Abstract
Frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U) is a common neuropathological subtype of frontotemporal dementia. Although this subtype of frontotemporal dementia is defined by the presence of ubiquitin-positive but tau- and α-synuclein-negative inclusions, it is unclear whether all cases of FTLD-U have the same underlying pathogenesis. Examination of tissue sections from FTLD-U brains stained with anti-ubiquitin antibodies revealed heterogeneity in the morphological characteristics of pathological inclusions among subsets of cases. Three types of FTLD-U were delineated based on morphology and distribution of ubiquitin-positive inclusions. To address the hypothesis that FTLD-U is pathologically heterogeneous, novel monoclonal antibodies (mAbs) were generated by immunization of mice with high molecular mass (Mr > 250 kd) insoluble material prepared by biochemical fractionation of FTLD-U brains. Novel mAbs were identified that immunolabeled all of the ubiquitin-positive inclusions in one subset of FTLD-U cases, whereas other mAbs stained the ubiquitin-positive inclusions in a second subset of cases. These novel mAbs did not stain inclusions in other neurodegenerative disorders, including tauopathies and α-synucleinopathies. Therefore, ubiquitin immunohistochemistry and the immunostaining properties of the novel mAbs generated here suggest that FTLD-U is pathologically he-terogeneous. Identification of the disease proteins recognized by these mAbs will further advance understanding of molecular substrates of FTLD-U neurodegenerative pathways.
Frontotemporal dementia (FTD) is the second most common cause of neurodegenerative dementia in those under the age of 65, after Alzheimer’s disease (AD).1,2 Clinical presentations of FTD include several behavioral variants of FTD, in which patients experience profound changes in personality and social function, as well as language disorders of expression and comprehension, known as progressive aphasia and semantic dementia, respectively.3 Motor manifestations, including signs and symptoms of motor neuron disease (MND) or parkinsonism, also occur with FTD.4,5 The diagnostic gold standard for FTD remains neuropathological examination of the brain. Grossly, the brains of FTD patients are characterized predominantly by circumscribed atrophy of the frontal and temporal lobes, hence the pathological designation frontotemporal lobar degeneration (FTLD), and neuronal loss and gliosis are apparent on microscopic examination of affected regions.6,7
Immunohistochemistry reveals the presence of abnormal proteinaceous inclusion bodies in some of the remaining neurons in affected areas of most FTD brains. Pathological categories of FTD defined by immunohistochemistry include cases without detectable inclusions (ie, dementia lacking distinctive histology), cases with tau-positive inclusions as exemplified by Pick’s disease (PiD), corticobasal degeneration, progressive supranuclear palsy, and neurofibrillary tangle dementia, cases with neurofilament-positive inclusions (neuronal intermediate filament inclusion disease), and cases with ubiquitin-positive, tau and α-synuclein-negative inclusions, known as FTLD with ubiquitin-positive inclusions, or FTLD-U.6 Recent studies suggest that FTLD-U is the most common neuropathological subtype of FTD.8–12
Although most FTLD-U cases are sporadic, several families exhibiting autosomal dominant inheritance patterns of FTLD-U neuropathology have been linked to chromosomes 9 and 17.13–17 No genetic mutations on chromosomes 9 and 17 responsible for the disease in these families, however, have been found to date, and the molecular pathogenesis underlying FTLD-U remains unknown. In the present study, examination of ubiquitin-immunostained sections from 36 postmortem-confirmed FTLD-U cases was performed to gain insights into the pathological basis of FTLD-U. Three patterns of FTLD-U pathology were delineated based on the morphological characteristics and cortical distribution of ubiquitin-positive inclusions in these cases. To test the hypothesis that FTLD-U is pathologically heterogeneous, novel monoclonal antibodies (mAbs) were generated using immunogens consisting of high molecular mass (Mr > 250 kd) insoluble material from cortical gray matter of two FTLD-U cases with different patterns of ubiquitin-positive pathology. The selective staining of pathological inclusions by these novel mAbs in subsets of FTLD-U cases corresponded to different patterns of ubiquitin-positive pathology, thereby suggesting that there may be multiple pathways of neurodegeneration leading to FTLD-U.
Materials and Methods
Brain Tissue Collection and Neuropathological Assessment
Frozen brain tissues and fixed, paraffin-embedded tissue blocks were obtained from the Center for Neurodegenerative Disease Research brain bank at the University of Pennsylvania School of Medicine, Philadelphia, PA, and from the Center for Neuropathology and Prion Research brain bank at the University of Munich, Munich, Germany. Diagnostic assessment of frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U), PiD, AD, dementia with Lewy bodies (DLB), and neuropathologically normal (NL) cases was performed by a trained neuropathologist in accordance with published guidelines.6,18,19 Although there are no formal consensus criteria for the diagnosis of FTLD-U, cases were pathologically diagnosed as FTLD-U when the predominant neuropathological abnormalities were the presence of ubiquitin-positive but tau- and α-synuclein-negative inclusions as well as neuronal loss and gliosis in the frontal and temporal cortices, based on the recommendations of the “Work Group on Frontotemporal Dementia and Pick’s Disease.”6
Antibodies
Anti-ubiquitin antibodies used in this study included the mouse monoclonal antibody (mAb) 1510 (Chemicon, Temecula, CA), a rabbit polyclonal anti-ubiquitin antibody (DAKO, Carpinteria, CA), and Ub1B4, a mouse mAb raised against a synthetic peptide corresponding to amino acid residues 26 to 54 of human ubiquitin. mAbs 26, 132, 137, 164, 244, and 471 are novel mAbs generated as described below. Rabbit polyclonal anti-tau antibody 17025 made against human recombinant tau and rabbit polyclonal anti-α-synuclein antibody (SNL-1) made against a peptide corresponding to residues 104 to 119 in human α-synuclein were also used.20,21
Immunohistochemical Staining
The harvesting, fixation, and further processing of the tissue specimens used in this study were conducted as previously described.22,23 Briefly, tissue blocks from frontal cortex, temporal cortex, hippocampus, and striatum of FTLD-U brains were fixed with either 70% ethanol in 150 mmol/L NaCl or phosphate-buffered 3.65% formaldehyde and infiltrated with paraffin. Immunohistochemistry was performed using the avidin-biotin complex detection system (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine as described.22 Briefly, sections were deparaffinized and rehydrated, endogenous peroxidases were quenched with 5% H2O2 in methanol for 30 minutes, and sections were blocked in 0.1 mol/L Tris with 2% fetal bovine serum (Tris/fetal bovine serum) for 5 minutes. Primary antibodies were incubated either for 1 to 2 hours at 37°C or overnight at 4°C. After washing, sections were sequentially incubated with biotinylated secondary antibodies for 1 hour and avidin-biotin complex for 1 hour. Bound antibody complexes were visualized by incubating sections in a solution containing 100 mmol/L Tris, pH 7.6, 0.1% Triton X-100, 1.4 mmol/L diaminobenzidine, 10 mmol/L imidazole, and 8.8 mmol/L H2O2. Sections were then lightly counterstained with hematoxylin, dehydrated, and coverslipped. Double-labeling immunofluorescence analyses were performed as previously described24 using Alexa Fluor 488- and 594-conjugated secondary antibodies (Molecular Probes, Eugene, OR) and coverslipped with Vectashield-DAPI mounting medium (Vector Laboratories).
