Abstract
UVB irradiation of the skin induces erythema, epidermal hyperplasia, vascular hyperpermeability, and edema formation. Previous studies have revealed that the cutaneous blood vasculature plays a critical role in the mediation of photodamage. In contrast, the role of lymphatic vessels, which play an essential role in the maintenance of tissue fluid balance, in the response to UVB irradiation has remained unknown. We report here that both acute and chronic UVB irradiation of murine skin results in prominent enlargement of lymphatic vessels. Surprisingly, these enlarged lymphatic vessels were functionally impaired and hyperpermeable, as detected by intravital lymphangiography. The expression levels of vascular endothelial growth factor (VEGF)-A but not of the known lymphangiogenesis factors VEGF-C or VEGF-D, were enhanced in UVB-irradiated epidermis. Targeted overexpression of VEGF-A in the epidermis of transgenic mice led to increased enlargement and leakage of lymphatic vessels after acute UVB irradiation, whereas systemic blockade of VEGF-A signaling largely prevented lymphatic vessel abnormalities and photodamage induced by UVB. Together, these findings identify lymphatic vessels as novel targets for UVB-induced cutaneous photodamage and suggest that VEGF-A mediates impairment of lymphatic vessel function, thereby contributing to the adverse effects of UVB irradiation on the skin.
Chronic UVB irradiation (290 to 320 nm) of the skin results in the degradation of matrix macromolecules, elastosis,1,2 and enhanced risk for skin cancer.3 In contrast, acute exposure of human skin to UVB leads to epidermal hyperplasia, erythema, vascular hyperpermeability, and edema formation.3,4 Our previous studies have revealed that pronounced angiogenesis is induced by acute UVB irradiation of human and mouse skin.5,6 Several angiogenesis factors, including vascular endothelial growth factor-A (VEGF-A), basic fibroblast growth factor, and interleukin-8 have been found to be up-regulated in UVB-irradiated skin,7–9 whereas the expression of thrombospondin-1, a potent endogenous angiogenesis inhibitor, was strongly inhibited by UVB irradiation.10 Recently, we have shown that targeted overexpression of VEGF-A enhances sensitivity to UVB-induced cutaneous photodamage,11 whereas transgenic overexpression of the angiogenesis inhibitor thrombospondin-1 in the epidermis completely prevented UVB-induced damage.6 Together, these findings indicate that the cutaneous blood vasculature plays a critical role in the mediation of photodamage. In contrast, the role of lymphatic vessels in the response to UVB irradiation has remained completely unknown.
The lymphatic vascular system is composed of a dense network of thin-walled capillaries that drain protein-rich lymph from the extracellular space. Its major roles include the maintenance of tissue fluid homeostasis and mediation of the afferent immune response.12,13 Recent studies have identified VEGF-C and VEGF-D as specific lymphangiogenesis factors, acting via activation of the VEGF receptor-3 (VEGFR-3), which is specifically expressed on lymphatic endothelial cells.14 Targeted overexpression of soluble VEGFR-3, which prevents VEGF-C and VEGF-D from reaching their receptor on lymphatic endothelium, in the epidermis of transgenic mice leads to lymphedema.15 More recently, VEGF-A and hepatocyte growth factor have also been shown to promote lymphatic vessel formation.16–18 In addition, several specific lymphatic markers have been recently identified, including the homeobox transcription factor Prox1, the hyaluronan receptor LYVE-1, and the mucin-type transmembrane glycoprotein podoplanin.12 Because one of the major functions of lymphatic vessels is the drainage of tissue fluid from normal and inflamed tissues,19 we hypothesized that they might also play a functional role in the cutaneous response to UVB-induced skin damage.
Here, we report that both acute and chronic UVB irradiation of the skin results in prominent enlargement of lymphatic vessels. Surprisingly, these enlarged lymphatic vessels are functionally impaired and are hyperpermeable. Expression levels of VEGF-A, but not of VEGF-C or VEGF-D, were enhanced in UVB-irradiated epidermis. Targeted overexpression of VEGF-A in the epidermis of transgenic mice led to increased enlargement and leakiness of lymphatic vessels after acute UVB irradiation, whereas systemic blockade of VEGF-A signaling largely prevented lymphatic vessel abnormalities and photodamage induced by UVB. Together, these findings indicate that lymphatic vessels may serve as novel targets for UVB-induced cutaneous photodamage and that VEGF-A-mediated impairment of lymphatic vessel function contributes to the adverse effects of UVB irradiation on the skin.
