Abstract
Oxidative and carbonyl stress leads to generation of Nε-carboxymethyllysine-modified proteins (CML-mps), which are known to bind the receptor for advanced glycation end products (RAGE) and induce nuclear factor (NF)-κB-dependent proinflammatory gene expression. To determine the impact of CML-mps in vivo, RAGE-dependent sustained NF-κB activation was studied in resection gut specimens from patients with inflammatory bowel disease. Inflamed gut biopsy tissue demonstrated a significant up-regulation of RAGE and increased NF-κB activation. Protein extracts from the inflamed zones, but not from noninflamed resection borders, caused perpetuated NF-κB activation in cultured endothelial cells, which was mediated by CML-mps including CML-modified S100 proteins. The resulting NF-κB activation, lasting 5 days, was primarily inhibited by either depletion of CML-mps or by the addition of sRAGE, p44/42 and p38 MAPKinase-specific inhibitors. Consistently, CML-mps isolated from inflamed gut areas and rectally applied into mice caused NF-κB activation, increased proinflammatory gene expression, and histologically detectable inflammation in wild-type mice, but not in RAGE−/− mice. A comparable up-regulation of NF-κB and inflammation on rectal application of CML-mps was observed in IL-10−/− mice. Thus, CML-mps generated in inflammatory lesions have the capacity to elicit a RAGE-dependent intestinal inflammatory response.
Inflammatory bowel diseases (IBD) such as Crohn’s disease (CD) and ulcerative colitis (UC) provide relevant model systems to study chronic inflammation mediated at least in part by sustained activation of the proinflammatory transcription factor nuclear factor (NF)-κB.1–6 Because of the strong positive correlation between NF-κB activation in gut tissue and the clinical course of CD,4–6 NF-κB is regarded as one of the main targets of anti-inflammatory therapies in human chronic active IBD.7
A critical question, however, concerns mechanisms capable of converting a pulse of proinflammatory cytokines and the transitory burst of reactive oxygen species and arachidonic acid metabolites1–6,8,9 into a sustained inflammatory stimulus resulting in long-lasting NF-κB activation. Cytokines and cytokine-driven activation of NF-κB play a crucial role not only in initiation of inflammation10 but also in its termination.10 This implies the existence of additional pathways able to convert the short-lasting initiation of NF-κB activation by reactive oxygen species or cytokines into sustained inflammation. One mechanism that potentially transforms short-lasting reactive oxygen species into a sustained cellular response could be post-translational modifications of proteins, leading to slowly degradable ligands that might cause long-lasting cell activation. Post-translationally modified proteins such as carboxymethyllysine-modified proteins (CML-mps) are known to occur in settings of oxidative and carbonyl stress such as inflammatory lesions.11–13 This led to the hypothesis that not only de novo synthesis but also posttranslational modification might participate in the pathophysiology of inflammation.
The receptor for advanced glycation end products (RAGE) is a pattern-recognition receptor14,15 that binds a variety of ligands released on inflammation, such as members of the S100/calgranulin family, HMGB1, and advanced glycation end products (AGEs).16–23 CML-mps are one of the earliest and most abundant markers of AGE formation in vivo.12,13,24
Engagement of RAGE results in NF-κB activation. One unique feature of RAGE-mediated NF-κB activation is the prolonged time course that appears to overwhelm endogenous autoregulatory feedback inhibition loops and to induce perpetuated NF-κB activation.25 Because, in turn, RAGE expression is induced by NF-κB,26 sustained activation of NF-κB results in up-regulation of the receptor and further ensures maintenance and amplification of the signal. In the study presented here, we demonstrate for the first time that post-translationally modified proteins such as CML-mps, which also include S100A8, S100A9, and S100A8/9, are direct mediators of inflammation in predisposed tissue and are able to convert a short-lasting cytokine-driven initiation into a longer-lasting sustained inflammation.
Materials and Methods
Patients
Six patients with CD (matched for CDAI and characterized as previously described in detail6) and five patients with UC were included in the study; clinical characteristics are given in Table 1. Because the part of the inflamed intestine removed during surgery was different in different patients, resection border and inflamed area of the same patient were compared with each other throughout the study. All patients included were under medical treatment in the Department of Visceral Surgery in Bruchsal, Germany, and gave informed consent for study participation. The study was approved by the ethics committee of the University of Heidelberg according to the guidelines of the Helsinki Declaration.
TABLE 1.
Clinical Characteristics of the Patients Studied
| Crohn’s disease (CD) | Ulcerative colitis (UC) | |
|---|---|---|
| Sex (M/F) | 3/3 | 3/2 |
| Age | 34.2 ± 7.2 | 38.2 ± 4.2 |
| Duration of disease (years) | 8.5 ± 1.4 | 6.2 ± 2.8 |
| Hemoglobin (g/dl) | 12.4 ± 2.6 | 11.4 ± 2.4 |
| Leucocytes (×109/L) | 14.3 ± 2.3 | 13.7 ± 1.9 |
| Time of glucocorticoid therapy (months) | 3.4 ± 1.2 | 1.4 ± 0.8 |
| CDAI | 289 ± 35 | |
| Van Hees index | 190 ± 25 |
Reagents
[γ-32P]ATP (3000 Ci/mmol at 10 Ci/ml); Hybond-N nylon filter, enhanced chemiluminescence (ECL)-nitrocellulose membranes, ECL detection reagents, and Hyperfilm X-ray-films were obtained from Amersham, Braunschweig, Germany. Poly(dI/dC) was from Pharmacia, Freiburg, Germany. Monoclonal anti-NF-κBp65 antibodies, specific for activated NF-κBp65, were obtained from Boehringer Mannheim, Mannheim, Germany. Anti-p65-antibodies (no. sc-109) were obtained from Santa Cruz Inc., Heidelberg, Germany. Anti-CML antibodies were produced and characterized as described recently.13 Antibodies for S100A8/9, S100A8, S100A9, and S100A1 were purchased from BMA, Basel, Switzerland. PD 98059 and SB203580 were purchased from Sigma-Aldrich, Deisenhofen, Germany, and MG132 was from Biomol (Darmstadt, Germany).
CML-Enzyme-Linked Immunosorbent Assay (ELISA)
CML detection by ELISA (Roche Diagnostics, Penzberg, Germany) was performed according to the manufacturer’s instructions as recently described in detail.27
Preparation of Tissues and Immunohistochemistry
Preparation and immunohistochemistry of the gut samples were performed as previously described.6,28 Resection gut specimens of patients with CD and UC were taken during operation and snap-frozen in liquid nitrogen. Cryostat sections (5 μm) were mounted and fixed in 2% paraformaldehyde (Merck, Darmstadt, Germany). Acetone-treated slides were postfixed with 2% paraformaldehyde before this step. For detection of RAGE, sections were washed in Earl’s balanced salt solution (Gibco BRL, Eggenstein, Germany) plus 0.01 mol/L HEPES and 0.1% Saponin (Riedel deHaen, Seelze, Germany).28 Anti-human RAGE antibodies were diluted 1:50 and incubated overnight in a humidified chamber in the presence of 2.5 mg/ml human immunoglobulins (Gamma-venin, Marburg, Germany) to reduce nonspecific background.28 After washing in Earl’s balanced salt solution/HEPES/Saponin-buffer (2 × 3 minutes) and incubating with 5% inactivated goat serum for 20 minutes, the biotinylated secondary antibody (goat anti-rabbit, 1:20; Sigma, Deisenhofen, Germany) was added for 30 minutes, followed by a 30-minute incubation with streptavidin complexed with alkaline phosphatase (DAKO, Copenhagen, Denmark).29 After subsequent washes with Earl’s balanced salt solution/HEPES/Saponin (3 × 2 minutes) and a final wash in distilled water, the color reaction was developed using naphthol AS-bi phosphate and new fuchsin as chromogen. For detection of NF-κBp65, a monoclonal mouse antibody for activated NF-κBp65 (1:10 diluted in Tris-buffered saline, pH 7.4, containing 0.2% bovine serum albumin) was applied to the cryostat sections for 90 minutes at room temperature followed by washing in Tris-buffered saline/0.2% bovine serum albumin (2 × 3 minutes). Thereafter, a biotin-sheep anti-mouse antibody (1:100; Amersham, Freiburg, Germany) was added for 30 minutes, before detection was performed using the alkaline phosphatase anti-alkaline phosphatase immunostaining method as above. After counterstaining with hematoxylin, sections were mounted in glycerol/gelatin. The immunohistochemical results were evaluated according to a score depicted by Thiele and colleagues.6 In brief, the score refers to the staining result of mononuclear cells and endothelial cells. Zero means no positive cells and no staining of endothelia, whereas 8 means 81 to 100% positive cells with a strong staining, up to 81 to 100% of vessel circumference stained strongly positive.6 One investigator (K.T.) blinded to the clinical diagnosis analyzed coded slides. Expression of NF-κBp65 and RAGE was determined on consecutive serial sections in each case. In each slide, the number of positive mononuclear cells was determined in at least five visual fields (magnification, ×160) in five different representative areas of the lamina propria as well as in the submucosa. For determination of the endothelial expression, 40 vessels in the lamina propria and the submucosa were analyzed in each case. Median values of the respective results were obtained for statistical evaluation.