Sequential Biochemical Fractionation
Gray matter from FTLD-U postmortem cortex was dissected and weighed. Tissue was homogenized in 5 ml/g low-salt (LS) buffer (10 mmol/L Tris, pH 7.5, 5 mmol/L ethylenediaminetetraacetic acid, 1 mmol/L dithiothreitol, 10% sucrose, and a cocktail of protease inhibitors) and sedimented at 25,000 × g for 30 minutes at 4°C. Supernatants were saved as the LS fraction, and pellets were washed by re-extraction in LS buffer and sedimentation. Resulting pellets were subjected to two sequential extractions in 5 ml/g Triton-X (TX) buffer (LS + 1% Triton X-100 + 0.5 mol/L NaCl) and sedimented at 180,000 × g for 30 minutes at 4°C. Supernatants from the first of these TX buffer extractions were saved as the TX fraction. An additional step with homogenization in TX buffer containing 30% sucrose followed by centrifugation was performed after the TX buffer extractions to float and remove myelin. Pellets were then homogenized in 5 ml/g sarkosyl (SARK) buffer (LS + 1% N-lauroyl-sarcosine + 0.5 mol/L NaCl) and incubated at 22°C on a shaker for 1 hour before sedimentation at 180,000 × g for 30 minutes at 22°C. Supernatants were saved as the SARK fraction. Remaining pellets were extracted in either 1 ml/g sodium dodecyl sulfate (SDS) buffer (2% SDS, 50 mmol/L Tris, pH 7.6, and a cocktail of protease inhibitors) or 1 ml/g urea buffer (7 mol/L urea, 2 mol/L thiourea, 4% 3-[(3-cholamidopro-pyl)dimethylammonio]-1-propanesulfonate (CHAPS), 30 mmol/L Tris-HCl, pH 8.5) before centrifugation at 25,000 × g for 30 minutes at 22°C. Supernatants were saved as the SDS and urea fractions, respectively. SDS sample buffer (10 mmol/L Tris, pH 6.8, 1 mmol/L ethylenediaminetetraacetic acid, 40 mmol/L dithiothreitol, 1% SDS, and 10% sucrose) was added to samples followed by heating at 100°C for 5 minutes, with the exception of the urea fraction, which was not heated to avoid carbamoylation of proteins.
Generation of Novel mAbs
Murine mAbs 26, 132, 137, 164, 244, and 471 were raised using high Mr (>250 kd) material from the urea fraction (prepared as described above) of FTLD-U brain (frontal cortex of case 11 for mAbs 26, 132, 164, and 244; frontal cortex of case 17 for mAbs 137 and 471) as immunogen. The urea fraction (100 to 150 μg protein/mouse) was separated by 5 to 20% gradient SDS-polyacrylamide gel electrophoresis, and the portion of the gel containing proteins with Mr > 250 kd (including the stacking gel; Supplementary Figure 1, see http://ajp.amjpathol.org) was minced, homogenized in phosphate-buffered saline, emulsified with incomplete Freund’s adjuvant, and injected subcutaneously into BALB/c mice. Immunogens prepared similarly from the urea fraction (except using 25 to 50 μg protein/mouse) were used for subcutaneous boost injections on days 21, 35, and 49, followed by intraperitoneal injection of immunogens without adjuvant on day 63. On day 66, the spleen was removed and spleen lymphocytes were fused to Sp2 myeloma cells to produce hybridomas. Resulting hybridoma supernatants were screened by immunohistochemistry on paraffin-embedded sections of FTLD-U cortex known to contain ubiquitin-positive inclusions. All of the mAbs that immunostained FTLD-U inclusions were determined to be of the IgM class using standard light and heavy chain antibody subtype analysis.
