Abstract
Subcutaneous injection of sodium arsenite (NaAs, 12.5 mg/kg) into BALB/c [wild-type (WT)] mice causes acute renal dysfunction characterized by severe hemorrhages, acute tubular necrosis, and cast formation, with increases in serum blood urea nitrogen and creatinine levels. Concomitant enhancement in intrarenal interferon (IFN)-γ expression prompted us to examine its roles in this pathology. IFN-γ-deficient (IFN-γ−/−) mice exhibited higher serum blood urea nitrogen and creatinine levels and exaggerated histopathological changes, compared with WT mice. Eventually, IFN-γ−/− mice exhibited a high mortality (87.5%) within 24 hours after NaAs challenge, whereas most WT mice survived. The intrarenal arsenic concentration was significantly higher in IFN-γ−/− mice later than 10 hours after NaAs treatment, with attenuated intrarenal expression of multidrug resistance-associated protein (MRP) 1, a main transporter for NaAs efflux, compared with WT mice. NF-E2-related factor (Nrf) 2 protein, a transcription factor crucial for MRP1 gene expression, was similarly increased in the kidneys of both strains of mice after NaAs treatment. In contrast, the absence of IFN-γ augmented transforming growth factor-β-Smad3 signal pathway and eventually enhanced the expression of activating transcription factor 3, which is presumed to repress Nrf2-mediated MRP1 gene expression. Thus, IFN-γ can protect against NaAs-induced acute renal injury, probably by maintaining Nrf2-mediated intrarenal MRP1 gene expression.
Arsenic inhibits the biological functions of various proteins by reacting with their sulfhydryl groups.1 Acute exposure to arsenic can cause profound injury to kidney, liver, intestine, and brain,2,3 frequently resulting in acute mortality. Chronic exposure causes dysfunctions in renal and nervous systems.4,5 Moreover, arsenic is a potent carcinogen to various organs including skin, lung, bladder, liver, and kidney.4,5 Arsenic is ubiquitously present in the natural environment in soil, water, and air. Furthermore, groundwater and/or soil are frequently contaminated with a high concentration of arsenic, which is generated during the refinement of various ores such as copper and lead and the consumption of coal. Thus, arsenic intoxication in an acute or a chronic form still remains a serious threat to public health in areas where groundwater and/or soil is contaminated with arsenic.
On the contrary, accumulating evidence has revealed that As2O3 may be efficacious for acute promyelocytic leukemia without causing bone marrow suppression.6–8 Moreover, As2O3 might be effective also for androgen-independent prostate cancer.9 Its efficaciousness may come from the capacity of As2O3 to induce apoptotic and/or autophagic cell death in vitro. Because As2O3 can exhibit severe toxicities to various organs, the clinical application of As2O3 necessitates the development of the measures to suppress or minimize its side effects.
Because active transport for extrusion is the most common mechanism by which cells can reduce stresses arising from exposure to heavy metals, active transport systems are well conserved in various species from bacteria to mammals. Accumulating evidence indicates that ATP-binding cassette (ABC) transporter proteins such as multidrug resistance gene protein (MDR)/P-glycoprotein and multidrug resistance-associated protein (MRP) family can transport arsenic in mammalian cells.10–15 Several lines of evidence demonstrate that arsenite is conjugated with glutathione and that the resultant conjugate is transported mainly by MRP1.16,17 Consistent with this notion, we previously observed that C57BL/6 mice were more resistant than BALB/c mice to sodium arsenic (NaAs)-induced renal injury together with a higher expression level of MRP1 but not other ABC transporters.18
Given that interferon (IFN)-γ was presumed to regulate the expression of ABC transporters,19–21 we explored the roles of IFN-γ in the pathogenesis of NaAs-induced renal injury. Here, we have provided definitive evidence that NaAs induces intrarenal production of IFN-γ, which can protect against NaAs-induced acute renal injury by regulating MRP1 expression in the renal tubular cells.
Materials and Methods
Reagents and Antibodies
NaAs was purchased from Wako Chemical Industries (Osaka, Japan). The following monoclonal antibodies (mAbs) or polyclonal antibodies (pAbs) were used for immunohistochemical or Western blotting analysis: rat anti-IFN-γ mAb (clone XMG 1.2; BD PharMingen, San Diego, CA), rabbit anti-MRP1 pAb (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and rat anti-mouse MRP1 mAb (clone MRPr1; Alexis, San Diego, CA), rabbit anti-transforming growth factor (TGF)-β pAb, rabbit anti-phosphorylated Smad3 pAb, rabbit anti-NF-E2-related factor (Nrf)2 pAb, and rabbit anti-activating transcription factor (ATF)3 pAb (Santa Cruz Biotechnology). For immunoneutralization, rat anti-mouse IFN-γ mAb (clone R4-6A2) was kindly provided by Dr. H. Fujiwara (Osaka University School of Medicine, Osaka, Japan). Recombinant mouse IFN-γ and recombinant human TGF-β1 were purchased from PeproTech (London, UK), and mouse IL-10 was obtained from R&D Systems, Inc. (Minneapolis, MN).
Mice
Male IFN-γ−/− mice, which were backcrossed to BALB/c mice for more than eight generations, were used in the experiments as described previously.22,23 Age-matched, male BALB/c mice (Sankyo Laboratories, Tokyo, Japan) were used as wild-type (WT) mice in the present experiments. All mice were housed individually in cages under the specific pathogen-free conditions during the experiments. All animal experiments were approved by the Committee on Animal Care and Use in Wakayama Medical University.
Sodium Arsenite (NaAs)-Induced Renal Injury
NaAs-induced renal injury was established as described previously.18 Briefly, NaAs was dissolved in phosphate-buffered saline (PBS, pH 7.4) at a concentration of 2.5 mg/ml. NaAs at a dose of 12.5 mg/kg or an equivalent volume of PBS was administered subcutaneously. In some experiments, anti-IFN-γ antibody (clone R4-6A2, 250 μg/mouse) was intraperitoneally injected to WT mice at 1 hour before NaAs challenge. In another series of experiments, WT mice received subcutaneously recombinant IFN-γ (4000 U/mouse) at 1 hour before or 30 minutes after NaAs challenge.
Determination of Blood Urea Nitrogen (BUN) and Creatinine (CRE) in the Serum
At the indicated time intervals, sera were collected to determine BUN and CRE levels with a Fuji DRI-CHEM 5500V (Fuji Medical System, Tokyo, Japan), according to the manufacturer’s instructions.
Histopathological and Immunohistochemical Analyses
Kidney tissues were obtained at the indicated time intervals after NaAs challenge and were fixed in 4% formaldehyde buffered with PBS (pH 7.2) to prepare paraffin-embedded sections (6 μm thick). Thereafter, the sections were subjected to hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) staining. In addition, deparaffinized sections were used to detect IFN-γ or MRP1 protein immunohistochemically, as described previously.18,22 Briefly, the sections were reacted with rat anti-mouse IFN-γ mAb (clone XMG 1.2) or rabbit anti-MRP1 pAb at a concentration of 1 μg/ml at 4°C overnight. After the incubation with biotinylated secondary antibodies (2.5 μg/ml) at room temperature for 30 minutes, immune complexes were visualized using a catalyzed signal amplification system (DAKO, Kyoto, Japan) according to the manufacturer’s instructions. Histopathological and immunohistochemical evaluation was performed by an examiner without previous knowledge of the experimental procedure.
