Abstract
Reduced expression of p27 has been associated with poor prognosis in most human cancers, including pancreatic adenocarcinoma. Jun activation domain-binding protein 1 (JAB1), an activator protein (AP-1) coactivator, previously implicated in p27 degradation, is overexpressed in various tumors and correlates with low p27 expression. We examined JAB1 and p27 in normal and neoplastic pancreatic tissues. Increased JAB1 expression was seen in pancreatic carcinoma samples but not in paired normal pancreatic tissues. Immunohistochemical analysis using tissue microarrays showed that JAB1 was overexpressed in all 32 (100%) pancreatic adenocarcinoma samples tested, predominantly nuclear in 23 (72%) samples and predominantly cytoplasmic in 9 (28%) tumors. When 10% was used as a cutoff for p27 positivity, p27 was expressed in 11 (34%) of tumors; however, p27 expression was localized in the nuclei of tumor cells in only 4 (13%) of the samples. Overexpression of the JAB1 in the pancreatic carcinoma cell lines Panc-1, Mia PaCa-2, and Panc-28 resulted in decreased p27 expression. Conversely, down-regulation of JAB1 by short interfering RNA substantially increased p27 expression and inhibited progression from G1 to S phase of the cell cycle. Interestingly, JAB1-mediated p27 degradation was not impaired when S-phase kinase-interacting protein 2 (Skp2), an F-box protein required for the ubiquitination and consequent degradation of p27, was silenced. Thus, JAB1 may have an Skp2-independent p27 degradation mechanism in pancreatic cancer cells. These findings suggest that JAB1 overexpression is involved in the pathogenesis of pancreatic cancer through JAB1-mediated p27 degradation and that control of JAB1 expression is a novel therapeutic target in patients with pancreatic adenocarcinomas.
Introduction
Pancreatic carcinoma represents the fourth leading cause of cancer-related deaths in the United States, carrying one of the most dismal prognoses of all cancers (1). In the United States, ∼32,180 new cases of pancreatic cancer are expected in 2006, and 5-year survival rates are only 4% regardless of disease stage, with a median survival time of <6 months (1). Even after potentially curative resection, 5-year survival rates do not exceed 20%, and ∼50% of patients treated with surgery and chemoradiation experience recurrence, predominantly as liver metastases (2). Given the resistance of pancreatic cancer to chemotherapy, understanding the molecular basis of pancreatic oncogenesis and applying this knowledge to early diagnosis and development of novel therapeutic strategies is critical (1, 3). Pancreatic adenocarcinoma arising from the pancreatic duct cells of the exocrine pancreas is the most common histologic type of pancreatic cancer. The etiology and pathogenesis of the disease remains unknown, although heterogeneous genetic alterations have been reported in pancreatic carcinomas. The most frequent genetic alterations reported in pancreatic carcinomas include point mutations of KRAS2 and inactivation of CDKN2A/p16/MTS1, p53, and MADH4/SMAD4/DPC4. In particular, loss of function of the cyclin-dependent kinase (CDK) inhibitor p16 by mutation, deletion, or promoter hypermethylation occurs in 80% to 95% of sporadic pancreatic cancer and represents a relatively early genetic event in pancreatic oncogenesis (4). Recently, it has been reported that the phosphatidylinositol 3-kinase/Akt pathway is also activated in pancreatic adenocarcinomas due to aberrant expression of phosphatase and tensin homologue (5). However, additional molecular abnormalities resulting in deregulation of cell cycle progression may occur.
The human Jun activation domain-binding protein 1 (JAB1) was originally identified as a coactivator of the gene-regulatory activator protein (AP-1) proteins (Jun/Fos proto-oncogenes) involved in the control of cell proliferation (6). JAB1, also known as CSN5, is also present in the COP9 signalosome (CSN), a multiprotein complex involved in modulating signal transduction, gene transcription, and protein stability (7, 8). Recent evidence suggests that JAB1/CSN5 specifically interacts with the CDK inhibitor p27Kip1 (p27; ref. 9) and induces nuclear export and subsequent degradation of p27 (9, 10). p27 is a universal CDK inhibitor that directly inhibits the enzymatic activity of cyclin-CDK complexes resulting in cell cycle arrest at G1 (11). p27 protein levels are increased in quiescent cells and rapidly decrease after cells are stimulated with mitogens (12). Although transcriptional regulation is possible, cellular abundance of p27 is primarily regulated at the posttranslational level by the ubiquitin-proteasome pathway (13). To be degraded, p27 must be phosphorylated at its T187 residue by the cyclin E/CDK2 or cyclin A/CDK2 complexes (14-16), although a T187-independent proteolytic pathway that functions during mid-G1 has been suggested as well (17). JAB1 has been shown to shuttle p27 from the nucleus to the cytoplasm and to decrease the amount of p27 in the cell by accelerating p27 degradation via the ubiquitin-proteasome system (9, 10).
The role of JAB1 in human oncogenesis is currently under investigation. Overexpression of JAB1 has been reported in epithelial, lymphoid, and other malignancies (18-25). We have previously shown that, in breast cancer, JAB1 and p27 expression are inversely correlated and overexpression of JAB1 results in increased p27 degradation in breast carcinoma cells (20).
Previous studies have shown that pancreatic carcinomas express little or no p27, suggesting that p27 may contribute to pancreatic oncogenesis (26, 27). In addition, low p27 expression has been associated with higher tumor grades (poorly or moderately differentiated pancreatic carcinomas) and advanced tumor stage (26, 27). However, the mechanisms leading to p27 down-regulation in these neoplasms are undefined. In this study, we hypothesized that JAB1 functions as a negative regulator of p27 and, as such, JAB1 may play a role in the pathogenesis of pancreatic cancer.
We assessed JAB1 and p27 expression in a series of pancreatic carcinoma and adjacent normal pancreatic tissue specimens and we found that JAB1 overexpression was associated with absent or low expression of p27 in pancreatic adenocarcinoma. To further elucidate the role of JAB1 overexpression in p27 degradation in pancreatic cancer, we infected pancreatic carcinoma cell lines with an adenoviral vector to overexpress JAB1 and found that p27 levels were significantly decreased after JAB1 gene transfer. Direct physical interaction between JAB1 and p27 was detected in pancreatic cancinoma cells. Furthermore, inhibition of endogenous JAB1 expression with specific short interfering RNAs (siRNAs) resulted in a substantial increase of p27 levels and inhibition of cell proliferation, indicating that, in pancreatic cancer cells, JAB1 controls the stability of p27 by targeting it for degradation. Interestingly, JAB1-mediated p27 degradation was not impaired when S-phase kinase-interacting protein 2 (Skp2), an F-box protein required for the ubiquitination and consequent degradation of p27, was silenced. This may suggest a potential Skp2-independent degradation mechanism of p27 by JAB1 in pancreatic cancer cells.
