Abstract
Objective.
Xylitol, given as two grams orally five times-a-day, significantly reduces the incidence of acute otitis media (AOM) in children. A less frequent dosing schedule, if tolerable and efficacious, would promote the more widespread use of this treatment. We sought to determine the tolerability and acceptability in young children of oral xylitol solution at doses of 5 grams three times-a-day (TID) and 7.5 grams once daily (QD).
Methods.
The study was a three-month randomized placebo-controlled trial of the tolerability and acceptability of oral xylitol solution in 120 children 6-36 months of age performed in the SCOR Network.
Results.
Study withdrawals and unscheduled medical visits for gastrointestinal complaints did not differ significantly among the study groups. The proportions of subjects in the xylitol TID group who experienced excessive gas or diarrhea at months one, two, and three were 22.7%, 10.0%, and 14.3%, respectively, and in the xylitol QD group were 27.3%, 17.4%, and 14.3%, respectively, and these did not differ from the placebo groups. The proportions who accepted the study solution easily or with only minor difficulty at one, two, and three months in the xylitol TID group were 77.3%, 90.0%, and 90.5% and in the xylitol QD group, 77.3%, 82.6%, and 90.5%, respectively.
Conclusions.
Oral xylitol solution at dosages of 5 grams TID and 7.5 grams QD is well-tolerated by young children. Given the potential for xylitol as a safe, inexpensive option for AOM prophylaxis, clinical trials using these dosages of xylitol can be conducted.
Keywords: xylitol, otitis media, tolerability, compliance
INTRODUCTION
Xylitol is a five-carbon sugar alcohol (also known as a polyol) found naturally in a wide variety of plants, including fruits and vegetables such as plums, strawberries, raspberries, and cauliflower, and produced in small amounts by human metabolism.(1)It is used widely as a bulking agent in foods and as a low-calorie sweetener in medications, dental care products, chewing gums, and candies.
Xylitol has long been known to suppress the growth of Streptococcus mutans, a major cause of dental caries, and xylitol chewing gum has been shown to prevent dental decay in children.(2-4) Randomized trials conducted in Finland have also demonstrated that xylitol taken in a regimen of 1.67 grams five times-a-day as a chewing gum or 2 grams five times-a-day as a solution reduces the incidence of acute otitis media (AOM) by 35-40% in young children,(5, 6) presumably because of its antibacterial effects against Streptococcus pneumoniae and Haemophilus influenzae.(7-9)
In dosages used in foods and candies, xylitol and other sugar alcohols appear to have few side effects. The main limitation of the use of sugar alcohols is a dose-dependent osmotic laxative effect, and in adults, xylitol is well tolerated in doses up to 100 grams per day.(1, 10, 11) However, the tolerability of xylitol in children is not well understood, and this is particularly so in infants and toddlers. In a dose-ranging study of xylitol in 13 children seven to 16 years of age, some increase in flatulence was reported at doses of 45 grams per day, but no diarrhea was seen with doses below 65 grams per day(12); in another study of xylitol tolerability in diabetic children, only one subject out of 24 experienced diarrhea requiring discontinuation of xylitol at a dose of 30 grams per day.(13) The Finnish studies of xylitol for AOM prevention have also shown that 10 grams of xylitol daily, given as two grams five times-a-day, is well tolerated in children as young as nine months of age.(5, 6, 14)
For xylitol to be used more widely for AOM prevention, alternatives to the previously studied five times-a-day regimen are needed.(15, 16) One possible alternative would be to deliver a total daily dose similar to that which has previously been shown effective in the Finnish studies, but divided into fewer doses per day. While there are no dose-response data for xylitol in AOM prevention, data on dental plaque prevention suggest a linear dose-response relationship.(17, 18) We therefore conducted, in infants and young children, a placebo-controlled tolerability trial of oral xylitol solution at doses of 5 grams three times-a-day (TID) and 7.5 grams once daily (QD).