Materials and Methods
UVB Irradiation Regimen
Female hairless Skh1 mice (8 weeks old) were exposed to UVB irradiation using a bank of four equally spaced fluorescence lamps (Southern New England UV, Bradford, CT), as described.20 Mice (n = 7 per group) were sham-irradiated or exposed to UVB irradiation three times a week for 10 weeks with a starting dose of 40 mJ/cm2 (0.5 minimal erythema dose; MED) and gradual increases in increments of 0.5 MED to a maximum dose of 4.5 MED. The total cumulative dose of UVB was 5.46 J/cm2. No acute sunburn reactions were observed. In additional studies, 8-week-old female Skh1 mice, FVB transgenic mice with skin-specific overexpression of murine VEGF164 under control of the K14 promoter,21 or wild-type (WT) FVB mice (n = 5 per group) were irradiated with a single dose of 40 mJ/cm2 (0.5 MED) or 80 mJ/cm2 (1 MED) UVB. In addition, WT mice (n = 5/group) were treated with 50 μg of an anti-mouse VEGF neutralizing antibody (R&D Systems, Minneapolis, MN) or with 50 μg of control IgG by intraperitoneal injection at 24 hours before exposure to 54 mJ/cm2 UVB irradiation as described.11 All animal studies were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Intravital Lymphangiography
Mice (n = 7/group) were anesthetized by using avertin (0.4 g/kg; Sigma, St. Louis, MO), and 1 μl of a 1% solution of Evans blue dye in 0.9% NaCl was injected intradermally at the inner surface of the rim of the ear, using a 10-μl Hamilton syringe to visualize the lymphatic vessels. Injection of Evans blue is a standard method to macroscopically visualize cutaneous lymphatic vessels and lymphatic drainage from the skin.22–24 Mouse ears were photographed at 1, 3, and 5 minutes after the dye injection. We chose these time points because previous studies had revealed that widespread filling of the lymphatic network of the ear skin occurred within 4 minutes after tracer injection.25
Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total cellular RNA was isolated from the whole back skin of mice at 2 days after the last UVB irradiation (n = 7/group), using the TRIzol reagent (Invitrogen, Carlsbad, CA) followed by treatment with RQ1 RNase-free DNase (Promega, Madison, WI), and the whole skin was homogenized using Tissue Lyser (Qiagen GmbH, Hilden, Germany). The expression levels of VEGF-A, VEGF-C, and VEGF-D mRNA were investigated by quantitative real-time RT-PCR, using the ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) as described.26 The primers and probes for murine VEGF-A, VEGF-C, and VEGF-D were described previously.19 Each reaction was multiplexed with glyceralaldehyde-3-phosphate dehydrogenase (GAPDH) primers (forward 5′-TCACTGGCATGGCCTTCC-3′, reverse 5′-GGCGGGCACGTCAGATCCA-3′), and probe (5′-JOE-TTCCTACCCCCAATGTGTCCGTCG-TAMRA-3′) as an internal control.
Immunostains and Computer-Assisted Morphometric Vessel Analysis
Immunofluorescence analyses were performed on 6-μm cryostat sections of mouse tissues using polyclonal antibodies against murine LYVE-1 (kindly provided by Dr. D. Jackson, Oxford, UK), murine CD31 (BD Biosciences, Pharmingen, San Diego, CA), and corresponding secondary antibodies labeled with Alexa Fluor488 or Alexa Fluor594 (Molecular Probes, Eugene, OR).17 Sections were examined by a Nikon E-600 microscope (Nikon, Melville, NY), and images were captured with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI). Morphometric analyses were performed using IP-LAB software (Scanalytics, Fairfax, VA) as described.11 Three different fields of each section were examined, and the number of vessels per square micrometer, the average vessel size, and the relative tissue area occupied by lymphatic vessels were determined in the dermis in an area within 200-μm distance from the epidermal-dermal junction. The unpaired Student’s t-test was used to analyze differences in microvessel density and size. In addition, routine hematoxylin and eosin stains and immunohistochemistry with a polyclonal anti-rat VEGF-C antibody (Abcam, Cambridge, UK) were performed as described previously.10,27
Results
Chronic UVB Irradiation Leads to Enlargement of Cutaneous Lymphatic Vessels
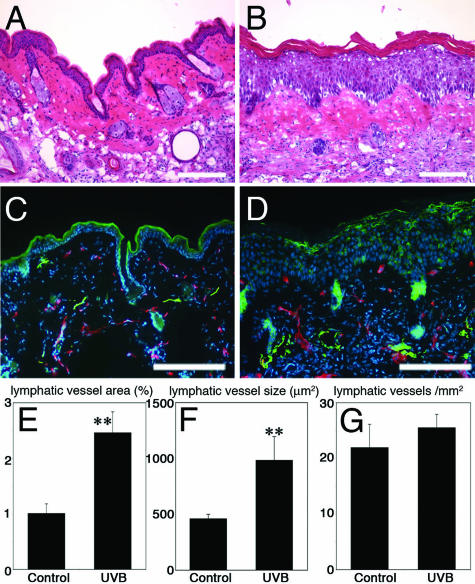
To investigate the effect of UVB irradiation on the lymphatic vasculature, we first performed chronic UVB irradiation of Skh 1 hairless mice28 with a cumulative dose of 5.46 J/cm2 throughout a period of 10 weeks. Chronic UVB irradiation resulted in pronounced wrinkle formation as previously reported,6 whereas no such changes were detected in sham-irradiated mice (data not shown). Histological examination revealed the typical signs of chronic UVB damage, including epidermal hyperplasia, inflammatory cell infiltration, and edema formation (Figure 1, A and B). Double-immunofluorescence stains for the panvascular marker CD31 and for the lymphatic-specific hyaluronan receptor LYVE-1 revealed moderate enlargement of CD31+/LYVE-1− blood vessels (Figure 1, C and D), in agreement with previous studies.5 Surprisingly, LYVE-1-positive cutaneous lymphatic vessels were dramatically increased in size, with irregular shapes and sometimes incomplete endothelial cell lining of the vessel walls, whereas only normal, collapsed lymphatic vessels were found in sham-irradiated mice (Figure 1, C and D). Computer-assisted morphometric analyses confirmed that the average dermal area occupied by lymphatic vessels (2.5-fold increase over sham-irradiated mice, P < 0.01) and the average size of dermal lymphatic vessels (2.1-fold increase, P < 0.01) were significantly increased in UVB-irradiated skin compared with sham-irradiated mice (Figure 1, E and F). In contrast, the density of lymphatic vessels was comparable in both groups (Figure 1G).