Preparation of Cell Extracts for Electrophoretic Mobility Shift Assays (EMSAs)
Frozen tissue was homogenized mechanically under liquid nitrogen using a mortar and pestle and transferred into ice-cold buffer A (10 mmol/L Hepes/KOH, pH 7.9, at 4°C, 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.5 mmol/L dithiothreitol, 0.2 mmol/L phenylmethyl sulfonyl fluoride, and 0.6% Nonidet-P40). Insoluble material was removed by centrifugation (30 seconds at 2000 rpm, 4°C), and the supernatant was incubated on ice for 10 minutes before being centrifuged for 5 minutes at 8000 rpm, 4°C. The supernatant was discarded, and the nuclear pellet was resuspended in 100 μl of buffer B (25% glycerol, 20 mmol/L HEPES/KOH, pH 7.9, at 4°C, 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L ethylenediaminetetraacetic acid, 0.5 mmol/L dithiothreitol, 0.2 mmol/L phenylmethyl sulfonyl fluoride, 2 mmol/L benzamidine, and 5 mg/ml leupeptin) and incubated on ice for 20 minutes. Cellular debris was removed by 2 minutes of centrifugation at 14,000 rpm, 4°C, and the supernatant was quick-frozen at −80°C.6,25
EMSAs
Nuclear proteins were harvested, and 10 μg of nuclear proteins were assayed for NF-κB binding activity using radioactive labeled oligonucleotides for the defined NF-κB consensus sequence (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) at ∼50,000 cpm (Cerénkov). Binding reaction was performed for 20 minutes at room temperature in 10 mmol/L HEPES, pH 7.5, 0.5 mmol/L ethylenediaminetetraacetic acid, 100 mmol/L KCl, 2 mmol/L dithiothreitol, 2% glycerol, 4% Ficoll, 0.25% Nonidet P-40, 1 mg/ml bovine serum albumin, and 0.1 μg/μl poly(dI/dC) as previously described.25 Protein-DNA complexes were separated from the unbound DNA by electrophoresis through 5% native polyacrylamide gels containing 2.5% glycerol and 0.5× TBE. Gels were dried under vacuum and exposed for 48 to 64 hours to Amersham Hyperfilms (Amersham) at −80°C with intensifying screens. Specificity of binding was ascertained by competition with a 160-fold molar excess of unlabeled consensus oligonucleotides.25 Each EMSA signal was quantified by densitometry, and the mean ± SD is given.6,29
Western Blot Analysis
Cytoplasmic and nuclear fractions were prepared as previously described.6,25 Twenty μg of cytoplasmic extracts or 10 μg of nuclear extracts were separated onto 10 to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, followed by electroblotting to ECL-nitrocellulose (CML-mps) or polyvinylidene difluoride (S100 proteins) membranes, respectively. Membranes were incubated with primary antibodies for CML (1:8000), S100A1, S100A8, and S100A9 (1:10,000) for 60 minutes at room temperature. After washing (2 × 7 minutes in Tris-buffered saline, 0.05% Tween), the secondary antibody (horseradish peroxidase-coupled rabbit IgG, 1:2000) was added, and incubation was continued for 30 minutes at room temperature. Membranes were washed 3 × 15 minutes as above followed by a final 5-minute wash in Tris-buffered saline. Immunoreactive proteins were detected with the ECL-Western blot system and subsequent autoradiography for 2 to 10 minutes.
Immunoprecipitation of S100A8 and S100A8/9 Proteins
Immunoprecipitation was performed using protein A/G PLUS agarose immunoprecipitation reagent according to the guidelines of Santa Cruz Biotechnology, Inc., Santa Cruz, CA. In brief, 400 μg of total gut protein lysates derived from representative patients with CD and colitis were incubated with 1 μg of S100A8 or 1 μg of S100A8/9-specific antibodies respectively for 2 hours at 4°C. The resulting S100A8 and S100A8/9 immunoprecipitates were incubated with 20 μl of resuspended protein A/G PLUS-agarose for an additional 2 hours at 4°C. Immunoprecipitates were collected by careful centrifugation (1000 × g) for 5 minutes at 4°C and pellet was washed four times with phosphate-buffered saline before resuspension in electrophoresis buffer.
Elution of CML-mps and S100 Proteins from Tissues
Isolation of CML-mps and S100 proteins was performed as previously described.30 In brief, tissue from resection borders or inflammatory regions were homogenized using a pestle and liquid nitrogen. Homogenized tissue was transferred to Sepharose lysis buffer (0.9% NaCl, 0.1 mmol/L leupeptin, 2 mmol/L benzamidine, 20 μg/ml soybean inhibitor, and 1.5 mmol/L phenylmethyl sulfonyl fluoride), cleared by centrifugation, and lysed by four freeze-thaw cycles, followed by pulsed ultrasonication for 3 minutes and a final freeze-thaw cycle. Insoluble material was removed by centrifugation, and the supernatant was loaded on Sepharose columns, onto which either anti-CML, anti-S100, or anti-phosphatidylinositol-4-phosphate (PIP)-kinase antibodies had been coupled.30 Fifteen mg of the supernatant were incubated with 750 μl of each, CML-, S100-, or PIP-Sepharose (1 mg/ml each), while continuously shaking for 2 hours at room temperature. At the end of incubation, the supernatant was removed (=depleted material). After extensive washing (7 × 10 ml 0.9% NaCl), proteins were eluted from the column for 10 to 15 minutes using 500 μl of 1 mol/L glycine, pH 3.0, neutralized and dialyzed against 4 L of 0.9% NaCl (=eluted material). Thereafter, protein concentration was determined using the BCA assay system (Pierce, Rockford, IL) and depleted and eluted samples were used in the respective experiments.
Determination of Endotoxin Contamination
The endotoxin content in protein fractions isolated from gut tissue was determined using a Limulus amoebocyte lysate (E-Toxate; Sigma, Deisenhofen, Germany). The undiluted material and three additional dilutions (1:10, 1:50, 1:100) of each tissue extract were tested, demonstrating that almost all extracts had undetectable levels of lipopolysaccharides at a protein concentration of 5 mg/ml. In both resection borders and inflamed areas, only occasional endotoxin, but less than 0.03 U/ml, was found. This amount has no influence on NF-κB binding activity and NF-κB-dependent gene expression (data not shown).
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
RT-PCR was performed as previously described25,31 using 1 μg of total RNA as starting material and the following conditions: β-actin (forward 5′-AGAGGTATCCTGACCCTGAAGTACC-3′, reverse 5′CCACCAGACAACAC-TGTGGTGTTGGCAT-3′): 1× 95°C for 360 seconds; 1× 94°C, 60 seconds; 55°C, 180 seconds; 72°C, 90 seconds; 1× 94°C, 45 seconds; 60°C, 45 seconds; 72°C, 90 seconds, 33× 94°C, 45 seconds; 65°C, 45 seconds; 72°C, 90 seconds; 1× 72°C, 600 seconds. IL-6: (forward: 5′-GATGCTACCAAACTGGATATAATC-3′; reverse: 5′-GGTCCTTAGCCACT CCTTCTGTG-3′): 1× 94°C, 240 seconds; 1× 94°C, 30 seconds; 55°C, 120 seconds; 72°C, 60 seconds); 2× 94°C, 30 seconds; 60°C, 45 seconds; 72°C, 45 seconds; 32× 94°C, 30 seconds; 65°C, 45 seconds; 72°C, 45 seconds; 1× 72°C, 600 seconds. The PCR products were separated on 1.5 to 2% agarose gels and visualized by ethidium bromide staining. Amplification of β-actin served as control for sample loading and integrity. Reactions lacking template RNA or AMV reverse transcriptase served as internal controls.