Quantification of Intrarenal Arsenic Contents
Intrarenal arsenic contents were analyzed as described previously.18 Briefly, a portion of the kidney (∼100 mg) was digested in nitric acid (100 mg/ml). Total arsenic was determined using graphite furnace atomic absorption spectrometry ACF-6800 (Shimadzu Corp., Kyoto, Japan). Certified arsenic standard solution (As 100: lot RWN9791; Wako Chemical Industries) was used for the calibration. Each sample was measured in triplicate with less than 1% coefficient of variance. The data were expressed as arsenic (μg)/sample weight (g).
Determination of Intrarenal IFN-γ Contents by Enzyme-Linked Immunosorbent Assay
A portion of the kidney was homogenized with a lysis buffer (0.1 mg tissue/μl) (10 mmol/L PBS, 1% Nonidet P-40, and 5 mmol/L ethylenediaminetetraacetic acid) containing Complete protease inhibitor mixture (Roche Diagnostics, Tokyo, Japan). The homogenates were centrifuged at 12,000 rpm for 15 minutes. IFN-γ levels in the supernatant were measured with a commercial enzyme-linked immunosorbent assay kit (R&D Systems) according to the manufacturer’s recommendation. Total protein in the supernatant was measured with a BCA protein assay kit (Pierce, Rockford, IL). The data were expressed as IFN-γ (pg)/total protein (mg) for each sample.
Cell Culture
A murine renal proximal tubular epithelial cell line (mProx24), derived from C57BL/6J adult mouse kidney,24 was maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum at 37°C in 5% CO2. mProx24 cells were seeded at 50% confluence on a 25-cm2 collagen-coated culture flask (Iwaki Glass, Tokyo, Japan) and grown to confluence throughout 24 hours. Then, the various combinations of NaAs (5 μmol/L), murine IFN-γ (500 U/ml), human TGF-β1 (10 ng/ml), or mouse IL-10 (10 ng/ml) were added to culture medium. Six hours later, the cells were collected, and membrane-rich fractions were obtained for Western blotting analysis.
Western Blotting Analysis for MRP1
Membrane-rich fractions were prepared from mouse kidney samples or cultured mProx24 cells as described previously.18 Membrane-rich fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis in a 7.5% slab and electrotransferred onto nitrocellulose membranes. After being immersed with the blocking buffer (10 mmol/L Tris-HCl, pH 7.4, containing 0.5 mol/L NaCl, 5% skim milk, and 0.05% Tween 20), the membranes were incubated with rat anti-mouse MRP1 mAb at 4°C overnight. After the incubation with horseradish peroxidase-conjugated secondary antibody (Ab) for 1 hour at room temperature, the antigen-antibody complexes were detected by using an enhanced chemiluminescence-Western blotting detection system (Amersham Bioscience Japan, Tokyo, Japan) according to the manufacturer’s instructions.
Western Blotting Analysis for TGF-β1, Phosphorylated Smad3, ATF3, and Nrf2
At the indicating time intervals after NaAs treatment, kidney tissues were homogenized with a lysis buffer (10 mmol/L PBS, pH 7.4, containing 0.01% Triton X-100, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) containing Complete protease inhibitor mixture, and phosphatase inhibitor cocktails for serine/threonine protein phosphatases and tyrosine protein phosphatases (P2850 and P5726, Sigma-Aldrich, Tokyo, Japan) and centrifuged to obtain lysates. The lysates were electrophoresed in a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred onto a nitrocellulose membrane. The membrane was then incubated with Abs to TGF-β1, phosphorylated Smad3, ATF3, or Nrf2 diluted at 1:1000. After the incubation of horseradish peroxidase-conjugated secondary Abs, the immune complexes were visualized as mentioned above. In some experiments, the cell lysates from kidney tissues were immunoprecipitated with anti-Nrf2 Ab. The resultant immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred onto nitrocellulose membranes. Thereafter, the membranes were incubated with anti-ATF3 Ab at 4°C overnight. After the incubation with secondary Ab, the antigen-antibody complexes were detected similarly as described above.
Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
A semiquantitative RT-PCR was conducted as described previously.22,23 Total RNAs were extracted from renal samples using Isogene (Nippon Gene, Toyama, Japan). Five μg of total RNA were reverse-transcribed to obtain cDNA, and the resultant cDNA was amplified together with Taq polymerase (Nippon Gene, Toyama, Japan) using specific sets of primers with an optimal number of cycles at 94°C for 1 minute, optimal annealing temperature for 1 minute, and 72°C for 1 minute, followed by incubation at 72°C for 3 minutes (Table 1). The PCR products were fractionated on a 2% agarose gel and visualized by ethidium bromide staining. The band intensities of ethidium bromide fluorescence were measured using NIH Image Analysis Software Version 1.61 (National Institutes of Health, Bethesda, MD). The relative intensities of the bands were determined, and the ratios to β-actin were calculated.
TABLE 1.
Sequences of the Primers Used for RT-PCR
| Transcript | Sequence | Annealing temperature (°C) | Cycles | Product size (bp) |
|---|---|---|---|---|
| IFN-γ | (F) 5′-ACTGAAGCCAGCTCTCTCTTCCTC-3′ | 60 | 36 | 274 |
| (R) 5′-TTCCTTCTTGGGGTCAGCACAGAC-3′ | ||||
| (F) 5′-CTTGTACCTTTACTTCACTG-3′* | 60 | 36 | 197 | |
| (R) 5′-AAGCACCAGGTGTCAAGTCT-3′† | ||||
| MRP1 | (F) 5′-GTTCCCTCCGCATGAACTTG-3′ | 55 | 30 | 550 |
| (R) 5′-CTGGCTCATGCCTGGACTCTG-3′ | ||||
| MDR1 | (F) 5′-CCCATCATTGCGATAGCTGG-3′ | 55 | 30 | 167 |
| (R) 5′-TTTCAAACTTCTGCTCCCGA-3′ | ||||
| MT-1 | (F) 5′-AGTGAGTTGGGACACCTTGG-3′ | 60 | 30 | 357 |
| (R) 5′-GCTGGGTTGGTCCGATACTA-3′ | ||||
| β-Actin | (F) 5′-TTCTACAATGAGCTGCGTGTGGC-3′ | 60 | 28 | 456 |
| (R) 5′-CTCATAGCTCTTCTCCAGGGAGGA-3′ |
(F), Forward primer; (R), reverse primer.
Primers were used for nested-PCR to prepare RNA probes for in situ hybridization.