FIGURE 1.
Enlargement of lymphatic vessels after chronic UVB irradiation of the skin. A and B: Histological examination revealed epidermal hyperplasia, inflammatory cell infiltration, and edema in UVB-irradiated skin (B) but not in sham-irradiated skin (A). H&E stains. C and D: Double-immunofluorescence stains for CD31 (red) and for LYVE-1 (green) revealed moderate enlargement of CD31+/LYVE-1− blood vessels in UVB-irradiated skin (D). LYVE-1-positive cutaneous lymphatic vessels were greatly increased in size, with irregular shapes and sometimes incomplete endothelial cell lining, whereas only normal, collapsed lymphatic vessels were found in sham-irradiated mice (C). Morphometric analyses revealed that the area covered by lymphatic vessels (E) and the average size of the lymphatic vessels (F) were significantly increased in UVB-irradiated skin as compared with sham-irradiated skin (P < 0.01), whereas the density of lymphatic vessels was comparable in both groups (G). Scale bars = 100 μm.
Impaired Function and Increased Leakiness of Lymphatic Vessels after Chronic UVB Irradiation
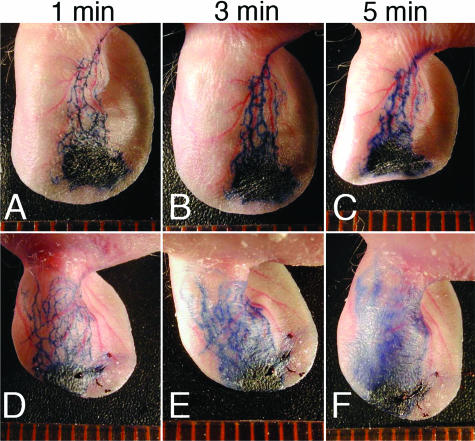
To further investigate the effects of chronic UVB irradiation on the function of cutaneous lymphatic vessels, Evans blue dye was injected intradermally into the rim of mouse ears. At 1 and 3 minutes after ink injection, markedly dilated lymphatic vessels were visualized in chronically UVB-irradiated mouse skin compared with sham-irradiated mice (Figure 2, A, B, D, and E). After 5 minutes, Evans blue dye had extravasated from lymphatic vessels in chronically UVB-irradiated skin, whereas no such leakage was observed in sham-irradiated mice (Figure 2, C and F). These findings indicate that the enlarged lymphatic vessels after UVB irradiation are leaky and functionally impaired.
FIGURE 2.
Enhanced leakiness of lymphatic vessels in chronic UVB-irradiated skin. Evans blue dye was injected intradermally into the rim of mouse ears. At 1 and 3 minutes after ink injection, markedly dilated lymphatic vessels were visualized in chronically UVB-irradiated mouse skin (D, E), as compared with sham-irradiated mice (A, B). After 5 minutes, Evans blue dye had extravasated from lymphatic vessels in chronically UVB-irradiated skin (F), whereas no such leakage was observed in sham-irradiated mice (C).