Materials and Methods
Patients and tissue samples
The study group consisted of 17 men and 16 women with pancreatic carcinoma (median age, 63; range, 30-75 years) who underwent surgical treatment in the Department of Surgical Oncology at The University of Texas M.D. Anderson Cancer Center from December 1997 to May 2001. Surgical treatment included pancreaticoduodenectomy (27 cases), total pancreatectomy (3 cases), and distal pancreatectomy (3 cases). Resection margins were free of tumor in 22 patients; the remaining 11 patients had evidence of microscopic invasion at the retroperitoneal margin. Twenty-seven of the 33 patients had received preoperative chemoradiation therapy. Patients were selected for this study based on the availability of archived paraffin blocks for immunohistochemical analysis. Surgical staging of tumors was done according to the American Joint Committee on Cancer tumor-nodesmetastasis system; 8 (24%) were stage I, 24 (73%) were stage II, and 1 (3%) was stage III. Tumor grading was based on currently used histopathologic criteria; 4 tumors (12%) were well differentiated, 17 (52%) were moderately differentiated, 11 (33%) were poorly differentiated, and differentiation (grade) was not reported for 1 tumor. Histologically, 32 tumors were ductal adenocarcinomas and 1 was papillary adenocarcinoma. The clinicopathologic characteristics of the patients are summarized in Table 1.
Table 1.
Characteristics of 33 patients included in the study
| Characteristics | No. patients |
|---|---|
| Gender (male/female) | 17/16 |
| Median age, y | 63 |
| Tumor markers at diagnosis | |
| Elevated carcinoembryonic antigen | 7 of 29 |
| Elevated CA-19-9 | 19 of 25 |
| Preoperative diagnosis (yes/no) | 26/7 |
| Preoperative chemoradiation treatment (yes/no) | 27/6 |
| Type of operation | |
| Pancreaticoduodenectomy | 27 of 33 |
| Total pancreatectomy | 3 of 33 |
| Distal pancreatectomy | 3 of 33 |
| Tumor histologic type | |
| Ductal adenocarcinoma | 32 of 33 |
| Papillary adenocarcinoma | 1 of 33 |
| Differentiation | |
| Well | 4 of 33 |
| Moderate | 17 of 33 |
| Poor | 11 of 33 |
| Not reported | 1 of 33 |
| Stage | |
| I | 8 of 33 |
| II | 24 of 33 |
| III | 1 of 33 |
| IV | 0 |
Tissue microarray and immunohistochemical analysis
A tissue microarray was constructed by using a manual tissue microarrayer (Beecher Instruments, Silver Springs, MD). The microarray included 33 pancreatic carcinomas and adjacent normal pancreatic tissue samples from 14 of those 33 patients. Consecutive 5-μm paraffin-embedded sections were cut from the tissue microarray block and processed for immunohistochemical analysis as described below.
The monoclonal antibodies used were specific for JAB1 (diluted 1:400; Zymed, South San Francisco, CA), p27 (diluted 1:200; DakoCytomation, Carpinteria, CA), and Ki-67 (MIB-1, diluted 1:120; Immunotech, Westbrook, ME). The specificity of the JAB1 antibodies was tested in normal tonsil tissue samples by competition with a specific JAB1 peptide and an unspecific peptide as previously described (21). The immunohistochemical method used in this study was previously described (20). Briefly, heat-induced retrieval of JAB1 and p27 antigens was done and, subsequently, the slides were incubated with the primary antibody. Immunoreaction was detected with the LSAB+ kit from Dakocytomation. We used 3,3′-diaminobenzidine as the chromogen and hematoxylin as the counterstain. Expression levels of JAB1 and p27 were evaluated by counting at least 500 tumor cells in representative high-power fields. Tumor cells were considered positive for JAB1 or p27 when nuclear or cytoplasmic staining was present, regardless of the intensity. For the purpose of statistical analysis, a 10% cutoff was used to define p27 positivity. The percentage of Ki-67-positive tumor cells was designated as the proliferation index of the tumors.
In a group of 10 pancreatic adenocarcinomas with adjacent normal pancreatic tissues with available pretreatment fresh-frozen tissue, JAB1 and p27 were also detected with a double immunofluorescence method. Briefly, sections from fresh-frozen tissues were fixed in 70% acetone and 30% ethanol for 15 minutes at -20°C and then incubated with the primary antibodies overnight at 4jC. Detection was achieved by incubation with a fluorescent antimouse antibody (Alexa Fluor 488, Molecular Probes, Eugene, OR) at 1:200 dilution for 1 hour at room temperature. 4′,6-Diamidino-2-phenylindole (Molecular Probes) was used as counterstain.
Statistical analysis
The Fisher’s exact test was used to compare expression and localization of JAB1 and p27 proteins with various clinicopathologic variables. The Mann-Whitney test was used to compare proliferation index with JAB1 localization. Progression-free survival was defined as time from diagnosis to last clinical follow-up or relapse. Overall survival was defined as time from diagnosis to death. All computations were carried out with the StatView statistical program (Abacus Concepts, Inc., Berkeley, CA).
Cell cultures
The Panc-1, Mia PaCa-2, and Panc-28 cell lines were cultured in high-glucose DMEM (Life Technologies, Inc., Gaithersburg, MD) containing 10% fetal bovine serum and penicillin-streptomycin sulfate at 37°C in an atmosphere of 5% CO2 and 95% oxygen.
Cell extracts, tissue samples, and immunoblotting
Cells in log-phase growth were collected, washed twice in cold PBS, and lysed at 4°C in lysis buffer [25 mmol/L HEPES (pH 7.7), 400 mmol/L NaCl, 0.5% Triton X-100, 1.5 mmol/L MgCl2, 2 mmol/L EDTA, 2 mmol/L DTT, 0.1 mmol/L phenylmethylsulfonyl fluoride (PMSF), protease inhibitors (10 μg/mL leupeptin, 2 μg/mL pepstatin, 50 μg/mL antipain, 2 μg/mL aprotinin, 20 μg/mL chymostatin, and 2 μg/mL benzamidine), and phosphatase inhibitors (50 mmol/L NaF, 0.1 mmol/L Na3VO4, and 20 mmol/L β-glycerophosphate)]. Total cell lysates were resolved in 10% SDS-PAGE gels, transferred to nitrocellulose membranes, and probed with anti-JAB1 (Zymed), anti-p27 (BD Biosciences PharMingen, San Diego, CA) and anti-Myc (Cell Signaling Technology, Beverly, MA) antibodies using enhanced chemiluminescence reagents (Amersham Pharmacia, Piscataway, NJ). β-Actin (Sigma-Aldrich, St. Louis, MO) was used as the internal positive control for all immunoblots.
Adenoviral vectors and gene transduction
A recombinant adenoviral vector expressing a doxycycline-regulated (Tet-Off) form of human JAB1 (Ad-Myc-JAB1) was constructed as we previously described (20). Pancreatic carcinoma cells were transduced for 48 hours with a regulatory virus (adeno-X Tet-Off, Clontech, Palo Alto, CA) and Ad-Myc-JAB1 and Ad-Myc-p27 or Ad-Myc-p27T187A mutant at a multiplity of infection of 50 in a growth medium containing 10% serum in the presence or absence of 1 μg/mL doxycycline, a tetracycline analogue.
Proteasome inhibition assays
Panc-28 cells were treated with the 26S proteasome inhibitors LLnL (35 μmol/L) and MG132 (30 μmol/L) and the 20S inhibitor LLM (25 μmol/L) and DMSO as controls. After 16 hours of treatment, the cells were harvested, whole-cell lysates were prepared, and immunoblotting was done with antibodies against p27. h-Actin antibodies were used as a loading control. Panc-28 cells were transduced with Ad-Myc-JAB1 as described above. At 32 hours after infection, LLnL, MG132, and DMSO (control) were added to the medium for 16 hours. At 48 hours after infection, whole-cell lysates were prepared and proteins were analyzed by immunoblotting.
siRNA transfection and bromodeoxyuridine analysis
For siRNA analysis, JAB1 siRNA and control siRNA oligonucleotides were cloned into an RNAi vector system (BD Biosciences PharMingen) according to the instructions of the manufacturer and as described by Dr. Korapati and colleagues.5 Briefly, oligonucleotides encoding the human JAB1 siRNA were cloned into the RNAi-Ready vector using BamH1 and EcoR1 restriction sites. Panc-1, Mia PaCa-2, and Panc-28 cells were transfected with either JAB1-siRNA vector DNA or control-siRNA vector DNA using the Lipofectamine PLUS reagent (Invitrogen, Carlsbad, CA) following the protocol of the manufacturer. At 48 hours after transfection, protein lysates were prepared and immunoblotted.
