implanted with indwelling cannulas aimed at the right lateral ventricle under anesthesia with 100 mg/kg ketamine hydrochloride and 10 mg/kg xylazine hydrochloride injected intramuscularly (i.m.). Rats were placed in a stereotaxic apparatus (Harvard Apparatus, Holliston, MA), and a guide cannula cut to 4.2 mm below pedestal (Plastics One Inc., Roanoke, VA) was implanted in the right lateral ventricle using standard coordinates (0.8 mm posterior and 1.5 mm lateral from bregma) (Paxinos and Watson, 1986). One stainless steel miniature machine screw (Small Parts Inc., Miami Lakes, FL) was inserted in the skull, and the cannula and screw were secured using Ortho-Jet dental resin (Lang Dental Mfg. Co. Inc., Wheeling, IL). The resin was allowed to dry for several minutes, and then a dummy cannula (Plastics One Inc.) cut to the length of the guide cannula was inserted to maintain cannula patency. Rats were allowed to recover for at least 3 days before experimentation.
4.4. Intravenous catheter implantation
Catheters were made from approximately 15 cm of Micro-Renathane tubing (MRE-040, Braintree Scientific, Inc., Braintree, MA). Rats were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine hydrochloride (10 mg/kg, i.p.). The right jugular vein was isolated ventrally through an incision in the neck. Approximately 3 cm of the catheter was inserted into the right jugular vein, and the tubing was sutured to the vein and to the surrounding tissues at multiple points in order to secure the catheter placement. The remaining tubing was threaded s.c. to an intrascapular incision and secured in place by sutures to the musculature directly below the incision. Two to three centimeters of tubing remained exposed outside the rat's body. The catheter was closed with a stainless steel pin (McMaster-Carr, Cleveland, OH). Immediately after and every day following the surgery, the catheters were flushed with approximately 0.5 ml of heparinized saline (50 U/ml). On average, rats were allowed 5 days of recovery from surgery before being used in an experiment.
4.5. Experimental treatment
Forced swim test experiments were carried out by injecting DPDPE, deltorphin II, and H-Dmt-Tic-NH-CH2-Bid i.c.v. and JOM-13 intravenously (i.v.) because JOM-13 crosses the blood–brain barrier (unpublished observations). The range of doses studied for each drug were chosen based on their efficacy in other behavioral assays found in the literature (usually doses that were effective in antinociceptive assays) or based on relative efficacy in in vitro preparations when compared to known compounds.
I.c.v. injections were given by administering 10 μL of the drug treatment or vehicle (saline or sterile water) into the cannula using a Hamilton syringe (Hamilton Instruments, Reno, NV). Injections were given at a rate of 1 μL/15 s, and injection cannulas were kept in the cannula for at least 30 s after injection to prevent drug reflux. Intravenous injections were given as a single injection infused over 20 s. Thirty minutes after the initiation of the i.c.v. or i.v. injection, the animals were assayed in the forced swim test (described below). In the antagonist studies, using a 2 × 2 experimental design, 1 or 10 mg/kg naltrindole, 0.1 mg/kg naltrexone, or vehicle was injected subcutaneously (s.c.) 15 (naltrexone) or 30 (naltrindole) min prior to injection of the test compound or its vehicle. The forced swim test was performed 30 min after the injection. Accurate cannula placement was verified by injecting 30 μL of methylene blue dye into the cannula followed by visual inspection of dye in the ventricles after brain dissection.
BDNF mRNA expression studies were performed by injecting the appropriate vehicle, DPDPE, deltorphin II, or H-Dmt-Tic-NH-CH2-Bid, or a combination of antagonist and test compound, as described above. Doses were chosen based on efficacy in the forced swim test. Animals were sacrificed by rapid decapitation, and their brains were dissected and placed in isopentane on dry ice. The brains were sectioned on a cryostat at a thickness of 20 μm, and the slides were stored at −80 °C until in situ hybridization histochemistry was performed (described below). Cannula placement was verified by sectioning the brain through the lateral ventricles to verify that the right lateral ventricle was punctured by the cannula. Data from inaccurately placed cannulas were discarded.
The number of animals in each treatment group varied due to elimination of animals with inaccurately placed cannula and because control animals were tested when each dose of drug was tested, therefore creating a larger pool of control animals. In addition, in the BDNF expression studies, some animals were eliminated who had excessive BDNF mRNA expression around the injection site that may have altered the amount of BDNF expression in the brain regions of interest.
4.6. Convulsion observation
Immediately after i.v. or i.c.v. injection, rats were returned to their home cage for a 20-min observation period of convulsion and catalepsy. Time to onset of convulsion, duration of convulsions, and duration of catalepsy were recorded. Catalepsy duration was defined as the time to remove forelimbs from a rod elevated approximately 2 in. off the ground.