Chronic UVB Irradiation Leads to Up-Regulation of VEGF-A, but Not of VEGF-C or VEGF-D Expression
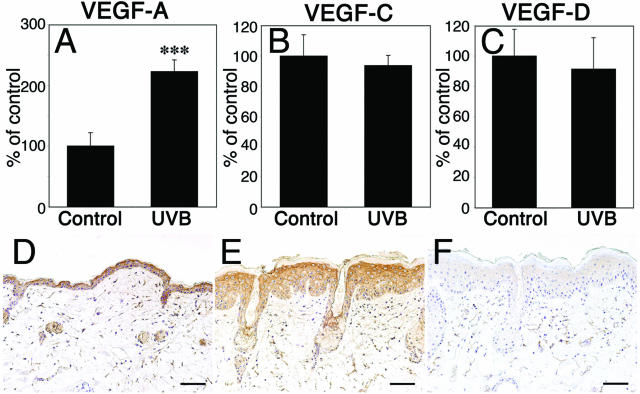
To investigate whether chronic UVB irradiation resulted in up-regulation of any of the known lymphangiogenesis factors, VEGF-A,25 VEGF-C, or VEGF-D14 by epidermal keratinocytes, we next isolated total RNAs from the epidermis of the ear skin, followed by quantitative real-time TaqMan RT-PCR analysis of mRNA expression. We found a 2.2-fold up-regulation of VEGF-A mRNA expression in UVB-irradiated epidermis, compared with sham-irradiated controls (P < 0.01; Figure 3A). In contrast, VEGF-C and VEGF-D mRNA expression levels were comparable in both groups (Figure 3, B and C). Comparable results were obtained when total RNA isolated from whole skin lysates, including both epidermis and dermis, was examined (data not shown). By immunohistochemistry, VEGF-C protein expression was detected at comparable levels in the normal and the UVB-irradiated epidermis (Figure 3, D–F).
FIGURE 3.
Up-regulation of VEGF-A, but not of VEGF-C or VEGF-D mRNA expression in chronic UVB-irradiated epidermis. A–C: Quantitative real-time TaqMan RT-PCR analysis of total RNAs isolated from the epidermis of the ear skin at 48 hours after the chronic UVB irradiation. VEGF-A mRNA expression was up-regulated in UVB-irradiated epidermis compared with sham-irradiated controls (A, P < 0.01). In contrast, VEGF-C and VEGF-D mRNA expression levels were comparable in both groups (B, C). D–F: By immunohistochemistry, VEGF-C protein was detected at comparable levels in the sham-irradiated (D) and the UVB-irradiated epidermis (E). F: Absence of specific staining after omission of the primary antibody. Scale bars = 100 μm.
Acute UVB Irradiation Induces Enlargement and Hyperpermeability of Cutaneous Lymphatic Vessels
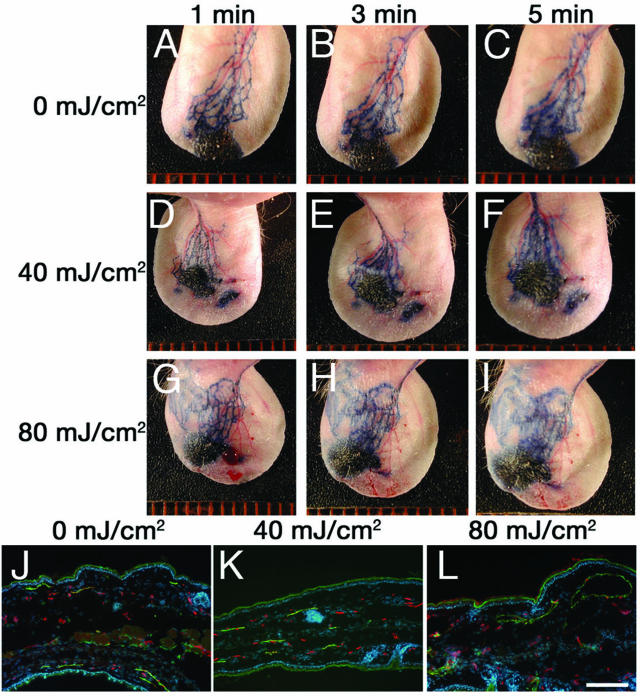
We next investigated whether a single dose of acute UVB irradiation might be sufficient to impair lymphatic vessel function. We studied lymphatic function at 2 days after irradiation. This time point was chosen because our previous studies with this acute UVB irradiation model revealed that up-regulation of VEGF-A and down-regulation of thrombospondin-1, as well as the UVB-induced histological alterations, were most pronounced at 2 days after irradiation.10 Two days after a single dose of 40 mJ/cm2 UVB irradiation (0.5 minimal erythema dose; MED), visualization of cutaneous lymphatic vessels by intradermal Evans blue dye injection revealed that the lymphatic vessels were indistinguishable from those in nonirradiated skin and that they were not hyperpermeable (Figure 4, A–F). In contrast, irradiation with a dose of 80 mJ/cm2 of UVB (1 MED) resulted in enlargement of cutaneous lymphatic vessels (Figure 4, G and H), and pronounced leakiness was observed (Figure 4I). Differential immunofluorescence analyses of skin sections revealed a dramatic enlargement of LYVE-1+ lymphatic vessels, but only moderate enlargement of CD31+/LYVE-1− blood vessels, after UVB irradiation with 80 mJ/cm2 (Figure 4L). However, no such changes were observed in nonirradiated skin or in skin irradiated with a UVB dose of 40 mJ/cm2 (Figure 4, J and K).