Quantitative Real-Time PCR
Real-time-PCR was performed on a LightCycler (Roche, Mannheim, Germany) as described31,32 using the following primers: GAPDH forward: 5′-AACGAC-CCCTTCATTGAC-3′; GAPDH reverse: 5′-TCCACGACATACTCAGCAC-3′; interleukin (IL)-6 forward: 5′-GATGCTACCAAACTGGATATAATC-3′ and IL-6 reverse: 5′-GGTCCTTAGCCACT CCTTCTGTG-3′). Cycling conditions were 95°C, 5 minutes; 94°C, 1 minute; 50°C, 2 minutes; 72°C, 2 minutes; 94°C, 1 minute; 52°C, 30 seconds; 72°C, 75 seconds, ×25; 72°C, 6 minutes; for mGAPDH and IL-6.31 The expected PCR products were 191 bp for GAPDH and 267 bp for IL-6, respectively. Serial dilutions of each purified product were made and standard curves obtained on a real-time PCR cycler (LightCycler; Roche) using the SYBR Green I kit (Roche) according to the manufacturer’s instructions. The resulting standard curves were exported into the RelQuant software (version 1.01; Roche) to establish individual standard curves (coefficient file) allowing evaluation of the final data including normalization to GAPDH and PCR efficiency correction.31 Using the standard curves for each gene, the relative number of copies was determined using the RelQuant software and expressed as standardized units.31
Mouse Models
IL-10−/− mice, C57BL6 wild-type mice (WT), and RAGE−/− mice15,33–35 (10 to 12 weeks old) have recently been described in detail. Mice were housed in groups of four with a 12-hour light/dark cycle and free access to food and water. Procedures in this study were approved by the Animal Care and Use Committee at the Regierungspraesidium Tuebingen and Karlsruhe.
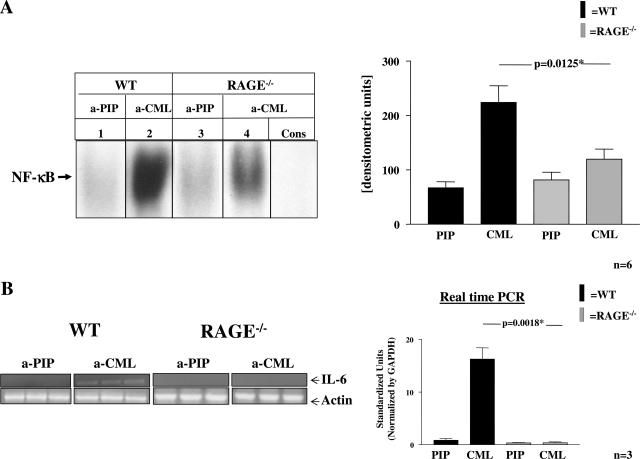
For determination of CML as a direct mediator of inflammation, 75 μg of PIP control proteins or CML-mps, isolated from guts of patients with CD via Sepharose adsorption (see above), were rectally applied into WT and RAGE−/− mice as previously described in detail.36 In some experiments, proteins were applied into WT and RAGE−/− mice for 30 minutes before mice were sacrificed, and rectosigmoid colon was retrieved for preparation of nuclear extracts (Figure 7). In other experiments, WT, RAGE−/−, and IL-10−/− mice were treated for 7 days, whereas proteins were rectally applied at days 0, 2, 4, and 6. Mice were sacrificed at day 7 and rectosigmoid colon was taken for preparation of nuclear extracts and for immunohistochemical evaluation for signs of inflammation (Figure 8).
FIGURE 7.
CML-containing CD-derived gut extracts induce NF-κB activation in murine rectosigmoid colon—effects of RAGE deletion. Protein extracts (15 mg) from inflamed colon of six CD patients were incubated with immobilized antibodies to either CML or PIP kinase (PIP) before adsorbed proteins were eluted as above. Seventy-five μg of each eluted material were applied topically to the rectosigmoid colon of WT or RAGE−/− mice for 30 minutes. A: Nuclear extracts were prepared and studied for NF-κB binding activity in EMSA. Lanes 1 and 3: NF-κB binding activity induced with 75 μg of material eluted from Sepharose-coupled PIP antibodies in WT and RAGE−/− mice. Lanes 2 and 4: NF-κB binding activity with 75 μg eluted from Sepharose-coupled CML antibodies in WT and RAGE−/− mice. Specificity of NF-κB binding was demonstrated by including a 160-fold molar excess of unlabeled consensus NF-κB oligonucleotide (Cons.) along with CML-enriched material treated nuclear extracts. The experiment was performed in six mice per group with identical results. The results of one representative mouse per group are shown on the left. The bar graphs on the right summarize the results and densitometric analysis of all mice tested. The mean ± SD is reported. B: Total RNA was prepared from the above-described colonic samples and IL-6 transcription was determined in both, RT-PCR (left) and real-time PCR (right). Left: RT-PCR for IL-6 and actin transcripts (serving as internal control) in WT and RAGE−/− mice treated with PIP- or CML-eluted proteins. Right: Results for IL-6 transcription determined by real-time PCR with a relative quantitative approach.31 After normalization with GAPDH and a PCR efficiency correction, the relative amount of IL-6 was determined in WT and RAGE−/− mice and is summarized in the bar graph. The mean ± SD is reported.
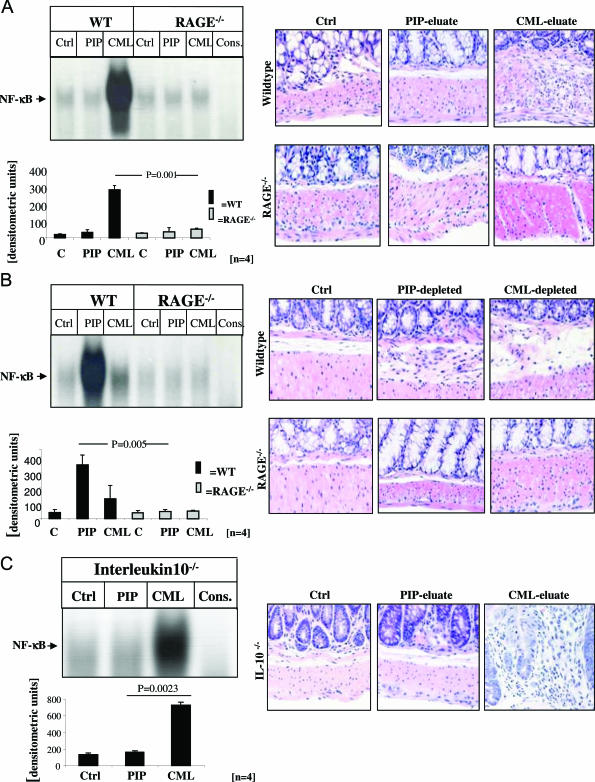
FIGURE 8.
CML-mps derived from highly inflamed CD gut cause prominent activation of NF-κB in the gut from WT and IL-10−/− mice. A and B: Left panels: Protein extracts (15 mg) from highly inflamed CD gut from four representative CD patients were incubated with Sepharose-immobilized antibodies to either PIP kinase or CML-mps and adsorbed proteins were eluted as above. Seventy-five μg of the resulting eluates (A, C) and 75 μg of the resulting supernatants (comprising the solution from which proteins had been eluted) (B) were rectally applied into the colon of WT (A and B, lanes 2 and 3), RAGE−/− (A and B, lanes 5 and 6) mice, and of IL-10−/− mice (C, lanes 2 and 3) at days 0, 2, 4, and 6. Untreated gut extracts from WT (A and B, lane 1), RAGE−/− mice (A and B, lane 4), and IL-10−/− mice (C, lane 1) served as an additional control. Specificity of NF-κB binding (using the same sample as in lane 7 in A and B and lane 4 in C) was shown by including a 160-fold molar excess of unlabeled consensus NF-κB oligonucleotide (A and B, lane 7). At day 7, mice were sacrificed and gut tissue was collected for EMSA. Nuclear extracts were prepared and assayed for NF-κB binding activity as shown for representative mice (A–C: top left), and the bar graphs summarize the densitometric analysis of all experiments (A–C: bottom left). The corresponding representative immunohistochemical analyses are shown on the right.
Densitometric Quantification
Densitometry was performed using a GS700 densitometer (Bio-Rad, München, Germany). Determination of the signal area to be measured and quantitative evaluation was performed, and the mean of two measurements was taken for the statistical analysis.6
Statistical Analysis
All values are given as mean with the bars showing SD. Student’s two-tailed t-test was used to determine significance. P < 0.05 was considered to be statistically significant.