For bromodeoxyuridine (BrdUrd) analysis, Panc-1, Mia PaCa-2, and Panc-28 cells were transfected as described above. At 48 hours, BrdUrd (BD Biosciences PharMingen) at a final concentration of 10 μmol/L was added for 45 minutes and the cells were stained with FITC-anti-BrdUrd antibodies according to the instructions of the manufacturer. The cells were then analyzed with flow cytometry to detect and quantify BrdUrd-stained cells as an indicator of the proportion of cells in S phase of the cell cycle.
For Skp2 siRNA experiments, Panc-28 cells were transfected with 50 and 100 nmol/L of siSkp2 (Ambion, Austin, TX) and with 100 nmol/L of control siRNA using Oligofectamine (Invitrogen). In addition, Panc-28 cells that were transfected with siSkp2 for 5 hours were then transduced with Ad-Myc-JAB1 as described above. Forty-eight hours later, cell lysates were prepared and analyzed by immunoblotting with anti-Skp2 (Zymed), anti-p27 (BD Biosciences PharMingen), and β-actin (Sigma-Aldrich) antibodies.
Glutathione S-transferase pulldown and coimmunoprecipitation assays
In vitro pulldown assays were done with glutathione S-transferase (GST)-p27 and GST-p27T187A fusion proteins or GST proteins coupled with glutathione-Sepharose beads, and in vitro pulldown assays were done with 35S-methionine-labeled JAB1. pcDNA3.1-JAB1 was transcribed and translated in vitro and labeled with 35S-methionine using the TNT T7/T3-coupled reticulocyte lysate system according to the instructions of the manufacturer (Promega, Madison WI). Binding conditions and the GST pulldown assays were done as previously described (6). The bound proteins were visualized by 10% SDS-PAGE and stained with Coomassie blue, and the resulting gel was dried and submitted to autoradiography.
GST pulldown assays were done with 800 μg of Panc-1 protein cell lysate expressing Ad-Myc-JAB or Ad-Myc-p27 mixed with 5 μg of GST-JAB1 or GST, immobilized on glutathione-Sepharose beads for 2 hours at 4°C as previously described (6). The GST pulldown products were run on an SDS-PAGE gel, stained with Coomassie blue, and immunoblotted with anti-Myc antibody (Cell Signaling Technology).
Coimmunoprecipitation assays were done with the total cell lysates from Panc-28 or Panc-1 cells transduced with Ad-Myc-p27 or Ad-Myc-p27T187A mutant in the presence or absence of Ad-Myc-JAB1. For coimmunoprecipitations of endogenous JAB1 and p27, cells were treated with MG132 (30 Amol/L) for 16 hours before harvesting. Cells were lysed on plates at 4°C in radioimmunoprecipitation assay (RIPA) buffer [20 mmol/L Tris-HCl (pH 7.4), 0.5% NP40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L PMSF, 1 μg/mL aprotinin, 1 μg/mL leupeptin, 1 μg/mL pepstatin, 1 mmol/L Na3VO4, 1 mmol/L NaF]. Immunoprecipitation was done in RIPA buffer for 4 hours at 4°C with either anti-p27 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) or nonimmune rabbit serum as a control. Protein A/G beads (Santa Cruz Biotechnology) were washed thrice with RIPA buffer followed by addition of Laemmli sample buffer. Proteins were resolved by 10% SDS-PAGE, transferred to nitrocellulose membranes, and analyzed by immunoblotting.
Results
JAB1 and p27 expression patterns in normal and neoplastic pancreatic tissues
Immunoblotting showed strong JAB1 expression in pancreatic carcinoma samples but not in paired normal pancreatic tissues, where weak or no JAB1 expression was detected (Fig. 1). In contrast, p27 levels were significantly higher in normal pancreatic tissues than in the pancreatic carcinoma samples obtained from the same patients (data not shown).
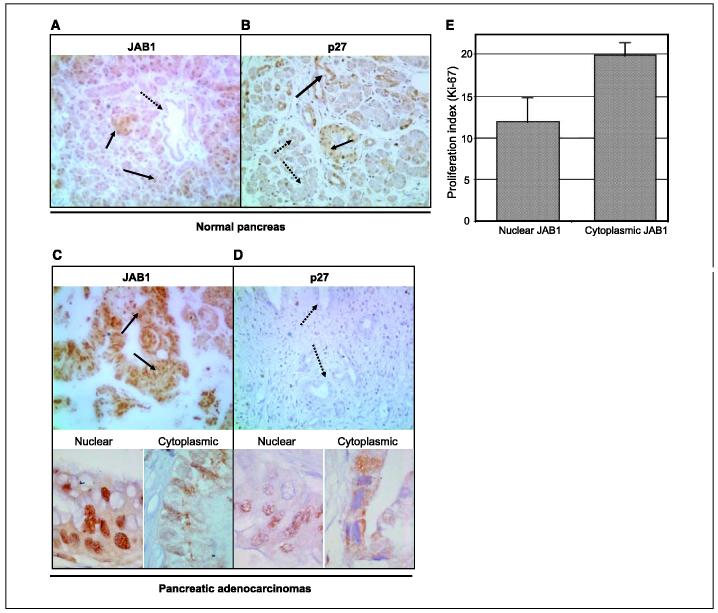
Figure 1.
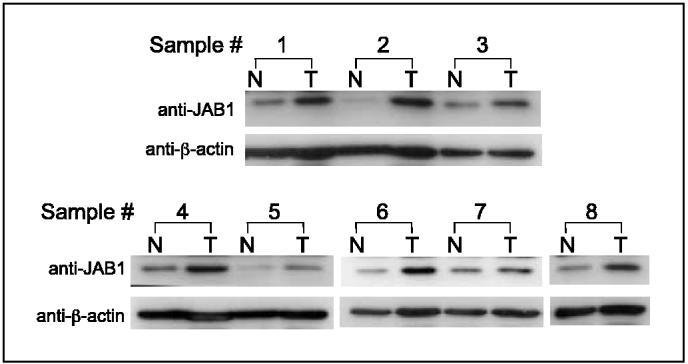
JAB1 is overexpressed in pancreatic adenocarcinoma but not in paired normal pancreatic tissues. Western blots of eight representative paired samples of nonneoplastic pancreatic tissue (N)and pancreatic adenocarcinoma tissue (T)immunoblotted against JAB1. Whole-cell lysates were prepared from tissue specimens obtained from pancreatic carcinoma and adjacent normal pancreas. In all samples tested, JAB1 expression levels were significantly higher in pancreatic cancer than in paired normal pancreatic tissues. β-Actin was used as a control for protein load and integrity.