FIGURE 4.
Acute UVB irradiation induces enlargement and hyperpermeability of cutaneous lymphatic vessels. Two days after a single dose of 40 mJ/cm2 UVB irradiation (0.5 MED), visualization of cutaneous lymphatic vessels by intradermal Evans blue dye injection (D–F) revealed that the lymphatic vessels were indistinguishable from those in nonirradiated skin (A–C). Irradiation with a dose of 80 mJ/cm2 UVB (1 MED) resulted in enlargement of cutaneous lymphatic vessels (G, H), and pronounced leakiness (I). Differential immunofluorescence analyses of skin sections for LYVE-1 (green) and CD31 (red) revealed marked enlargement of LYVE-1+ lymphatic vessels after UVB irradiation with 80 mJ/cm2 (L). No such changes were observed in nonirradiated skin (J) or in skin irradiated with 40 mJ/cm2 (K). Scale bar = 100 μm.
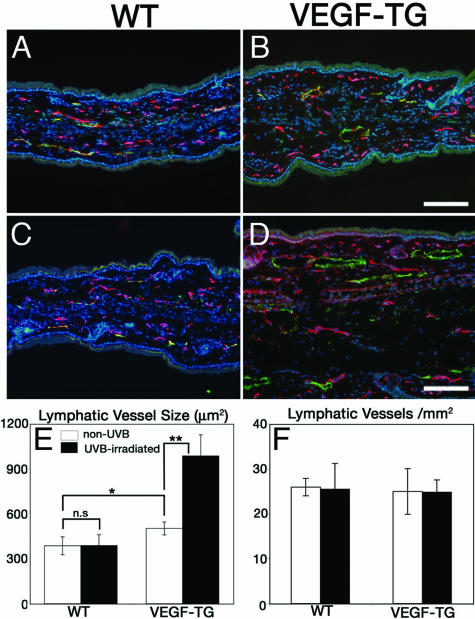
Increased Enlargement of Lymphatic Vessels in UVB-Irradiated VEGF-A Transgenic Mice
Because we found that the mRNA expression of VEGF-A was up-regulated by epidermal keratinocytes after UVB irradiation, we next investigated whether epidermis-derived VEGF-A might directly promote the UVB-induced enlargement and leakiness of lymphatic vessels. Therefore, transgenic FVB mice that overexpress murine VEGF-A164 under control of the keratin 14 promoter21 and FVB WT mice were exposed to a single dose of 40 mJ/cm2 UVB—previously shown to induce ear swelling in VEGF-A transgenic mice11—or were sham-irradiated. Differential immunofluorescence analyses of ear sections at 2 days after UVB irradiation revealed comparable numbers and sizes of CD31+/LYVE-1− blood vessels and of LYVE-1+ lymphatic vessels in sham-irradiated and UVB-irradiated WT mice (Figure 5, A and C). Sham-irradiated VEGF-A transgenic mice already showed a slight increase in lymphatic vessel size compared with WT mice (Figure 5B). Importantly, lymphatic vessels were dramatically enlarged after UVB irradiation of VEGF-A transgenic mice (Figure 5D), associated with increased ear thickness and edema formation. Computer-assisted morphometric analyses confirmed a 1.3-fold increase of lymphatic vessel size in sham-irradiated VEGF transgenic mice compared with WT mice (P < 0.05) and a further significant 1.9-fold enlargement after UVB irradiation (P < 0.01; Figure 5E). The density of lymphatic vessels was comparable in all groups (Figure 5F).
FIGURE 5.
Increased enlargement of lymphatic vessels in UVB-irradiated VEGF-A transgenic mice. VEGF-A transgenic mice and WT mice were exposed to a single dose of 40 mJ/cm2 UVB irradiation (C, D) or were sham-irradiated (A, B). At 2 days after UVB irradiation, double-immunofluorescence stains of ear sections for CD31 (red) and LYVE-1 (green) revealed comparable numbers and sizes of CD31+/LYVE-1− blood vessels and of LYVE-1+ lymphatic vessels in sham-irradiated and UVB-irradiated WT mice (A, C). Sham-irradiated VEGF transgenic mice showed a slight increase in lymphatic vessel size (B) as compared with WT mice (A). Lymphatic vessels were dramatically enlarged in UVB-irradiated VEGF transgenic mice (D), associated with edema formation. E: Morphometric analyses showed a 1.3-fold increase of lymphatic vessel size in sham-irradiated VEGF transgenic mice compared with WT mice (P < 0.05), and a 1.9-fold enlargement after UVB irradiation (P < 0.01). F: The density of lymphatic vessels was comparable in all groups. Scale bars = 100 μm.