Results
Overlapping Distribution of Activated NF-κB and RAGE Epitopes in Inflamed Gut from Patients with CD
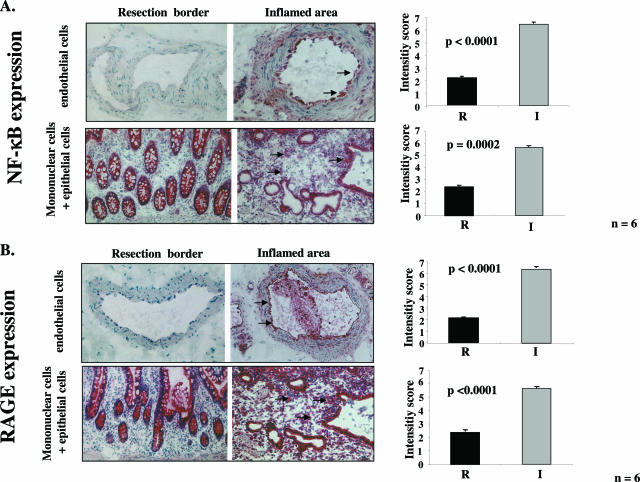
Consistent with previous studies,5,6 immunohistochemistry with an antibody specific for activated NF-κBp65 displayed strong staining in almost all infiltrating mononuclear/epithelial and endothelial cells in highly inflamed areas of gut from patients with CD. The observation, that NF-κB seemed to be activated in almost all cells was indicative of sustained NF-κB (Figure 1A, right). In contrast, staining for activated NF-κBp65 was significantly lower in mononuclear/epithelial (P = 0.0002) and endothelial cells (P = 0.000016) in the resection border (Figure 1A, left). The distribution of RAGE epitopes closely paralleled that of activated NF-κB. RAGE was up-regulated in mononuclear/epithelial (P = 0.00002) and endothelial cells (P = 0.0000006) present in highly inflamed zones (Figure 1B, right) but not in the resection area (Figure 1B, right). In situ hybridization with RAGE-specific riboprobes confirmed increased levels of transcription in mononuclear/epithelial and endothelial cells of the highly inflamed zones (data not shown).
FIGURE 1.
Activated NF-κBp65 and RAGE expression are significantly higher in highly inflamed zones compared with resection borders of gut specimens of patients with CD. Alkaline phosphatase anti-alkaline phosphatase immunohistochemical staining of activated NF-κBp65 antigen (A) and RAGE (B) expression in endothelial cells and mononuclear/epithelial cells in resection gut specimens from patients with CD comparing resection border (left) and the highly inflamed zone (right). Representative positively stained cells are marked with an arrow. The right panels show the results of semiquantification of the histological results according to an intensity score (R, resection border; I, inflamed zone). Original magnifications, ×40.
NF-κB Activation Is Induced in CD-Derived Gut Tissue, and Gut Tissue-Derived Extracts Activate NF-κB in Cultured Endothelial Cells
Consistent with previous results,1,2,4,5 nuclear NF-κB binding activity was significantly higher in tissue of the highly inflamed area than in tissue of the resection margin (data not shown). Most of these studies examined activation of inflammatory cells derived from patients with IBD.1,2,4,5,37,38 Besides, mucosal endothelium has become well recognized to play an active role in the pathogenesis of both CD and UC.39,40 Endothelial cells regulate immune homeostasis by controlling leukocyte accumulation in the intestinal mucosa, and endothelial cell dysfunction might thereby primarily contribute to IBD.40 Because the endothelium of patients with IBD demonstrated a strong increase in both RAGE and NF-κB (Figure 1) we focused on endothelial cells.
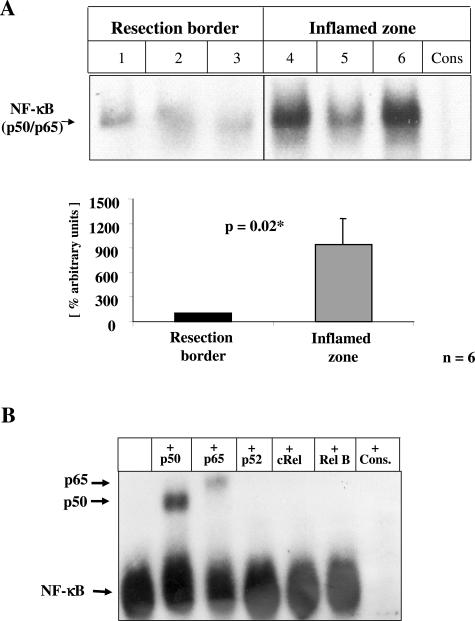
To identify factors responsible for NF-κB activation in CD and UC gut tissue, protein extracts were prepared from the inflamed zone and the border of the normal-appearing respected area. Thereafter, bovine aortic endothelial cells (BAECs; Figure 2) were incubated with 100 μg of isolated protein extract for 5 days, before NF-κB activation was determined. Cytokine or lipopolysaccharide-dependent NF-κB activation is generally limited to 48 hours at the most.41 On the contrary, RAGE-dependent NF-κB activation41 is sustained and can be followed for more than 5 days in cell culture.25 When nuclear extracts from BAECs were assayed for NF-κB binding activity by EMSA (Figure 2), resection border-derived extracts induced only marginal NF-κB binding activity (Figure 2A, lanes 1 to 3), whereas extracts derived from the highly inflamed zone resulted in strong NF-κB binding activity (Figure 2A, lanes 4 to 6). Densitometric evaluation of the results obtained in all patient-derived extracts confirmed a strong and highly significant induction of NF-κB binding activity in BAECs stimulated with extracts derived from the inflamed zone (P = 0.02, Figure 2B). The long-lasting NF-κB activation in vitro implies involvement of RAGE ligands rather than cytokines or endotoxin. Moreover, heat treatment of the gut-derived extract abrogated the NF-κB-inducing activity, whereas the addition of polymyxin B had no effect on the induction of NF-κB binding activity. These data point to a protein-derived mediator capable of inducing sustained NF-κB activation.
FIGURE 2.
Induction of NF-κB activation in cultured endothelial cells by CD-derived gut extracts from inflamed areas. BAECs (106) were incubated with 100 μg of total protein extracts isolated from either resection borders or inflamed gut tissue of patients with CD. After 5 days, BAECs were harvested, and nuclear extract was prepared as described under Materials and Methods, before 10 μg of nuclear extracts were assayed for NF-κB binding activity in EMSA. Three representative patients are shown. A: Top: NF-κB binding activity in BAECs incubated with total gut protein of resection border (lanes 1 to 3) and highly inflamed zones (lanes 4 to 6). Specificity of NF-κB binding activity was shown by including a 160-fold molar excess of unlabeled consensus NF-κB oligonucleotide (Cons). The position of NF-κB is indicated by an arrow. A: Bottom: Densitometric analysis of signal intensity of the NF-κB binding activity in all samples studied (n = 6). The mean ± SD is given. B: Characterization of the NF-κB subunits forming the NF-κB heterodimer was performed by including 2.5 μg of anti-p50 (lane 2), anti-p65 (lane 3), anti-p52 (lane 4), anti-cRel (lane 5), or anti-relB (lane 6) antibodies in the binding reactions.
CML-Modified S-100/Calgranulins Are Present in CD Gut Extracts
Two potential mediators known to bind to RAGE42,43 and to be associated with chronic inflammation and sustained NF-κB activation15,19,25,34,42 (closely correlating with the clinical course in gut samples of patients with CD5,6) are S100/calgranulins and CML-mps. The S100 proteins S100A, S100A9, and S100A12 have recently been demonstrated to be strongly up-regulated in chronic, active IBD.37,38 Furthermore, immunohistochemistry studies of colons from NSAID-treated IL10−/− mice have shown increased CML-mps in experimental IBD.44
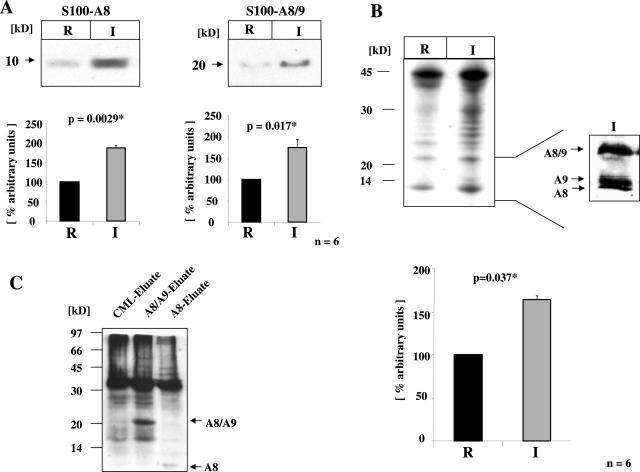
To confirm the presence of S100 proteins and CML-mps in the inflamed tissue, protein extracts were prepared from resection borders (R) and the inflamed area (I). Immunoblotting demonstrated increased levels of S100A8 (note that antibodies to S100A8 and S100A8/9 display cross-reactivity) (P = 0.0029, Figure 3A) and S100A8/9 (P = 0.017, Figure 3A) in extracts from inflamed regions, but not the resection borders. No change was observed in S100A1 antigen levels (P = 0.107, data not shown).
FIGURE 3.