Expression of JAB1 and p27 was further studied by immunohistochemical analysis of tissue microarrays containing 33 pancreatic carcinomas and 14 samples of normal pancreatic tissue. In normal pancreatic tissue, JAB1 was detected only in the acinar cells, which were predominantly p27 negative (Fig. 2A). The staining pattern in those cells was strongly nuclear and weakly cytoplasmic. Normal ductal and islet cells were usually JAB1 negative or faintly positive, and those cells strongly expressed p27 (Fig. 2B).
Figure 2.
Expression patterns of JAB1 and p27 in normal (A and B)and neoplastic (C and D)pancreatic tissues. Paraffin-embedded tissue sections cut from a tissue microarray were stained with monoclonal antibodies for JAB1 and p27 and counterstained with hematoxylin. The tissue microarray included cores from pancreatic adenocarcinomas and normal pancreatic tissues. A, in normal pancreas, JAB1 immunoreactivity was detected in acinar cells, with staining predominant in the nucleus and weaker in the cytoplasm; most ductal and islet cells were JAB1 negative. B, by contrast, p27 was strongly expressed in most ductal and islet cells whereas most acinar cells were p27 negative or expressed p27 only weakly (black and dotted arrows, positive and negative, respectively). Original magnification, ×200. C, all pancreatic adenocarcinomas tested expressed JAB1. A case of pancreatic carcinoma with the predominant staining pattern of strongly nuclear and weakly cytoplasmic is shown. bottom, cases of pancreatic carcinoma with a predominant nuclear (left)and cytoplasmic (right)staining pattern for JAB1. D, most pancreatic adenocarcinomas were p27-negative. bottom, nuclear (left)and cytoplasmic (right)expression of p27 in pancreatic carcinomas expressing p27 (black and dotted arrows, positive and negative, respectively). Original magnification, ×200 (top); ×1,000 (bottom). E, difference in proliferation index (% Ki-67-positive tumor cells) between tumors with cytoplasmic and nuclear expression of JAB1.
Data on JAB1 and p27 expression in tumors were available for 32 of the 33 pancreatic tumors as one tumor core was lost during the histology and staining process. In pancreatic adenocarcinomas, JAB1 was overexpressed in all 32 tumors tested (Fig. 2C). The staining pattern was predominantly nuclear, with weaker cytoplasmic immunoreactivity in 23 (72%) tumors and predominantly cytoplasmic with a weaker nuclear immunoreactivity in 9 (28%) tumors (Fig. 2C). The nucleoli were often negative for JAB1 regardless of nuclear/cytoplasmic expression pattern. The proportion of JAB1-positive tumor cells ranged from 60% to 100%. When 10% was used as the cutoff for positivity, p27 was expressed in 11 (34%) of the pancreatic carcinomas (Fig. 2D). Of those 11 tumors, p27 expression was exclusively nuclear in 4 (13%) and predominantly cytoplasmic in the remaining 7 (22%) tumors (Fig. 2D). Intensity of staining was generally stronger for nuclear p27 compared with cytoplasmic p27 expression. The proportion of nuclear p27-positive tumor cells ranged from 0% to 55%, whereas the proportion of cytoplasmic p27-positive cells ranged from 0% to 90%. Of note, all 9 tumors with predominantly cytoplasmic JAB1 did not expressed p27. The mean proliferation index was 20.2% in tumors with predominantly cytoplasmic JAB1 (all p27-negative) compared with 11.6% in tumors with predominantly nuclear JAB1 expression (P = 0.04, Mann-Whitney test; Fig. 2E).
To further confirm the results of our immunohistochemical analysis, we used a double immunofluorescence method to detect JAB1 and p27 in the same fresh-frozen tissue sections obtained from previously untreated patients with pancreatic cancer. Using this method, JAB1 was overexpressed in tumor cells of all 10 tumors tested but not in cells of adjacent normal pancreatic ducts (Supplementary Fig. S1). By contrast, p27 was not expressed in the majority (8 of 10) of these tumors using a 10% cutoff.
Adenoviral JAB1 gene transfer reduces p27 levels in pancreatic cancer cell lines
Low expression of p27 protein is associated with excessive cell proliferation and has been linked to many types of human tumors, including pancreatic adenocarcinoma (26, 27). We screened a number of pancreatic adenocarcinoma cells and found that most of them had very low expression of p27. Treatment of pancreatic adenocarcinoma cell lines Mia PaCa-2 and Panc-28 with specific proteasome inhibitors resulted in a significant increase in total p27 levels (Fig. 3A). To investigate whether JAB1 overexpression in pancreatic cancer cells would down-regulate p27 levels, we transduced three pancreatic cancer cell lines, Panc-1, Mia PaCa-2, and Panc-28, with JAB1 adenovirus in the absence (-) or presence (+) of doxycycline and measured JAB-Myc and p27 levels 48 hours after infection. Using this inducible (Tet-Off) system, JAB1 overexpression led to a significant decrease in p27 levels in all cell lines tested (Fig. 3B). Downregulation of p27 by JAB1 overexpression was inhibited in cells that had been treated with inhibitors of the 26S proteasome (LLnL and MG132) but not in those treated with a control DMSO (Fig. 3C), indicating that JAB1 promotes p27 degradation through a proteolysis and is sensitive to 26S proteasome inhibitors. These data provide direct evidence of a functional relationship between JAB1 and p27 in pancreatic carcinoma cells. These findings support our tissue microarray results showing an inverse correlation between JAB1 and p27 expression in pancreatic adenocarcinomas.
Figure 3.
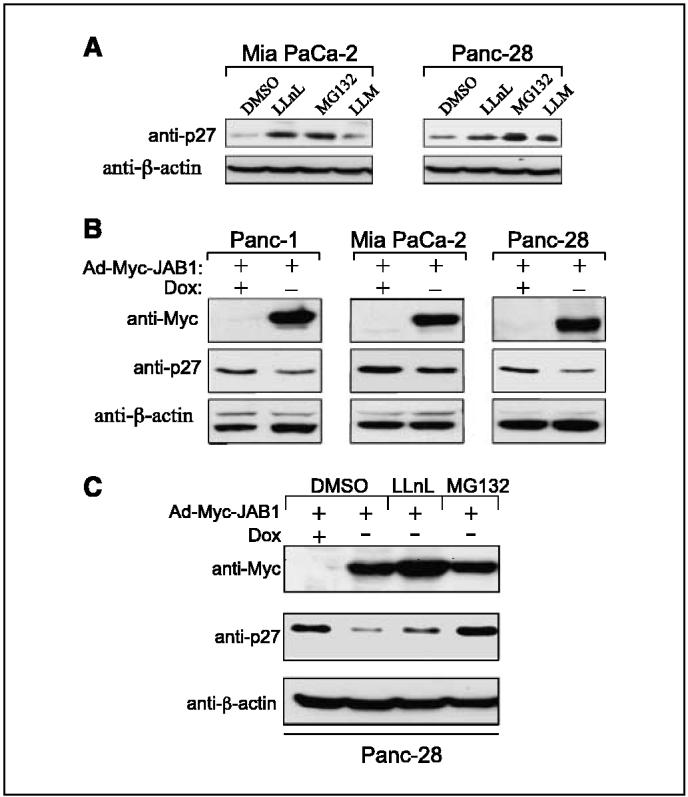
JAB1 regulates p27 levels in pancreatic cancer cells. A, degradation of p27 was inhibited in Mia PaCa-2 and Panc-28 cells treated with proteasome inhibitors LLnL (35 μmol/L)and MG132 (30 μmol/L)but not in cells treated with LLM (25 μmol/L)or control DMSO. B, inducible expression of Ad-Myc-JAB1 down-regulated endogenous p27 levels in three pancreatic cancer cell lines as indicated. Lysates were prepared from Ad-Myc-JAB1-infected cells in the absence (-)or presence (+)of doxycycline (Dox)and proteins were immunoblotted with anti-Myc and anti-p27 antibodies. Anti-h-actin antibodies were used as a loading control. C, down-regulation of p27 by Ad-Myc-JAB1 is sensitive to 26S proteasome inhibitors. Panc-28 cells were transduced with the Ad-Myc-JAB1 virus and, 32 hours postinfection, 26S proteasome inhibitors LLnL and MG132 and DMSO as control were added for 16 hours. At 48 hours postinfection, whole-cell lysates were prepared and proteins analyzed by immunoblotting.