Results
Ubiquitin Immunohistochemistry Suggests Pathological Heterogeneity Among FTLD-U Cases
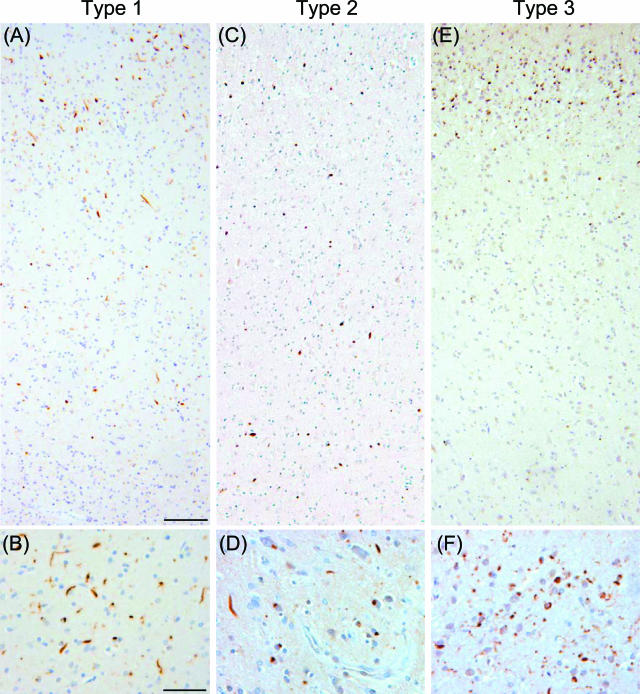
Tissue sections of hippocampus, frontal cortex, temporal cortex, and striatum from each of 36 FTLD-U cases (Table 1) were stained with an anti-ubiquitin antibody (mAb 1510) and examined by light microscopy. All of the FTLD-U cases demonstrated ubiquitin-positive, tau- and α-synuclein-negative inclusions, especially in the dentate gyrus and frontotemporal cortical regions. Closer examination of cortical sections, however, revealed heterogeneity in the morphology and laminar distribution of neuronal ubiquitin-positive inclusions among the FTLD-U cases (Figure 1 and Supplementary Figure 2; see http://ajp.amjpathol.org). Three patterns of inclusion pathology could be defined based on ubiquitin immunohistochemistry as follows: 1) type 1 (cases 1 thru 11, Table 1), cases with a relative abundance of ubiquitin-positive pathology in superficial cortical layers (Figure 1A) and a predominance of long neuritic profiles over cytoplasmic inclusions (Figure 1B and Supplementary Figure 2, A and B; see http://ajp.amjpathol.org); 2) type 2 (cases 12 thru 17, Table 1), cases with ubiquitin-positive pathology in both superficial and deep cortical layers (Figure 1C) with a predominance of cytoplasmic inclusions and only rare neuritic inclusions (Figure 1D and Supplementary Figure 2, C and D; see http://ajp.amjpathol.org); and 3) type 3 (cases 18 thru 36, Table 1), cases with a predominance of ubiquitin-positive pathology in superficial cortical layers (Figure 1E) and an abundance of cytoplasmic inclusions that were often ring-shaped, short neuritic profiles, and ubiquitin-positive dots in the gray matter (Figure 1F and Supplementary Figure 2, E and F; see http://ajp.amjpathol.org). Thus, ubiquitin immunohistochemistry of cortical sections suggests that FTLD-U is pathologically heterogeneous. Of note, most type 2 cases had a positive family history of dementia or MND but without known linkage to reported familial FTD genetic loci (Table 1).
TABLE 1.
FTLD-U Patients Used in This Study
| Case no. | Sex | Age | Duration | FH | 26/132/164 | 244 | 137/471 | |
|---|---|---|---|---|---|---|---|---|
| Type 1 | 1 | F | 62 | 5 | NR | Yes | Yes | No |
| 2 | M | 71 | 8 | NR | Yes | Yes | No | |
| 3 | M | 92 | 3 | NR | Yes | Yes | No | |
| 4 | M | 77 | 12 | NR | Yes | Yes | No | |
| 5 | F | 69 | 6 | Yes§ | Yes | Yes | No | |
| 6 | M | 77 | NR | NR | Yes | Yes | No | |
| 7 | F | 76 | 11 | NR | Yes | Yes | No | |
| 8 | F | 68 | 7 | NR | Yes | Yes | No | |
| 9 | M | 64 | 10 | NR | Yes | Yes | No | |
| 10 | F | 81 | 2 | NR | Yes | Yes | No | |
| 11 | M | 54 | 7 | NR | Yes | Yes | † | |
| Type 2 | 12* | M | 57 | 3 | Yes§ | No | † | Yes |
| 13* | M | 54 | 2 | NR | No | † | Yes | |
| 14 | F | 54 | 7 | Yes‡ | No | † | Yes | |
| 15 | F | 61 | 4 | Yes§ | No | † | Yes | |
| 16* | M | 67 | 10 | Yes‡ | No | No | Yes | |
| 17 | M | 41 | 6 | Yes‡ | No | No | Yes | |
| Type 3 | 18 | F | NR | NR | NR | No | No | No |
| 19 | F | 75 | 3 | NR | No | No | No | |
| 20 | F | 62 | 5 | Yes‡ | No | No | No | |
| 21* | M | 65 | 6 | Yes§ | No | No | No | |
| 22* | F | 79 | 5 | Yes‡ | No | No | No | |
| 23 | F | 76 | 7 | Yes§ | No | No | No | |
| 24 | F | 77 | 11 | Yes‡ | No | No | No | |
| 25 | F | 69 | 7 | Yes§ | No | No | No | |
| 26 | M | 55 | 2 | NR | No | No | No | |
| 27 | F | 73 | 6 | Yes‡ | No | No | No | |
| 28 | M | 76 | 7 | NR | No | No | No | |
| 29* | F | 63 | 11 | NR | No | No | No | |
| 30 | F | 49 | 3 | Yes§ | No | No | No | |
| 31 | F | 68 | 9 | NR | No | No | No | |
| 32* | M | 59 | 10 | NR | No | No | No | |
| 33* | M | 48 | 2 | NR | No | No | No | |
| 34* | F | 53 | 2 | NR | No | No | No | |
| 35* | M | 53 | 3 | NR | No | No | No | |
| 36 | F | 72 | 3 | NR | No | No | No |
Frontotemporal degeneration with ubiquitin-positive inclusion (FTLD-U) cases used in this study are numbered in the left-most column. Indicated is the ability of mAbs 26/132/164, 244, and 137/471 to immunohistochemically stain pathological inclusions in the FTLD-U cases. Sections of frontal cortex and hippocampus were examined from all of the FTLD-U cases with all of the mAbs, and temporal cortex and striatum were additionally examined in some of the cases for confirmation of the results. Disease duration and age are given in years. M, male; F, female; FH, family history; NR, not recorded.
Cases that exhibited MND in addition to dementia.
