Abstract
Alzheimer’s disease is the most common cause of the loss of cognitive function among the elderly, and the aggregation and deposition of misfolded β–amyloid protein (Aβ) contribute to this progressive central nervous system decline. Therefore, compounds that inhibit or even reverse Aβ aggregation might be useful for the treatment or prevention of Alzheimer’s disease. To identify potential therapeutic agents for the treatment of Alzheimer’s disease, a mass spectrometry-based screening assay was developed to identify and rank order compounds that inhibit the aggregation of Aβ. To carry out this assay, Aβ was incubated with a test compound at 37°C for 20 h followed by ultrafiltration to separate the monomeric Aβ from its aggregates. Aliquots of the ultrafiltrate were analyzed for monomeric Aβ using positive ion electrospray mass spectrometry based on the abundance the quadruply protonated molecule of Aβ at m/z 1083. The calibration curve for Aβ was linear with a correlation coefficient (r2) > 0.99 over the range of at least 11 to 110 μM. The limit of detection was 0.224 ng (5.18 nM, 10 μL injection) and the limit of quantitation was 0.747 ng (17.2 nM, 10 μL injection). Based on previous reports of compounds that either bind to Aβ or are useful in treating Alzheimer’s disease, melatonin, methysticin, 3-indolepropionic acid, and daunomycin were assayed and ranked in order of inhibition of Aβ aggregation. The most effective inhibitor of aggregation of Aβ protein was daunomycin followed in descending order by 3-indolepropionic acid, melatonin, and then methysticin. These data suggest that this ultrafiltration LC-MS screening assay may be used to identify potential therapeutic agents for the treatment of Alzheimer’s disease based on the prevention of Aβ aggregation.
Keywords: β-amyloid protein, Alzheimer’s disease, aggregation, electrospray mass spectrometry, screening
Alzheimer’s disease is a progressive neurodegenerative disease of the brain that is the most common form of age-related dementia. Currently, approximately 4 million Americans and 17–25 million people worldwide suffer from Alzheimer’s disease, and by the middle of the 21st century, the number of Americans with this form of dementia is expected to grow to at least 13 million unless preventive or curative medications are discovered.1,2 The cost of Alzheimer’s disease in the United States has been estimated to be $100 billion annually in the United States based on lost business productivity of the affected individuals and their caregivers as well as the costs of health care.3 Therefore, finding treatments that can delay, prevent or reverse Alzheimer’s disease would have a substantial impact.
The primary cause of the inflammation, cytotoxicity and neurotoxicity associated with the progression of Alzheimer’s disease is probably related to be the formation of Aβ aggregates and plaques.4 Aβ is produced by endoproteolysis of the amyloid precursor protein and can form non-covalent fibrillar aggregates in the brain that have been related to Alzheimer’s disease neurotoxicity.5,6 Factors that increase the production of Aβ, facilitate amyloid aggregation, facilitate deposition, or inhibit the clearance of amyloid deposits either cause Alzheimer’s disease or increase the risk of Alzheimer’s disease.2,7 Enzymes known as secretases are responsible for the cleavage of amyloid precursor protein that leads to Aβ formation. The normal route of amyloid precursor protein processing is cleavage by α-secretase between residues 16 and 17 forming a soluble and non-toxic product.8 However, β- and γ-secretases cleave amyloid precursor protein between amino acid residues 596 and 597 and 637, 638, or 639, respectively, forming Aβ peptides which are strongly related to the development of Alzheimer’s disease.9,10 Therefore, one of the strategies currently being investigated for preventing Aβ formation and Alzheimer’s disease consists of inhibiting γ- and β-secretases.