4.7. Forced swim test
The forced swim test was conducted as described previously (Torregrossa et al., 2004). Briefly, rats were videotaped from above during a 15-min swim period in a cylindrical container (46 cm tall × cm diameter), filled to 30 cm with 25 °C (±1) water. The swim period began 30 min after injection of test compound. An observer blind to treatment scored the videotapes, classifying behaviors every 5 s for the entire 15-min period. The behaviors observed were immobility, swimming, and climbing, which were defined as described by Broom et al. (2002b,c) and Detke et al. (1997).
We employed a 1-day forced swim test rather than the more commonly used 2-day test because previous work in our laboratory has shown that a single 15-min forced swim test is sufficient to observe antidepressant-like effects of both known antidepressant drugs and novel compounds, including delta agonists (Broom et al., 2002c).
4.8. In situ hybridization histochemistry
BDNF mRNA expression levels were determined by a double label in situ hybridization with a [35S]-labeled BDNF cRNA probe as described previously (Torregrossa et al., 2004). The rat BDNF cDNA (Isackson et al., 1991) was donated by Drs. Gall and Lauterborn (University of California, Irvine). The [35S]-labeled BDNF probe was hybridized to brain sections in hybridization buffer containing 1.5 million cpms radiolabeled probe per 80 μL. The slides were exposed on Kodak XAR film (Eastman Kodak, Rochester, NY) for 14 days.
4.9. Quantification of radioactive signal
BDNF mRNA levels were quantified using NIH Image (Scion Image Corp., Frederick, MD) software. BDNF mRNA expression was examined in the frontal cortex, the CA1, the CA3, and the dentate gyrus regions of hippocampus. All of these regions with the exception of the dentate gyrus have been shown to at least modestly increase BDNF mRNA expression after acute administration of the nonpeptidic delta agonist (+)BW373U86 (Torregrossa et al., 2004). Each brain region was analyzed by creating an outline around the region and measuring both the left and right sides of the brain and from rostral–caudal sections 100–200 μm apart. At least four sections per region per rat were quantified. The signal measurements were corrected for background and were determined as the mean radioactive intensity per pixel for that region. These signal values for each section were then averaged to obtain the mean signal for each region in each rat. These data points were then averaged per group and compared statistically.
4.10. Statistical analysis
Comparisons between two groups were made using a Student's t test. All comparisons between multiple groups were conducted by one-way ANOVA. Tukey's post-hoc test was used to determine differences between groups, where P < 0.05 was considered significant.
Acknowledgments
The authors would like to thank Ryan Cole, Prasanth Navarasala, and Melissa Spencer for their technical assistance. This research was supported by USPHS grants DA00254, DA07281, DA13386, and MH42251. We would also like to thank Italian COFIN 2004.
References
- Aydemir O, Deveci A, Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:261–265. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Baamonde A, Dauge V, Ruiz-Gayo M, Fulga IG, Turcand S, Fournie-Zaluski MC, Roques BP. Antidepressant-type effects of endogenous enkephalins protected by systemic RB101 are mediated by opioid delta and dopamine D1 receptor stimulation. Eur J Pharmacol. 1992;216:157–166. doi: 10.1016/0014-2999(92)90356-9. [DOI] [PubMed] [Google Scholar]
- Balboni G, Guerrini R, Salvadori S, Bianchi C, Rizzi D, Bryant SD, Lazarus LH. Evaluation of the Dmt-Tic pharmacophore: conversion of a potent delta-opioid receptor antagonist into a potent delta agonist and ligands with mixed properties. J Med Chem. 2002;45:713–720. doi: 10.1021/jm010449i. [DOI] [PubMed] [Google Scholar]
- Broom DC, Nitsche JF, Pintar JE, Rice KC, Woods JH, Traynor JR. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002a;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002b;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Convulsant activity of a non-peptidic delta-opioid receptor agonist is not required for its antidepressant effects in Sprague–Dawley rats. Psychopharmacology. 2002c;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Comer SD, Hoenicke EM, Sable AI, McNutt RW, Chang KJ, DeCosta BR, Mosberg HI, Woods JH. Convulsive effects of systemic administration of the delta opioid agonist BW373U86 in mice. J Pharmacol Exp Ther. 1993;215:127–134. [PubMed] [Google Scholar]
- Coppell AL, Pei Q, Zetterstrom TSC. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44:903–910. doi: 10.1016/s0028-3908(03)00077-7. [DOI] [PubMed] [Google Scholar]
- DeFoubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA, Murray TK, Gaillard JP, Deville C, Xhenseval V, Thomas CE, O'Neill MJ, Zetterstrom TSC. Fluoxetine-induced change in rat brain expression of brain-derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–112. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Shoenbaum GM, Yarbrough J, McNutt R, Chang KJ. A novel delta opioid agonist, BW373U86, in squirrel monkeys responding under a schedule of shock titration. J Pharmacol Exp Ther. 1993;267:875–882. [PubMed] [Google Scholar]