Cases in which the mAbs detected only a small percentage of the ubiquitin-positive inclusions.
Two or more first degree relatives with dementia or MND.
One first degree relative or two or more second degree relatives with dementia or MND.
FIGURE 1.
Patterns of FTLD-U pathology based on ubiquitin immunohistochemistry. Frontal cortex in FTLD-U cases. A and B: Type 1 is characterized by abundance of long neuritic profiles predominantly in the superficial cortical layers (case 11). C and D: Type 2 is characterized by numerous cytoplasmic inclusions in superficial and deep cortical layers as well as infrequent neuritic profiles (case 17). E and F: Type 3 is characterized by pathology predominantly in the superficial cortical layers with numerous, often ring-shaped cytoplasmic inclusions, and frequent small neuritic profiles (case 18). Scale bars: 100 μm (A, C, E); 50 μm (B, D, F).
Novel mAbs Demonstrate the Existence of Distinct Patterns of Inclusion Pathology Among FTLD-U Cases
To gain further evidence for the pathological heterogeneity of FTLD-U that was observed by ubiquitin immunolabeling, novel mAbs that could detect FTLD-U inclusions by immunohistochemistry were generated. Ubiquitin immunoblots of sequential biochemical fractions from an FTLD-U case revealed a relative abundance of high Mr (>250 kd) ubiquitin-immunoreactive proteins in the urea fraction when compared with the NL control (Supplementary Figure 1, see http://ajp.amjpathol.org). Based on the assumption that ubiquitinated forms of the protein(s) within FTLD-U inclusions may be accounting for at least some of this immunoreactivity, attempts were made to generate antibodies against this high Mr material, with the goal of identifying antibodies that would be reactive against FTLD-U inclusion pathology. Therefore, for each of the three FTLD-U types, high Mr material from the urea fraction of FTLD-U cortical gray matter was used as an immunogen, and hybridoma supernatants were screened by immunohistochemistry for their ability to stain pathological inclusions in ethanol- and formalin-fixed, paraffin-embedded tissue sections from the same case used as an immunogen. For some fusions, frozen sections were used for the immunohistochemical screening as well. Briefly, for type 1, 12 fusions were conducted from the spleens of 18 mice immunized using high Mr insoluble material from case 11 and ∼37,000 hybridoma supernatants were screened, resulting in the generation of 14 mAbs that detected the inclusions in case 11 by immunohistochemistry. Likewise, for type 2, two fusions were conducted from the spleens of four mice immunized with material from case 17, and screening of ∼5000 hybridoma supernatants resulted in seven mAbs that detected inclusions in case 17. Despite screening ∼10,000 hybridoma supernatants generated from seven fusions using the spleens of 11 mice immunized with high Mr insoluble material from an FTLD-U type 3 case, no mAbs that detected inclusions in type 3 cases were identified.
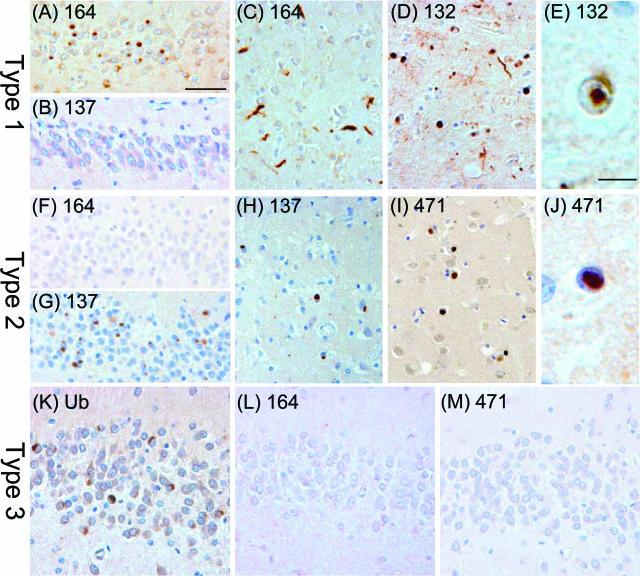
Four of the mAbs generated from case 11 (mAbs 26, 132, 164, and 244) and two of the mAbs generated from case 17 (mAbs 137 and 471) were further characterized for their ability to stain inclusions in a panel of 36 FTLD-U cases (Table 1). Tissue sections from each FTLD-U case were stained with an anti-ubiquitin antibody (mAb 1510 or DAKO anti-Ub) and with mAbs 26, 132, 164, 244, 137, and 471. Sections of frontal cortex and hippocampus were examined from all of the FTLD-U cases with all of the mAbs, whereas temporal cortex and striatum were additionally examined in some of the cases for confirmation of the results. Although ubiquitin-positive inclusions were present in all 36 FTLD-U cases examined, the mAbs 26, 132, 164, and 244 stained all of the pathological inclusions only in 11 of 36 of the FTLD-U cases (cases 1 thru 11, Table 1), which correspond to the cases categorized as type 1 by ubiquitin immunohistochemistry. In FTLD-U type 1 cases, mAbs 26, 132, 164, and 244 immunolabeled cytoplasmic inclusions in the dentate gyrus (Figure 2A) as well as cytoplasmic and neuritic inclusions in the cortex (Figure 2C) and striatum (Figure 2D); rare intranuclear inclusions in small neurons of the striatum were also seen (Figure 2E). No inclusions were labeled by mAbs 26, 132, and 164 in the remaining 25 FTLD-U cases belonging to types 2 and 3, but mAb 244 stained a small subset of the ubiquitin-positive inclusions in dentate granule cells of the hippocampus (<25%) and in cortical neurons (<5%) of four type 2 cases (cases 12 thru 15, Table 1); mAb 244 stained none of the ubiquitin-positive inclusions present in frontal cortex, temporal cortex, hippocampus, and striatum of the remaining 21 FTLD-U cases examined (cases 16 thru 36, Table 1). The mAbs 137 and 471 stained the ubiquitin-positive pathological inclusions in all six FTLD-U type 2 cases (cases 12 thru 17), and additionally stained a small subset of inclusions in case 11, which belonged to type 1 (Table 1). In FTLD-U type 2 cases, mAbs 137 and 471 labeled cytoplasmic inclusions in the dentate gyrus (Figure 2G), cortex (Figure 2H), and striatum (Figure 2I), and rare intranuclear inclusions were also seen (Figure 2J). None of the novel mAbs generated stained the ubiquitin-positive inclusions in cases demonstrating type 3 pathology (Figure 2, K–M).