SUBJECTS AND METHODS
The study was performed in the Slone Center Office-based Research (SCOR) Network, a national pediatric research network of approximately 500 pediatricians and family physicians. Physicians were recruited through a mailed invitation. In the course of their routine office practices, participating physicians in turn recruited eligible subjects for the study. Inclusion criteria were: age six to 36 months; history of at least two episodes of AOM in the previous 12 months; good general health; and English-speaking. Exclusion criteria were: history of myringotomy tubes; intestinal malabsorption or chronic diarrhea; diabetes mellitus; any inborn errors of metabolism; parent (or guardian) unreachable by telephone; current use of any oral prescription medication; or a diagnosis of AOM in the previous two weeks. Informed consent was obtained by the enrolling physicians and faxed to the investigators.
Subjects were randomized to one of four groups: xylitol 5 grams TID; placebo TID; xylitol 7.5 grams QD; or placebo QD. A dilute solution of sorbitol (5%) was chosen as the placebo because it provided a similar taste to the xylitol solution but contained a sorbitol content below that expected to cause any gastrointestinal effects.(19, 20) Dosing for the TID groups consisted of 20 mL TID of a 25% aqueous xylitol solution or a 5% aqueous sorbitol solution given after a morning meal/bottle, a mid-day meal/bottle, and at bedtime by oral syringe or clean bottle or cup. Dosing for the QD group consisted of 20 mL QD of a 37.5% aqueous xylitol solution or a 5% sorbitol solution given at bedtime by oral syringe or clean bottle or cup. Parents were instructed that no food or fluids should be offered to the subject for 30 minutes after the study solution was given. If the child refused to take the study solution after multiple attempts, parents were instructed that they could mix the study solution with up to an equal volume of formula, milk, juice, or water.
Subjects were to be given the study solution daily for three months. Parents were contacted by telephone monthly for a structured, computer-assisted interview to determine tolerability of the study solution, as well as the occurrence of adverse effects, unscheduled medical visits, and hospitalizations.
The study was approved by the Boston University School of Medicine Institutional Review Board and was performed under IND #66,964 from the U.S. Food and Drug Administration.
Statistical Analyses
Comparisons of proportions between the xylitol and placebo groups were performed by Fisher’s exact test. SPSS version 11.0 and SAS version 9.1 were used for statistical analyses.
RESULTS
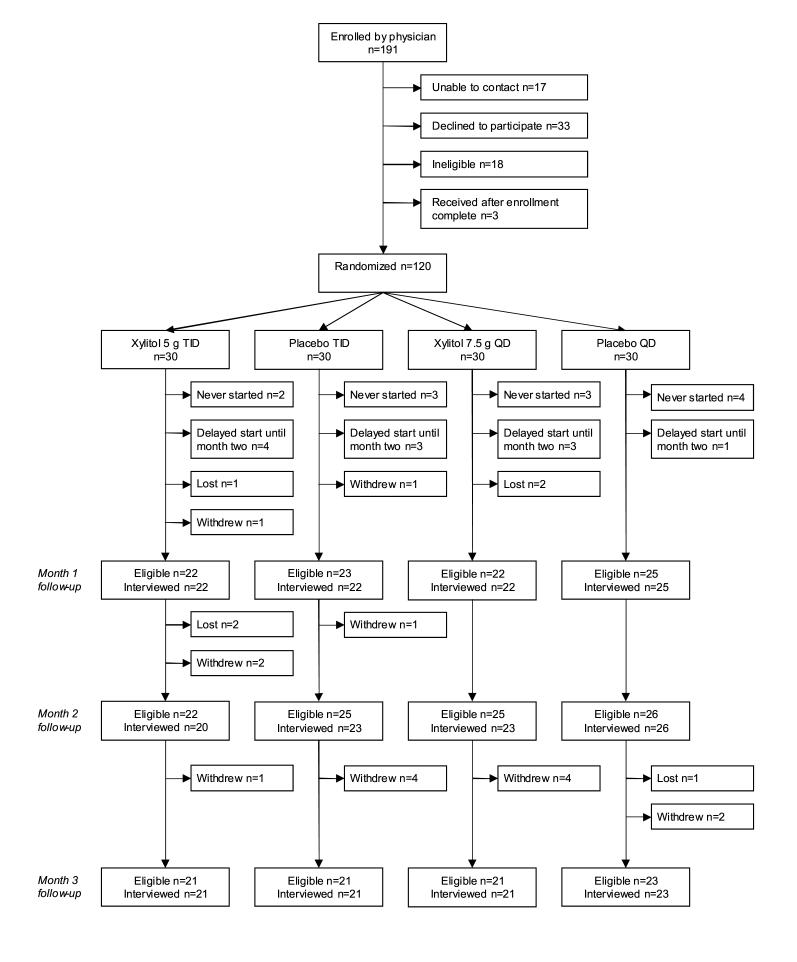
Twenty-four physicians enrolled 191 subjects for the study and 120 subjects were randomized. Study enrollments, completion, withdrawals, and losses to follow-up are summarized in Figure 1. Subject demographics are shown in Table 1.