In Situ Detection of IFN-γ mRNA in the Kidneys
In situ hybridization analyses were performed to detect IFN-γ mRNA in kidney, as described previously.25 RT-PCR product of IFN-γ was obtained using the pair of primers with the addition of T7- and Sp6-RNA polymerase promoter to the 5′ end of each sense and anti-sense primer of IFN-γ, respectively (Table 1). Digoxigenin-labeled sense and anti-sense probes were obtained by using DIG RNA labeling kit (Boehringer Mannheim Biochemica, Mannheim, Germany) according to the manufacturer’s instructions. The sense probe was used as a negative control. Deparaffinized sections were further fixed with 4% paraformaldehyde in PBS for 15 minutes and incubated with 10 μg/ml proteinase K in TE buffer (10 mmol/L Tris-HCl and 1 mmol/L ethylenediaminetetraacetic acid) at 37°C for 10 minutes. After washing with 5× standard saline citrate at room temperature for 15 minutes, the sections were prehybridized at 55°C for 1 hour with a buffer containing 50% deionized formamide, 5× standard saline citrate, and 40 μg/ml salmon sperm DNA. After the RNA probes were added to the prehybridization buffer to 400 ng/ml, the slides were incubated under a cover at 55°C for 16 hours in a moist chamber. After the section was incubated with anti-digoxigenin Abs for 16 hours, positive signals were visualized with a color-substrate solution containing nitro blue tetrazolium salt and 5-bromo-4-chloro-3-indolyl phosphate toluidinium salt.
Statistical Analysis
The means and SEMs were calculated for all parameters determined in this study. Statistical significance was evaluated using analysis of variance or Mann-Whitney’s U-test. P < 0.05 was accepted as statistically significant. The survival curve by the Kaplan-Meier procedure was analyzed by a log-rank test.
Results
Intrarenal IFN-γ Expression in WT Mice after NaAs Challenge
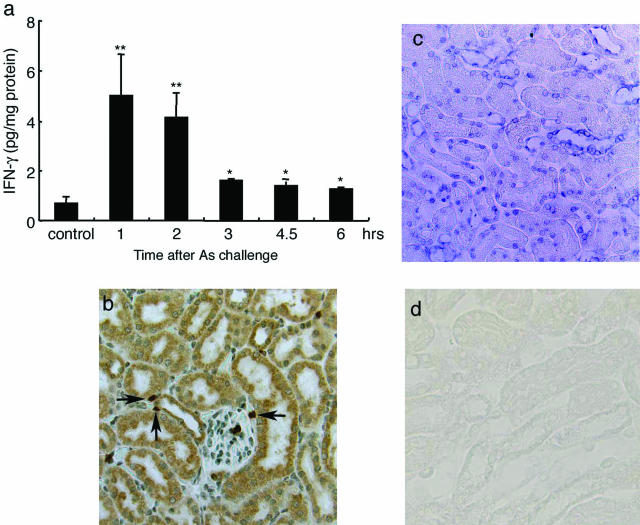
We previously observed that NaAs caused severe renal dysfunction.18 Because IFN-γ can regulate ABC transporter expression,19–21 we determined intrarenal IFN-γ contents after the subcutaneous administration of NaAs. NaAs challenge increased intrarenal IFN-γ contents remarkably even at 1 and 2 hours, declining thereafter (Figure 1a). Immunohistochemical analysis demonstrated that most tubular epithelial cells and some interstitial cells expressed IFN-γ protein even at 1 hour after NaAs challenge (Figure 1b). Consistently, in situ hybridization analyses detected IFN-γ mRNA exclusively in tubular epithelial cells at 1 hour after NaAs challenge (Figure 1c). Sense probes showed no positive reactions for IFN-γ mRNA, indicating the specificity of the reaction (Figure 1d). These observations indicated that IFN-γ might be locally produced by tubular epithelial cells, the main target cells in NaAs-induced renal injury.
FIGURE 1.
Analysis on IFN-γ expression in the kidney of WT mice. a: Intrarenal IFN-γ contents were determined at the indicated time intervals after NaAs challenge as described in Materials and Methods. All values represent means ± SEM (n = 6 animals). **P < 0.01, *P < 0.05; NaAs-treated WT mice versus control mice. b: Immunohistochemical detection of IFN-γ in the kidneys of WT mice after NaAs challenge. Representative results from six individual animals are shown here. At 1 hour after NaAs challenge, IFN-γ protein was immunohistochemically detected in most renal tubular cells and some interstitial cells (arrows). c: In situ hybridization analysis to detect IFN-γ mRNA in the kidneys of WT mice at 1 hour after NaAs challenge. In situ hybridization analysis was performed using either anti-sense (c) or sense probe (d) as described in Materials and Methods. Representative results from six independent experiments are shown here. Original magnifications, ×200.
Exaggerated NaAs-Induced Renal Injury in the Absence of IFN-γ
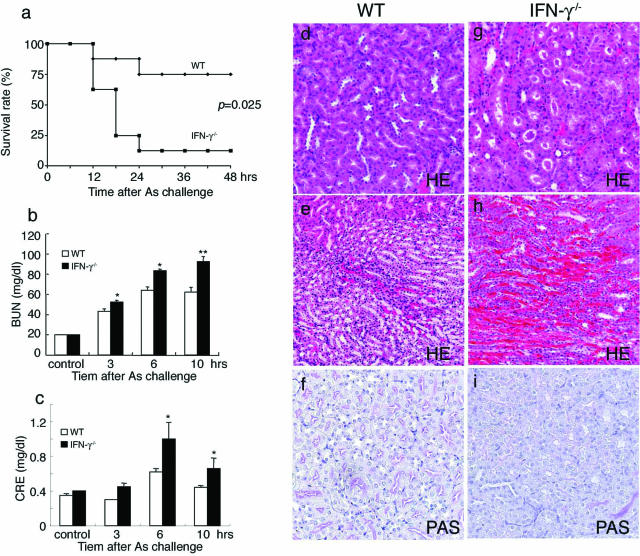
Acute exposure to arsenic can cause profound injury to kidney.2,3 Thus, enhanced intrarenal expression of IFN-γ prompted us to examine its role in NaAs-induced renal injury. On subcutaneous administration of NaAs into WT and IFN-γ−/− mice, both strains started to die later than 10 hours after the injection. However, IFN-γ−/− mice exhibited a markedly higher mortality rate (14 deaths/16 mice) than WT mice (two deaths/16 mice) within 24 hours after NaAs injection (Figure 2a). There was no significant difference in serum BUN and CRE levels between untreated WT and IFN-γ−/− mice (Figure 2, b and c). Serum BUN and CRE levels were increased after NaAs treatment in both strains, but the increments were greater in IFN-γ−/− than WT mice. Moreover, IFN-γ−/− mice exhibited more severe pathological changes in terms of hemorrhages, acute tubular necrosis, cast formation, and disappearance of PAS-positive brush borders, than WT mice (Figure 2, d–i). Although NaAs can cause damages to other organs such as liver, intestines, and brain,2,3 we failed to detect apparent morphological differences in these organs between WT and IFN-γ−/− mice at 24 hours after NaAs injection (data not shown). These observations implied that IFN-γ has a protective role in NaAs-induced renal injury.
FIGURE 2.