FIGURE 2.
Immunoreactivity profile for newly generated antibodies in FTLD-U cases with different patterns of inclusion pathology. A–E: Type 1: mAbs 164 and 132 label numerous cytoplasmic and neuritic inclusions in dentate gyrus (A; case 4, mAb 164), frontal cortex (C; case 4, mAb 164), and striatum (D; case 11, mAb 132) as well as intranuclear inclusions in striatal neurons (E; case 11, mAb 132). Inclusions in the dentate gyrus of a type 1 case are not labeled with mAb 137 (B; case 4, mAb 137). F–J: Type 2: Cytoplasmic inclusions are labeled with mAbs 137 and 471 in dentate gyrus (G; case 13, mAb 137), frontal cortex (H; case 13, mAb 137), and striatum (I; case 14, mAb 471). Intranuclear neuronal inclusion in temporal cortex labeled with mAb 471 (J, case 14). Inclusions in the dentate gyrus of a case with type 2 pathology are negative with mAb 164 (F, case 13). K–M: Type 3: Ubiquitin-positive inclusions in the dentate gyrus (K) of case 35, type 3, are negative for mAbs 164 (L) and 471 (M). Scale bars: 50 μm (A–D, F–I, K–M); 10 μm (E, J).
Novel mAbs Specifically Detect Ubiquitin-Positive Inclusions in FTLD-U but Not Inclusions in Other Neurodegenerative Disorders
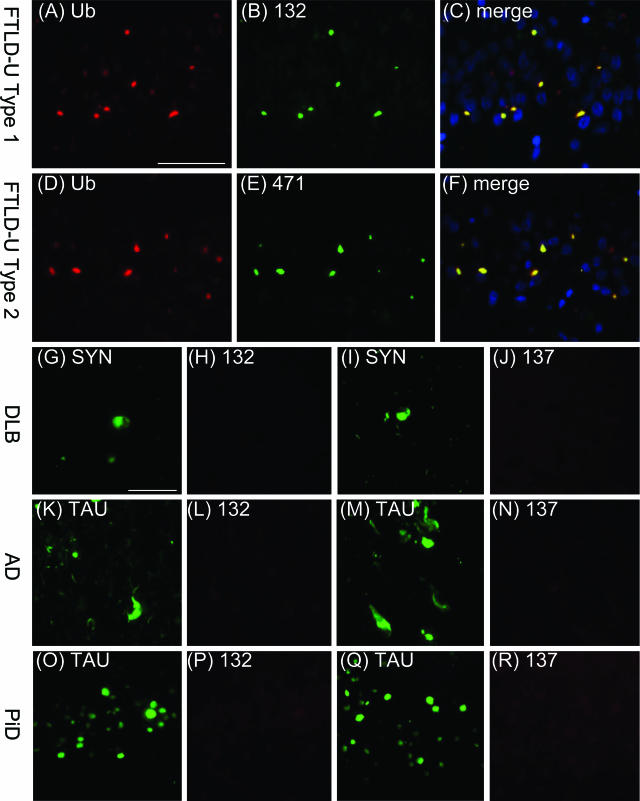
Double-labeling immunofluorescence experiments on FTLD-U tissue sections were performed to verify that the novel mAbs detect the same pathological structures immunolabeled by anti-ubiquitin antibodies (Figure 3, A–F). In FTLD-U type 1 cases, cytoplasmic and neuritic inclusions in the frontotemporal cortices (data not shown) as well as cytoplasmic inclusions in hippocampal dentate granule cells (Figure 3, A–C) were double-labeled by mAb 132 and a rabbit anti-ubiquitin antibody. Similarly, in FTLD-U type 2 cases, immunofluorescent staining of cytoplasmic inclusions in hippocampal dentate granule cells and in frontotemporal cortices revealed co-localization of mAb 471 staining with ubiquitin immunostaining (Figure 3, D–F; and data not shown).
FIGURE 3.
Specificity of newly generated antibodies for FTLD-U inclusions. A–C: FTLD-U type 1: ubiquitin-positive inclusions in the dentate gyrus (A) stain positive using mAb 132 (B). D–F: FTLD-U type 2: ubiquitin-positive inclusions in the dentate gyrus (D) stain positive using mAb 137. G–J: α-Synuclein-positive Lewy bodies (G, I) in DLB are not recognized by mAb 132 (H) or mAb 137 (J). K–R: Tau-positive neurofibrillary tangles, neuropil threads, and dystrophic neurites in the hippocampus in AD (K, M) as well as Pick bodies in the dentate gyrus in PiD (O, Q) are not labeled by mAb 132 (L, P) or mAb 137 (N, R). Scale bars = 50 μm.
Furthermore, to determine the specificity of the novel mAbs for FTLD-U inclusions, double-labeling immunofluorescence experiments were performed using tissue sections from other neurodegenerative disorders that exhibit pathological protein inclusions, including DLB, AD, and PiD. Although Lewy bodies in DLB were labeled with an anti-α-synuclein antibody, no labeling of these inclusions was seen using any of the novel mAbs (Figure 3, G–J; and data not shown). Each of the novel mAbs also failed to label neurofibrillary tangles, neuropil threads, dystrophic neurites, and senile plaques in AD (Figure 3, K–N; and data not shown) and Pick bodies in PiD (Figure 3, O–R; and data not shown) in immunofluorescence experiments.