Figure 1.
Diagram of study enrollments and follow-up
Table 1.
Demographics of study subjects
| Xylitol 5g TID n=30 | Placebo TID n=30 | Xylitol 7.5g QD n=30 | Placebo QD n=30 | |
|---|---|---|---|---|
| Number Female (%) | 13 (43.3) | 15 (50.0) | 14 (46.7) | 17 (56.7) |
| Age in months, median (range) | 14.9 (6.5-32.6) | 19.8 (8.3-31.5) | 17.9 (7.8-26.4) | 15.9 (6.3-29.2) |
| Weight in kg, median (range) | 10.8 (6.8-15.0) | 11.1 (3.6-14.6) | 10.5 (8.4-15.0) | 11.4 (6.4-13.6) |
Sixteen subjects withdrew from the study, seven due to gastrointestinal side effects (Table 2). Three withdrawals due to gastrointestinal side effects occurred in the xylitol TID group (10.0%, 95%CI 0-20.7) compared to one in the placebo TID group (3.3%, 95%CI 0-9.8; p for comparison=0.6) and two occurred in the xylitol QD group (6.7%, 95%CI 0-15.6) compared to one in the placebo QD group (3.3%, 95%CI 0-9.8; p for comparison=1.0). The median age of the five children from the xylitol groups who withdrew due to gastrointestinal side effects was 9.7 months (range: 8.2-12.7) and their median weight was 9.1 kg (range: 7.9-11.4).
Table 2.
Reasons for study withdrawals
| Xylitol 5g TID n=30 | Placebo TID n=30 | Xylitol 7.5g QD n=30 | Placebo QD n=30 | |
|---|---|---|---|---|
| Gastrointestinal side effects | 3 | 1 | 2 | 1 |
| Too much work/child wouldn’t take | 1 | 3 | 0 | 0 |
| Myringotomy tubes placed | 0 | 0 | 2 | 0 |
| Other | 0 | 2 | 0 | 1 |
Unscheduled medical visits for gastrointestinal complaints (abdominal pain, excessive gas, diarrhea, or vomiting) did not differ significantly among groups for months one, two, or three of the study (Table 3).
Table 3.
Unscheduled medical visits for gastrointestinal complaints, number (%)
| Xylitol 5g TID | Placebo TID | p | Xylitol 7.5g QD | Placebo QD | p | |
|---|---|---|---|---|---|---|
| Month 1 | 1 (4.6) | 1 (4.6) | 1.0 | 1 (4.6) | 2 (8.0) | 1.0 |
| Month 2 | 2 (10.0) | 0 (0.0) | 0.2 | 1 (4.4) | 1 (3.9) | 1.0 |