Analysis of NaAs-induced renal injury in WT and IFN-γ−/− mice. a: Survival rates of WT (n = 16 animals) and IFN-γ−/− mice (n = 16 animals) after administration of NaAs (12.5 mg/kg). b and c: Determination of serum BUN (b) and serum CRE (c) levels in WT (open bars) and IFN-γ−/− mice (closed bars) at indicated time intervals after NaAs challenge. All values represent means ± SEM (n = 15). *P < 0.05, **P < 0.01 WT versus IFN-γ−/− mice. d–i: Histopathological observations of the kidneys from WT (d–f) and IFN-γ−/− mice (g–i). Representative results from six individual animals are shown here. The specimens were obtained from mice at 10 hours after NaAs challenge and were stained with H&E (d, e, g, h) or PAS (f, i). In IFN-γ−/− mice, massive tubular necrosis with cast formation and severe hemorrhages (g and h), and disappearance of PAS-positive brush borders (i) were observed. In contrast, the histopathological changes were less evident in WT mice, compared with IFN-γ−/− mice (d, e), and PAS-positive brush borders were retained in WT mice (f).
Different Intrarenal Arsenic Contents between WT and IFN-γ−/− Mice
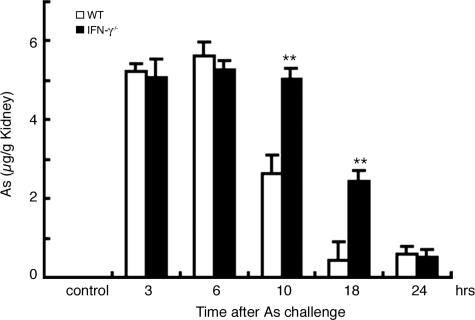
Because differences in intrarenal arsenic contents can result in different sensitivities to NaAs-induced renal injury,18 we measured intrarenal arsenic contents in WT and IFN-γ−/− mice after NaAs challenge. Before NaAs injection, arsenic was barely detected in kidney of both WT and IFN-γ−/− mice (Figure 3). NaAs administration caused a rapid and massive increase in intrarenal arsenic contents, to similar extents in WT and IFN-γ−/− mice until 6 hours after the treatment (Figure 3). WT mice exhibited a progressive decrease thereafter, but the decrease was delayed in IFN-γ−/− mice until 10 hours after the injection (Figure 3), when most IFN-γ−/− mice succumbed. These observations imply that the lack of IFN-γ would delay arsenite efflux from the kidney, resulting in enhanced sensitivities to NaAs-mediated nephrotoxicity.
FIGURE 3.
Arsenic contents in the kidney after NaAs challenge. Arsenic contents in the kidneys from WT (open bars) and IFN-γ−/− (filled bars) mice were analyzed by atomic absorption spectrometry at 3, 6, 10, 18, and 24 hours after NaAs challenge (12.5 mg/kg) and expressed as the arsenic amount (μg) divided by the sample weight (g). All values represent means ± SEM (n = 6 animals). **P < 0.01, WT versus IFN-γ−/− mice.
Expression of MRP1, Metallothionein-1 (MT-1), and MDR1 in the Kidneys after NaAs Challenge
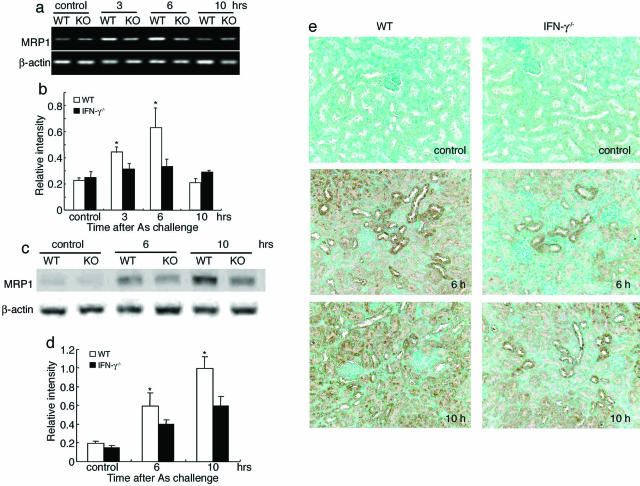
We next examined intrarenal expression levels of MRP1, which is essentially involved in the efflux of arsenic from the kidneys.18 Under the used experimental conditions, MRP1 mRNA was faintly detected in the kidneys without any significant differences between WT and IFN-γ−/− mice (Figure 4, a and b). WT but not IFN-γ−/− mice exhibited an enhancement in intrarenal MRP1 gene expression significantly 3 and 6 hours after NaAs treatment (Figure 4, a and b). Consistent with RT-PCR analysis, Western blotting analysis demonstrated that MRP1 protein levels were significantly higher in the kidneys of WT mice after NaAs treatment, than IFN-γ−/− mice (Figure 4, c and d). An immunohistochemical analysis demonstrated that MRP1 protein was localized in the renal tubular cells and that its expression levels were higher in WT than those of IFN-γ−/− mice until 10 hours after NaAs treatment (Figure 4e). Although MDR1 and metallothionein-1 (MT-1) can contribute to the efflux or detoxification of arsenite,3,26 there was no significant difference in the gene expression of MDR1 and MT-1 between IFN-γ−/− and WT mice even after NaAs injection (data not shown). These observations suggest that the absence of IFN-γ may counteract MRP1 expression induced by NaAs treatment.
FIGURE 4.
a and b: MRP1 mRNA expression in the kidneys of WT (open bars) and IFN-γ−/− mice (filled bars) were determined by RT-PCR at 3, 6, and 10 hours after NaAs challenge. Representative results from six independent experiments are shown in a. The ratios of MRP1 to β-actin were calculated and are shown in b (open bars, WT; filled bars, IFN-γ−/−). All values represent means ± SEM (n = 6 animals). *P < 0.05, WT versus IFN-γ−/− mice. c and d: MRP1 protein expression in kidneys of WT and IFN-γ−/− mice. Western blotting analysis was performed on the membrane-rich fraction obtained from the kidney tissues as described in Materials and Methods. Under the used condition, MRP1 protein was faintly detected in the kidneys of untreated WT and IFN-γ−/− mice. Western blotting analysis using anti-β-actin antibody confirmed that an equal amount of protein was loaded onto each lane. Representative results from six individual animals in each group are shown in c. The ratios of MRP1 protein to β-actin protein were calculated and are shown in d (open bars, WT; filled bars, IFN-γ−/−). All values represent means ± SEM (n = 6 animals). *P < 0.05, WT versus IFN-γ−/− mice. e: Immunohistochemical detection of MRP1 protein in the kidneys before, at 6 hours, or 10 hours after NaAs challenge. Representative results from six individual animals in each group are shown here. Original magnifications, ×200.