S100A8, S100A8/9, and CML-mps are increased in inflamed gut tissue. A: Top: Total protein extracts were prepared from gut tissue of CD patients (n = 6) as described under Materials and Methods. Total protein extracts isolated from either the resection border or the inflamed area were separated by SDS-PAGE and immunoblotted with antibodies for S100A8 and S100A8/9. A: Bottom: Densitometric analysis and the mean ± SD is indicated. Signal intensity of the band in the sample derived from the resection border was arbitrarily set at 100%. B: Top: Total protein extracts isolated from either the resection border or the inflamed area were separated by SDS-PAGE and immunoblotted with an antibody specific for CML modifications. Immunoblots showed a significant increase of a variety of CML-mps in the inflamed zone. The inset indicates the presence of CML-modified S100A8, S100A9, and S100A8/9 proteins in the inflamed zone. B: Bottom: Densitometric analysis and the mean ± SD is indicated. Signal intensity of the band in the sample derived from the resection border was arbitrarily set at 100%. C: CML-specific immunoblots of total protein extracts from highly inflamed zone enriched for CML-mps, S100A8/A9, or S100A8 via affinity chromatography with immobilized antibodies: S100A8/9- and S100A8-enriched protein fractions both contain CML-modified proteins.
In addition, analysis of the tissue protein extracts demonstrated a significant increase in a variety of CML-mps in the inflamed zone when compared with extracts from the resection border (P = 0.037, Figure 3B). These data were confirmed in CML-specific ELISA (P = 0.04, data not shown). Longer exposure of the immunoblots revealed the presence of smaller proteins in tissue extracts from the highly inflamed zone with an apparent size of 8, 14, and 20 kd (Figure 3B, inset). Thus, we hypothesized that S100 proteins might also be targets of CML modifications.
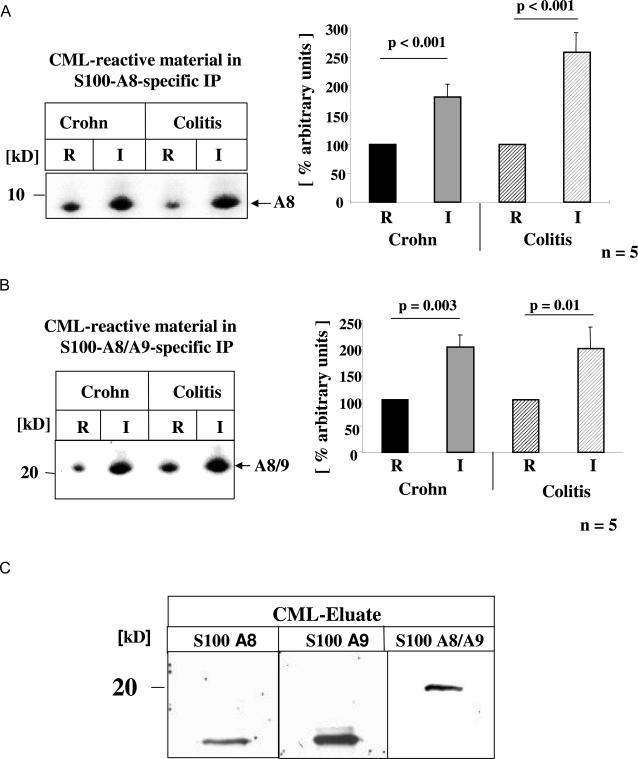
To further define the nature of the CML-mps, protein extracts from the highly inflamed zone were subjected to affinity chromatography using immobilized antibodies for either CML-mps, S100A8, or S100A8/9. Thereafter, protein extracts eluted from these three columns were subjected to CML-specific immunoblot analysis (Figure 3C), and demonstrated that S100A8- and S100A8/A9-enriched protein fractions both contained CML-modified proteins. These data were validated by performing S100A8- and S100A8/A9-specific immune precipitations using protein extracts from highly inflamed tissue, before the immune-precipitated material was reprobed for the presence of CML-mps. Both S100A8 (Figure 4A) and S100A8/A9 (Figure 4B) precipitated from the inflamed tissue (I) demonstrated a strong increase in CML modifications when compared with resection border material (R), further implying the presence of posttranslational CML-modified S100 proteins in the inflamed tissue. To confirm further this concept, immunoblotting was performed with protein extracts eluted from the anti-CML column using antibodies specific for the S100 proteins A8, A9, and A8/9 (Figure 4C). The CML-enriched material demonstrated strong immunoreactivity with each of the S100-specific antibodies and thus confirmed that S100 proteins in inflamed gut extracts are subject to carboxymethylation.
FIGURE 4.
Posttranslational CML modification of S100A8 and S100A8/9 proteins in inflamed CD and colitis gut. Total gut protein extracts were isolated from resection borders and inflamed tissue areas from representative patients with CD (n = 6) and UC (n = 5) as described under Materials and Methods. For immunoprecipitation equal amounts of total protein extracts were immunoprecipitated with S100A8-specific (A) or/and S100A8/9-specific (B) antibodies, respectively. Thereafter, S100A8 and S100A8/9 immunoprecipitates were separated by SDS-PAGE and subjected to immunoblotting with CML-specific antibodies. Signals obtained in all samples were quantified by densitometry as summarized in the bar graphs on the right. The mean ± SD is reported. C: CML-mps, enriched via CML-specific affinity chromatography were separated by SDS-PAGE followed by immunoblotting with antibodies to S100A8, S100A9, and S100A8/9, respectively.
S100 Proteins and CML-mps from Highly Inflamed IBD Gut Tissue Induce Activation of NF-κB in Cultured Endothelial Cells
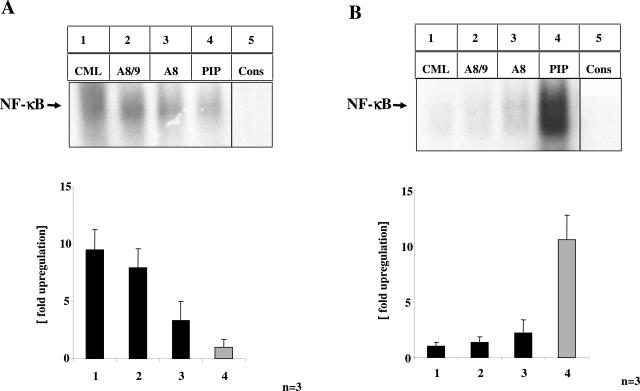
To study the functional relevance of S100 proteins and CML-mps eluted from inflamed gut tissue, proteins eluted from CML-, S100A8-, S100A8/9-, and phosphatidylinositol 4-phosphate-kinase (PIP)- (serving as control) specific affinity columns were incubated with BAECs for 5 days, before NF-κB activating capacity was determined by EMSA (Figure 5).30 Enriched proteins from the immobilized anti-CML and anti-S100A8/9 antibodies strongly induced NF-κB binding activity (Figure 5A, lanes 1 and 2), whereas the material eluted from the anti-A8 column induced moderate NF-κB binding activity (Figure 5A, lane 3). In contrast, proteins eluted from the anti-PIP column resulted only in marginal NF-κB activation (Figure 5A, lane 4). Material eluted from Sepharose-coupled CML antibodies induced a 9.5-fold increase (P < 0.001) in NF-κB binding activity in BAECs, whereas material eluted from immobilized S100A8/9 antibodies resulted in a 7.9-fold (P < 0.0001) and S100A8 in a 3.3-fold increase (P = 0.004) in NF-κB binding activity (Figure 5A, right) compared with PIP-eluted material. Consistently, the supernatant depleted either from CML or S100 proteins showed no residual NF-κB binding activity (Figure 5B), whereas the supernatant from columns enriching for PIP was still effective in inducing NF-κB binding activity. Because depletion of S100A8 and S100A8/A9 proteins from gut extracts using specific antibodies also removed important CML-mps and vice versa, these results further confirmed that S100 proteins in inflamed gut extracts are the subject of carboxymethylation.
FIGURE 5.
CML-mps and S100 proteins isolated from highly inflamed CD gut induce activation of NF-κB in cultured endothelial cells. A and B: Protein extracts (15 mg) from highly inflamed CD gut from three representative CD patients were incubated with Sepharose-immobilized antibodies to CML-mps (lane 1), S100A8/9 (lane 2), S100A8 (lane 3), or PIP-kinase (lane 4). Adsorbed proteins were eluted from the respective columns under acidic conditions, followed by neutralization of the eluted material. Eluted material (100 μg) (A) or supernatant (100 μg, comprising the solution from which proteins had been eluted) (B) were incubated with cultured BAECs for 5 days before nuclear extracts were prepared and 10 μg were assayed for NF-κB binding activity by EMSA. Specificity of NF-κB binding (using the same sample as in lane 1 in A and lane 4 in B) was shown by including a 160-fold molar excess of unlabeled consensus NF-κB oligonucleotide (lane 5). Signals obtained in all samples were quantified by densitometry as summarized in the bar graphs on the bottom. The mean ± SD is reported.