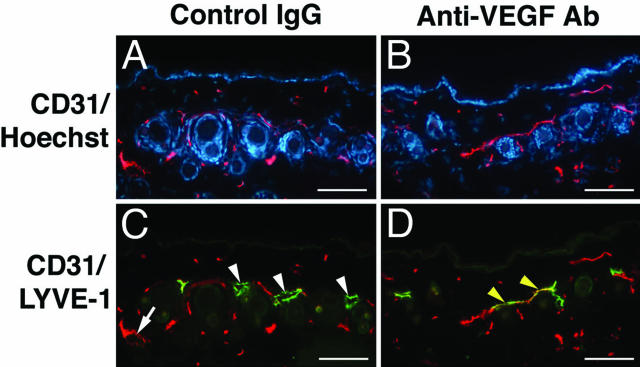
Systematic Blockade of VEGF-A Inhibits the UVB-Induced Enlargement of Lymphatic Vessels
We next investigated whether systemic blockade of VEGF-A might prevent the UVB-induced lymphatic vessel enlargement. WT FVB mice were exposed to a single dose of 54 mJ/cm2 UVB irradiation11 1 day after intraperitoneal injection of a blocking antibody against VEGF-A or of an equal amount of control IgG. Double-immunofluorescence stains for CD31 and LYVE-1 at 2 days after UVB irradiation demonstrated pronounced enlargement of LYVE-1-positive lymphatic vessels, as well as moderate dilation of CD31+/LYVE-1− blood vessels in mice treated with control IgG (Figure 6, A and C). In contrast, systemic treatment with a VEGF-A blocking antibody completely prevented the enlargement of lymphatic and blood vessels (Figure 6, B and D).
FIGURE 6.
Blockade of VEGF-A inhibits the UVB-induced enlargement of lymphatic vessels. WT mice were exposed to a single dose of 54 mJ/cm2 UVB irradiation 1 day after intraperitoneal injection of a blocking antibody against VEGF-A or control IgG. A and C: Double-immunofluorescence stains for CD31 (red) and LYVE-1 (green) at 2 days after UVB irradiation reveal enlargement of LYVE-1-positive lymphatic vessels (arrowheads), as well as moderate dilation of CD31+/LYVE-1− blood vessels (arrow) in mice treated with control IgG. B and D: Anti-VEGF-A treatment prevented the enlargement of lymphatic and blood vessels. Scale bars = 100 μm.
Discussion
Our results reveal that UVB irradiation of the skin leads to enlargement and leakage of lymphatic vessels and that UVB-induced VEGF-A plays an important role in mediating the impairment of lymphatic function that is associated with edema formation—in addition to its known function as a permeability factor for blood vessels. Thus, VEGF-A appears to promote UVB-induced edema formation by exerting two distinct but synergistic effects, ie, promotion of fluid leakage from cutaneous blood vessels, and impairment of fluid drainage via lymphatic vessels. In support of these conclusions, systemic blockade of VEGF-A inhibited enlargement of lymphatic and blood vessels and reduced the UVB-induced cutaneous damage.
UVB-induced cutaneous photodamage is characterized by epidermal hyperplasia, degradation of matrix proteins, and dermal elastosis.29 Edema formation is one of the most prominent features of UVB-induced photodamage and has been attributed to the increased permeability of blood vessels. Surprisingly, we also found enhanced leakiness of the lymphatic vascular system in UVB-irradiated skin. Under normal conditions, most lymphatic vessels of the skin are collapsed. In the presence of increased interstitial fluid pressure, often caused by increased leakage from blood vessels, lymphatic vessels are pulled open by anchoring filaments that directly connect the lymphatic endothelial cells with elastic fibers in the extracellular matrix.30 This is thought to facilitate fluid drainage via the lymphatic vascular system. However, overextension of lymphatic vessels can also cause impaired fluid transport function,30 therefore contributing to enhanced edema formation. Our studies indicate that both vascular hyperpermeability and reduced lymphatic drainage can be caused by the same molecule, VEGF-A, that has been shown to be potently up-regulated in epidermal keratinocytes, both in vitro and in vivo, after UVB irradiation.5,9 These findings are in agreement with our recent results that cultured lymphatic endothelial cells react to VEGF-A stimulation via VEGFR-218 and that VEGF-A transgenic mice develop persistent lymphatic vessel enlargement after experimental induction of skin inflammation.19 They are also in agreement with previous findings that adenoviral delivery of the murine VEGF-A164 gene to mouse skin results in the increased formation of enlarged, nonfunctional lymphatic vessels.25 It is of interest that injection of Evans blue dye into UVB-irradiated skin resulted in the visualization of more superficial lymphatic vessels than in the nonirradiated skin. This is likely attributable to impaired Evans blue drainage via deeper lymphatic vessels, with a resulting backflow to superficial lymphatics.24 Taken together, our results suggest a new concept for the biological mediation of UVB-induced damage, with an important functional role of lymphatic vessels, in addition to blood vessels.