Another approach to the treatment or prevention of Alzheimer’s disease is the discovery of drugs that prevent or perhaps reverse Aβ aggregation. Several assays have been developed to help identify compounds that inhibit β-amyloid protein aggregation. For example, affinity-based screening for ligands to Aβ have been reported that use methods such as surface plasmon resonance.11 Affinity based screening is based on the assumption that ligands to Aβ might prevent its aggregration, but additional assays are required to determine to extent to which these ligands actually inhibit Aβ aggregation. To address this need cell based assays have been reported to screen for the prevention of Aβ aggregation or amyloid toxicity. However, cell based assays are low in throughput.12
Some alternative assays for the screening of compounds of Aβ aggregation have been reported. For example, Ahn et al.13 developed an electrophoresis assay in which the mobility of DNA incubated with aggregated Aβ1–40 was shifted compared to DNA incubated with monomeric Aβ. However, this assay provides a non-quantitative and indirect measure of Aβ aggregation, relies on electrophoresis which is low in throughput, and requires a high concentration (4 mM) of Aβ. A fluorescence-based assay has also been reported to screen for inhibitors of β–amyloid peptide aggregation, which uses the immobilized Aβ aggregation-core peptide, KLVFF, and solution-phase Aβ10–35 containing a fluorescent tag.14 This fluorescence-based assay is suitable for high throughput screening but relies on the specialized reagents immobilized KLVFF and fluorescent tagged Aβ10–35.
Here we report the development of a mass spectrometry assay to screen for compounds that prevent Aβ aggregation, which is based on the measurement of the concentration of monomeric Aβ. Our approach overcomes the limitations of previous assays in that it is high throughput, sensitive, and measures directly the extent of aggregation instead of a related property. Melatonin, daunomycin, and 3-indolepropionic acid were used to validate our new assay since they have been reported to inhibit the formation of β amyloid protein aggregates.15–17 The kavalactone methysticin was assayed since kava (Piper methysticum) dietary supplements have been reported to relieve benefit Alzheimer’s sufferers, and kavalactones such as methysticin are the active ingredients in kava responsible for its anxiolytic effects.18
EXPERIMENTAL SECTION
Materials
HPLC-grade acetonitrile and ammonium acetate were purchased from Fisher Scientific (Fair Lawn, NJ). Melatonin, 3-indolepropionic acid, daunomycin hydrochloride (see structures in Figure 1), and Aβ1–40 were purchased from Sigma-Aldrich (St. Louis, MO). Methysticin was purchased from Standardherbs (Chesterfield, MO). All additional chemicals were reagent or HPLC grade and were used without further purification.
Figure 1.
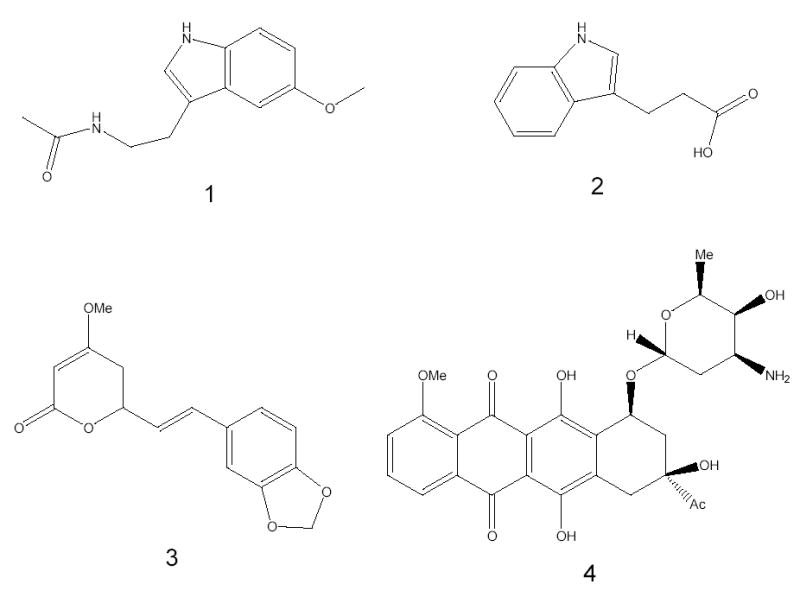
Structures of (1) melatonin, (2) 3-indolepropionic acid, (3) methysticin, and (4) daunomycin
Stock solutions
A stock solution containing 500 mg (116 μmol) Aβ1–40 was prepared in 5 μL acetic acid and 1 mL double-distilled water. Stock solutions of melatonin, 3-indolepropionic acid, daunomycin, and methysticin were prepared in double-distilled water each containing a single compound at 20 mM. Standard solutions of Aβ1–40 were prepared by spiking Aβ1–40 into double-distilled water, and then the solutions were used immediately for the determination of the limit of detection, limit of quantitation, and for the determination of the linearity of the mass spectrometer response (see methods for the mass spectrometric analysis below). Standard solutions of Aβ1–40 were prepared by spiking Aβ1–40 stock solution into double distilled water, giving final concentrations of 11.5, 34.5, 57.5, 80.5, and 114 μM Aβ1–40.