In addition, the specificity of mAbs 26, 132, 137, 164, 244, and 471 was confirmed in conventional immunohistochemistry experiments using adjacent sections stained with an anti-ubiquitin antibody. None of the novel mAbs immunolabeled ubiquitin-positive inclusions in other neurodegenerative diseases, including AD, PiD, DLB, and multiple system atrophy, nor did the mAbs stain any structures resembling inclusions in neuropathologically normal brains (data not shown).
Discussion
FTLD-U, a form of frontotemporal lobar degeneration characterized by the presence of ubiquitin-positive but tau- and α-synuclein-negative inclusions, appears to be the most common pathological subgroup of FTD, ranging from 26 to 62% in various series of neuropathologically confirmed FTLD cases.8–12 The purpose of the present study was to gain more insights into the pathological basis of FTLD-U. Examination of ubiquitin-immunolabeled sections from a panel of 36 FTLD-U cases allowed delineation of these cases into three patterns of pathology. To test the hypothesis that these varying patterns of ubiquitin-positive pathology in FTLD-U cases represent pathological heterogeneity, novel mAbs that detect FTLD-U inclusions were generated by immunizing mice with insoluble high Mr material derived from FTLD-U cortical gray matter. Although the mAbs demonstrated some overlap in staining of pathological inclusions among the cases in FTLD-U types 1 and 2, the mAbs generated from case 11 (26, 132, 164, and 244) predominantly stained pathological inclusions in FTLD-U cases with type 1 pathology, whereas the mAbs generated from case 17 (137 and 471) more predominantly stained inclusions in type 2 FTLD-U cases. The existence of cases in which all of the inclusions were stained by mAbs from case 11 (26, 132, 164, and 244) but none of the mAbs from case 17 (137 and 471), and vice versa, in addition to the fact that none of the pathological inclusions in FTLD-U cases with type 3 pathology were detected by any of the novel mAbs, indicates that FTLD-U is pathologically heterogeneous. The selective staining by these novel mAbs of pathological inclusions in subsets of FTLD-U cases, all of which exhibit ubiquitin-positive pathology, as well as failure of the mAbs to detect free ubiquitin and ubiquitinated proteins by immunoblot, indicates that the mAbs are not anti-ubiquitin antibodies (Supplementary Figure 3, see http://ajp.amjpathol.org). These mAbs do not stain ubiquitin-positive inclusions in other neurodegenerative disorders, including AD, DLB, and PiD, further reinforcing that these antibodies are not recognizing ubiquitin and that they demonstrate specificity for FTLD-U pathology.
The molecular basis of the pathological heterogeneity among FTLD-U cases is unclear. One possibility is that each of the FTLD-U types is characterized by inclusions with a distinct protein composition. Alternatively, the pathological inclusions in all FTLD-U cases may be composed of the same protein building block, with the variation among types reflecting differences in the structural properties of the protein within the inclusions. Such structural variation may represent different stages of inclusion formation and/or may be the result of different posttranslational modifications occurring in the three FTLD-U types. There was some overlap in staining characteristics in a few type 1 and 2 cases, raising the possibility that inclusions in FTLD-U types 1 and 2 share a common protein component, although this is speculative.
The ubiquitin-positive inclusions now regarded to be characteristic of FTLD-U were first identified in patients with clinical and pathological MND, and FTLD-U is commonly referred to as FTLD with MND-type inclusions or MND-inclusion dementia.6,25 Okamoto and colleagues26–28 first described the presence of ubiquitin-positive tau-negative intraneuronal inclusions in the dentate gyrus and cortex of several MND patients, and subsequently, ubiquitin-positive inclusions were described in the brains of patients with both FTD and MND, as well as in patients who suffered from FTD but without any clinical or pathological signs of MND. These findings of pathological overlap suggest that FTLD-U and MND may be part of a clinicopathological spectrum in which the same molecular pathogenesis occurs in different populations of neurons (ie, frontal and temporal cortical neurons in FTD, motor neurons in MND) to cause different clinical presentations. The existence of families with ubiquitin-positive inclusions in which first-degree relatives have presented with FTD, MND, or both FTD and MND, lends support to this concept.29
Recent studies have closely examined the ubiquitin-positive pathology of FTLD-U, FTLD-U with MND, and MND, and have demonstrated pathological heterogeneity as well as overlap among these three disease entities. A previous report suggested that the morphological characteristics of neuronal inclusions in the dentate gyrus may help in differentiating FTLD-U (described as having Pick body-like, or crescentic/ring-shaped inclusions) from FTLD-U with MND (described as having granular inclusions).30 Although the number of cases with MND is limited in the present cohort (three cases from type 2 and seven cases from type 3), the dentate gyrus inclusions immunostained by ubiquitin and novel mAbs (type 2 cases) in the cases with MND did not appear significantly different from the inclusions in cases without MND. Another recent report suggested that FTLD-U, FTLD-U with MND, and MND represent a clinicopathological spectrum, based on the degree of overlap seen in these entities by ubiquitin immunohistochemistry, with the only finding restricted to a subset of patients being the presence of neuronal intranuclear inclusions in some familial FTLD-U cases.31 In the present study, extremely rare neuronal intranuclear inclusions were observed in several sporadic FTLD-U cases, suggesting that intranuclear pathology is not a specific marker for familial FTLD-U.
Familial forms of FTD with genetic linkage and in which FTLD-U is the underlying pathology have been recently reported, and identification of specific mutations in these families may provide insights into the pathogenesis of FTLD-U. Several reports have suggested linkage for autosomal dominantly inherited FTLD-U to a region within chromosome 17q21-22 in some of these families.14–17 Although this is the same chromosomal region in which the tau gene is located, no tau mutations have been found in any of these families, and patients from these families have not been found to have tauopathy on neuropathological examination.15–17 To date, no genetic mutation responsible for FTLD-U in these chromosome 17-linked families has been found. Three genetic loci on chromosome 9 have also been linked to familial forms of FTD. Families linked to chromosome 9p13.3-p12 have been reported to exhibit autosomal dominant inheritance of a unique disorder that clinically presents with a variable combination of FTD, Paget disease of bone, and myopathy,32 and responsible mutations have been identified in the gene that encodes valosin-containing protein (VCP).33 Neuropathological examination of one patient with a VCP mutation has revealed the presence of ubiquitin-positive intranuclear inclusions, with an absence of cytoplasmic inclusions, suggesting that cases with VCP mutations may represent an atypical form of FTLD with ubiquitin-positive inclusions.34 Two distinct loci on chromosome 9 (9q21-22 and 9p13.2-21.3) have been linked to autosomal dominantly inherited disease in families in which patients develop both FTD and MND.13,35 Genetic mutations on chromosome 9 have not yet been identified in these families, but neuropathological examination of patients with linkage to 9p13.2-21.3 has revealed the presence ubiquitin-positive tau-negative inclusions.13 Of note, most type 2 cases in the current study had a family history positive for dementia or MND, although the significance of this is unclear.