CML-mps-Induced Sustained NF-κB Activation Is Dependent on RAGE
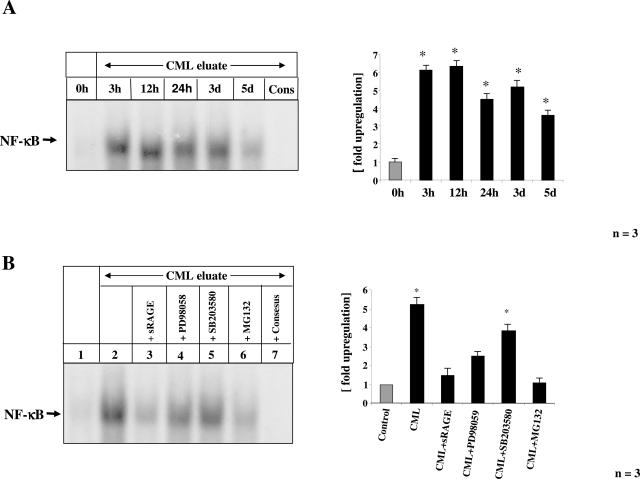
Exposure of BAECs to CML-eluted material resulted in NF-κB activation lasting for up to 5 days (Figure 6A), thus strongly indicating an engagement of RAGE.25 Consistently, CML-mps-induced NF-κB activation was reduced in the presence of sRAGE (25 μg/ml), a truncated form of the receptor comprising the extracellular domain of RAGE (Figure 6B, lane 3). Furthermore, specific inhibitors of p44/42-MAPKinases (PD98059, 30 μmol/L) and p38MAPKinase (SB203580, 10 nmol/L), both known to be required for CML-dependent RAGE-mediated NF-κB activation,45 reduced CML-mps-induced NF-κB binding activity (Figure 6B, lanes 4 and 5). The proteasome inhibitor MG132 (N-cbz-Leu-Leu-leucinal, 1 μmol/L), which blocks IκB protein degradation and prevents p65/p50 dimer nuclear translocation, inhibited CML-mps-induced NF-κB activation (Figure 6B, lane 6).
FIGURE 6.
Time course of CML-mps induced activation of NF-κB in cultured endothelial cells. Cultured BAECs were incubated with CML-mps (100 μg) eluted from highly inflamed areas from CD patients for the time points indicated in the figure legend before cells were harvested and nuclear extracts were prepared. NF-κB binding activity was determined by EMSA. Specificity of NF-κB binding (using the same sample as in lane 5 in A) was shown by including a 160-fold molar excess of unlabeled consensus NF-κB oligonucleotide (Cons). The experiment was repeated three times with identical results and the results of one representative patient are shown on the left. The bar graphs on the right summarize the results and densitometric analysis of all extracts tested. The mean ± SD is reported. B: Cultured BAECs were preincubated with either sRAGE (25 μg), the p44/p42 MAPKinase inhibitor PD98058 (30 μmol/L), the p38 MAPKinase inhibitor SB203580 (10 nmol/L), and the proteasome inhibitor MG132 (1 μmol/L) 2 hours before stimulation with CML-mps eluates isolated from highly inflamed areas from CD patients for 3 days. NF-κB binding activity was studied in EMSA. The experiment was repeated three times with identical results, and the results of one representative patient are shown on the left. Specificity of NF-κB binding (using the same sample as in lane 2) was shown by including a 160-fold molar excess of unlabeled consensus NF-κB oligonucleotide (lane 7). The bar graphs on the right summarize the results and densitometric analysis of all extracts tested. The mean ± SD is reported.
To assess the role of CML-mps-RAGE interaction in colonic inflammation in vivo, protein extracts from inflamed colons of six CD patients were incubated with Sepharose-coupled CML or PIP antibodies as above. Thereafter, the CML-enriched material was applied to the rectosigmoid colon of RAGE−/− mice and strain-matched controls (WT) for 30 minutes, before mice were sacrificed and colonic tissue was prepared for determination of NF-κB binding activity by EMSA. Strongest NF-κB binding activity was observed in colonic tissue from WT mice treated with CML-enriched material (Figure 7A, lane 2). In contrast, NF-κB binding activity induced by CML-enriched material was significantly lower in colonic tissues of RAGE−/− mice (P = 0.0125) (Figure 7A, lane 4). Material eluted from immobilized PIP-kinase antibodies did not induce NF-κB activation in WT or RAGE−/− mice (Figure 7A, lanes 1 and 3).
The functional relevance of the NF-κB binding activity induced by CML-enriched material was confirmed by RT-PCR and quantitative real-time PCR for the NF-κB regulated cytokine interleukin-6. RT-PCR demonstrated a significant increase in IL-6 transcription in colonic samples of WT mice exposed to CML-eluted material, but not in extracts of RAGE−/− mice (Figure 7B, left). Real-time PCR analysis for IL-6 validated these results (P = 0.0018; Figure 7B, right). Thus, NF-κB activation in vivo by proteins derived from inflamed intestinal tissue is at least in part mediated by RAGE.
We next asked whether long-term application of CML-mps had the potential to induce sustained NF-κB activation paralleled by colitis-like morphological changes in murine colonic tissue. CML and PIP eluates, prepared from highly inflamed areas of four patients with CD were topically applied to the rectosigmoid colon of healthy WT and RAGE−/− mice for 7 days. Activation of NF-κB was determined by EMSA, confirming increased NF-κB binding activity in colonic extracts from WT mice exposed to CML-eluted material (Figure 8A, left). In contrast, CML-eluate-induced NF-κB activation was missing in RAGE−/− mice (Figure 8A, left). Histological analysis confirmed a strong increase in tissue inflammation evident in WT, but not in RAGE−/− mice (Figure 8A, right). PIP-kinase eluates serving as control had no effect on NF-κB binding activity or the histological degree of inflammation, neither in WT nor in RAGE−/− mice (Figure 8A). To confirm that inflammation was mediated by CML-mps, CML- and PIP-depleted supernatants were applied. Although PIP-depleted material (still containing CML-mps) strongly induced NF-κB binding activity and inflammation in colonic tissue of WT but not of RAGE−/− mice (Figure 8B), CML-depleted material did not cause any significant inflammatory reaction in either WT or RAGE−/− mice (Figure 8B). Consistently, healthy IL-10−/− mice, genetically susceptible to inflammation, demonstrated an exaggerated inflammatory response on exposure to eluate containing CML-mps including CML-modified S100 proteins (Figure 8C).
Discussion
The data presented here extend previous concepts of inflammation by providing in vitro and in vivo evidence that CML-modified proteins (CML-mps) including CML-modified members of the S100/calmodulin family, contribute to perpetuation of inflammatory responses. CML modification occurs in an environment characterized by oxidative and carbonyl stress.11–13 Accumulation and infiltration by myeloperoxidase (MPO)-secreting neutrophil granulocytes are prominent features in local inflammatory processes in IBD.46 Because CML is rapidly generated when proteins are exposed to the myeloperoxidase system of activated human neutrophils,12 production of glycolaldehyde and other reactive aldehydes by myeloperoxidase promotes the formation of CML-mps at sites of inflammation.12 Because activated neutrophils infiltrating inflamed bowel tissue in IBD strongly express and secrete S100 proteins, they might represent a preferential target for CML modifications.37 Because of their prolonged half-life and resistance to protecting mechanisms,11,47–50 CML-mps thus provide tissue with a memory for an inflammatory response when cells bearing appropriate receptors populate that area.
Although the spectrum of CML adducts in tissues is quite diverse,13,27,30,43,51–60 immunoblotting of CD- and UC-derived gut extracts indicated that only specific proteins rather than a large number of proteins underlie CML modification during inflammation (Figure 3). This suggests that these protein modifications might not occur at random, but at especially sensitive target sites. In addition to as yet unidentified CML modification-sensitive target proteins, CML-modified S100 proteins may be important contributors in the progression of inflammation. As demonstrated here, S100A8, S100A9, and S100A8/A9 isolated from inflamed gut tissue carry CML modifications (Figures 3 and 4). Because the cellular receptors for S100A8/A9 have not been identified, these observations imply that CML-modified S100 proteins mediate at least some of their effects via RAGE. A further matter of complexity is added by the recent characterization of carbohydrate modifications of glycoproteins in endothelial cells and macrophages.61 These carboxylated N-glycans are recognized by S100A8, S100A9, and S100A12 and mediate RAGE-ligand binding.38,61 Carboxylated N-glycans also mediate colitis through activation of NF-κB.38 This implies that posttranslational modifications on both ligands such as S100 proteins and cellular receptors such as RAGE further promote and enhance inflammation.