The Effects of IFN-γ Deficiency on the TGF-β-Mediated Signaling Pathway
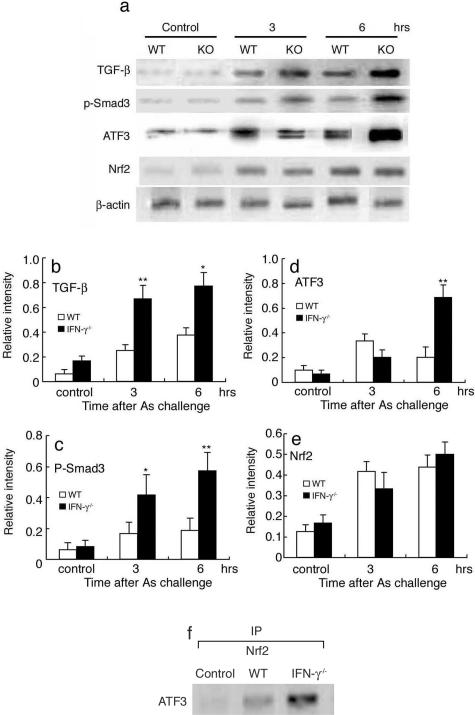
Several lines of evidence demonstrated that Smad3-ATF3 can suppress the Nrf2-mediated gene expression, by association with Nrf2, an essential transcription factor for MRP1 gene expression.27,28 Moreover, several independent groups including ours demonstrated that IFN-γ can inhibit TGF-β/Smad signaling pathway.29,30 These observations suggest that a crosstalk between IFN-γ and TGF-β/Smad signaling pathways can modulate the expression and activity of Nrf2, thereby regulating MRP1 expression. Hence, we examined these molecules in NaAs-treated kidneys (Figure 5, a–e). Intrarenal TGF-β protein contents were increased after NaAs treatment, and the increment was greater in IFN-γ−/− mice than in WT mice, as we previously observed on skin wound sites in IFN-γ−/− mice.30 Concomitantly, phosphorylated Smad3 and ATF3 contents were increased in IFN-γ−/− and WT mice after NaAs treatment, and the gain was more evident in IFN-γ−/− than WT mice. In contrast, NaAs challenge increased the amounts of Nrf2 protein to similar extents in the kidneys of WT and IFN-γ−/− mice. Moreover, immunoprecipitation analyses demonstrated that the absence of IFN-γ increased the amount of ATF3 protein associated with Nrf2 protein (Figure 5f), which represents an inactive form of Nrf2. These observations implied that the lack of IFN-γ augmented the TGF-β signal pathway and eventually enhanced the expression of ATF3, a potent repressor of Nrf2, and the association of these two proteins, thereby repressing Nrf2, an essential transcription factor for MRP1 gene expression.27,28
FIGURE 5.
a–e: Western blotting analysis to detect TGF-β, phosphorylated Smad3, ATF3, and Nrf2 proteins in the kidney. Under the conditions used, these molecules were detected faintly but to a similar extent, in the kidneys of untreated WT and IFN-γ −/− mice. Western blotting analysis using anti-β-actin antibody confirmed that an equal amount of protein was loaded onto each lane. Representative results from six individual animals in each group are shown in a. The ratios of each molecule to β-actin were calculated and are shown in b to e (open bars, WT; filled bars, IFN-γ−/−). All values represent means ± SEM (n = 6 animals). **P < 0.01; *P < 0.05, WT versus IFN-γ−/− mice. f: The association of ATF3 protein with Nrf2 proteins. Cell extracts of the kidneys were immunoprecipitated with anti-Nrf2 antibody, followed by Western blotting analysis using anti-ATF3 antibody. Representative results from six individual animals are shown here (6 hours after NaAs challenge).
Induction of MRP1 Expression by NaAs and IFN-γ in Renal Tubular Cells
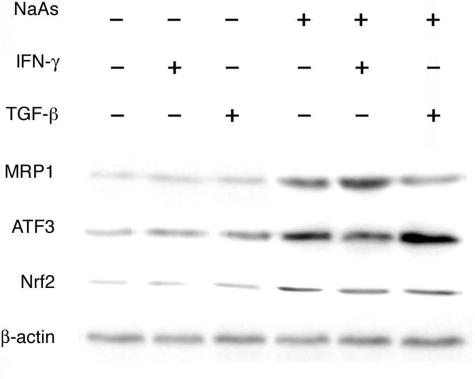
To clarify more in detail whether IFN-γ can regulate intrarenal MRP1 expression through TGF-β/Smad3/ATF3 signal pathway, we examined the effects of NaAs, IFN-γ, and/or TGF-β on MRP1, ATF3, and Nrf2 expression in a renal tubular cell line, mProx24 cells.24 In untreated mProx 24 cells, MRP1 protein was faintly detected (Figure 6). NaAs enhanced MRP1 and Nrf2 as well as ATF3 expression in mProx24 cells. On the contrary, IFN-γ or TGF-β alone had little effect on MRP1, Nrf2, and ATF3 expression. IFN-γ augmented NaAs-induced MRP1 expression with a concomitant reduction in ATF3 expression, whereas TGF-β attenuated NaAs-induced MRP1 expression with a concomitant enhancement in ATF3 expression (Figure 6). However, neither IFN-γ nor TGF-β further augmented NaAs-induced Nrf2 expression. Another anti-inflammatory cytokine, interleukin (IL)-10, had no effect on MRP1 expression (data not shown). Thus, these observations further support our hypothesis that IFN-γ regulates MRP1 expression through TGF-β/Smad3/ATF3 signal pathway.
FIGURE 6.
Effects of the various combinations of NaAs, IFN-γ, and TGF-β on MRP1, ATF3, and Nrf2 protein expression in mProx24 cells. The membrane-rich fractions were obtained from mProx24 cells at 6 hours after the treatment. Western blotting analysis was performed as described in Materials and Methods. Western blotting analysis using anti-β-actin antibody confirmed that an equal amount of protein was loaded onto each lane. Representative results from six independent experiments are shown here.
The Efficacy of Anti-IFN-γ Antibody or Exogenous IFN-γ on NaAs-Induced Renal Injury
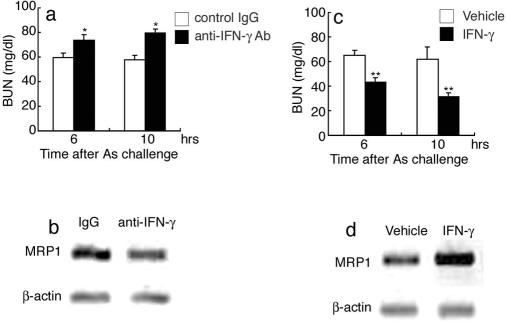
We examined the efficacy of anti-IFN-γ antibody or exogenous IFN-γ on NaAs-induced renal injury. Anti-IFN-γ antibody aggravated renal injury as judged by increased serum BUN levels, together with attenuated intrarenal MRP1 protein expression (Figure 7, a and b). Concomitantly, anti-IFN-γ treatment increased the mortality within 24 hours after NaAs challenge (anti-IFN-γ antibody, six deaths/10 mice; control IgG, two deaths/10 mice). On the contrary, when administered with IFN-γ at 1 hour before NaAs challenge, all WT mice survived (IFN-γ, no deaths/eight mice; vehicle, two deaths/eight mice). Moreover, IFN-γ treatment reduced the elevation of serum BUN levels with an enhanced MRP1 expression in WT mice, compared with vehicle-treated WT mice (Figure 7, c and d). In contrast, when WT mice were administered with IFN-γ at 30 minutes after NaAs challenge, IFN-γ enhanced MRP1 expression but marginally attenuated NaAs-induced increases in serum BUN levels (vehicle versus posttreatment of IFN-γ, 63.7 ± 2.8 versus 56.7 ± 2.6 at 6 hours; 62.1 ± 3.1 versus 50.1 ± 4.3 at 10 hours) and did not improve the associated mortality (IFN-γ, one death/eight mice; vehicle, two deaths/eight mice). These observations may be explained by an assumption that IFN-γ can augment the MRP1 levels sufficiently to prevent an increase in intrarenal arsenite concentration, only with a time lag.