JAB1 specifically interacts with p27 wild-type and p27T187A
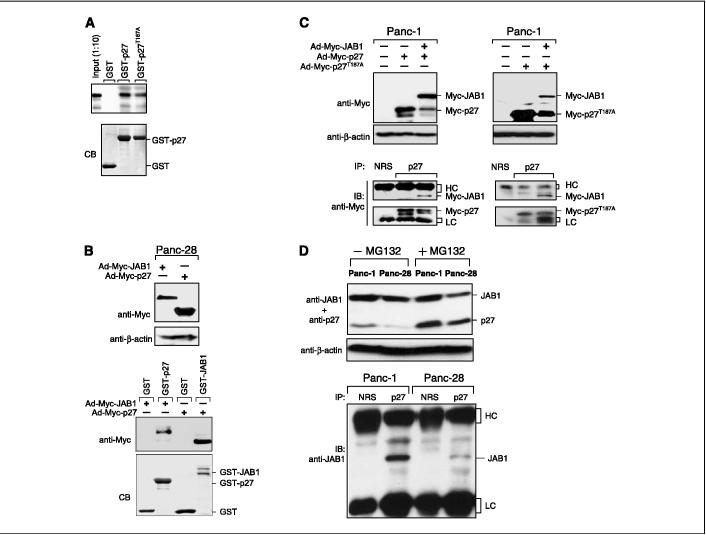
We next determined whether JAB1 interacts with p27 in vitro and in vivo. We have also examined the interaction of JAB1 with a proteolysis-resistant mutant of p27 containing a Thr187 to Ala mutation (T187A). The p27T187A mutant was not degraded by the ubiquitin-mediated proteasome pathway. in vitro translated and radioactively labeled JAB1 protein was incubated with GST or GST-p27 or GSTp27T187A fusion proteins bound to glutathione beads. Figure 4A shows that both GST-p27 and GST-p27T187A proteins were able to pull down 35S-labeled JAB1, as opposed to the GST protein alone. The interaction between JAB1 and p27 was also assessed in a pulldown assay with the use of cell lysates from Panc-28 cells transduced with Ad-Myc-JAB1 or Ad-Myc-p27. Expression of the Myc-recombinant proteins was analyzed (Fig. 4B, top) and binding assays were done by incubating GST, GST-p27, or GST-JAB1 immobilized on glutathione beads. The bound proteins were analyzed by immunoblotting with antibodies against Myc. As shown in Fig. 4B (bottom), we observed the strongest association between Myc-JAB1 and GST-p27 or Myc-p27 and GST-JAB1. However, GST-recombinant protein alone did not pull down Myc-JAB1 nor Myc-p27.
Figure 4.
JAB1 specifically interacts with p27. A, physical interaction of JAB1 with p27. Top, GST, GST-p27, or GST-p27T187A, bound to glutathione beads, was incubated with in vitro translated, 35S-methionine-labeled JAB1 proteins. Bound 35S-JAB1 was detected by autoradiography. bottom, GST-recombinant proteins were localized by Coomassie blue staining (CB). B, interactions of Myc-JAB1 with GST-p27 and Myc-p27 with GST-JAB1. Top, lysates from Panc-28 cells transduced with either Ad-Myc-JAB1 virus or Ad-Myc-p27 virus in the absence of doxycycline were prepared and analyzed for Myc-recombinant protein expression by Western blotting with anti-Myc antibodies and with anti-β-actin antibodies as a loading control. GST pulldown assays were done with lysates incubated with 5 μg of GST, GST-JAB1, or GST-p27 immobilized on glutathione beads. The bound proteins were analyzed by immunoblotting with antibodies against Myc. bottom, Coomassie blue staining of GST-recombinant proteins. C, in vivo interaction of JAB1 with p27 or p27T187A mutant. Panc-1 cells were transduced with either Ad-Myc-p27 or Ad-Myc-p27T187A with or without Ad-Myc-JAB1 in the absence of doxycycline. Forty-eight hours after transduction, cells were lysed, which was followed by immunoblotting with anti-Myc and anti-β-actin antibodies. Expression of Myc-JAB1 along with Myc-p27 or Myc-p27T187A protein showed degradation of the Myc-p27 protein level. bottom, cell lysates from the transduced cells described above were immunoprecipitated (IP)with antibodies against p27 and with a nonimmune rabbit serum (NRS)as a control. Immunoblotting (IB)was done with anti-Myc antibodies. Myc-p27 and Myc-p27T187A associated with Myc-Jab1 in Panc-1 cells. Immunoglobulin G heavy chain (HC)and light chain (LC)were indicated. D, in vivo interaction of endogenous JAB with endogenous p27 proteins. Panc-1 and Panc-28 cells were treated with or without proteasome inhibitor MG132 and cell lysates were analyzed by Western blotting with anti-JAB1 and anti-p27 antibodies (top). Cell lysates treated with MG132 were immunoprecipitated with either nonimmune rabbit serum or p27 and immunoblotted with anti-JAB1 (bottom).
Finally, to confirm the physical interactions between JAB1 and p27, coimmunoprecipitations analyses were done in vivo. Panc-1 cell extract was transduced with Ad-Myc-p27 or Ad-Myc-p27T187A with or without Ad-Myc-JAB1. Cell lysates were prepared and Western blotting was done with anti-Myc (Fig. 4C, top). Myc-p27 and Myc-p27T187A degradation was observed with Myc-JAB1 (Fig. 4C, top). Lysates were immunoprecipitated with p27 antibodies and immunoblotted with anti-Myc antibodies. Figure 4C (bottom) shows that both Myc-p27 and Myc-p27T187A indeed interact with Myc-JAB1. This interaction was specific because JAB1-containing lysates were not immunoprecipitated with non-immune rabbit serum. These data strongly indicate that JAB1 associates with p27 in pancreatic adenocarcinomas. Furthermore, endogenous JAB1 and p27 interaction was studied in vivo. Panc-1 and Panc-28 cells were treated with or without the proteasome inhibitor MG132 to stabilize p27 protein level (Fig. 4D, top). Panc-1 and Panc-28 cell lysates treated with MG132 were immunoprecipated with anti-p27 or nonimmune rabbit serum as control and Western blotting with anti-JAB1 (Fig. 4D, bottom). Results indicate that endogenous p27 interacts with endogenous JAB1 (Fig. 4D, bottom), suggesting a direct interaction between the two proteins.