| Month 3 | 1 (4.8) | 1 (4.8) | 1.0 | 0 (0.0) | 0 (0.0) | 1.0 |
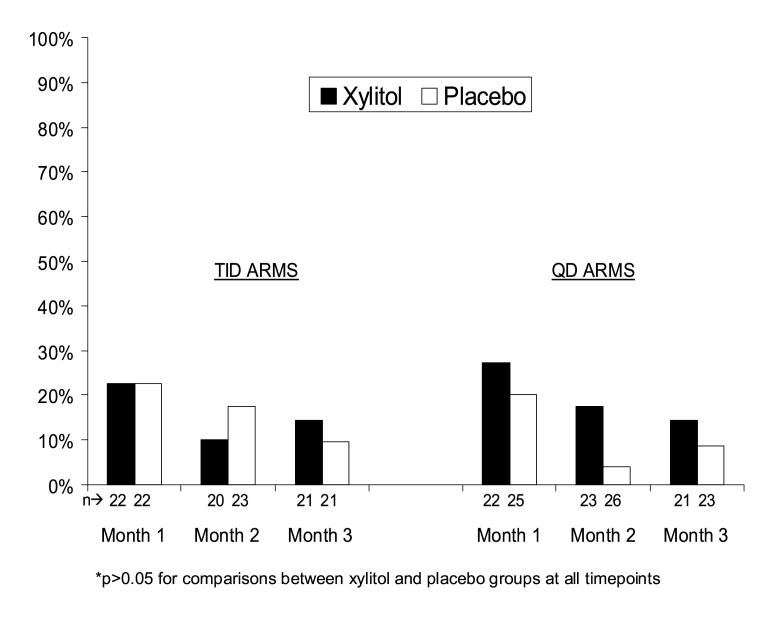
The proportion of subjects in the each study group who experienced excessive gas or loose stools/diarrhea at months one, two, and three are shown in Figure 2. The relative risks (95% confidence intervals) for the occurrence of excessive gas or loose stools/diarrhea at months one two, and three for subjects receiving TID xylitol versus TID placebo were 1.0 (0.3-3.0), 0.6 (0.1-2.8), and 1.5 (0.3-8.1), respectively. The corresponding relative risks for those receiving QD xylitol versus QD placebo were 1.4 (0.5-3.9), 4.5 (0.5-37.6), and 1.6 (0.3-8.9), respectively. The median age and weight of those in the xylitol groups reporting excessive gas or loose stools/diarrhea was 16.1 months (range: 7.2-28.2) and 10.4 kg (range: 7.9-15.0).
Figure 2.
Proportion of subjects reporting excessive gas and/or loose stools by month and study group
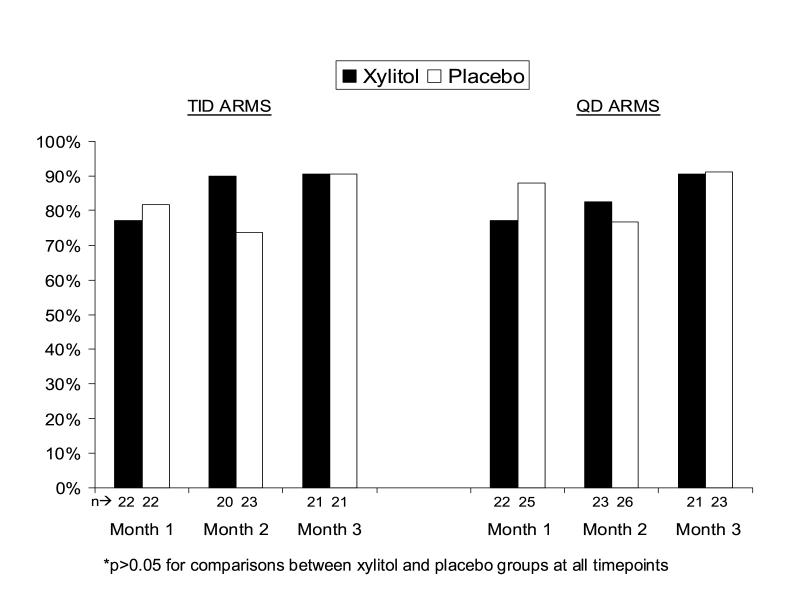
Most subjects accepted the study solutions easily or with only minor difficulty, with little difference between xylitol and placebo groups (Figure 3). The proportions who accepted the study solution easily or with only minor difficulty at one, two, and three months in the xylitol TID group were 77.3%, 90.0%, and 90.5%, respectively and in the xylitol QD group, 77.3%, 82.6%, and 90.5%, respectively.
Figure 3.
Proportion of subjects who took the study solution easily or with only minor difficulty by month and study group
As noted, parents were offered the option of mixing the study solution with no more than an equal volume of formula, milk, juice, or water if the child repeatedly refused to take the study solution. The mixing option was used by two subjects in the xylitol TID group, five subjects in the xylitol QD group, nine subjects in the placebo TID group, and six subjects in the placebo QD group.