Sample preparation
For the screening of each compound a 1 μL aliquot of the corresponding 20 mM stock solution was mixed with 99 μL of the 114 μM Aβ1–40 stock solution and then incubated at 37 °C for 20 h. The mixture was filtered through a 10,000 molecular weight cut-off (Millipore, Bedford, MA) regenerated cellulose ultrafiltration membrane, and a 10 μL aliquot of the ultrafiltrate was removed for quantitative analysis of the Aβ1–40 monomer concentration using flow injection electrospray mass spectrometry.
Mass spectrometric analysis
Flow-injection positive ion electrospray mass spectrometry was carried out using a Micromass (Manchester, UK) Quattro II triple quadrupole mass spectrometer equipped with a Waters 2690 HPLC system. The mobile phase consisted of water (containing 0.1% formic acid) and acetonitrile (1:1, v/v) at a flow rate of 200 μL/min. Positive ion electrospray was used to form quadruply protonated molecules of the Aβ1–40 monomer at m/z 1083, using the optimized ion source conditions of a capillary voltage of 3000 V, cone voltage 35 eV, source block temperature 150 °C, and a desolvation temperature of 300 °C. Selected ion monitoring (SIM) with a dwell time of 1.00 s was used during flow-injection to monitor the Aβ1–40 signal of m/z 1083 for test samples, control solutions and standards. A scheme summarizing the mass spectrometry-based screening assay for the discovery of compounds that inhibit the aggregation of Aβ1–40 is summarized in Figure 2.
Figure 2.
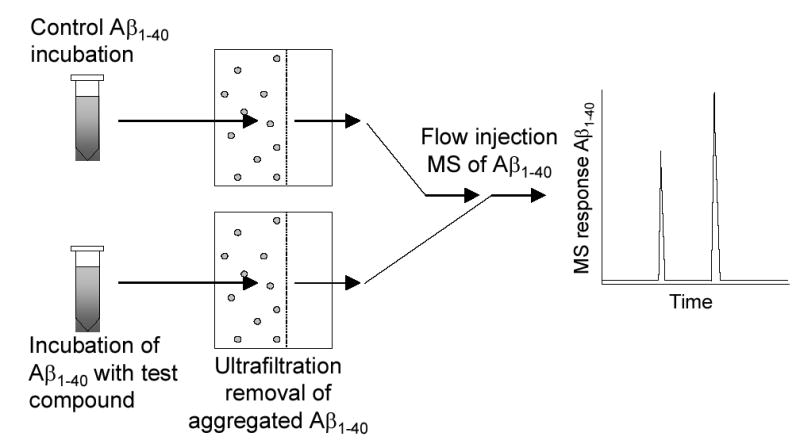
Design of the ultrafiltration mass spectrometric screening assay for the discovery of ligands that prevent Aβ aggregation. After incubation of Aβ1–40 with either a blank control solution or a series of test compounds, aggregated Aβ1–40 is removed by ultrafiltration, and the relative amount of monomeric Aβ1–40 in the control (untreated) solution is compared to that in each of the treated solutions using flow injection electrospray mass spectrometry. Compounds that prevent Aβ1–40 aggregation produce solutions containing the highest concentrations of monomeric Aβ1–40.