In summary, this study demonstrates that FTLD-U is a pathologically heterogeneous entity. Ubiquitin immunohistochemistry allowed categorization of FTLD-U into three types based on the morphology and cortical distribution of ubiquitin-positive inclusions. Novel mAbs, generated by immunization of mice with insoluble high Mr material from FTLD-U cortical gray matter, selectively immunostained inclusions in subsets of FTLD-U cases, providing further objective evidence for pathological heterogeneity in FTLD-U. Additional studies are required to determine the basis for this pathological variation in FTLD-U as well as the precise molecular nature of pathological lesions in FTLD-U, which may be clarified using the novel mAbs developed here as probes in protein biochemical approaches currently underway.
Note Added in Proof
Since the submission of this article, mutations in the PRGN gene-encoding progranulin were shown to be pathogenic for familial FTLD-U.36,37 However, progranulin is not found in the ubiquitin inclusions of FTLD-U syndromes caused by PRGN mutations, so the disease protein in the inclusion of FTLD-U remains to be indentified.
Supplementary Material
Acknowledgments
We thank the families of patients who made this research possible.
Footnotes
Address reprint requests to Virginia M.-Y. Lee, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 3600 Spruce St., 3rd Floor Maloney Bldg., Philadelphia, PA 19104. E-mail: vmylee@mail.med.upenn.edu.
Supported by the National Institutes of Health (AG10124, AG17586, and a training grant T32 AG 00255 to D.M.S.) and the German Brain Bank Brain-Net by the German Federal Ministry of Education and Research (grant 01GI0299 to M.N.).
D.M.S. and M.N. contributed equally to this study.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
V.M.-Y.L. is the John H. Ware III Chair of Alzheimer’s Research.
References
- Harvey RJ, Skelton-Robinson M, Rossor MN. The prevalence and causes of dementia in people under the age of 65 years. J Neurol Neurosurg Psychiatry. 2003;74:1206–1209. doi: 10.1136/jnnp.74.9.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–1621. doi: 10.1212/wnl.58.11.1615. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–1079. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Munoz D. Relationship between frontotemporal dementia and corticobasal degeneration/progressive supranuclear palsy. Dementia Geriatric Cognitive Disorders. 2004;17:282–286. doi: 10.1159/000077155. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Tolnay M, Probst A. Frontotemporal lobar degeneration. An update on clinical, pathological and genetic findings. Gerontology. 2001;47:1–8. doi: 10.1159/000052763. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, Kril JJ, Halliday GM. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Holton JL, Rossor MN, Godbolt AK, Ozawa T, Strand K, Khan N, Al Sarraj S, Revesz T. Frontotemporal lobar degeneration and ubiquitin immunohistochemistry. Neuropathol Appl Neurobiol. 2004;30:369–373. doi: 10.1111/j.1365-2990.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- Lipton AM, White CL, III, Bigio EH. Frontotemporal lobar degeneration with motor neuron disease-type inclusions predominates in 76 cases of frontotemporal degeneration. Acta Neuropathol (Berl) 2004;108:379–385. doi: 10.1007/s00401-004-0900-9. [DOI] [PubMed] [Google Scholar]
- Johnson JK, Diehl J, Mendez MF, Neuhaus J, Shapira JS, Forman M, Chute DJ, Roberson ED, Pace-Savitsky C, Neumann M, Chow TW, Rosen HJ, Forstl H, Kurz A, Miller BL. Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol. 2005;62:925–930. doi: 10.1001/archneur.62.6.925. [DOI] [PubMed] [Google Scholar]
- Shi J, Shaw CL, Du PD, Richardson AM, Bailey KL, Julien C, Stopford C, Thompson J, Varma A, Craufurd D, Tian J, Pickering-Brown S, Neary D, Snowden JS, Mann DM. Histopathological changes underlying frontotemporal lobar degeneration with clinicopathological correlation. Acta Neuropathol (Berl) 2005;110:501–512. doi: 10.1007/s00401-005-1079-4. [DOI] [PubMed] [Google Scholar]
- Vance C, Al Chalabi A, Ruddy D, Smith BN, Hu X, Sreedharan J, Siddique T, Schelhaas HJ, Kusters B, Troost D, Baas F, de Jong V, Shaw CE. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2-21.3. Brain. 2006;129:868–876. doi: 10.1093/brain/awl030. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Kawarai T, Rogaeva E, George-Hyslop P, Poorkaj P, Bird TD, Munoz DG. Familial frontotemporal dementia with ubiquitin-positive, tau-negative inclusions. Neurology. 2000;54:818–827. doi: 10.1212/wnl.54.4.818. [DOI] [PubMed] [Google Scholar]
- Rosso SM, Kamphorst W, de Graaf B, Willemsen R, Ravid R, Niermeijer MF, Spillantini MG, Heutink P, van Swieten JC. Familial frontotemporal dementia with ubiquitin-positive inclusions is linked to chromosome 17q21-22. Brain. 2001;124:1948–1957. doi: 10.1093/brain/124.10.1948. [DOI] [PubMed] [Google Scholar]
- Rademakers R, Cruts M, Dermaut B, Sleegers K, Rosso SM, Van den BM, Backhovens H, van Swieten J, van Duijn CM, Van Broeckhoven C. Tau negative frontal lobe dementia at 17q21: significant finemapping of the candidate region to a 4.8 cM interval. Mol Psychiatry. 2002;7:1064–1074. doi: 10.1038/sj.mp.4001198. [DOI] [PubMed] [Google Scholar]
- Froelich FS, Axelman P, Almkvist A, Basun H, Lannfelt L. Extended investigation of tau and mutation screening of other candidate genes on chromosome 17q21 in a Swedish FTDP-17 family. Am J Med Genet B Neuropsychiatr Genet. 2003;121:112–118. doi: 10.1002/ajmg.b.20067. [DOI] [PubMed] [Google Scholar]