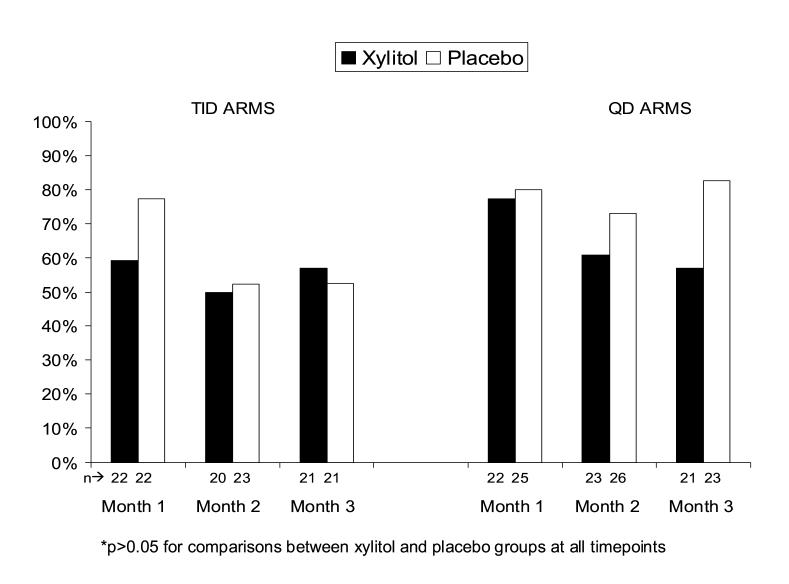
Acceptable adherence, defined as taking at least half of the recommended doses of study solution over the previous month, was achieved at one, two, and three months by 59.1%, 50.0%, and 57.1%, respectively in the xylitol TID group, and by 77.3%, 60.9%, and 57.1% respectively in the xylitol QD group; none of these proportions differed significantly from the relevant placebo groups (Figure 4).
Figure 4.
Proportion of subjects who took all or most of the recommended doses of study solution by month and study group
DISCUSSION
Xylitol offers an attractive option for prevention of AOM in young children and two studies have demonstrated its efficacy when given in a regimen of 1.67 or 2 grams five times-a-day.(5, 6) Despite this evidence, xylitol is not used widely for AOM prevention for perhaps two key reasons: the five times-a-day dosing schedule is impractical for most families and concerns remain about possible gastrointestinal side effects, especially among infants. This pilot study was designed to test whether alternative regimes of 5 grams TID and 7.5 grams QD would be tolerated by children between the ages of six months and three years old. The regimens were chosen to approximate the total daily dosage used in the successful five times-a-day AOM prevention trials (i.e., 8.35 to 10 grams per day) but delivered in fewer daily doses. They were also based on the limited published evidence of the tolerability of xylitol in children.(5, 6, 12-14) Subjects generally tolerated the xylitol solution well, with only three withdrawals due to gastrointestinal side effects out of the 30 subjects assigned to TID xylitol and two out of 30 subjects assigned to QD xylitol. While the number of subjects withdrawing due to gastrointestinal side effects in the xylitol groups were not significantly different from those in the placebo groups, it is notable that the median age of those who withdrew from both xylitol groups was 9.7 months compared to 17.1 months for all study subjects, and the median weight of the withdrawals was 9.1 kg compared to 10.9 kg for all study subjects. This suggests that infants may be more prone to significant gastrointestinal side effects of a given dose of xylitol than slightly older children, but further research would be needed to confirm this preliminary observation.
The milder and expected side effects of excessive gas or loose stools/diarrhea did not occur with a significantly greater frequency among subjects receiving xylitol compared to placebo, assessed either by unscheduled medical visits for gastrointestinal symptoms or parental report of symptoms. Furthermore, the rates of reported diarrhea among the study subjects were similar to baseline rates of parent-reported diarrhea in healthy young children.(21)
The median age and weight of those in the xylitol groups whose parents reported mild gastrointestinal side effects was not materially different from the entire study cohort (16.1 months vs. 17.1 months and 10.4 kg vs. 10.9 kg, respectively), so these side effects do not appear to be particularly age- or weight-dependent. It has been shown that adaptation to xylitol and other sugar alcohols occurs rapidly, such that the laxative effect diminishes within several days of beginning regular use(1, 11, 12); we saw a suggestion of this phenomenon in the declining rate of gastrointestinal side effects in the xylitol groups from month one compared to months two and three.