Depletion of JAB1 by siRNA increases accumulation of p27 and induces cell cycle arrest in pancreatic cancer cell lines
To assess the effect of silencing JAB1 in human pancreatic carcinoma cells, we transfected Panc-1, Mia PaCa-2, and Panc-28 cells with JAB1 siRNA oligonucleotides or control siRNA oligonucleotides cloned into the RNAi-vector system. At 48 hours after transfection, JAB1 protein levels were substantially decreased (by >60%) in all three JAB1 siRNA-transfected cell lines when compared with cells transfected with the control siRNA oligonucleotides (Fig. 5A, top). The decrease in endogenous JAB1 protein expression was associated with a significant increase in p27 levels (Fig. 5A), suggesting the biological importance of JAB1 in regulation of p27 proteolysis in pancreatic cancer.
Figure 5.
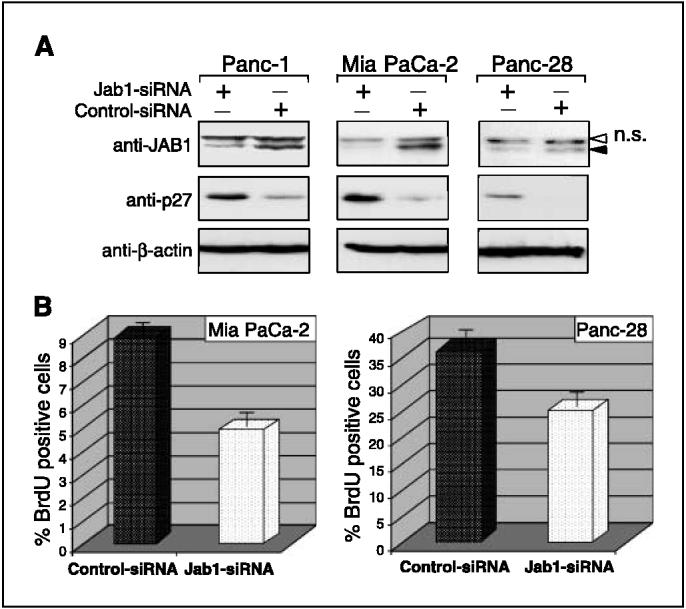
Down-regulation of JAB1 increases p27 levels and induces cell cycle arrest. A, knockdown of JAB1 with siRNA enhanced p27 stability. Cells were transfected with siRNA targeting either JAB1 or a scrambled sequence (control); 48 hours later, proteins were isolated and immunoblotted with anti-JAB1 and anti-p27 antibodies. Anti-β-actin antibodies were used as loading controls. The specific JAB1 bands (filled arrow)and nonspecific bands (n.s., white arrow) are indicated. B, siRNA ablation of JAB1 induced G1 arrest. Mia PaCa-2 and Panc-28 cells were transfected as described in (A); 48 hours later, BrdUrd (BRdU)was added and the cells were stained with FITC-anti-BrdUrd antibodies. Cells that stained positively for BrdUrd were then analyzed by flow cytometry. Columns, percentage of BrdUrd-positive (i.e., S-phase)cells.
Next, to determine whether knockdown of JAB1 gene would lead to an increase in p27 activity, as indicated by inhibition of G1-to-S phase progression, we used BrdUrd staining to analyze the proportion of cells that were in S phase. In the Mia PaCa-2 cell line, 8% of the control siRNA-transfected cells were in S phase and 4% of the JAB1 siRNA-transfected cells were in S phase (Fig. 5B). Similar results were obtained for the Panc-28 cells (32% S phase for control cells versus 22% S phase for JAB1 siRNA cells; Fig. 5B). These data suggest that down-regulation of JAB1 induces stabilization of p27 and silencing of JAB1 increases p27 protein levels and activity.
Loss of Skp2 does not impair JAB1-mediated p27 degradation
The CDK inhibitor p27Kip1 is degraded at the G0-G1 transition of the cell cycle by the ubiquitin-proteasome pathway (13, 28). Although the nuclear ubiquitin ligase (E3) SCFSkp2 is implicated in p27Kip1 degradation (29-31), proteolysis of p27Kip1 at the G0-G1 transition proceeds normally and is sensitive to proteasome inhibitors in Skp2-/- cells (32, 33), indicating that p27 is degraded at G0-G1 by a proteasome-dependent, but Skp2-independent, mechanism. Recent identification of a ubiquitin ligase, Kip1 ubiquitylation-promoting complex (KPC), which consists of KPC1 and KPC2 subunits, was shown to be responsible for the rapid degradation of p27 at the transition from G0 to G1 (34, 35). Therefore, we decided to investigate whether loss of Skp2 impairs JAB1-mediated p27 degradation. Panc-28 cells were transfected with either control siRNA (100 nmol/L) or Skp2 siRNA (50 and 100 nmol/L) oligonucleotides alone (Fig. 6A) or transfected with Skp2 siRNA oligonucleotides and then transduced with adenovirus JAB1 (Fig. 6B). The data showed that targeted silencing of Skp2 decreased endogenous Skp2 protein levels in a dose-dependent manner whereas no decrease was seen in control siRNA-transfected cells. Skp2 protein was decreased by ∼20% to >70% with 50 mmol/L and 100 nmol/L of Skp2 siRNA oligonucleotides, respectively. In Skp2 knockdown cells, the level of endogenous p27 was increased, unlike in control cells, suggesting that inhibition of Skp2 inhibits p27 degradation. To verify whether JAB1-mediated degradation of p27 is Skp2 dependent or Skp2 independent, the endogenous Skp2 was silenced in Panc-28 cells and then transduced with the doxycyclin-inducible adeno-JAB1 virus. Unlike in Fig. 6A, a dramatic knockdown of Skp2 was observed in Fig. 6B, wherein the level of endogenous Skp2 was decreased by 90% with 100 nmol/L Skp2 siRNA transfection followed by infection of adeno-JAB1. Exogenous Myc-JAB1 expression was seen only in the absence of doxycyclin. Skp2 knockdown increased the level of p27 protein significantly in these cells but, on expression of exogenous Myc-JAB1, p27 protein was degraded as indicated by low p27 levels. This suggests that JAB1-mediated p27 degradation could also occur in a Skp2-independent manner and probably involved another F-box protein (e.g. KPC; refs. 34, 35).
Figure 6.
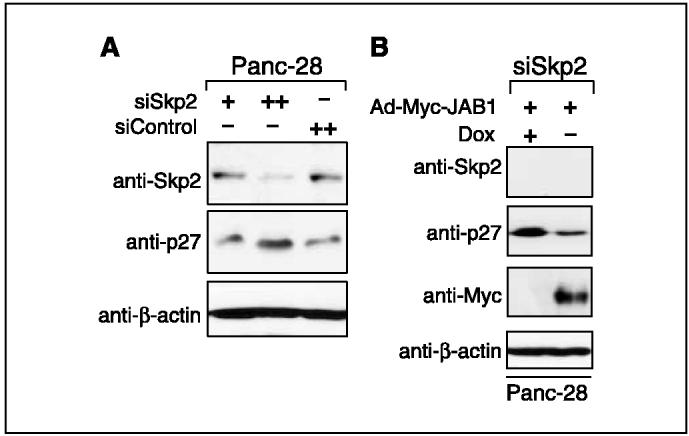
Down-regulation of p27 by JAB1 in Skp2 knockdown Panc-28 cells. A, Skp2 siRNA induced p27 accumulation. Panc-28 cells were transfected with 50 nmol/L (+)and 100 nmol/L (++)of siSkp2 and with 100 nmol/L (++)of scrambled sequence as a control siRNA. Western blot analysis of Skp2 and p27 was done 48 hours after transfection. Anti-β-actin antibodies were used as loading controls. B, effect of Skp2 depletion on JAB1-mediated p27 degradation. Panc-28 cells were transfected with siSkp2 as described above. Cells were then transduced with Ad-Myc-JAB1 virus and subjected to Western blot analysis with the antibodies indicated on the left.