FIGURE 7.
The effects of anti-IFN-γ antibody (a, b) or exogenous IFN-γ (c, d) on serum BUN levels and intrarenal MRP1 expression. WT mice were treated with anti-IFN-γ antibody or recombinant IFN-γ, as described in Materials and Methods. a and c: Serum BUN levels were determined at 6 and 10 hours after NaAs challenge. All values represent means ± SEM (n = 8). *P < 0.05, control IgG versus anti-IFN-γ antibody, **P < 0.01; vehicle versus recombinant IFN-γ. b and d: Intrarenal MRP1 expression was examined at 10 hours after NaAs challenge, as described in Materials and Methods. Western blotting analysis using anti-β-actin antibody confirmed that an equal amount of protein was loaded onto each lane. Representative results from six independent experiments are shown here.
Discussion
Accumulating evidence demonstrated that renal tubular epithelial cells also produced IFN-γ in several types of renal injuries.31,32 In line with these previous reports, immunohistochemical and in situ hybridization analyses detected IFN-γ in renal tubular and interstitial cells in WT mice treated with NaAs. On NaAs treatment, IFN-γ−/− mice exhibited exaggerated renal dysfunction, compared with WT mice, suggesting the protective roles of IFN-γ against NaAs-induced renal injury. This notion is further supported by the observation that pretreatment of anti-IFN-γ antibody and that of IFN-γ aggravated and reduced NaAs-induced renal injury as judged by serum BUN levels, respectively.
Intrarenal arsenic contents are a major factor to determine the sensitivities to NaAs-induced renal injury.18 The return of intrarenal arsenic contents to basal levels was impeded in IFN-γ−/− mice, suggesting the delayed efflux of arsenic in these mice. Active extrusion executed by ABC transporter proteins, has major roles in arsenic detoxification, among several detoxifying systems.33 Among ABC transporter proteins, P-glycoprotein encoded by MDR1 gene and MRP1 are presumed to be involved in arsenic transport in mammalian cells. Mice lacking both mdr1a and mdr1b showed an increased accumulation of arsenic in the tissues, resulting in higher susceptibility to arsenic toxicity.26 IFN-γ-induced MDR1 expression was documented but with controversy.19–21 Because NaAs injection augmented MDR1 gene expression to similar extents in both IFN-γ−/− and WT mice (data not shown), it is unlikely that MDR1 gene was responsible for different sensitivities to NaAs between these two strains.
Tumor cells overexpressing MRP1 were resistant to arsenical salts, and its inhibitor, MK-571,34 can in vitro block the arsenic efflux.35 Moreover, we previously provided definitive evidence that differences in NaAs-induced MRP1 expression levels were responsible for different sensitivities to NaAs-induced renal injury between C57BL/6 and BALB/c mice.18 Furthermore, we observed here that the NaAs-induced increase in MRP-1 expression was abrogated by the absence of IFN-γ. These observations suggest that endogenous IFN-γ could regulate the expression of MRP1, a transporter essentially involved in arsenic extrusion. This notion was supported by the observation that IFN-γ could enhance the expression of MRP1 in a renal tubular cell line, mProx24, in the presence of arsenic. Given that both IFN-γ and MRP1 were mainly expressed by tubular cells, IFN-γ may act to enhance MRP1 expression in an autocrine and/or paracrine manner.
Kala and colleagues36 demonstrated that mice deficient in MRP1 exhibited urinary arsenic levels similar to WT mice, when arsenite was subcutaneously injected up to 5 mg/kg. Moreover, there was no significant difference in the intrarenal arsenic contents between MRP1−/− and WT mice after intraperitoneal injection of 1 mg/kg NaAs.37 In these experiments, they administered much lower doses than ours. Thus, the discrepancies can be explained by the assumption that MRP1 can extrude arsenic mainly at relatively higher doses and that other molecule(s) such as MDR1 may extrude arsenic at lower doses.
In addition to ABC transporter proteins, the low-molecular weight, cysteine-rich metal-binding protein metallothionein is presumed to be involved in detoxification of heavy metals including arsenic. The absence of metallothionein exaggerated hepatic and renal injury caused by chronic exposure to arsenic.3 However, we could not detect a significant difference in intrarenal metallothionein gene expression during the course of NaAs-induced renal injury between WT and IFN-γ−/− mice. Thus, metallothionein levels alone cannot explain the different sensitivities.
Of interest is that arsenic augmented intrarenal MRP1 expression at transcriptional levels and that IFN-γ further enhanced its expression. The promoter region of the human MRP1 gene contains two potential IFN-γ activation site elements, which are presumed to be involved in IFN-γ-mediated transcription of the target genes.38 However, IFN-γ alone failed to enhance MRP1 gene expression in vitro in mProx24 cells. Thus, it is unlikely that IFN-γ can directly enhance intrarenal MRP1 gene expression. Evidence is accumulating to indicate that a transcription factor, Nrf2, can regulate MRP1 gene expression.27 The activities of Nrf2 are under the control of various signal pathways.28 ATF3 is a member of the activating transcription factor/cAMP-responsive element binding protein of transcription factors,39 which is up-regulated by TGF-β-Smad3 signal pathway. Bakin and colleagues28 demonstrated that TGF-β/Smad3-induced ATF3 represses the Nrf2-mediated gene expression, by association with Nrf2. Moreover, several independent groups including us have revealed that IFN-γ can negatively affect the TGF-β1 signaling pathway.29,30 Thus, we examined TGF-β1/Smad3/ATF3 in kidney of IFN-γ−/− mice after NaAs treatment. As we previously observed on skin wound sites of IFN-γ−/− mice,30 the amounts of TGF-β1 and phosphorylated Smad3 were significantly elevated in the kidney of IFN-γ−/− mice, compared with WT mice. Enhanced TGF-β1/Smad3 signaling pathway has resulted in enhanced expression of ATF3 but not Nrf2 protein level, which may eventually depress MRP1 expression in IFN-γ−/− mice. Supporting this notion, in vitro, IFN-γ enhanced NaAs-induced MRP1 expression with a concomitant reduction in ATF3 expression, whereas TGF-β reduced NaAs-induced MRP1 expression with a concomitant increase in ATF3 expression. Moreover, both IFN-γ and TGF-β had little effects on Nrf2 expression. Thus, IFN-γ can enhance MRP1 expression mainly by counteracting the negative effects of TGF-β/Smad3/ATF3 signaling pathway.