- Ball M, Braak H, Coleman P, Dickson D, Duyckaerts C, Gambetti P, Hansen L, Hyman B, Jellinger K, Markesbery W, Perl D, Powers J, Price J, Trojanowski JQ, Wisniewski H, Phelps C, Khachaturian Z. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosako K, Lee VM-Y, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetove-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies. Third report of the DLB consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM-Y. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, Lee VM-Y. A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson’s disease. J Neurosci Res. 2000;59:528–533. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Gur TL, Montine TJ, Robertson D, Biaggioni I, Hurtig HI, Stern MB, Gollomp SM, Grossman M, Lee VM-Y, Trojanowski JQ. Immunohistochemical and biochemical studies demonstrate a distinct profile of alpha-synuclein permutations in multiple system atrophy. J Neuropathol Exp Neurol. 2000;59:830–841. doi: 10.1093/jnen/59.9.830. [DOI] [PubMed] [Google Scholar]
- Schmidt ML, Murray J, Lee VM-Y, Hill WD, Wertkin A, Trojanowski JQ. Epitope map of neurofilament protein domains in cortical and peripheral nervous system Lewy bodies. Am J Pathol. 1991;139:53–65. [PMC free article] [PubMed] [Google Scholar]
- Forman MS, Schmidt ML, Kasturi S, Perl DP, Lee VM-Y, Trojanowski JQ. Tau and alpha-synuclein pathology in amygdala of Parkinsonism-dementia complex patients of Guam. Am J Pathol. 2002;160:1725–1731. doi: 10.1016/s0002-9440(10)61119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M, Lennox G, Lowe J. Motor neurone disease-inclusion dementia. Neurodegeneration. 1996;5:339–350. doi: 10.1006/neur.1996.0046. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Hirai S, Yamazaki T, Sun XY, Nakazato Y. New ubiquitin-positive intraneuronal inclusions in the extra-motor cortices in patients with amyotrophic lateral sclerosis. Neurosci Lett. 1991;129:233–236. doi: 10.1016/0304-3940(91)90469-a. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Murakami N, Kusaka H, Yoshida M, Hashizume Y, Nakazato Y, Matsubara E, Hirai S. Ubiquitin-positive intraneuronal inclusions in the extramotor cortices of presenile dementia patients with motor neuron disease. J Neurol. 1992;239:426–430. doi: 10.1007/BF00856806. [DOI] [PubMed] [Google Scholar]
- Yaguchi M, Okamoto K, Nakazato Y. Frontotemporal dementia with cerebral intraneuronal ubiquitin-positive inclusions but lacking lower motor neuron involvement. Acta Neuropathol (Berl) 2003;105:81–85. doi: 10.1007/s00401-002-0611-z. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Feldman H. Neuronal intranuclear inclusions distinguish familial FTD-MND type from sporadic cases. Acta Neuropathol (Berl) 2003;105:543–548. doi: 10.1007/s00401-003-0678-1. [DOI] [PubMed] [Google Scholar]
- Katsuse O, Dickson DW. Ubiquitin immunohistochemistry of frontotemporal lobar degeneration differentiates cases with and without motor neuron disease. Alzheimer Dis Assoc Disord. 2005;19(Suppl 1):S37–S43. doi: 10.1097/01.wad.0000183889.61421.a8. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Feldman HH. Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia, and frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. J Neuropathol Exp Neurol. 2005;64:730–739. doi: 10.1097/01.jnen.0000174335.27708.0a. [DOI] [PubMed] [Google Scholar]
- Kovach MJ, Waggoner B, Leal SM, Gelber D, Khardori R, Levenstien MA, Shanks CA, Gregg G, Al Lozi MT, Miller T, Rakowicz W, Lopate G, Florence J, Glosser G, Simmons Z, Morris JC, Whyte MP, Pestronk A, Kimonis VE. Clinical delineation and localization to chromosome 9p13.3-p12 of a unique dominant disorder in four families: hereditary inclusion body myopathy, Paget disease of bone, and frontotemporal dementia. Mol Genet Metab. 2001;74:458–475. doi: 10.1006/mgme.2001.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts GD, Wymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–381. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- Schroder R, Watts GD, Mehta SG, Evert BO, Broich P, Fliessbach K, Pauls K, Hans VH, Kimonis V, Thal DR. Mutant valosin-containing protein causes a novel type of frontotemporal dementia. Ann Neurol. 2005;57:457–461. doi: 10.1002/ana.20407. [DOI] [PubMed] [Google Scholar]
- Hosler BA, Siddique T, Sapp PC, Sailor W, Huang MC, Hossain A, Daube JR, Nance M, Fan C, Kaplan J, Hung WY, McKenna-Yasek D, Haines JL, Pericak-Vance MA, Horvitz HR, Brown RH., Jr Linkage of familial amyotrophic lateral sclerosis with frontotemporal dementia to chromosome 9q21–q22. JAMA. 2000;284:1664–1669. doi: 10.1001/jama.284.13.1664. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadnovick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey A, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M: Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006, (in press) [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vanderberghe R, Dermaut B, Martin J, van Duijn C, Peeters K, Sciot R, Santerns P, De Pooter T, Mattheijssens M, van den Broeck M, Cuijt I, Vennekens K, De Deyn P, Kumar-Singh S, Van Broeckhoven C: Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006, (in press) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.