The xylitol solution was readily accepted by the majority of subjects despite the fact that it consisted simply of xylitol dissolved in distilled water. Adherence to the three-month treatment regimen was reasonable, with half to three-quarters of subjects reportedly taking all or most of the recommended doses at each time point. This adherence rate compares favorably to adherence rates for children on chronic medications in general.(22) Better palatability and therefore adherence could likely be achieved with added flavoring and/or coloring to appeal to young children.
Overall, this pilot study demonstrates that oral xylitol solution at dosages of 5 grams TID and 7.5 grams QD is reasonably well-tolerated by and acceptable to children in the age range of highest risk for recurrent AOM. Given these results and the potential for xylitol as a safe, inexpensive option for AOM prophylaxis, formal clinical trials using these dosages of xylitol can be conducted to determine their clinical efficacy.
ACKNOWLEDGEMENTS
The authors wish to thank the following members of the SCOR Network who enrolled subjects in the study: Charles Anderson, M.D. (San Marcos, TX), Barbara P. Belcher, M.D. (San Antonio, TX), Michael A. Blum, D.O. (Overland Park, KS), Paul M. Douthitt, M.D. (Springfield, TN), Patricia Edwards, M.D. (Concord, NH), Michael F. Grossberg, M.D. (Chambersburg, PA), Daniel E. Halm, M.D. (Bellevue, NE), James Horwitz, M.D. (Hendersonville, NC), Shahina R. Khan, M.D. (Gaffney, SC), George M. McCormick, M.D. (State College, PA), John Mulawka, D.O. (Silver Creek, NY), Sree K. Mulpuru, M.D. (Fairmont, WV), Beth Nauert, M.D. (Austin, TX), Jo Anne Nielsen, M.D. (Oregon City, OR), Jagdish Patel, M.D. (Bronx, NY), Suzanne W. Schuessler, M.D. (Lagrange, GA), Onkar Sharma, M.D. (Mattoon, IL), Merl W. Simmons, M.D. (Oklahoma City, OK), Michael W. Simon, M.D. (Lexington, KY), Lyle D. Smith, M.D. (Dodge City KS), Neil J. Stalker, M.D. (Peru, IN), Jeb S. Teichman, M.D. (Jeffersonville, IN), Louis Vernacchio, M.D. (Boston, MA), Shaila N. Williston, M.D. (Orange Park, FL), and Michael F. Yeiser, M.D. (Owensboro, KY).
Footnotes
Financial Support: This study was supported by funding from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health through grant R21-DC058582-01. Xylitol and sorbitol were provided for the study by Danisco Sweeteners (Thomson, IL).
REFERENCES
- 1.Wang YM, van Eys J. Nutritional significance of fructose and sugar alcohols. Annu Rev Nutr. 1981;1:437–75. doi: 10.1146/annurev.nu.01.070181.002253. [DOI] [PubMed] [Google Scholar]
- 2.Trahan L. Xylitol: a review of its action on mutans streptococci and dental plaque--its clinical significance. Int Dent J. 1995;45(1 Suppl 1):77–92. [PubMed] [Google Scholar]
- 3.Van Loveren C. Sugar alcohols: what is the evidence for caries-preventive and caries-therapeutic effects? Caries Res. 2004;38:286–93. doi: 10.1159/000077768. [DOI] [PubMed] [Google Scholar]
- 4.Ly KA, Milgrom P, Rothen M. Xylitol, sweeteners, and dental caries. Pediatr Dent. 2006;28:154–63. [PubMed] [Google Scholar]
- 5.Uhari M, Kontiokari T, Koskela M, Niemela M. Xylitol chewing gum in prevention of acute otitis media: double blind randomised trial. BMJ. 1996;313:1180–4. doi: 10.1136/bmj.313.7066.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhari M, Kontiokari T, Niemela M. A novel use of xylitol sugar in preventing acute otitis media. Pediatrics. 1998;102(4 Pt 1):879–84. doi: 10.1542/peds.102.4.879. [DOI] [PubMed] [Google Scholar]