One of the unique properties of RAGE, potentially linking it to chronic inflammatory situations such as IBD, is its ability to trigger long-lasting activation of NF-κB.25 It is important to note that although the receptor RAGE appears to be involved in the inflammatory response triggered by CML-mps,43 attenuation of this response in RAGE−/− mice was only partial. This indicates that other cell surface receptor molecules are likely to participate in mediating the effects of CML-mps on vulnerable cellular targets. A number of receptors have been identified that bind RAGE ligands62 and therefore might contribute to CML-mps-mediated inflammatory responses in IBD. Our future studies will address this question.
The data presented here lead us to propose a two-hit model for chronic inflammation. First, accumulation of CML-mps as well as, potentially, other types of posttranslational modifications induced by oxidant and carbonyl stress prime subsequent proinflammatory mechanisms. Second, inflammation is triggered when cells bearing receptors capable of interacting with posttranslational modified adducts, such as RAGE, are present.19,20,23,42,43 These findings extend the concept of inflammation, classically consisting of de novo synthesis of cytokines and release of arachidonic acid metabolites, to the posttranslational generation of CML-mps able to perpetuate inflammation because of their interaction with RAGE.25,27,34,42,43,57,58 We therefore suggest that preventing the formation of CML adducts and/or blockade of their interaction with cellular receptors may impact positively in settings of destructive chronic inflammation. Further studies are required to identify substances able to inhibit CML-mps-dependent cellular activation that might eventually be implemented into a pathogenetically oriented treatment of IBD.
Acknowledgments
We thank B.S. Andrassy for editorial support.
Footnotes
Address reprint requests to Angelika Bierhaus, Ph.D., Department of Medicine I, University of Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany. E-mail: angelika_bierhaus@med.uni-heidelberg.de.
Supported by the Deutsche Forschungsgemeinschaft [grant DFG-Na 138 and DFG/SFB 405 (to P.P.N.)], Stiftung Verum (to P.P.N.), and the Deutsche Crohn/Colitis Ulcerosa Vereinigung (to P.P.N.).
References
- Ellis RD, Limb GA, Thompson RP, Punchard NA. NF kappa B in Crohn’s disease. Biochem Soc Trans. 1997;25:178S. doi: 10.1042/bst025178s. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Pettersson S. Predominant role of NF-kappa B p65 in the pathogenesis of chronic intestinal inflammation. Immunobiology. 1997;198:91–98. doi: 10.1016/s0171-2985(97)80030-7. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Scholmerich J, Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484. doi: 10.1136/gut.42.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele K, Bierhaus A, Autschbach F, Hofmann M, Stremmel W, Thiele H, Ziegler R, Nawroth PP. Cell specific effects of glucocorticoid treatment on the NF-kappaBp65/IkappaBalpha system in patients with Crohn’s disease. Gut. 1999;45:693–704. doi: 10.1136/gut.45.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbach E, Vieth M, Roessner A, Neumann M, Malfertheiner P, Naumann M. Inhibition of RICK/nuclear factor-kappaB and p38 signaling attenuates the inflammatory response in a murine model of Crohn disease. J Biol Chem. 2005;280:14981–14988. doi: 10.1074/jbc.M500966200. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Nawroth PP. Modulation of the vascular endothelium during infection—the role of NF-kappa B activation. Contrib Microbiol. 2003;10:86–105. doi: 10.1159/000068133. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Manning AM. NF-kappaB as a primary regulator of the stress response. Oncogene. 1999;18:6163–6171. doi: 10.1038/sj.onc.1203174. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW, Colville-Nash PR, Willoughby DA. Possible new role for NF-kappaB in the resolution of inflammation. Nat Med. 2001;7:1291–1297. doi: 10.1038/nm1201-1291. [DOI] [PubMed] [Google Scholar]
- Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- Anderson MM, Requena JR, Crowley JR, Thorpe SR, Heinecke JW. The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J Clin Invest. 1999;104:103–113. doi: 10.1172/JCI3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher ED, Wagner E, Nerlich AG. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J Clin Invest. 1997;99:457–468. doi: 10.1172/JCI119180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liliensiek B, Weigand MA, Bierhaus A, Nicklas W, Kasper M, Hofer S, Plachky J, Grone HJ, Kurschus FC, Schmidt AM, Yan SD, Martin E, Schleicher E, Stern DM, Hammerling GG, Nawroth PP, Arnold B. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucciarelli LG, Wendt T, Rong L, Lalla E, Hofmann MA, Goova MT, Taguchi A, Yan SF, Yan SD, Stern DM, Schmidt AM. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell Mol Life Sci. 2002;59:1117–1128. doi: 10.1007/s00018-002-8491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt AM, Hofmann M, Taguchi A, Yan SD, Stern DM. RAGE: a multiligand receptor contributing to the cellular response in diabetic vasculopathy and inflammation. Semin Thromb Hemost. 2000;26:485–493. doi: 10.1055/s-2000-13204. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Stern DM. Receptor for age (RAGE) is a gene within the major histocompatibility class III region: implications for host response mechanisms in homeostasis and chronic disease. Front Biosci. 2001;6:D1151–D1160. doi: 10.2741/schmidt. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- Stern D, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv Drug Deliv Rev. 2002;54:1615–1625. doi: 10.1016/s0169-409x(02)00160-6. [DOI] [PubMed] [Google Scholar]
- Stern DM, Yan SD, Yan SF, Schmidt AM. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res Rev. 2002;1:1–15. doi: 10.1016/s0047-6374(01)00366-9. [DOI] [PubMed] [Google Scholar]
- Wendt T, Tanji N, Guo J, Hudson BI, Bierhaus A, Ramasamy R, Arnold B, Nawroth PP, Yan SF, D’Agati V, Schmidt AM. Glucose, glycation, and RAGE: implications for amplification of cellular dysfunction in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1383–1395. doi: 10.1097/01.asn.0000065100.17349.ca. [DOI] [PubMed] [Google Scholar]
- Nawroth P, Bierhaus A, Marrero M, Yamamoto H, Stern DM. Atherosclerosis and restenosis: is there a role for RAGE? Curr Diab Rep. 2005;5:11–16. doi: 10.1007/s11892-005-0061-9. [DOI] [PubMed] [Google Scholar]
- Schleicher ED, Bierhaus A, Haring HU, Nawroth PP, Lehmann R. Chemistry and pathobiology of advanced glycation end products. Contrib Nephrol. 2001:1–9. doi: 10.1159/000060056. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Schiekofer S, Schwaninger M, Andrassy M, Humpert PM, Chen J, Hong M, Luther T, Henle T, Kloting I, Morcos M, Hofmann M, Tritschler H, Weigle B, Kasper M, Smith M, Perry G, Schmidt AM, Stern DM, Haring HU, Schleicher E, Nawroth PP. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappaB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation end products. J Biol Chem. 1997;272:16498–16506. doi: 10.1074/jbc.272.26.16498. [DOI] [PubMed] [Google Scholar]
- Morcos M, Sayed AA, Bierhaus A, Yard B, Waldherr R, Merz W, Kloeting I, Schleicher E, Mentz S, Abd el Baki RF, Tritschler H, Kasper M, Schwenger V, Hamann A, Dugi KA, Schmidt AM, Stern D, Ziegler R, Haering HU, Andrassy M, van der Woude F, Nawroth PP. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes. 2002;51:3532–3544. doi: 10.2337/diabetes.51.12.3532. [DOI] [PubMed] [Google Scholar]
- Autschbach F, Braunstein J, Helmke B, Zuna I, Schurmann G, Niemir ZI, Wallich R, Otto HF, Meuer SC. In situ expression of interleukin-10 in noninflamed human gut and in inflammatory bowel disease. Am J Pathol. 1998;153:121–130. doi: 10.1016/S0002-9440(10)65552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MA, Schiekofer S, Isermann B, Kanitz M, Henkels M, Joswig M, Treusch A, Morcos M, Weiss T, Borcea V, Abdel Khalek AK, Amiral J, Tritschler H, Ritz E, Wahl P, Ziegler R, Bierhaus A, Nawroth PP. Peripheral blood mononuclear cells isolated from patients with diabetic nephropathy show increased activation of the oxidative-stress sensitive transcription factor NF-kappaB. Diabetologia. 1999;42:222–232. doi: 10.1007/s001250051142. [DOI] [PubMed] [Google Scholar]