How can we improve lymphatic function in the skin and/or prevent UVB-induced lymphatic impairment? Previously, VEGF-C and VEGF-D have been identified as potent lymphangiogenesis factors, acting via VEGFR-3 and, likely, VEGFR-2 on lymphatic endothelium.14 In fact, blockade of VEGF-C and VEGF-D via transgenic overexpression of a soluble VEGFR-3 resulted in lymphedema, and transgenic overexpression of VEGF-C or of a mutant VEGF-C that only activates VEGFR-3 led to increased lymphatic vascularization of the skin.15,31 Results obtained in tissue repair models indicate that VEGF-C indeed promotes the enhanced formation of functional lymphatic vessels that efficiently drain lymph fluid from the skin,32 although a recent report found no long-term effects of VEGF-C application.33 Because we did not detect any major changes of VEGF-C or VEGF-D expression after UVB irradiation, the predominant influence of VEGF-A over VEGF-C and VEGF-D signaling might lead to the functional lymphatic impairment observed after UVB irradiation of the skin. This concept is further supported by our recent findings that blockade of VEGFR-3 prolonged UVB-induced edema formation and inflammation.34 Further studies are needed to investigate whether enhanced production of VEGF-C (or VEGF-D) may counteract these effects of VEGF-A in cutaneous photodamage or in skin inflammation and might ameliorate UVB-induced lymphatic impairment and edema formation. Moreover, additional modulators of lymphatic function, including hepatocyte growth factor, angiopoietin-1 and -2, ephrin B2, and podoplanin, have been recently identified,17,35–38 and it will be of great interest to investigate their modulation after UVB irradiation and their biological impact on the photodamage reaction. Finally, future investigations are needed to study whether UVB-induced impairment of lymphatic vessel function might facilitate the metastatic spread of skin cancers, such as malignant melanomas, to sentinel lymph nodes and beyond.18,39
Acknowledgments
We thank B. Ma, M. Min, and M. Constant for expert technical assistance.
Footnotes
Address reprint requests to Michael Detmar, M.D., Institute of Pharmaceutical Sciences, Swiss Federal Institute of Technology, ETH Zurich, Wolfgang-Pauli-Str. 10, HCI H303, CH-8093 Zurich, Switzerland. E-mail: michael.detmar@pharma.ethz.ch.
Supported by the National Institutes of Health (grants CA69184, CA86410, and CA92644 to M.D.), the American Cancer Society (research project grant 99-23901 to M.D.), the Swiss National Fund (to M.D.), the Austrian Science Foundation (grant S9408-B11 to M.D.), and the Cutaneous Biology Research Center through the Massachusetts General Hospital/Shiseido Co. Ltd. Agreement (to M.D.).
References
- Kligman AM. The treatment of photoaged human skin by topical tretinoin. Drugs. 1989;38:1–8. doi: 10.2165/00003495-198938010-00001. [DOI] [PubMed] [Google Scholar]
- Leyden JJ, Grove GL, Grove MJ, Thorne EG, Lufrano L. Treatment of photodamaged facial skin with topical tretinoin. J Am Acad Dermatol. 1989;21:638–644. doi: 10.1016/s0190-9622(89)70231-0. [DOI] [PubMed] [Google Scholar]
- Kripke ML. Ultraviolet radiation and immunology: something new under the sun—presidential address. Cancer Res. 1994;54:6102–6105. [PubMed] [Google Scholar]
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315–319. doi: 10.1111/j.1365-2133.1992.tb00671.x. [DOI] [PubMed] [Google Scholar]
- Yano K, Kadoya K, Kajiya K, Hong YK, Detmar M. Ultraviolet B irradiation of human skin induces an angiogenic switch that is mediated by upregulation of vascular endothelial growth factor and by downregulation of thrombospondin-1. Br J Dermatol. 2005;152:115–121. doi: 10.1111/j.1365-2133.2005.06368.x. [DOI] [PubMed] [Google Scholar]
- Yano K, Oura H, Detmar M. Targeted overexpression of the angiogenesis inhibitor thrombospondin-1 in the epidermis of transgenic mice prevents ultraviolet-B-induced angiogenesis and cutaneous photo-damage. J Invest Dermatol. 2002;118:800–805. doi: 10.1046/j.1523-1747.2002.01752.x. [DOI] [PubMed] [Google Scholar]
- Kramer M, Sachsenmaier C, Herrlich P, Rahmsdorf HJ. UV irradiation-induced interleukin-1 and basic fibroblast growth factor synthesis and release mediate part of the UV response. J Biol Chem. 1993;268:6734–6741. [PubMed] [Google Scholar]