RESULTS AND DISCUSSION
Although several Aβ proteins are produced by the γ- and β-secretases including Aβ1–40, Aβ1–41, and Aβ1–42, Aβ1–40 was selected for use in the mass spectrometry-based screening assay due to its higher water solubility, lower cost, and higher abundance in vivo. During positive ion electrospray, the most abundant ion of Aβ1–40 corresponded to [M+H]4+ and was detected at m/z 1083 (see Figure 3). Therefore, selected ion monitoring of m/z 1083 was used for the determination of the concentration of monomeric Aβ1–40 remaining in solution after the removal of aggregates using ultrafiltration.
Figure 3.
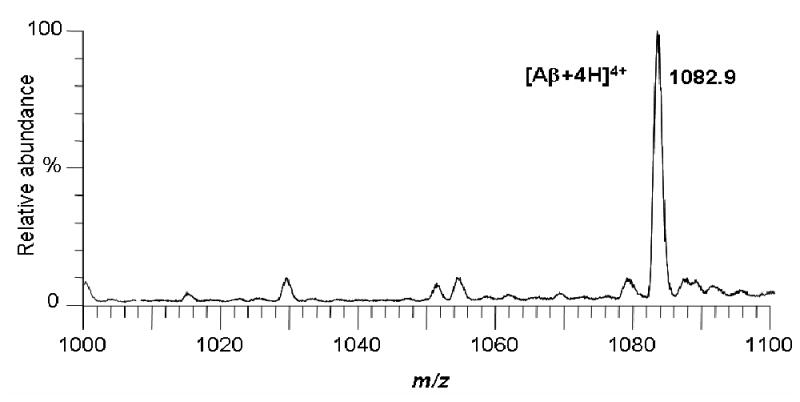
Positive ion electrospray mass spectrum of amyloid β protein 1–40 (Aβ1–40). The most abundant ion of m/z 1083 corresponds to [Aβ1–40 +4H]4+. During screening analyses, the ion of m/z 1083 was monitored during flow-injection for the quantitative analysis of monomeric Aβ1–40.
The calibration curve for Aβ1–40 was linear (r2 > 0.990) up to the maximum concentration used in the screening assay (114 μM) and was described by the equation, y = 1.55x − 10. The limit of detection of Aβ1–40 was 0.224 ng (5.18 nM, 10 μL injection) based on a signal-to-noise ratio of 3:1, and the limit of quantitation was 0.747 ng (17.2 nM, 10 μL injection), based on a signal-to-noise ratio of 10:1. Since a linear response was obtained for monomeric Aβ1–40 over the range of concentrations that were anticipated (between 11.5 and 114 μM), mass spectrometric detection was possible for the comparison of the concentrations of Aβ1–40 in test solutions as part of a screening assay for inhibitors of Aβ1–40 aggregation.
After incubation of Aβ1–40 with or without test compounds, Aβ1–40 aggregates were removed by ultrafiltration through a membrane with a molecular weight cut-off of 10,000. Since the mass of Aβ1–40 is 4,328, aggregates consisting of 3 or more molecules of Aβ1–40 did not pass through the ultrafiltration membrane. Therefore, the Aβ1–40 in the ultrafiltrate represented unaggregated protein.
Since melatonin, methysticin, 3-indolepropionic acid, and daunomycin have been reported to be either useful in the treatment of Alzheimer’s disease or to prevent Aβ aggregation, these compounds were screened to establish the feasibility of the new screening assay and to evaluate the potential of each of these compounds to prevent aggregation of Aβ1–40. After incubation of each compound with Aβ1–40, the solutions were ultrafiltered. The concentrations of monomeric Aβ1–40 in the ultrafiltrates were measured using flow injection mass spectrometry and compared to a control incubation containing no test compound. In the sample flow injection mass chromatogram for the screening of these compounds shown in Figure 4, the area of each peak represents the concentration of monomeric Aβ1–40. The largest peaks (daunomycin and 3-indolepropionic acid incubations) corresponded to the greatest inhibition of Aβ1–40 aggregation, and the smallest peak (in this case the control incubation without any ligand) represented the most extensive aggregation of Aβ1–40.
Figure 4.