- 7.Kontiokari T, Uhari M, Koskela M. Effect of xylitol on growth of nasopharyngeal bacteria in vitro. Antimicrob Agents Chemother. 1995;39:1820–3. doi: 10.1128/aac.39.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kontiokari T, Uhari M, Koskela M. Antiadhesive effects of xylitol on otopathogenic bacteria. J Antimicrob Chemother. 1998;41:563–5. doi: 10.1093/jac/41.5.563. [DOI] [PubMed] [Google Scholar]
- 9.Tapiainen T, Kontiokari T, Sammalkivi L, Ikaheimo I, Koskela M, Uhari M. Effect of xylitol on growth of Streptococcus pneumoniae in the presence of fructose and sorbitol. Antimicrob Agents Chemother. 2001;45:166–9. doi: 10.1128/AAC.45.1.166-169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asano T, Levitt MD, Goetz FC. Xylitol absorption in healthy men. Diabetes. 1973;22:279–81. doi: 10.2337/diab.22.4.279. [DOI] [PubMed] [Google Scholar]
- 11.Forster H, Quadbeck R, Gottstein U. Metabolic tolerance to high doses of oral xylitol in human volunteers not previously adapted to xylitol. Int J Vitam Nutr Res Suppl. 1982;22:67–88. [PubMed] [Google Scholar]
- 12.Akerblom HK, Koivukangas T, Puukka R, Mononen M. The tolerance of increasing amounts of dietary xylitol in children. Int J Vitam Nutr Res Suppl. 1982;22:53–66. [PubMed] [Google Scholar]
- 13.Forster H, Boecker S, Walther A. [Use of xylitol as sugar substitute in diabetic children] Fortschr Med. 1977;95:99–102. [PubMed] [Google Scholar]
- 14.Tapiainen T, Luotonen L, Kontiokari T, Renko M, Uhari M. Xylitol administered only during respiratory infections failed to prevent acute otitis media. Pediatrics. 2002;109:e19. doi: 10.1542/peds.109.2.e19. [DOI] [PubMed] [Google Scholar]
- 15.Wright PF. Xylitol sugar and acute otitis media. Pediatrics. 1998;102(4 Pt 1):971–2. doi: 10.1542/peds.102.4.971. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell AA. Xylitol prophylaxis for acute otitis media: tout de suite? Pediatrics. 1998;102(4 Pt 1):974–5. doi: 10.1542/peds.102.4.974. [DOI] [PubMed] [Google Scholar]
- 17.Ly KA, Milgrom P, Roberts MC, Yamaguchi DK, Rothen M, Mueller G. Linear response of mutans streptococci to increasing frequency of xylitol chewing gum use: a randomized controlled trial [ISRCTN43479664] BMC Oral Health. 2006;6:6. doi: 10.1186/1472-6831-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milgrom P, Ly KA, Roberts MC, Rothen M, Mueller G, Yamaguchi DK. Mutans streptococci dose response to xylitol chewing gum. J Dent Res. 2006;85:177–81. doi: 10.1177/154405910608500212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corazza GR, Strocchi A, Rossi R, Sirola D, Gasbarrini G. Sorbitol malabsorption in normal volunteers and in patients with coeliac disease. Gut. 1988;29:44–8. doi: 10.1136/gut.29.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoekstra JH, van Kempen AA, Kneepkens CM. Apple juice malabsorption: fructose or sorbitol? J Pediatr Gastroenterol Nutr. 1993;16:39–42. doi: 10.1097/00005176-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Vernacchio L, Vezina RM, Mitchell AA, Lesko SM, Plaut AG, Acheson DW. Diarrhea in American infants and young children in the community setting: incidence, clinical presentation and microbiology. Pediatr Infect Dis J. 2006;25:2–7. doi: 10.1097/01.inf.0000195623.57945.87. [DOI] [PubMed] [Google Scholar]
- 22.Matsui DM. Drug compliance in pediatrics. Clinical and research issues. Pediatr Clin North Am. 1997;44:1–14. doi: 10.1016/s0031-3955(05)70459-4. [DOI] [PubMed] [Google Scholar]