Nrf2 can also regulate the expression of genes encoding phase II detoxifying proteins in addition to MRP1. Conjugation of arsenic with glutathione (GSH) is essential for the cellular extrusion by ABC transporter such as MRP1. The synthesis of GSH is a rate-limiting step regulated by γ-glutamylcysteinyl synthase (γ-GCS), a member of phase II detoxifying proteins. Nrf2 is an essential transcription factor for γ-GCS gene expression as evidenced by impaired γ-GCS expression in mice lacking Nrf2.40,41 However, we could not detect a significant difference in intrarenal γ-GCS gene expression between IFN-γ−/− and WT mice (unpublished data). Thus, IFN-γ may have negligible effects on GSH synthesis and subsequent arsenic conjugation by GSH in NaAs-induced renal injury model.
Acute exposure to arsenic sometimes causes severe damage to organs and with a high mortality. Our present observations on IFN-γ−/− mice suggest that IFN-γ can enhance arsenic excretion into urine by augmenting the expression of an essential transporter, MRP1. This assumption was supported by the observation that the pretreatment with recombinant IFN-γ or anti-IFN-γ antibody could modulate intrarenal MRP1 expression. Therefore, IFN-γ may be useful to prevent acute arsenic intoxication. In contrast, posttreatment with IFN-γ had marginal effects on NaAs-induced renal injury, suggesting that IFN-γ may increase MRP1 levels sufficient to prevent NaAs-induced intrarenal injury, only with a time lag.
Acknowledgments
We thank Dr. Yoichiro Iwakura (University of Tokyo) for providing us with IFN-γ−/− mice.
Footnotes
Address reprint requests to Toshikazu Kondo, M.D., Ph.D., Department of Forensic Medicine, Wakayama Medical University, 811-1 Kimiidera, Wakayama 641-8509, Japan. E-mail: kondot@wakayama-med.ac.jp.
Supported in part by grants-in-aid from the Ministry of Education, Culture, Science, and Technology of the Japanese Government.
References
- Goering PL, Aposhian HV, Mass MJ, Cebrian M, Beck BD, Waalkes MP. The enigma of arsenic carcinogenesis: role of metabolism. Toxicol Sci. 1999;49:5–14. doi: 10.1093/toxsci/49.1.5. [DOI] [PubMed] [Google Scholar]
- Klaassen CD. Heavy metals and heavy-metal antagonist. Hardman JG, Gilman AG, Limbird LE, editors. New York: McGraw-Hill,; Goodman and Gilman’s The Pharmacological Basis of Therapeutics, (ed 9) 1996:pp 1649–1672. [Google Scholar]
- Liu J, Liu YP, Goyer RA, Chanzar W, Waalkes MP. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of oral or injected inorganic arsenicals. Toxicol Sci. 2000;55:460–467. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- Snow E. Metal carcinogenesis: mechanistic implications. Pharmacol Ther. 1992;53:31–65. doi: 10.1016/0163-7258(92)90043-y. [DOI] [PubMed] [Google Scholar]
- Thompson DJ. A chemical hypothesis for arsenic methylation in mammals. Chem Biol Interact. 1993;88:89–114. doi: 10.1016/0009-2797(93)90086-e. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, Han ZG, Ni JH, Shi GY, Jia PM, Liu MM, He KL, Niu C, Ma J, Zhang TD, Paul P, Naoe T, Kitamura K, Miller W, Waxman S, Wang ZY, de Chen HSJ, Chen Z. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukaemia (APL): As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM, Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, Chen Y, Zhou L, Fang ZW, Wang YT, Ma J, Zhang P, Zhang TD, Chen SJ, Chen Z, Wang ZY. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukaemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- Soignet SL, Maslak PZ, Wang G, Jhanwar S, Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J, Scheinberg DA, Pandolfi PP, Warrell RP., Jr Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- Maeda H, Hori S, Nishitoh H, Ichijo H, Ogawa O, Kakehi Y, Kakizuka A. Tumor growth inhibition by arsenic trioxide (As2O3) in the orthotopic metastasis model of androgen-independent prostate cancer. Cancer Res. 2001;61:5432–5440. [PubMed] [Google Scholar]
- Zaman GJ, Flens MJ, van Leusden MR, de Haas M, Mulder HS, Lankelma HJ, Pinedo HM, Scheper RJ, Baas F, Broxterman HJ, Brost P. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci USA. 1994;91:8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P, Schinkel AH, Smit JJ, Wagenaar E, Van Deemter L, Smith AJ, Eijdems EW, Baas F, Zaman GJ. Classical and novel forms of multidrug resistance and the physiological functions of P-glycoproteins in mammals. Pharmacol Ther. 1993;60:289–299. doi: 10.1016/0163-7258(93)90011-2. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Liu J, Chen H, Miller DS, Saavedra JE, Keefer L, Johnson DR, Klaassen CD, Waalkes MP. Overexpression of glutathione S-transferase II and multidrug resistance transport proteins is associated with acquired tolerance to inorganic arsenic. Mol Pharmacol. 2001;60:302–309. doi: 10.1124/mol.60.2.302. [DOI] [PubMed] [Google Scholar]
- Vernhet L, Allain N, Bardiau C, Anger JP, Fardel O. Differential sensitivities of MRP1-overexpressing lung tumor cells to cytotoxic metals. Toxicology. 2000;142:127–134. doi: 10.1016/s0300-483x(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Chen ZS, Mutoh M, Sumizawa T, Furukawa T, Haraguchi M, Tani A, Saijo N, Kondo T, Akiyama S. An active efflux system for heavy metals in cisplatin-resistant human KB carcinoma cells. Exp Cell Res. 1998;240:312–320. doi: 10.1006/excr.1998.3938. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Li ZS, Lu YP, Rea PA. The GS-X pump in plant, yeast, and animal cells: structure, function, and gene expression. Biosci Rep. 1997;17:189–207. doi: 10.1023/a:1027385513483. [DOI] [PubMed] [Google Scholar]
- McCarver DG, Hines RN. The ontogeny of human drug-metabolizing enzymes: phase II conjugation enzymes and regulatory mechanisms. J Pharmacol Exp Ther. 2002;300:361–366. doi: 10.1124/jpet.300.2.361. [DOI] [PubMed] [Google Scholar]
- Kimura A, Ishida Y, Wada T, Yokoyama H, Mukaida N, Kondo T. MRP-1 expression levels determine strain-specific susceptibility to sodium arsenic-induced renal injury between C57BL/6 and BALB/c mice. Toxicol Appl Pharmacol. 2005;203:53–61. doi: 10.1016/j.taap.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Dixit SG, Zingarelli B, Buckley DJ, Buckley AR, Pauletti GM. Nitric oxide mediates increased P-glycoprotein activity in interferon-γ stimulated human intestinal cells. Am J Physiol. 2005;288:G533–G540. doi: 10.1152/ajpgi.00248.2004. [DOI] [PubMed] [Google Scholar]