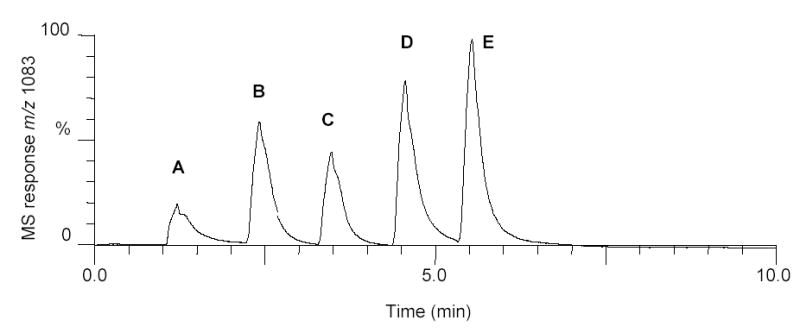
Selected ion monitoring mass chromatogram of m/z 1083 corresponding to the [M+4H]4+ ion of Aβ1–40 obtained during flow injection with positive ion electrospray of solutions containing Aβ1–40 incubated with or without test compounds. (A) Control (containing only Aβ1–40); (B) melatonin; (C) methysticin; (D) 3-indolepropionic acid; and (E) daunomycin. The area of each peak is proportional to the concentration of monomeric Aβ1–40 and indicates the relative efficacy of the test compound at preventing aggregation of Aβ1–40.
Based on a minimum of three assays normalized to the control incubation, the inhibition of aggregation of Aβ1–40 for each test compound was determined, and the results are summarized in Table 1. The interassay coefficient of variation for these measurements was 4.7%. The order of anti-aggregation activities for the four test compounds was daunomycin > 3-indolepropionic acid > melatonin > methysticin. Furthermore, a wide range of potencies was observed ranging from 90% inhibition of Aβ1–40 aggregation compared to control for methysticin to more than 260% inhibition of aggregation for daunomycin. Since methysticin was the only compound tested that was not already known to inhibit Aβ1–40 aggregation, it is not surprising that it was the least effective in this assay.
Table 1.
Comparison of inhibitors of Aβ1–40 aggregation. Values* are expressed as the concentration of monomeric Aβ1–40 or as a percent change in Aβ1–40 concentration relative to control.
| Sample | Concentration ±SD (μM) | Change in Aβ1–40aggregation relative to control |
|---|---|---|
| Control | 23.3 ±1.3 | 0 |
| Melatonin | 58.2 ±2.7 | 150 ±4 |
| Methysticin | 44.3 ±1.6 | 90.1 ±1.4 |
| 3-Indolepropionic acid | 74.4 ±2.6 | 219 ±6 |
| Daunomycin | 85.4 ±5.3 | 267 ±14 |
Mean ± std dev (N = 3)
Although different assays of the same compounds produced the same rank ordering and relative concentrations of Aβ1–40 for both test solutions and control solutions, the concentration of monomeric Aβ1–40 in the control solutions varied as much as 3-fold between different commercial lots of Aβ1–40 that were tested (data not shown). This variation was probably the result of different degrees of aggregation during the preparation of commercial Aβ1–40. Since the batch-to-batch shift in Aβ1–40 concentration of the control was also reflected in a shift in Aβ1–40 concentrations in the test solutions, values for compounds assayed using different batches of Aβ1–40 may still be compared by normalizing the test values to the control and expressing the values as a percentage of the control as shown in Table 1.
CONCLUSIONS
A mass spectrometry-based screening assay has been developed that may be used to discover compounds that inhibit Aβ protein aggregation for the potential treatment or prevention of Alzheimer’s disease. The scale of this assay (100 μL incubations) should be regarded as a proof of principle, and the incubation volumes could be reduced if desired to conserve reagents. Nevertheless, this assay in its present form exhibits excellent sensitivity so that only low levels of Aβ1–40 and test compounds are required. This is an advantage over many other assays that require much more Aβ which adds considerably to the cost of those assays. Other advantages of this mass spectrometry-based assay are that it exhibits good linearity of response and is quantitative.