- Fraser GL, Pradhan AA, Clarke PBS, Wahlestedt C. Supraspinal antinociceptive response to [D-Pen2,5]-enkephalin (DPDPE) is pharmacologically distinct from that to other delta-agonists in the rat. J Pharmacol Exp Ther. 2000a;295:1135–1141. [PubMed] [Google Scholar]
- Fraser GL, Parenteau H, Tu TM, Ducharme J, Perkins MN, Clarke PBS. The effects of [delta] agonists on locomotor activity in habituated and non-habituated rats. Life Sci. 2000b;67:913–922. doi: 10.1016/s0024-3205(00)00690-1. [DOI] [PubMed] [Google Scholar]
- Haffmans J, Dzoljic MR. Differential epileptogenic potentials of selective mu and delta opiate receptor agonists. J Neural Transm. 1983;57:1–11. doi: 10.1007/BF01250043. [DOI] [PubMed] [Google Scholar]
- Heyl DL, Omnaas JR, Sobczyk-Kojiro K, Medzihradsky F, Smith CB, Mosberg HI. Opioid receptor affinity and selectivity effects of second residue and carboxy terminal residue and carboxy terminal residue variation in a cyclic disulfide-containing opioid tetrapeptide. Int J Pept Protein Res. 1991;37:224–229. doi: 10.1111/j.1399-3011.1991.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Hong EJ, Rice KC, Calderon S, Woods JH, Traynor JR. Convulsive behavior of nonpeptide delta opioid ligands: comparison of SNC80 and BW373U86 in mice. Analgesia. 1998;3:269–276. [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Eller EB, Folk JE, Rice KC, Traynor JR, Woods JH. Delta-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague–Dawley rats. J Pharmacol Exp Ther. 2004;309:173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Walker NP, Folk JE, Rice KC, Portoghese PS, Woods JH, Traynor JR. Comparison of peptide and nonpeptidic delta-opioid agonists on [35S]GTPgammaS binding in brain slices from Sprague–Dawley rats. J Pharmacol Exp Ther. 2005a;312:1314–1320. doi: 10.1124/jpet.104.078741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Rice KC, Traynor JR, Woods JH. Separation of the convulsions and antidepressant-like effects produced by the delta opioid receptor agonist SNC80 in rats. Psychopharmacology. 2005b;182:588–596. doi: 10.1007/s00213-005-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkiewicz EM, Torregrossa MM, Sobczyk-Kojiro K, Mosberg HI, Folk JE, Rice KC, Watson SJ, Woods JH. Behavioral and neurobiological effects of the enkephalinase inhibitor RB101 relative to its antidepressant effects. Eur J Pharmacol. doi: 10.1016/j.ejphar.2005.12.002. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori N, Clifton GL, Dash PK. Altered expression of novel genes in the cerebral cortex following experimental brain injury. Mol Brain Res. 2002;104:148–158. doi: 10.1016/s0169-328x(02)00331-5. [DOI] [PubMed] [Google Scholar]
- Miro X, Perez-Torres S, Artigas F, Puigdomenech P, Palacios JM, Mengod G. Regulation of cAMP phosphodiesterase mRNAs expression in rat brain by acute and chronic fluoxetine treatment, an in situ hybridization study. Neuropharmacology. 2002;43:1148–1157. doi: 10.1016/s0028-3908(02)00220-4. [DOI] [PubMed] [Google Scholar]
- Negus SS, Butelman ER, Chang KJ, DeCosta B, Winger G, Woods JH. Behavioral effects of the systemically active delta opioid agonist BW373U86 in rhesus monkeys. J Pharmacol Exp Ther. 1994;270:1025–1034. [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and TrkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakarinen ED, Woods JH, Moerschbaecher JM. Repeated acquisition of behavioral chains in squirrel monkeys: comparisons of a mu, kappa, and delta opioid agonist. J Pharmacol Exp Ther. 1995;272:552–559. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd ed. Academic Press; San Diego: 1986. [Google Scholar]
- Saitoh A, Kimura Y, Suzuki T, Kawai K, Nagase H, Kamei J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen ACH, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Tejedor-Real P, Mico JA, Smadja C, Maldonado R, Roques BP, Gibert-Rahola J. Involvement of delta-opioid receptors in the effects induced by endogenous enkephalins on learned helplessness model. Eur J Pharmacol. 1998;354:1–7. doi: 10.1016/s0014-2999(98)00423-3. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Isgor C, Folk JE, Rice KC, Watson SJ, Woods JH. The delta opioid receptor agonist (+) BW373U86 regulates BDNF mRNA expression in rats. Neuropsychopharmacology. 2004;29:649–659. doi: 10.1038/sj.npp.1300345. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Folk JE, Rice KC, Watson SJ, Woods JH. Chronic administration of the delta opioid receptor agonist (+)BW373U86 and antidepressants on behavior in the forced swim test and BDNF mRNA expression in rats. Psychopharmacology. 2005;183:31–40. doi: 10.1007/s00213-005-0113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HN, Woods JH, Watson SJ, Ko MC. Antidepressant-like effects of endogenous opioid peptides in rats: behavioral and BDNF mRNA expression studies. FASEB J. 2005;19:A1542–A1543. [Google Scholar]