- Quehenberger P, Bierhaus A, Fasching P, Muellner C, Klevesath M, Hong M, Stier G, Sattler M, Schleicher E, Speiser W, Nawroth PP. Endothelin 1 transcription is controlled by nuclear factor-kappaB in AGE-stimulated cultured endothelial cells. Diabetes. 2000;49:1561–1570. doi: 10.2337/diabetes.49.9.1561. [DOI] [PubMed] [Google Scholar]
- Chen J, Kasper M, Heck T, Nakagawa K, Humpert PM, Bai L, Wu G, Zhang Y, Luther T, Andrassy M, Schiekofer S, Hamann A, Morcos M, Chen B, Stern DM, Nawroth PP, Bierhaus A. Tissue factor as a link between wounding and tissue repair. Diabetes. 2005;54:2143–2154. doi: 10.2337/diabetes.54.7.2143. [DOI] [PubMed] [Google Scholar]
- Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol. 2004;164:1935–1947. doi: 10.1016/S0002-9440(10)63754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constien R, Forde A, Liliensiek B, Grone HJ, Nawroth P, Hammerling G, Arnold B. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis. 2001;30:36–44. doi: 10.1002/gene.1030. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Haslbeck KM, Humpert PM, Liliensiek B, Dehmer T, Morcos M, Sayed AA, Andrassy M, Schiekofer S, Schneider JG, Schulz JB, Heuss D, Neundorfer B, Dierl S, Huber J, Tritschler H, Schmidt AM, Schwaninger M, Haering HU, Schleicher E, Kasper M, Stern DM, Arnold B, Nawroth PP. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest. 2004;114:1741–1751. doi: 10.1172/JCI18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, Roth J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52:847–853. doi: 10.1136/gut.52.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikrishna G, Turovskaya O, Shaikh R, Newlin R, Foell D, Murch S, Kronenberg M, Freeze HH. Carboxylated glycans mediate colitis through activation of NF-kappa B. J Immunol. 2005;175:5412–5422. doi: 10.4049/jimmunol.175.8.5412. [DOI] [PubMed] [Google Scholar]
- Bardin N, Reumaux D, Geboes K, Colombel JF, Blot-Chabaud M, Sampol J, Duthilleul P, Dignat-George F. Increased expression of CD146, a new marker of the endothelial junction in active inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:16–21. doi: 10.1097/01.mib.0000194181.46930.88. [DOI] [PubMed] [Google Scholar]
- Danese S, Semeraro S, Marini M, Roberto I, Armuzzi A, Papa A, Gasbarrini A. Adhesion molecules in inflammatory bowel disease: therapeutic implications for gut inflammation. Dig Liver Dis. 2005;37:811–818. doi: 10.1016/j.dld.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Chen J, Liliensiek B, Nawroth PP. LPS and cytokine-activated endothelium. Semin Thromb Hemost. 2000;26:571–587. doi: 10.1055/s-2000-13214. [DOI] [PubMed] [Google Scholar]
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Kislinger T, Fu C, Huber B, Qu W, Taguchi A, Du Yan S, Hofmann M, Yan SF, Pischetsrieder M, Stern D, Schmidt AM. N(epsilon)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- Narushima S, Spitz DR, Oberley LW, Toyokuni S, Miyata T, Gunnett CA, Buettner GR, Zhang J, Ismail H, Lynch RG, Berg DJ. Evidence for oxidative stress in NSAID-induced colitis in IL10−/− mice. Free Radic Biol Med. 2003;34:1153–1166. doi: 10.1016/s0891-5849(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Yeh CH, Sturgis L, Haidacher J, Zhang XN, Sherwood SJ, Bjercke RJ, Juhasz O, Crow MT, Tilton RG, Denner L. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-kappaB transcriptional activation and cytokine secretion. Diabetes. 2001;50:1495–1504. doi: 10.2337/diabetes.50.6.1495. [DOI] [PubMed] [Google Scholar]
- Kristjansson G, Venge P, Wanders A, Loof L, Hallgren R. Clinical and subclinical intestinal inflammation assessed by the mucosal patch technique: studies of mucosal neutrophil and eosinophil activation in inflammatory bowel diseases and irritable bowel syndrome. Gut. 2004;53:1806–1812. doi: 10.1136/gut.2003.036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagi R, Miyata T. Oxidative protein damage with carbohydrates and lipids in uremia: ‘carbonyl stress.’. Blood Purif. 1999;17:95–98. doi: 10.1159/000014380. [DOI] [PubMed] [Google Scholar]
- Suzuki D, Miyata T. Carbonyl stress in the pathogenesis of diabetic nephropathy. Intern Med. 1999;38:309–314. doi: 10.2169/internalmedicine.38.309. [DOI] [PubMed] [Google Scholar]
- Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001;36:1527–1537. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Baynes JW. The Maillard hypothesis on aging: time to focus on DNA. Ann NY Acad Sci. 2002;959:360–367. doi: 10.1111/j.1749-6632.2002.tb02107.x. [DOI] [PubMed] [Google Scholar]
- Kilhovd BK, Giardino I, Torjesen PA, Birkeland KI, Berg TJ, Thornalley PJ, Brownlee M, Hanssen KF. Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism. 2003;52:163–167. doi: 10.1053/meta.2003.50035. [DOI] [PubMed] [Google Scholar]
- Lieuw AFML, van Hinsbergh VW, Teerlink T, Barto R, Twisk J, Stehouwer CD, Schalkwijk CG. Increased levels of N(epsilon)-(carboxymethyl)lysine and N(epsilon)-(carboxyethyl)lysine in type 1 diabetic patients with impaired renal function: correlation with markers of endothelial dysfunction. Nephrol Dial Transplant. 2004;19:631–636. doi: 10.1093/ndt/gfg619. [DOI] [PubMed] [Google Scholar]
- Schwedler SB, Metzger T, Schinzel R, Wanner C. Advanced glycation end products and mortality in hemodialysis patients. Kidney Int. 2002;62:301–310. doi: 10.1046/j.1523-1755.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- Sebekova K, Podracka L, Heidland A, Schinzel R. Enhanced plasma levels of advanced glycation end products (AGE) and pro-inflammatory cytokines in children/adolescents with chronic renal insufficiency and after renal replacement therapy by dialysis and transplantation—are they inter-related? Clin Nephrol. 2001;56:S21–S26. [PubMed] [Google Scholar]
- Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, Lin J, Bierhaus A, Nawroth P, Hannak D, Neumaier M, Bergfeld R, Giardino I, Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- Haslbeck KM, Bierhaus A, Erwin S, Kirchner A, Nawroth P, Schlotzer U, Neundorfer B, Heuss D. Receptor for advanced glycation endproduct (RAGE)-mediated nuclear factor-kappaB activation in vasculitic neuropathy. Muscle Nerve. 2004;29:853–860. doi: 10.1002/mus.20039. [DOI] [PubMed] [Google Scholar]
- Haslbeck KM, Friess U, Schleicher ED, Bierhaus A, Nawroth PP, Kirchner A, Pauli E, Neundorfer B, Heuss D. The RAGE pathway in inflammatory myopathies and limb girdle muscular dystrophy. Acta Neuropathol (Berl) 2005;110:247–254. doi: 10.1007/s00401-005-1043-3. [DOI] [PubMed] [Google Scholar]
- Haslbeck KM, Schleicher E, Bierhaus A, Nawroth P, Haslbeck M, Neundorfer B, Heuss D. The AGE/RAGE/NF-(kappa)B pathway may contribute to the pathogenesis of polyneuropathy in impaired glucose tolerance (IGT). Exp Clin Endocrinol Diabetes. 2005;113:288–291. doi: 10.1055/s-2005-865600. [DOI] [PubMed] [Google Scholar]
- Schalkwijk CG, Baidoshvili A, Stehouwer CD, van Hinsbergh VW, Niessen HW. Increased accumulation of the glycoxidation product Nepsilon-(carboxymethyl)lysine in hearts of diabetic patients: generation and characterisation of a monoclonal anti-CML antibody. Biochim Biophys Acta. 2004;1636:82–89. doi: 10.1016/j.bbalip.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Schiekofer S, Andrassy M, Chen J, Rudofsky G, Schneider J, Wendt T, Stefan N, Humpert P, Fritsche A, Stumvoll M, Schleicher E, Haring HU, Nawroth PP, Bierhaus A. Acute hyperglycemia causes intracellular formation of CML and activation of ras, p42/44 MAPK, and nuclear factor kappaB in PBMCs. Diabetes. 2003;52:621–633. doi: 10.2337/diabetes.52.3.621. [DOI] [PubMed] [Google Scholar]
- Srikrishna G, Huttunen HJ, Johansson L, Weigle B, Yamaguchi Y, Rauvala H, Freeze HH. N-Glycans on the receptor for advanced glycation end products influence amphoterin binding and neurite outgrowth. J Neurochem. 2002;80:998–1008. doi: 10.1046/j.0022-3042.2002.00796.x. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]