- Belliard AM, Lacour B, Farinotti R, Leroy C. Effect of tumor necrosis factor-alpha and interferon-gamma on intestinal P-glycoprotein expression, activity, and localization in Caco-2 cells. J Pharm Sci. 2004;93:1524–1536. doi: 10.1002/jps.20072. [DOI] [PubMed] [Google Scholar]
- Kawaguchi H, Matsui Y, Watanabe Y, Takakura Y. Effect of interferon-gamma on the pharmacokinetics of digoxin, a P-glycoprotein substrate, intravenously injected into the mouse. J Pharmacol Exp Ther. 2004;308:91–96. doi: 10.1124/jpet.103.057521. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Maegawa T, Kondo T, Kimura A, Iwakura Y, Nakamura S, Mukaida N. Essential involvement of IFN-γ in Clostridium difficile toxin A-induced enteritis. J Immunol. 2004;172:3018–3025. doi: 10.4049/jimmunol.172.5.3018. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Ohshima T, Fujiwara H, Iwakura Y, Mukaida N. A pivotal involvement of IFN-γ in the pathogenesis of acetaminophen-induced acute liver injury. FASEB J. 2002;16:1227–1236. doi: 10.1096/fj.02-0046com. [DOI] [PubMed] [Google Scholar]
- Takaya K, Koya D, Isono M, Sugimoto T, Sugaya T, Kashiwagi A, Haneda M. Involvement of ERK pathway in albumin-induced MCP-1 expression in mouse proximal tubular cells. Am J Physiol. 2003;284:F1037–F1045. doi: 10.1152/ajprenal.00230.2002. [DOI] [PubMed] [Google Scholar]
- Sato Y, Ohshima T, Kondo T. Regulatory role of endogenous interleukin-10 in cutaneous inflammatory response of murine wound healing. Biochem Biophys Res Commun. 1999;265:194–199. doi: 10.1006/bbrc.1999.1455. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Powell DA, Waalkes MP, Klaassen CD. Multidrug-resistance mdr1a/1b double knockout mice are more sensitive than wild type mice to acute arsenic toxicity, with higher arsenic accumulation in tissues. Toxicology. 2002;170:55–62. doi: 10.1016/s0300-483x(01)00532-7. [DOI] [PubMed] [Google Scholar]
- Hayashi A, Suzuki H, Itoh K, Yamamoto M, Sugiyama Y. Transcription factor Nrf2 is required for the constitutive and inducible expression of multidrug resistance-associated protein 1 in mouse embryo fibroblasts. Biochem Biophys Res Commun. 2003;310:824–829. doi: 10.1016/j.bbrc.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Bakin AV, Stourman NV, Sekhar KR, Rinehart C, Yan X, Meredith MJ, Arteaga CL, Freeman ML. Smad3-ATF3 signaling mediates TGF-β suppression of genes encoding phase II detoxifying proteins. Free Radic Biol Med. 2005;38:375–387. doi: 10.1016/j.freeradbiomed.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-β/SMAD signalling by the interferon-γ/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-γ and TGF-β in the skin wound-healing process. J Immunol. 2004;172:1848–1855. doi: 10.4049/jimmunol.172.3.1848. [DOI] [PubMed] [Google Scholar]
- Kikawada E, Lenda DM, Kelley VR. IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-γ expression, and diminishes systemic pathology. J Immunol. 2003;170:3915–3925. doi: 10.4049/jimmunol.170.7.3915. [DOI] [PubMed] [Google Scholar]
- Timoshanko JR, Holdsworth SR, Kitching AR, Tipping PG. IFN-γ production by intrinsic renal cells and bone marrow-derived cells is required for full expression of crescentic glomerulonephritis in mice. J Immunol. 2002;168:4135–4141. doi: 10.4049/jimmunol.168.8.4135. [DOI] [PubMed] [Google Scholar]
- Rosen BP. Biochemistry of arsenic detoxification. FEBS Lett. 2002;529:86–92. doi: 10.1016/s0014-5793(02)03186-1. [DOI] [PubMed] [Google Scholar]
- Jones TR, Zamboni R, Belley M, Champion E, Charette L, Ford-Hutchinson AW, Frenette R, Gauthier JY, Leger S, Masson P, Macfarlane CS, Piechuta H, Rokach J, Williams H, Young NR. Pharmacology of L-660,711 (MK-571): a novel potent and selective leukotriene D4 receptor antagonist. Can J Physiol Pharmacol. 1989;67:17–28. doi: 10.1139/y89-004. [DOI] [PubMed] [Google Scholar]
- Vernhet L, Allain N, Payen L, Anger JP, Guillouzo A, Fardel O. Resistance of human multidrug resistance-associated protein 1-overexpressing lung tumor cells to the anticancer drug arsenic trioxide. Biochem Pharmacol. 2001;61:1387–1391. doi: 10.1016/s0006-2952(01)00606-2. [DOI] [PubMed] [Google Scholar]
- Kala SV, Kala G, Prater CI, Sartorelli AC, Lieberman MW. Formation and urinary excretion of arsenic triglutathione and methylarsenic diglutathione. Chem Res Toxicol. 2004;17:243–249. doi: 10.1021/tx0342060. [DOI] [PubMed] [Google Scholar]
- Lorico A, Bertola A, Baum C, Fodstad O, Rappa G. Role of the multidrug resistance protein 1 in protection from heavy metal oxyanions: investigations in vitro and in MRP1-deficient mice. Biochem Biophys Res Commun. 2002;291:617–622. doi: 10.1006/bbrc.2002.6489. [DOI] [PubMed] [Google Scholar]
- Decker T, Kovarik P, Meinke A. GAS elements: a few nucleotides with a major impact on cytokine-induced gene expression. J Interferon Cytokine Res. 1997;17:121–134. doi: 10.1089/jir.1997.17.121. [DOI] [PubMed] [Google Scholar]
- Chen BP, Liang G, Whelan J, Hai T. ATF3 and ATF3 δ Zip. Transcriptional repression versus activation by alternatively spliced isoforms. J Biol Chem. 1994;269:15819–15826. [PubMed] [Google Scholar]
- Chan JY, Kwong M. Impaired expression of glutathione synthetic enzyme genes in mice with targeted deletion of the Nrf2 basic-leucine zipper protein. Biochim Biophys Acta. 2000;1517:19–26. doi: 10.1016/s0167-4781(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Chanas SA, Jiang Q, McMahon M, McWalter GK, McLellan LI, Elcombe CR, Henderson CJ, Wolf CR, Moffat GJ, Itoh K, Yamamoto M, Hayes JD. Loss of the Nrf2 transcription factor causes a marked reduction in constitutive and inducible expression of the glutathione S-transferase Gsta1, Gsta2, Gstm1, Gstm2, Gstm3 and Gstm4 genes in the livers of male and female mice. Biochem J. 2002;365:405–416. doi: 10.1042/BJ20020320. [DOI] [PMC free article] [PubMed] [Google Scholar]