- Strickland I, Rhodes LE, Flanagan BF, Friedmann PS. TNF-alpha and IL-8 are upregulated in the epidermis of normal human skin after UVB exposure: correlation with neutrophil accumulation and E-selectin expression. J Invest Dermatol. 1997;108:763–768. doi: 10.1111/1523-1747.ep12292156. [DOI] [PubMed] [Google Scholar]
- Bielenberg DR, Bucana CD, Sanchez R, Donawho CK, Kripke ML, Fidler IJ. Molecular regulation of UVB-induced cutaneous angiogenesis. J Invest Dermatol. 1998;111:864–872. doi: 10.1046/j.1523-1747.1998.00378.x. [DOI] [PubMed] [Google Scholar]
- Yano K, Kajiya K, Ishiwata M, Hong YK, Miyakawa T, Detmar M. Ultraviolet B-induced skin angiogenesis is associated with a switch in the balance of vascular endothelial growth factor and thrombospondin-1 expression. J Invest Dermatol. 2004;122:201–208. doi: 10.1046/j.0022-202X.2003.22101.x. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Fujii S, Kajiya K, Yano K, Detmar M. Vascular endothelial growth factor promotes sensitivity to ultraviolet B-induced cutaneous photodamage. Blood. 2005;105:2392–2399. doi: 10.1182/blood-2004-06-2435. [DOI] [PubMed] [Google Scholar]
- Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- Witte MH, Bernas MJ, Martin CP, Witte CL. Lymphangiogenesis and lymphangiodysplasia: from molecular to clinical lymphology. Microsc Res Tech. 2001;55:122–145. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- Jussila L, Alitalo K. Vascular growth factors and lymphangiogenesis. Physiol Rev. 2002;82:673–700. doi: 10.1152/physrev.00005.2002. [DOI] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Liersch R, Nay F, Lu L, Detmar M. Induction of lymphatic endothelial cell differentiation in embryoid bodies. Blood. 2006;107:1214–1216. doi: 10.1182/blood-2005-08-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, Wu Y, Hicklin D, Bohlen P, Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–1057. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- Kochevar IE, Moran M, Lyon N, Flotte T, Siebert E, Gange RW. Effects of systemic indomethacin, meclizine, and BW755C on chronic ultraviolet B-induced effects in hairless mouse skin. J Invest Dermatol. 1993;100:186–193. doi: 10.1111/1523-1747.ep12462804. [DOI] [PubMed] [Google Scholar]
- Detmar M, Brown LF, Schon MP, Elicker BM, Velasco P, Richard L, Fukumura D, Monsky W, Claffey KP, Jain RK. Increased microvascular density and enhanced leukocyte rolling and adhesion in the skin of VEGF transgenic mice. J Invest Dermatol. 1998;111:1–6. doi: 10.1046/j.1523-1747.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Heath TJ, Brandon RA, Norman ST. Drainage of lymph from the foreleg to the superficial cervical lymph node in sheep. Res Vet Sci. 1984;37:66–71. [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Foreman K, Shin JW, Hirakawa S, Curry CL, Sage DR, Libermann T, Dezube BJ, Fingeroth JD, Detmar M. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat Genet. 2004;36:683–685. doi: 10.1038/ng1383. [DOI] [PubMed] [Google Scholar]
- Prophet EMB, Arrington J, Sobin L. Laboratory Methods in Histotechnology. American Registry of Pathology: Washington, DC,; 1992:pp 53–55. [Google Scholar]
- Kligman LH. The hairless mouse model for photoaging. Clin Dermatol. 1996;14:183–195. doi: 10.1016/0738-081x(95)00154-8. [DOI] [PubMed] [Google Scholar]
- Kligman LH. Symposium on models for the study of human photoaging: American Society for Photobiology. Photochem Photobiol. 1989;50:903–905. doi: 10.1111/j.1751-1097.1989.tb02920.x. [DOI] [PubMed] [Google Scholar]
- Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J Invest Dermatol Symp Proc. 2000;5:14–19. doi: 10.1046/j.1087-0024.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Szuba A, Skobe M, Karkkainen MJ, Shin WS, Beynet DP, Rockson NB, Dakhil N, Spilman S, Goris ML, Strauss HW, Quertermous T, Alitalo K, Rockson SG. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 2002;16:1985–1987. doi: 10.1096/fj.02-0401fje. [DOI] [PubMed] [Google Scholar]
- Goldman J, Le TX, Skobe M, Swartz MA. Overexpression of VEGF-C causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circ Res. 2005;96:1193–1199. doi: 10.1161/01.RES.0000168918.27576.78. [DOI] [PubMed] [Google Scholar]
- Kajiya K, Detmar M. An important role of lymphatic vessels in the control of UVB-induced edema formation and inflammation. J Invest Dermatol. 2006;126:919–921. doi: 10.1038/sj.jid.5700126. [DOI] [PubMed] [Google Scholar]
- Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, Detmar M. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18:1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]