Correlation of JAB1/p27 expression with clinical outcome
In the entire study group of 33 patients, the median progressionfree survival was 12.4 months (95% confidence interval, 9-16). After a median follow-up of 35 months (range, 3-75 months) for survivors, 23 of the 30 (70%) patients with available clinical follow-up data had progression of disease or relapsed after curative surgery. Interestingly, 21 of the 26 patients with p27-negative pancreatic tumors experienced disease progression after surgery, whereas only 1 of the 3 patients with p27-positive tumors experienced progression; however, this apparent correlation was not statistically significant (P = 0.13, Fisher’s exact test) and a larger series of untreated patients is needed to confirm this observation. In addition, all 8 patients in whom JAB1 was expressed in the cytoplasm experienced disease progression compared with 14 of 22 patients with JAB1 localization predominantly in the nucleus; but again, this was not statistically significant (P = 0.14, Fisher’s exact test). The median overall survival was 24 months (95% confidence interval, 14-45 months). When this report was written, 11 (33.3%) patients were alive (7 patients with no evidence of disease and 4 with disease), 18 patients had died of disease, 3 patients had died of unknown causes, and 1 patient had died of an non-disease-related cause. However, the number of cases studied was small, and most (27 of 33) of the patients included in this study had undergone chemotherapy before the tissue samples were obtained, thus precluding definitive conclusions from survival analyses.
Discussion
The prognosis of pancreatic adenocarcinoma remains dismal even after tumor resection. The patterns of treatment failure remain unknown owing to heterogeneous genetic alterations and unknown mechanisms of pathogenesis. Therefore, identification of novel mechanisms involved in pancreatic oncogenesis is needed.
JAB1, originally identified as a AP-1 coactivator (6), is also an important negative regulator of p27 (9, 10), a CDK inhibitor that controls cell cycle progression from G1 to S phase, providing a novel mechanism by which JAB1 may contribute to human oncogenesis. In this study, we investigated the role of JAB1-mediated p27 degradation in pancreatic oncogenesis. First, we showed that JAB1 protein is overexpressed in pancreatic cancer but not in paired normal pancreatic tissue specimens. To confirm these findings, we used immunohistochemical analysis of a tissue microarray to assess JAB1 expression in normal and neoplastic pancreatic tissues. This is the first report on the distribution of JAB1 immunostaining among normal pancreatic cell types. JAB1 expression seems to be inversely associated with nuclear p27 expression patterns, suggesting that JAB1 has a physiologic role in controlling p27 levels, which most likely depends on the proliferative activity of normal epithelial cells. We also found that all pancreatic carcinomas overexpressed JAB1, which was localized in the nucleus in most cases. In contrast, nuclear p27 expression was found in 13% of pancreatic carcinomas, and in another 22% of pancreatic carcinomas, only cytoplasmic p27 expression was seen, indicating that the relative lack of activity of p27 in these tumor cells may have resulted from its mislocalization into the cytoplasm. Interestingly, all nine tumors with predominantly cytoplasmic JAB1 expression were p27 negative and were associated with a significantly higher proliferation index.
Our finding of an inverse correlation between JAB1 and p27 expression levels in pancreatic carcinomas is in agreement with our previously published results on breast cancer (20). Others have reported similar associations in other epithelial or lymphoid malignancies (19, 21). To our knowledge, only one previous study has been published on the expression of p27 and JAB1 proteins in pancreatic adenocarcinoma, and that report also showed JAB1 overexpression in all of the pancreatic cancer cases analyzed (36). Our findings on p27 expression also confirm those of previous reports. For instance, Feakins et al. (26) reported that pancreatic carcinomas expressed very low levels of p27, with the median percentage of p27-positive tumor cells being 6%. In our study, when 10% was used as the cutoff for p27 positivity, approximately one third of pancreatic carcinomas expressed either nuclear or cytoplasmic p27.
To further investigate the potential role of JAB1-mediated down-regulation of p27 in pancreatic cancer, we infected pancreatic cancer-derived cell lines with an inducible JAB1-expressing adenoviral vector and found that p27 levels were significantly reduced after JAB1 gene transfer, results similar to those we previously obtained in breast cancer cell lines (20). Furthermore, siRNA to knock down endogenous JAB1 expression in pancreatic carcinoma cell lines led to significant increases in total p27 levels and substantial decreases in the S-phase fraction. The p27-JAB1 interaction was confirmed by glutathione S-transferase pulldown assays in vitro and by coimmunoprecipitation assays in vivo. More importantly, a mutant of p27, p27T187A, which is not degraded by ubiquitin-proteasome pathway, also associated with JAB1 and expression of JAB1 decreased the level of p27T187A protein, suggesting that JAB1 accelerated degradation of both wild-type p27 and p27T187A mutant. In addition, this degradation of p27 by JAB1 is independent of Skp2 as loss of Skp2 by siRNA stabilized endogenous p27, which was degraded by expression of exogenous JAB1. These results provide additional evidence for JAB1-mediated posttranslational control of p27 levels by accelerating p27 degradation in pancreatic cancer.
High expression levels of both nuclear JAB1 and p27 were found only in four tumors in this study. In these cases, the possibility of abnormal nuclear accumulation of inactive p27/cyclin D3/CDK4 to CDK6 complexes, as has been suggested for other tumor types, cannot be excluded (37). In addition, the cytoplasmic localization of p27 observed in a subset of pancreatic tumors indicates that p27 may have been phosphorylated and inactivated in those cases. These findings support the concept that JAB1 shuttles phosphorylated p27 from the nucleus to the cytoplasm, where p27 gets degraded by the ubiquitin-proteasome system (9).
p27 proteolysis probably is mediated not only by JAB1 but also by the COP9 signalosome complex, of which JAB1 is a component (29). Ectopic expression of other components of the CSN complex, including CSN6, CSN7, and CSN8, is capable of inducing p27 down-regulation, and this function may require the nuclear export of p27 (10). In fact, regulation of p27 levels is complex. Other mechanisms, including Skp2, may contribute to p27 degradation (29). Malignant transformation and progression of pancreatic neoplasm is a complex multistage process (4). Overexpression of JAB1 leading to accelerated turnover and lower levels of a critical cell cycle regulator, such as p27, may contribute to pancreatic oncogenesis. Moreover, endogenous JAB1 is physiologically involved in the regulation of AP-1 transcriptional activity (6) and participates in COP9-mediated kinase activity in various transcription regulators (7). The significance of these functions of JAB1 in pancreatic cancer needs to be explored in future mechanistic studies.
In conclusion, we found JAB1 to be overexpressed and inversely correlated with p27 levels in pancreatic cancer. Low p27 levels associated with JAB1 overexpression or other factors might contribute to uncontrolled cell cycle progression and tumorigenesis. Modulation of the JAB1 gene product may be a novel target for experimental therapies in pancreatic cancer.
Acknowledgments
Grant support: NIH grants 1 R01 CA90853 and CA16672 and Susan G. Komen Foundation (F.X. Claret); Odyssey Special Fellowship by the University of Texas M.D. Anderson Cancer Center (M.A. Kouvaraki); and fellowship from Susan G. Komen Foundation (A.L. Korapati).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
M.A. Kouvaraki, A.L. Korapati, and G.Z. Rassidakis contributed equally to this work.