In addition, this simple assay utilizes flow-injection electrospray mass spectrometry instead of chromatography so that high throughput may be achieved. In the feasibility study reported here, samples were analyzed in less than 1 min each, and even faster throughput should be possible. Another advantage of flow-injection mass spectrometry is the ability to automate the assay, which was demonstrated here using a commercially available autoinjector. Finally, this assay has the potential to accelerate the discovery of compounds that inhibit Aβ aggregation which might lead to the control or prevention of the growing threat of Alzheimer’s disease in the aging population of developed countries.
Acknowledgments
Funding for these studies was provided by grant P50 AT00155 from the Office of Dietary Supplements, the National Institute of General Medical Sciences, the Office for Research on Women’s Health, and the National Center for Complementary and Alternative Medicine. Its contents are the responsibility of the authors and do not necessarily represent the official views of the sponsors.
References
- 1.Lahiri DK, Farlow MR, Greig NH, Sambamurfi K. Drug Develop Res. 2002;56:267–281. [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 3.National Institute on Aging. Progress report on Alzheimer’s disease. National Institute on Aging; Bethesda, MD, USA: 1998. [Google Scholar]
- 4.De Felice FG, Ferreira ST. Cell Molec Neurobiol. 2002;22:545–563. doi: 10.1023/A:1021832302524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA. Nat Med. 1998;4:827–831. doi: 10.1038/nm0798-827. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzo A, Yankner BA. Proc Natl Acad Sci U S A. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Citron M. Mol Med Today. 2000;6:392–397. doi: 10.1016/s1357-4310(00)01759-7. [DOI] [PubMed] [Google Scholar]
- 8.Verbeek MM, Ruiter DJ, de Waal RM. Biol Chem. 1997;378:937–950. doi: 10.1515/bchm.1997.378.9.937. [DOI] [PubMed] [Google Scholar]
- 9.Nunan J, Small DH. FEBS Lett. 2000;483:6–10. doi: 10.1016/s0014-5793(00)02076-7. [DOI] [PubMed] [Google Scholar]
- 10.Esler WP, Wolfe MS. Science. 2001;293:1449–1454. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- 11.Cairo CW, Strzelec A, Murphy RM, Kiessling LL. Biochemistry. 2002;41:8620–8629. doi: 10.1021/bi0156254. [DOI] [PubMed] [Google Scholar]
- 12.Apostol BL, Kazantsev A, Raffioni S, Illes K, Pallos J, Bodai L, Slepko N, Bear JE, Gertler FB, Hersch S, Housman DE, Marsh JL, Thompson LM. Proc Natl Acad Sci U S A. 2003;100:5950–5955. doi: 10.1073/pnas.2628045100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn BW, Song DU, Jung YD, Chay KO, Chung MA, Yang SY, Shin BA. Anal Biochem. 2000;284:401–405. doi: 10.1006/abio.2000.4719. [DOI] [PubMed] [Google Scholar]
- 14.Akikusa S, Nakamura K, Watanabe KI, Horikawa E, Konakahara T, Kodaka M, Okuno H. J Peptide Res. 2003;61:1–6. doi: 10.1034/j.1399-3011.2003.21028.x. [DOI] [PubMed] [Google Scholar]
- 15.Pappolla MA, Chyan YJ, Poeggeler B, Frangione B, Wilson G, Ghiso J, Reiter RJ. J Neural Transm. 2000;107:203–231. doi: 10.1007/s007020050018. [DOI] [PubMed] [Google Scholar]
- 16.Howlett DR, George AR, Owen DE, Ward RV, Markwell RE. Biochem J. 1999;343:419–423. [PMC free article] [PubMed] [Google Scholar]
- 17.Bendeim PE, Poeggeler B, Neria E, Ziv V, Pappolla MA, Chain DG. J Mol Neurosci. 2002;19:213–217. doi: 10.1007/s12031-002-0036-0. [DOI] [PubMed] [Google Scholar]
- 18.Wheatley D. Handbook Exper Pharmacol. 2004;157:325–351. [Google Scholar]