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Evans DB, Pisters PW, Lee JE, et al. Preoperative chemoradiation strategies for localized adenocarcinoma of the pancreas. J Hepatobiliary Pancreat Surg. 1998;5:242–50. doi: 10.1007/s005340050041. [DOI] [PubMed] [Google Scholar]
- 3.Hilgers W, Kern SE. Molecular genetic basis of pancreatic adenocarcinoma. Genes Chromosomes Cancer. 1999;26:1–12. [PubMed] [Google Scholar]
- 4.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 5.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The PI3-kinase/Akt signaling pathway is activated due to aberrant Pten expression and targets transcription factors NF-nB and c-Myc in pancreatic cancer cells. Oncogene. 2004;23:8571–80. doi: 10.1038/sj.onc.1207902. [DOI] [PubMed] [Google Scholar]
- 6.Claret FX, Hibi M, Dhut S, Toda T, Karin M. A new group of conserved coactivators that increase the specificity of AP-1 transcription factors. Nature. 1996;383:453–7. doi: 10.1038/383453a0. [DOI] [PubMed] [Google Scholar]
- 7.Chamovitz DA, Segal D. JAB1/CSN5 and the COP9 signalosome. A complex situation. EMBO Rep. 2001;2:96–101. doi: 10.1093/embo-reports/kve028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwechheimer C, Deng XW. COP9 signalosome revisited: a novel mediator of protein degradation. Trends Cell Biol. 2001;11:420–6. doi: 10.1016/s0962-8924(01)02091-8. [DOI] [PubMed] [Google Scholar]
- 9.Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160–5. doi: 10.1038/18230. [DOI] [PubMed] [Google Scholar]
- 10.Tomoda K, Kubota Y, Arata Y, et al. The cytoplasmic shuttling and subsequent degradation of p27Kip1 mediated by Jab1/CSN5 and the COP9 signalosome complex. J Biol Chem. 2002;277:2302–10. doi: 10.1074/jbc.M104431200. [DOI] [PubMed] [Google Scholar]
- 11.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 12.Polyak K, Lee MH, Erdjument-Bromage H, et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 13.Pagano M, Tam SW, Theodoras AM, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–5. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 14.Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–78. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 15.Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334–44. doi: 10.1093/emboj/16.17.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montagnoli A, Fiore F, Eytan E, et al. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181–9. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malek NP, Sundberg H, McGrew S, et al. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature. 2001;413:323–7. doi: 10.1038/35095083. [DOI] [PubMed] [Google Scholar]
- 18.Korbonits M, Chahal HS, Kaltsas G, et al. Expression of phosphorylated p27(Kip1) protein and Jun activation domain-binding protein 1 in human pituitary tumors. J Clin Endocrinol Metab. 2002;87:2635–43. doi: 10.1210/jcem.87.6.8517. [DOI] [PubMed] [Google Scholar]
- 19.Sui L, Dong Y, Ohno M, et al. Jab1 expression is associated with inverse expression of p27(kip1) and poor prognosis in epithelial ovarian tumors. Clin Cancer Res. 2001;7:4130–5. [PubMed] [Google Scholar]
- 20.Kouvaraki MA, Rassidakis GZ, Tian L, Kumar R, Kittas C, Claret FX. Jun activation domain-binding protein 1 expression in breast cancer inversely correlates with the cell cycle inhibitor p27(Kip1) Cancer Res. 2003;63:2977–81. [PubMed] [Google Scholar]
- 21.Rassidakis GZ, Claret FX, Lai R, et al. Expression of p27(Kip1) and c-Jun activation binding protein 1 are inversely correlated in systemic anaplastic large cell lymphoma. Clin Cancer Res. 2003;9:1121–8. [PubMed] [Google Scholar]
- 22.Esteva FJ, Sahin AA, Rassidakis GZ, et al. Jun activation domain binding protein 1 expression is associated with low p27(Kip1)levels in node-negative breast cancer. Clin Cancer Res. 2003;9:5652–9. [PubMed] [Google Scholar]
- 23.Shintani S, Li C, Mihara M, Hino S, Nakashiro K, Hamakawa H. Skp2 and Jab1 expression are associated with inverse expression of p27(KIP1) and poor prognosis in oral squamous cell carcinomas. Oncology. 2003;65:355–62. doi: 10.1159/000074649. [DOI] [PubMed] [Google Scholar]
- 24.Ivan D, Diwan AH, Esteva FJ, Prieto VG. Expression of cell cycle inhibitor p27Kip1 and its inactivator Jab1 in melanocytic lesions. Mod Pathol. 2004;17:811–8. doi: 10.1038/modpathol.3800123. [DOI] [PubMed] [Google Scholar]
- 25.Goto A, Niki T, Moriyama S, et al. Immunohistochemical study of Skp2 and Jab1, two key molecules in the degradation of P27, in lung adenocarcinoma. Pathol Int. 2004;54:675–81. doi: 10.1111/j.1440-1827.2004.01679.x. [DOI] [PubMed] [Google Scholar]
- 26.Feakins RM, Ghaffar AH. p27 Kip1 expression is reduced in pancreatic carcinoma but has limited prognostic value. Hum Pathol. 2003;34:385–90. doi: 10.1053/hupa.2003.23. [DOI] [PubMed] [Google Scholar]
- 27.Hu YX, Watanabe H, Li P, et al. An immunohistochemical analysis of p27 expression in human pancreatic carcinomas. Pancreas. 2000;21:226–30. doi: 10.1097/00006676-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Shirane M, Harumiya Y, Ishida N, et al. Down-regulation of p27(Kip1) by two mechanisms, ubiquitin-mediated degradation and proteolytic processing. J Biol Chem. 1999;274:13886–93. doi: 10.1074/jbc.274.20.13886. [DOI] [PubMed] [Google Scholar]
- 29.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–9. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 30.Sutterluty H, Chatelain E, Marti A, et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–14. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 31.Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–4. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 32.Hara T, Kamura T, Nakayama K, Oshikawa K, Hatakeyama S. Degradation of p27(Kip1) at the G(0)-G(1) transition mediated by a Skp2-independent ubiquitination pathway. J Biol Chem. 2001;276:48937–43. doi: 10.1074/jbc.M107274200. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama K, Nagahama H, Minamishima YA, et al. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell. 2004;6:661–72. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 34.Kamura T, Hara T, Matsumoto M, et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol. 2004;6:1229–35. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- 35.Kotoshiba S, Kamura T, Hara T, Ishida N, Nakayama KI. Molecular dissection of the interaction between p27 and Kip1 ubiquitylation-promoting complex, the ubiquitin ligase that regulates proteolysis of p27 in G1 phase. J Biol Chem. 2005;280:17694–700. doi: 10.1074/jbc.M500866200. [DOI] [PubMed] [Google Scholar]
- 36.Fukumoto A, Ikeda N, Sho M, et al. Prognostic significance of localized p27Kip1 and potential role of Jab1/CSN5 in pancreatic cancer. Oncol Rep. 2004;11:277–84. [PubMed] [Google Scholar]
- 37.Sanchez-Beato M, Camacho FI, Martinez-Montero JC, et al. Anomalous high p27/KIP1 expression in a subset of aggressive B-cell lymphomas is associated with cyclin D3 overexpression. p27/KIP1-cyclin D3 colocalization in tumor cells. Blood. 1999;94:765–72. [PubMed] [Google Scholar]